Abstract
Background:
Multiple sclerosis (MS) is a chronic autoimmune disease of the central nervous system, which often causes progressive neurological disability. In addition to motor and sensory dysfunction, cognitive decline and fatigue are frequent manifestations of the disease. Fatigue is probably the most common symptom, with up to 90% of MS individuals reporting fatigue at some point. Cognitive impairment affects about 50% of patients and may be present at all MS stages. The aim of this observational study was to evaluate changes in cognition, fatigue, and disability status in 300 relapsing–remitting MS (RRMS) patients, treated with subcutaneous (sc) interferon (IFN) β-1a over 2 years.
Methods:
The study was designed as an observational, multicentre, prospective, single-arm, phase IV study carried out in 13 MS centres in the Czech Republic. Cognition status was assessed using the Paced Auditory Serial Addition Task (PASAT), fatigue using the Fatigue Descriptive Scale (FDS), and disability using the Expanded Disability Status Scale (EDSS), at baseline, and after 6, 12 and 24 months. The percentage of patients with changed versus stable cognition, fatigue status and disability was calculated at each time point and the changes in these scores were evaluated.
Results:
The proportion of patients with cognitive improvement was higher compared with those with a stable or decreased PASAT scores at all time points, and the average cognitive performance improved during the follow-up period. Also the proportion of patients with stable or improved fatigue and EDSS scores was higher compared with those in which FDS or EDSS scores declined, this was found at all time points of the analysed sample. However, the direct effect of IFN β-1a on cognition and fatigue cannot be concluded from this study.
Conclusions:
The results of this observational study have demonstrated a stable or improved cognitive performance, fatigue status, and disability level in the majority of RRMS patients treated with sc IFN β-1a over a two-year follow-up period, in a real life setting, in the Czech Republic.
Keywords: cognitive impairment, fatigue, interferon β-1a, multiple sclerosis, treatment
Background
Multiple sclerosis (MS) is a chronic autoimmune disease of the central nervous system (CNS) leading to demyelination and axonal damage, with subsequent degeneration, which causes progressive neurological disability [Hartung et al. 2004]. In addition to motor and sensory dysfunctions, progression of cognitive decline and fatigue are frequent manifestations of the disease [Krupp et al. 2010; Lincoln et al. 2002].
Fatigue is probably the most common symptom of MS, with the majority of individuals (up to 90%) reporting fatigue at some point in their disease course [Krupp et al. 2010; Lerdal et al. 2007]. Fatigue is characterized by a lack of energy, feelings of exhaustion that are unaided by sleep, and the perception that one is unable to perform mental and physical activities [Harrington, 2012]; it is also frequently associated with anxiety and depression [Van Kessel and Moss-Morris, 2006].
One of the most characteristic features is the fluctuation of symptoms, which is usually exacerbated by exercise or an increased in body/ambient temperature (Uhthoff symptom). Fatigue is often chronic and may occur with varying degrees of severity between mild and severely disabling [Fisk et al. 1994]. It is a significant symptom of MS that can negatively affect the patient’s quality of life and socio-economic functions, including the ability to work, the effects of which are independent of the direct effects of disability [Bakshi et al. 2000; Lobentanz et al. 2004; Merkelbach et al. 2002]. Fatigue can be classified as physical or mental, with the mental part affecting working memory, processing speed, and other activities that require maintenance of mental effort [Ferreira, 2010]. The pathogenesis of chronic fatigue syndrome is unknown. Dobryakova and colleagues [Dobryakova et al. 2013] proposed that fatigue is caused by a dopamine imbalance within the CNS.
Cognitive impairment often occurs in patients with MS, affecting between 43% and 70% of patients [Rao, 1995] and may be present at stages ranging from clinically isolated syndrome (CIS) to relapsing or progressive forms of MS [Amato et al. 2006, 2010; Glanz et al. 2007; Langdon, 2011; Patti, 2009].
The pattern of cognitive impairment in MS is characterized by deficits in sustained attention, verbal and nonverbal memory, conceptual reasoning, and information processing speed, while language and intellectual functions are preserved [Amato et al. 2006; Rao, 1995]. The factors that have been shown to influence cognitive impairment in MS are disease course and duration [Amato et al. 2006], affective disturbance, and medication [Rogers and Panegyres, 2007]. It appears to be strongly related to brain magnetic resonance imaging (MRI) parameters, especially atrophy [Filippi et al. 2010], grey matter (GM) lesions and GM atrophy [Klaver et al. 2013], which are present from the earliest stages of the disease and increase with disease progression. Recent studies have demonstrated significant GM demyelination and microglia activation [Klaver et al. 2013]. Candidate genetic risk factors, accelerated inflammatory processes [Benedict and Zivadinov, 2011] and dysregulated signalling pathways [Dutta et al. 2011] have been implicated as contributing factors. Rahn and colleagues [Rahn et al. 2012] found that right hippocampal NAAG/creatine (Cr) concentrations positively correlated with cognitive function in MS patients. Some studies have also described an association between cognitive impairment and fatigue [Andreasen et al. 2010; Cameron et al. 2014].
The most useful measurement of disability in MS patients is the Expanded Disability Status Scale (EDSS) [Kurtzke, 1983]. However, the scale provides only a rudimentary estimate of cognitive function [Marrie and Goldman, 2007; Meyer-Moock et al. 2014] and in many studies no correlations were found between cognitive deficits, fatigue, and disability when assessed using the EDSS [Bakshi et al. 2000; Polman and Rudick, 2010]. The reason is that the EDSS primarily evaluates motor functions and has low sensitivity relative to cognitive deficits.
Therefore, other scales for the assessment of fatigue and cognitive functions have been developed and recommended. A widely used test is the Paced Auditory Serial Addition Task (PASAT). The PASAT is a measure of cognitive function that specifically assesses auditory information processing speed and flexibility, as well as calculation ability. It was initially developed by Gronwall [Gronwall, 1977] to monitor recovery of patients who had sustained mild head injuries. It was adapted for use in MS patients [Rao et al. 1991] and has been widely used in MS studies during the last decade. This test has been used to monitor therapeutic efficacy of disease-modifying drugs (DMDs) on cognition [Fischer et al. 1999; Kappos et al. 2009].
Several scales have been developed to measure fatigue. One of them, the Fatigue Descriptive Scale (FDS) was developed by Krupp and colleagues [Krupp et al. 1989] and has been tested in patients with MS and systemic lupus erythematosus. The FDS evaluates and measures the severity of MS related fatigue, which is defined by some of its characteristics, and provides information regarding the impact of fatigue on daily living [Iriarte et al. 1999]. The FDS has been validated.
Material and methods
Study purpose and design
The purpose of this study was to evaluate cognition, fatigue, and neurological disability in relapsing–remitting MS (RRMS) patients treated with sc IFN β-1a (Rebif®) over two years. The study was designed as an observational, multicentre, single-arm, phase IV study carried out in 13 MS centres in the Czech Republic between March 2009 and June 2013.
Patient characteristics
The baseline group consisted of 300 White patients fulfilling the McDonald [McDonald et al. 2001] criteria for MS with a mean (standard deviation [SD]) age of 36.33 (10.04) years, of whom 198 (66.6%) were females. The mean (SD) duration of the disease was 61.6 (5.63) months, the mean annualized relapse rate (SD) was 1.15 (0.91) during the 2 years prior to baseline. Two patients discontinued prior to month 6, seven by month 12, and 19 by month 24. Overall, 272 (90.7%) patients completed a 2-year follow-up period, and represent the analysed population. The reasons for early termination of Rebif treatment are summarized in Table 1. The frequency of the early discontinuation did not differ between the subgroups (p = 0.3592).
Table 1.
Summary of reasons for early termination of sc IFN β-1a treatment during the observational period, by subgroups.
| Reason for the early termination | Subgroup by sc IFN β-1a dosage |
Total | ||
|---|---|---|---|---|
| 22 µg thrice in week | 44 µg thrice in week | Dose escalation | ||
| Disease progression | 5 | 3 | 4 | 12 |
| Liver tests elevation | 2 | - | - | 2 |
| Pregnancy | 3 | - | - | 3 |
| Leukopenia | 1 | - | - | 1 |
| Tolerability issue: injection site reaction |
2 | 2 | 1 | 5 |
| Patient’s decision | 1 | 1 | - | 2 |
| Participation in a clinical trial | 1 | - | - | 1 |
| Change of location | 1 | - | - | 1 |
| Out of reimbursement criteria | 1 | - | - | 1 |
| Sum | 17 | 6 | 5 | 28 |
Patients were eligible for enrolment in the study if they fulfilled the following criteria: diagnosis of RRMS; 18–65 years of age; EDSS score < 4 and treatment with sc IFN β-1a starting no more than 3 months prior to their baseline visit. Enrolment of patients and follow-up visits were performed during routine neurological visits to MS centres.
All patients were treated with subcutaneous IFN β-1a (Rebif®). Therapy was initiated with a titration pack, 8.8 mcg, followed by 22 or 44 mcg 3x/week (tiw) thereafter.
The study was approved by the Ethics Committee of the local Association of Innovative Pharmaceutical Industry (AIFP), and the regulatory authority; the State Institution for Drug Control was also notified of the study. All persons gave their informed consent prior to enrolment in the study.
Primary objective and endpoint
The primary objective was to assess cognition and its changes. The cognition status was assessed using the PASAT at defined time points (baseline, months 6, 12 and 24) in all patients. The percentage of patients with either decreased, increased or stable cognition status was calculated for each time point. The PASAT score and its changes were also expressed in terms of descriptive statistics (mean, standard deviation) at each time point.
The PASAT was presented on an audiocassette tape or compact disc to control the rate of stimulus presentation. Single digits were presented every 3 seconds, and the patient was tasked with adding each new digit to the one immediately preceding it. The test score was the total number of correct responses and ranged from 0 to 60 on each trial.
In order to minimize the learning effect, two PASAT forms (A and B) were used alternatively on subsequent visits. Thus, the interval between testing with same form was 12 months for each patient. As a result, each form was never used more than twice per patient during the study period.
Secondary objective and endpoint
The secondary objective was to assess fatigue and its changes. We also assessed correlations between cognition, fatigue and disability status. In addition, we assessed differences in the cognition, fatigue, and disability in patients with different sc IFN β-1a dosages.
Fatigue level and disability were assessed using the FDS and EDSS, respectively, at the defined time points (baseline, months 6, 12 and 24) in all patients. The percentage of patients with either decreased, increased, or stable fatigue levels and disability status was evaluated for each time point. FDS and EDSS scores and changes in scores were assessed at baseline as well as months 6, 12 and 24. Results are presented using descriptive statistics for each time point as well as trend changes over the 2-year study period, relative to each time point.
Fatigue was evaluated using the FDS The FDS was designed specifically to measure the severity, and to define the characteristics of MS fatigue. The questionnaire contained 12 items and assesses four domains. It included information regarding the modality of fatigue: spontaneity in the narration of the symptoms, severity, frequency and Uhthoff’s phenomenon. Uhthoff’s phenomenon is a transient worsening of neurological symptoms in MS when the body gets overheated. Each of the 12 items was scored on a 4-point scale, except for the Uhthoff domain items, which was scored as either ‘yes’ or ‘no’ answers. The range of the scale was from 0–17 points. Zero points means that fatigue was not present with higher scores indicating progressively greater levels of fatigue.
Neurological disability was assessed using the EDSS score [Kurtzke, 1983]. The EDSS score assesses 8 functional systems with each system scored on a 0 (normal) to 10 (maximal impairment) scale. The EDSS (complete) is measured in half points on a 0.0 (normal neurologic exam) to 10.0 (death due to MS) scale.
Statistical analysis
Statistical analysis was performed using SAS software (SAS Institute Inc., Cary, NC, USA) and Statistica Software (StatSoft, Inc., Tulsa, OK, USA). Statistical analyses were performed on all available data (N = 300), and separately on those patients who completed the whole study period (n = 272), i.e. baseline, months 6, 12 and 24. The latter sample results are presented here.
For the primary endpoint analysis, changes in the PASAT score from baseline to months 6, 12 and 24 were calculated, along with the percentage of patients with decreased, increased or stable cognitive status. The PASAT score was also expressed in terms of descriptive statistics (mean, standard deviation) at each time point. Descriptive statistics [all data were evaluated descriptively by number (n), mean, SD for continuous variables, and by counts and percentages for categorical variables].
For the secondary endpoint analyses, the changes in FDS and EDSS scores from baseline to months 6, 12 and 24 were calculated, along with the percentage of patients with decreased, increased or stable fatigue or disability levels. The FDS and EDSS scores were also expressed using descriptive statistics (mean, SD) at each time point.
The relationship between the PASAT, FDS and EDSS scores was assessed using Spearman’s correlation coefficient at the given time points.
Differences from baseline to follow-up measurement points (months 6, 12 and 24) were evaluated using the paired Wilcoxon test. The development of PASAT, FDS and EDSS scores across all four time points was tested separately using the Friedman analysis of variance (ANOVA; one p value for each score) in all samples, and for each subgroup separately. The parametric repeated ANOVA was used for testing the interactions, that is, differences from baseline to each time point for each score separately in all samples, and for each subgroup separately.
For evaluation of differences relative to sc IFN β-1a dosage, the sample was divided into three subgroups: (1) low-dose, those treated with 22 µg tiw; (2) high-dose, 44 µg tiw during the whole follow-up period; and (3) dose-escalation, those who experienced dose escalation from 22 to 44 µg tiw. In each subgroup the following numbers were calculated at the defined time points:
proportion of patients with increased, stable, or decreased PASAT scores;
proportion of patients with decreased, stable, or increased FDS scores;
proportion of patients with improved, stable, or worsened EDSS scores;
descriptive statistics for the PASAT, FDS and EDSS, and comparison of its development and changes;
correlation among the PASAT, FDS and EDSS in all samples and in all three subgroups.
Differences between subgroups with regard to Rebif dosage were analysed nonparametrically using a nonparametric ANOVA (Kruskal–Wallis test, Wilcoxon Two-Sample Test) for numerically continuous variables, and the chi-square test for categorical ordinal variables. Values of p ⩽ 0.05 were considered statistically significant.
A correlation among PASAT, FDS and EDSS scores was calculated in total, and for each subgroup separately at each time point using Spearman’s correlation coefficient with the correlation size defined as follows: 0.90–1.00 very high; 0.70–0.89 high; 0.50–0.69 moderate; 0.30–0.49 low; and 0.00–0.29 little if any.
Results
Cognition
Overall, the proportion of patients with an increased or stable PASAT score was higher compared with those with decreased PASAT scores at all time points, and the average cognitive performance improved over the 2-year follow-up period.
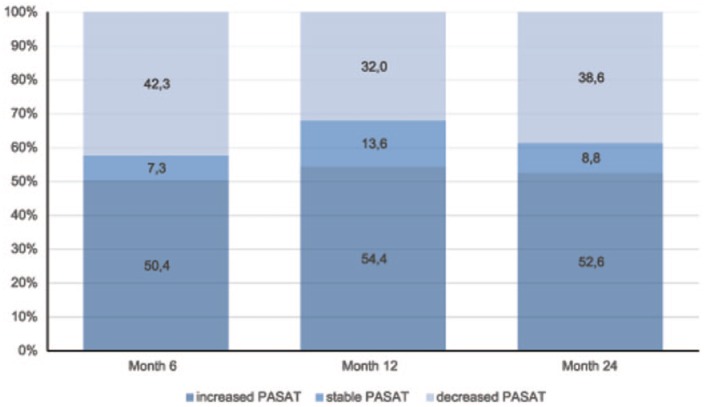
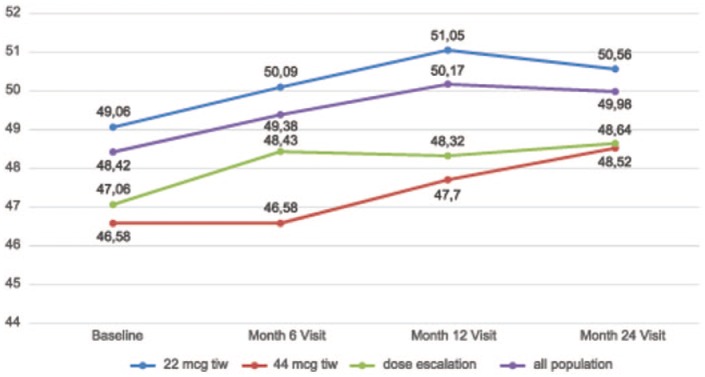
In the analysed population (n = 272), the proportion of patients with an increased or stable PASAT score vs baseline was 57.7% at 6 months, 68.0% at 12 months and 61.4% at 24 months (the end of the 2-year follow-up period). Additional information regarding patients with an increased, stable or decreased PASAT scores at each time point is shown in Figure 1. The mean (SD) PASAT score increased from a baseline value of 48.42 (9.09) to 49.98 (8.00) at month 24. The mean PASAT score by time points is detailed in Figure 2. The increase in the mean PASAT score was statistically significant throughout the follow-up period (p = 0.00026), and the pair test demonstrated a statistically significant increase in the PASAT score compared to the baseline at each time point, that is, at 6 months (p = 0.006), 12 months 12 (p = 0.001) and 24 months (p = 0.004).
Figure 1.
Overall proportion of patients with increased, stable or decreased Paced Auditory Serial Addition Task (PASAT) scores versus baseline for each time point.
Figure 2.
Total mean Paced Auditory Serial Addition Task (PASAT) scores and for subgroups for all time points.
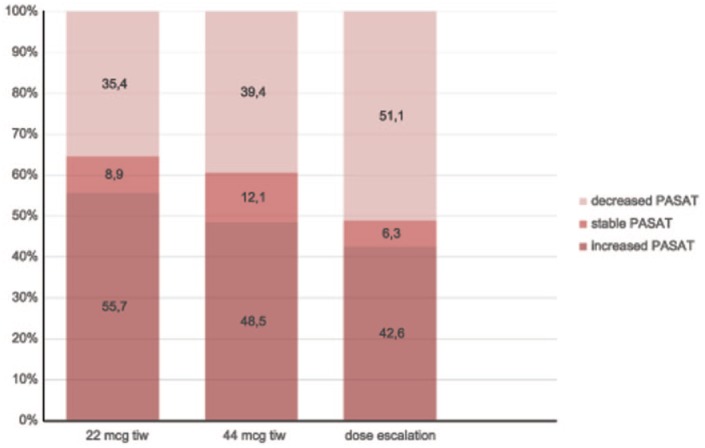
In subgroup analyses of the 24 months visit, a stable or improved PASAT score was reported in 64.6%, 60.6% and 48.9% of patients in the low-dose, high-dose and dose-escalation subgroups, respectively. The proportion of patients with an increased, stable or decreased PASAT score in each subgroup at the month 24 visit is detailed in Figure 3. The mean PASAT score at each time point in each subgroup is detailed in Figure 2. The increase in the mean PASAT score throughout the defined time points reached statistical significance in the low-dose subgroup (p = 0.0004), and was not significant in the high-dose (p = 0.293) and dose escalation (p = 0.798) subgroups. Comparisons of the mean PASAT scores at each time point showed an increase in all subgroups (p = 0.0001) with no difference in mean values (p = 0.06) or the change, over time, among subgroups (p = 0.52).
Figure 3.
Proportion of patients with increased, stable or decreased Paced Auditory Serial Addition Task (PASAT) scores at 24 months versus baseline, by subgroups.
Furthermore, comparisons of PASAT score differences from baseline among subgroups showed no statistical significance at month 6 (p = 0.40), month 12 (p = 0.53) or month 24 (p = 0.75).
Fatigue
In the analysed sample (n = 272), the proportion of patients with stable or improved fatigue status was higher compared with those with an increased FDS score at all time points.
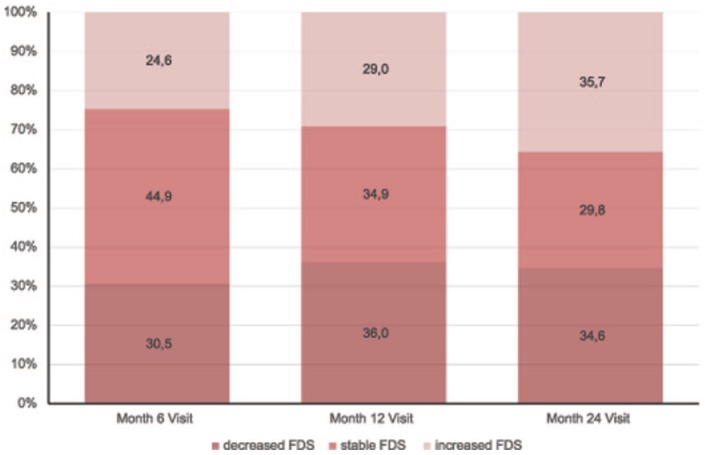
The proportion of patients with a decreased or stable FDS score versus baseline was 75.4% at 6 months, 71.0% at 12 months and 64.3% at 24 months. Additional information regarding patients with a decreased or stable or increased FDS score at each time point is shown in Figure 4.
Figure 4.
Overall proportion of patients with increased, stable or decreased Fatigue Descriptive Scale (FDS) score versus baseline for each time point.
The mean (SD) FDS score was 3.58 (3.66) at baseline and 3.68 (3.83) at 24 months (Figure 5). There was no statistically significant change during follow-up (p = 0.39036). No statistically significant change in the FDS score at baseline, compared with each time point, month 6 (p = 0.6609), month 12 (p = 0.6797) and month 24 (p = 0.2794) was observed.
Figure 5.
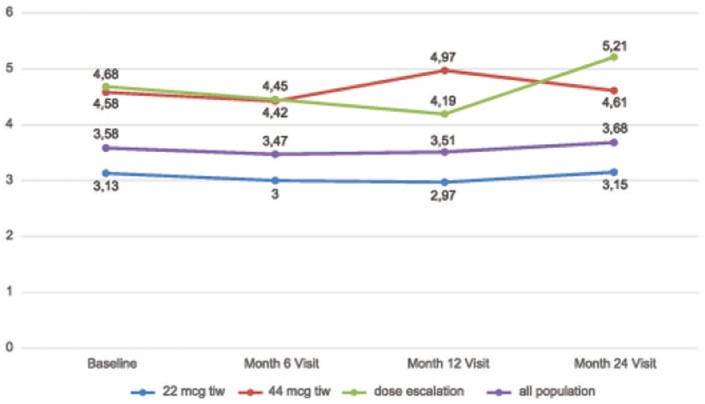
Total mean Fatigue Descriptive Scale (FDS) scores and for subgroups for all time points.
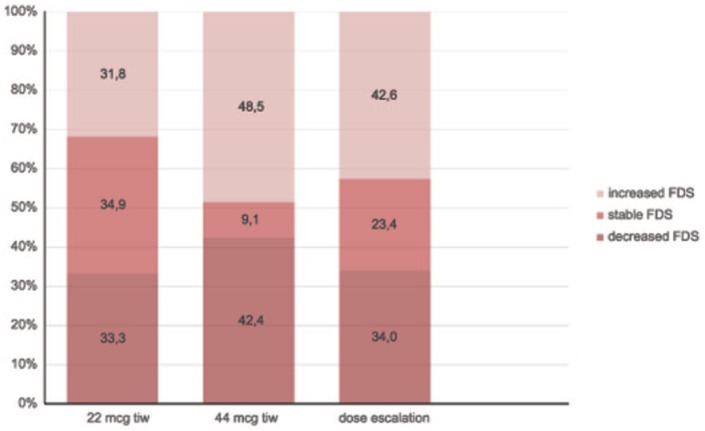
Subgroup analysis at month 24 versus baseline showed stable or improved FDS scores at the 2-year follow up in 68.2%, 51.5% and 57.4% of patients in the low-dose, high-dose and dose-escalation subgroups, respectively. Additional information regarding proportions of patients with decreased, stable, or increased FDS scores in each subgroup at 2 years are shown in Figure 6. The mean FDS score in subgroups at each time point is shown in Figure 5. No changes in mean FDS scores, over time, was seen in the three subgroups. There was a significant difference among the subgroups with a lower mean FDS in the low-dose subgroup at all time points (p = 0.0008), however, there was no difference in the time changes of mean FDS scores among the subgroups (p = 0.53). Comparison of FDS score differences from baseline among subgroups showed no statistical significance at month 6 (p = 0.65), month 12 (p = 0.20) or month 24 (p = 0.60). Thus, there was significantly lower fatigue in the low-dose subgroup from baseline through to the end of the study (24 months), however there were no differences in fatigue status changes among all three subgroups.
Figure 6.
Proportion of patients with increased, stable or decreased Fatigue Descriptive Scale (FDS) scores at 24 months versus baseline, by subgroups.
Disability
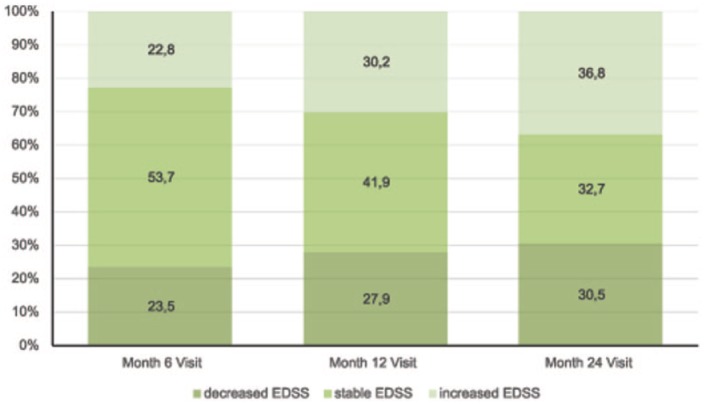
In total, the proportion of patients with stable or improved EDSS compared to baseline was higher than those with an EDSS that worsened at each time point: 77.2% after 6 months, 69.8% after 12 months and 63.2% after 24 months. Detailed proportions of patients with improved, stable or worsened EDSS scores at each time point are shown in Figure 7.
Figure 7.
Overall proportion of patients with increased, stable or decreased Expanded Disability Status Scale (EDSS) scores versus baseline for each time point.
The mean EDSS (SD) score is detailed in Figure 8. For the analysed population (n = 272), it was 2.85 (1.10) at baseline, and 2.98 (1.40) at 24 months, without significant development over time (p = 0.085); the paired test demonstrated no statistically significant change in mean EDSS scores compared with baseline and at month 6 (p = 0.73), month 12 (p = 0.56) and month 24 (p = 0.06), respectively.
Figure 8.
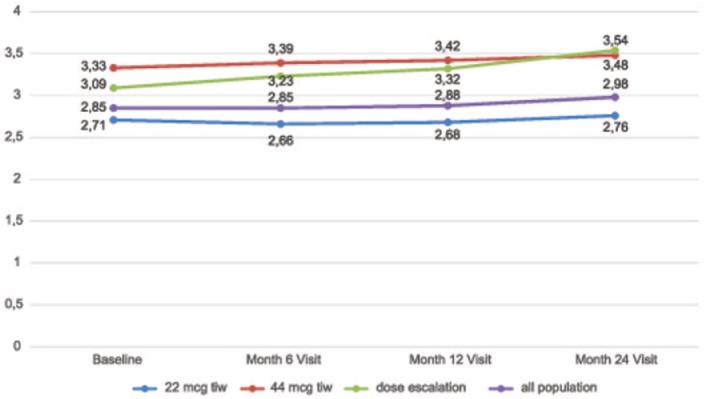
Total mean Expanded Disability Status Scale (EDSS) scores and for subgroups for all time points.
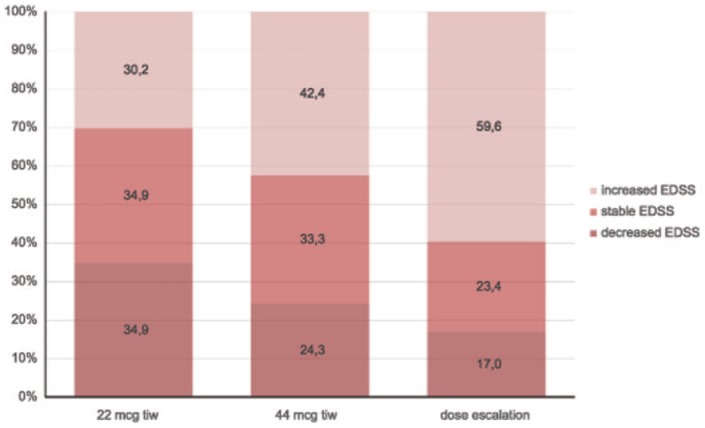
In subgroup analyses, stable or improved EDSS scores at 24 months was reported in 69.8%, 57.6% and 40.4% of patients in the low-dose, high-dose and dose-escalation subgroups, respectively. Additional information regarding mean EDSS scores at all time points in each subgroup, and the proportion of patients with improved, stable or worsening EDSS scores at 24 months are shown in Figures 8 and 9, respectively. The changes in mean EDSS scores over time were insignificant in the low-dose (p = 0.317) and high-dose (p = 0.356) subgroups, while there was a significant increase in mean EDSS scores in the dose-escalation subgroup (p = 0.00029). Comparison of mean EDSS at all time points in subgroups revealed a significant increase over time in the dose-escalation subgroup (p = 0.00063) due to the difference between baseline and month 24 values; and there was a significant difference in the mean EDSS (p = 0.00016) among all subgroups at all time points. A significant difference in EDSS scores from baseline was found at month 6 (p = 0.0247), month 12 (p = 0.0331) and month 24 (p = 0.0017) between the dose-escalation and stable-dosage subgroups. So, there was a significantly higher but stable mean EDSS in the high-dose subgroup, and a higher mean EDSS with more pronounced EDSS worsening in the dose escalation subgroup over the 2-year follow up. These findings are discussed in the following.
Figure 9.
Proportion of patients with total mean Fatigue Descriptive Scale (FDS) scores and for subgroups for all time points with improved, stable or worsened Expanded Disability Status Scale (EDSS) scores at 24 months versus baseline, by subgroups.
Correlation of cognition, fatigue and disability
Generally, none of the correlations among EDSS, PASAT and FDS scores reached the size of very high or high at any time point in the whole sample, or in any of the subgroups.
There was a low negative correlation between the PASAT and FDS scores at baseline [correlation coefficient (r) = −0.32148] and month 6 (r = −0.30236), that is, there was a higher probability of low fatigue in patients with better cognition and vice versa, p < 0.0001; there was also little, if any, negative correlation between PASAT and EDSS scores at baseline (r = −0.26292) and month 6 (r = −0.28649), that is, patients with higher disability had a higher probability of lower cognitive performance, p < 0.0001. There was a small positive correlation between EDSS and FDS scores at baseline (r = 0.44018) and month 6 (r = 0.43800), that is, a higher probability of more pronounced fatigue with more severe disability, p < 0.0001.
At months 12 and 24, there was a low negative correlation between PASAT and FDS scores (r = −0.38837 and r = −0.35189, respectively, p < 0.0001), a low negative correlation between PASAT and EDSS scores (r = −0.37947 and r = −0.41383, respectively, p < 0.0001), and a small positive correlation between EDSS and FDS scores (r = 0.49890 and r = 0.48975, respectively, p < 0.0001).
Only four patients (1.3%) experienced adverse reactions leading to early discontinuation of IFN β-1a treatment during the observational period. None of the reported adverse reactions were evaluated as serious, and no deaths were reported in this study.
Discussion
We have observed stable or improved cognitive performance in the majority of patients treated with sc IFN β-1a. In comparison, a significant decline in cognitive functions has been previously observed in the natural course of the disease. A longitudinal observation showed that the proportion of patients with cognitive impairment increased dramatically from 29% at baseline to 54% during the 5 years following a CIS, and it was partly predicted by the baseline T2 lesion load [Reuter et al. 2011]. Another prospective study showed a significant proportion of MS patients had a decline in verbal memory performance from the beginning of the disease through the 2 years of follow up; the decline was independent of other parameters of disease activity [Duque et al. 2008]. Compared with these observations, our findings could indicate that sc IFN β-1a may protect against cognitive decline in patients with MS.
Our results are in accordance with findings that show IFN β treatment influences cognitive maintenance in MS patients [Patti et al. 2010]. In addition, a significant benefit has been shown for 44 µg doses over 22 µg doses; higher-dose treatments have been predictive of better cognitive outcomes over 3- and 5-year periods [Bastianello et al. 2011; Patti et al. 2013]. It has also been found that IFN β-1a may have a dose-dependent influence on cognitive maintenance in mildly disabled patients with RRMS. These findings support the point of view that early initiation treatment with high-dose IFN β-1a treatment is more beneficial [Patti et al. 2010].
Other factors, such as cognitive and brain reserve capacity [Modica et al. 2016; Pinter et al. 2014], employment, social status and support [Baughman et al. 2015], or sleep disturbance [Braley et al. 2016] are possible influencing factors that contribute to the level of cognitive impairment in MS. It has been shown that patients with greater education are protected against disease-related cognitive inefficiency and memory problems [Modica et al. 2016; Pinter et al. 2014; Sumowski, 2015]. Benefits of leisure activities (e.g. reading, hobbies, etc.) have also been shown to be protective against cognitive impairment in MS [Schwartz et al. 2013; Sumowski et al. 2016]. Separate from the cognitive reserve hypothesis, the theory of brain reserve capacity proposes that cognitive impairment emerges when brain volume falls below a critical threshold [MacLullich et al. 2002]. Also sleep disturbances are significantly associated with diminished visual and verbal memory, executive function, attention, processing speed and working memory in patients with MS [Braley et al. 2016].
Our results also demonstrated stable or improved fatigue status in the majority of patients. There has been only one study evaluating fatigue and cognitive deficits in patients with RRMS receiving IFN β treatment [Melanson et al. 2010]. The outcome of the Melanson study suggests that IFN β may reduce fatigue and cognitive deficits in patients with RRMS which correlates with our results. The aetiology of fatigue remains unclear. Dobryakova and colleagues proposed that fatigue is caused by an imbalance of the modulatory neurotransmitter, dopamine, in both the CNS and the immune system, and that fatigue depends on the levels of dopamine in the CNS. This is supported by neuroimaging studies in clinical populations with fatigue that have shown the structural and functional impairments in regions innervated by dopaminergic neurons, namely the striatum and mesocorticolimbic pathway [Dobryakova et al. 2013]. It has been shown that proliferated CD4+ T cells in MS express the D3 receptor that contributes to the destruction of dopamine neurons in the substantia nigra and leads to the production of IFN γ, a compound that exacerbates inflammation and prevents dopamine synthesis [Cosentino et al. 2005, 2012; González et al. 2013; Pacheco et al. 2014]. IFN β increases dopamine synthesis in the brain [Cosentino et al. 2005, 2012; Zaffaroni et al. 2008], and it is possible that MS patients treated with the IFN β might benefit from its dopaminergic effect.
Both, cognition and fatigue could be influenced by comorbidities such as depression or endocrinological conditions [Langdon, 2011; Niino et al. 2014]. In our sample, the reported baseline frequency of psychological and/or psychiatric comorbidities was 6.9% and endocrinological conditions was 13.8%. However, the frequency of depression and endocrinological conditions were low and are unlikely to have affected cognition or fatigue functions.
We have also observed that about two-thirds of the patients treated using sc IFN β-1a experienced no progression in disability during the 2-year follow-up period. This is in agreement with many clinical studies that have demonstrated that IFN β significantly prevented relapses and disability progression [Comi et al. 1995, 2001; Durelli et al. 2002; Edan et al. 2014; Filippini et al. 2013; Freedman et al. 2003; Goodin et al. 2012; Jacobs et al. 2000; Kappos et al. 2007, 2009; Panitch et al. 2002]. Nevertheless, due to the fact that disability progresses gradually in most patients with RRMS, the relatively short 2-year observational period, and a lack of a control group in our study, we cannot conclude that the lack of disability progression was a treatment effect. We observed higher levels of disability in the 44 µg tiw subgroup and a more pronounced accumulation of disabilities in the dose escalation subgroup. These findings reflect the facts that: (a) based on the previous Czech national DMD reimbursement criteria, Rebif 22 µg tiw was used as a treatment for patients with less active disease, (b) Rebif 44 µg tiw was reimbursed in more active diseases and (c) a dose-escalation from 22 to 44 µg tiw was used in patients with suboptimal disease control (relapses or disability progression). Therapy optimization as first-line treatment is also supported by recent results coming from the CARE-MS II study in RRMS patients with suboptimal responses to IFN β or glatiramer acetate. Switching to sc IFN β-1a (44 µg tiw) resulted in only 21.13% of them having sustained accumulation of disability during the subsequent 2 years of follow up [Coles et al. 2012]. A similar treatment algorithm and significant disease stabilization after optimization of first-line DMD treatment to sc IFN-β-1a (44 µg tiw) has also been reported in a German observational study [Masri et al. 2010]. Furthermore, CIS and RRMS trials have shown a more pronounced benefit in prevention of disability progression in cases where sc IFN β-1a was started early using a high-dose, high-frequency regimen [Comi et al. 2011; Kappos et al. 2015; Cohen and Rivera, 2010]. Thus, our explanation for the observed difference in disability among subgroups relative to dose is that the higher level of disability and its progression had led to treatment with a higher dose of IFN β-1a as well as dose escalation. In other words, the higher dose and dose escalation was a response to and not the cause of disability progression.
The limitations of our study include a relatively short observation period of 24 months and the lack of control group, thus no conclusions regarding causality can be drawn from our results. Additional controlled, prospective studies using large RRMS populations with longer observation periods need to be performed to fully demonstrate the effect of sc IFN-β-1a on cognitive performance, disease progression and fatigue status.
Conclusion
The results of this observational, prospective, multicentre study in patients with RRMS, have demonstrated stable or improved cognitive performance and fatigue status in the majority of those treated with sc IFN-β-1a over the 2-year follow-up period, in a real-life setting, in the Czech Republic. Almost two-thirds of patients experienced improvement in their cognitive performance, and there was a significant increase in average cognitive performance; almost two thirds reported “stable or” improved fatigue levels with a stable average fatigue level, however, direct effects of IFN β-1a on cognition and fatigue cannot be concluded form this observational study. In addition, almost two thirds of the study group maintained stable disability levels or saw improvements in their disability status, but with no significant change in the mean EDSS score. The stable or improved disability levels observed in the majority of patients was in accordance with the proven efficacy of sc IFN β-1a in preventing disability progression.
Acknowledgments
Data collection and analysis were performed by Software Production spol. s r. o., Pilsen, the Czech Republic.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Merck spol. s r.o., Prague, Czech Republic, an affiliate of Merck KGaA, Darmstadt, Germany and MZ ČR - RVO (FNBr, 65269705).
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Yvonne Benešová has nothing to disclose. Aleš Tvaroh is an employee of Merck spol. s r. o., Prague, Czech Republic, an affiliate of Merck KGaA, Darmstadt, Germany.
Contributor Information
Yvonne Benešová, Department of Neurology, University Hospital Brno and Faculty of Medicine, Masaryk University, Jihlavská 20, Brno 625 00, Czech Republic.
Aleš Tvaroh, Merck spol. s r.o., Prague, Czech Republic.
References
- Amato M., Portaccio E., Goretti B., Zipoli V., Iudice A., Della Pina D., et al. (2010) Relevance of cognitive deterioration in early relapsing–remitting MS: a 3-year follow-up study. Mult Scler 6: 1474–1482. [DOI] [PubMed] [Google Scholar]
- Amato M., Zipoli V., Portaccio E. (2006) Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci 245: 41–46. [DOI] [PubMed] [Google Scholar]
- Andreasen A., Spliid P., Andersen H., Jakobsen J. (2010) Fatigue and processing speed are related in multiple sclerosis. Eur J Neurol 17: 212–218. [DOI] [PubMed] [Google Scholar]
- Bakshi R., Shaikh Z., Miletich R., Czarnecki D., Dmochowski J., Henschel K., et al. (2000) Fatigue in multiple sclerosis and its relationship to depression and neurologic disability. Mult Scler 6: 181–185. [DOI] [PubMed] [Google Scholar]
- Bastianello S., Giugni E., Amato M., Tola M., Trojano M., Galletti S., et al. (2011) Changes in magnetic resonance imaging disease measures over 3 years in mildly disabled patients with relapsing–remitting multiple sclerosis receiving interferon β-1a in the COGnitive Impairment in MUltiple Sclerosis (COGIMUS) study. BMC Neurol 11: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista S., Zivadinov R., Hoogs M., Bergsland N., Heininen-Brown M., Dwyer M., et al. (2012) Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol 259: 139–146. [DOI] [PubMed] [Google Scholar]
- Baughman B., Basso M., Sinclair R., Combs D., Roper B. (2015) Staying on the job: the relationship between work performance and cognition in individuals diagnosed with multiple sclerosis. J Clin Exp Neuropsychol 37: 630–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R., Zivadinov R. (2011) Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol 7: 332–342. [DOI] [PubMed] [Google Scholar]
- Braley T., Kratz A., Kaplish N., Chervin R. (2016) Sleep and cognitive function in multiple sclerosis. Sleep 39: 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron M., Peterson V., Boudreau E., Downs A., Lovera J., Kim E., et al. (2014) Fatigue is associated with poor sleep in people with multiple sclerosis and cognitive impairment. Mult Scler Int 2014: 872732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B., Rivera V. (2010) PRISMS: the story of a pivotal clinical trial series in multiple sclerosis. Curr Med Res Opin 26: 827–838. [DOI] [PubMed] [Google Scholar]
- Comi G., Barkhof F., Durelli L., Edan G., Fernandez O., Filippi M., et al. (1995) Early treatment of multiple sclerosis with Rebif (recombinant human interferon beta): design of the study. Mult Scler 1(Suppl. 1): S24–S27. [PubMed] [Google Scholar]
- Comi G., De Stefano N., Freedman M., Barkhof F., Polman C., Uitdehaag B., et al. (2011) Comparison of two dosing frequencies of subcutaneous interferon beta-1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): a phase 3 randomised controlled trial. Lancet Neurol 11: 33–41. [DOI] [PubMed] [Google Scholar]
- Comi G., Filippi M., Barkhof F., Durelli L., Edan G., Fernández O., et al. (2001) Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet 357: 1576–1582. [DOI] [PubMed] [Google Scholar]
- Cools R., D’Esposito M. (2011) Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69: e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino M., Marino F. (2013) Adrenergic and dopaminergic modulation of immunity in multiple sclerosis: teaching old drugs new tricks? J Neuroimmune Pharmacol 8: 163–179. [DOI] [PubMed] [Google Scholar]
- Cosentino M., Zaffaroni M., Ferrari M., Marino F., Bombelli R., Rasini E., et al. (2005) Interferon-gamma and interferon-beta affect endogenous catecholamines in human peripheral blood mononuclear cells: implications for multiple sclerosis. J Neuroimmunol 162: 112–121. [DOI] [PubMed] [Google Scholar]
- Cosentino M., Zaffaroni M., Trojano M., Giorelli M., Pica C., Rasini E., et al. (2012) Dopaminergic modulation of CD4 + CD25(high) regulatory T lymphocytes in multiple sclerosis patients during interferon-β therapy. Neuroimmunomodulation 19: 283–292. [DOI] [PubMed] [Google Scholar]
- De Felice B., Mondola P., Sasso A., Orefice G., Bresciamorra V., Vacca G., et al. (2014) Small non-coding RNA signature in multiple sclerosis patients after treatment with interferon-β. BMC Med Genomics 7: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobryakova E., DeLuca J., Genova H., Wylie G. (2013) Neural correlates of cognitive fatigue: cortico-striatal circuitry and effort-reward imbalance. J Int Neuropsychol Soc 19: 849–853. [DOI] [PubMed] [Google Scholar]
- Duque B., Sepulcre J., Bejarano B., Samaranch L., Pastor P., Villoslada P. (2008) Memory decline evolves independently of disease activity in MS. Mult Scler 14: 947–953. [DOI] [PubMed] [Google Scholar]
- Durelli L., Verdun E., Barbero P., Bergui M., Versino E., Ghezzi A., et al. (2002) Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet 359: 1453–1460. [DOI] [PubMed] [Google Scholar]
- Dutta R., Chang A., Doud M., Kidd G., Ribaudo M., Young E., et al. (2011) Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann Neurol 69: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edan G., Kappos L., Montalbán X., Polman C., Freedman M., Hartung H., et al. (2014) Long-term impact of interferon beta-1b in patients with CIS: 8-year follow-up of BENEFIT. J Neurol Neurosurg Psychiatry 85: 1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M. (2010) Cognitive deficits in multiple sclerosis: a systematic review. Arq Neuropsiquiatr 68: 632–641. [DOI] [PubMed] [Google Scholar]
- Filippi M., Rocca M., Benedict R., DeLuca J., Geurts J., Rombouts S., et al. (2010) Contribution of MRI in assessing cognitive impairment in multiple sclerosis. Neurology 75: 2121–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini G., Del Giovane C., Vacchi L., D’Amico R., Di Pietrantonj C., Beecher D., et al. (2013) Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev 6: CD008933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J., Rudick R., Cutter G., Reingold S. (1999) The Multiple Sclerosis Functional Composite measure (MSFC): an integrated approach to MS clinical outcome assessment. Mult Scler 5: 244–250. [DOI] [PubMed] [Google Scholar]
- Fisk J.D., Pontefract A., Ritvo P.G., Archibald C.J., Murray T.J. (1994) The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci 21: 9–14. [PubMed] [Google Scholar]
- Freedman M., King J., Oger J., Sharief M., Hartung H.; PRISMS Study Investigators. (2003) Interferons in relapsing remitting multiple sclerosis. Lancet 361: 545–552. [DOI] [PubMed] [Google Scholar]
- Glanz B., Holland C., Gauthier S., Amunwa E., Liptak Z., Houtchens M., et al. (2007) Cognitive dysfunction in patients with clinically isolated syndromes or newly diagnosed multiple sclerosis. Mult Scler 13: 1004–1010. [DOI] [PubMed] [Google Scholar]
- González H., Contreras F., Prado C., Elgueta D., Franz D., Bernales S., et al. (2013) Dopamine receptor D3 expressed on CD4+ T cells favors neurodegeneration of dopaminergic neurons during Parkinson’s disease. J Immunol 190: 5048–5056. [DOI] [PubMed] [Google Scholar]
- Goodin D., Traboulsee A., Knappertz V., Reder A., Li D., Langdon D., et al. (2012) Relationship between early clinical characteristics and long term disability outcomes: 16 year cohort study (follow-up) of the pivotal interferon β-1b trial in multiple sclerosis. J Neurol Neurosurg Psychiatry 83: 282–287. [DOI] [PubMed] [Google Scholar]
- Gronwall D. (1977) Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills 44: 363–373. [DOI] [PubMed] [Google Scholar]
- Harrington M. (2012) Neurobiological studies of fatigue. Prog Neurobiol 99: 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung H., Bar-Or A., Zoukos Y. (2004) What do we know about the mechanism of action of disease-modifying treatments in MS? J Neurol 251: v12–v19. [DOI] [PubMed] [Google Scholar]
- Iriarte J., Katsamakis G., de Castro P. (1999) The Fatigue Descriptive Scale (FDS): a useful tool to evaluate fatigue in multiple sclerosis. Mult Scler 5: 10–16. [DOI] [PubMed] [Google Scholar]
- Jacobs L., Beck R., Simon J., Kinkel R., Brownscheidle C., Murray T., et al. (2000) Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 343: 898–904. [DOI] [PubMed] [Google Scholar]
- Kappos L., Freedman M., Polman C., Edan G., Hartung H., Miller D., et al. ; BENEFIT Study Group. (2007) Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet 370: 389–397. [DOI] [PubMed] [Google Scholar]
- Kappos L., Freedman M., Polman C., Edan G., Hartung H., Miller D., et al. (2009) Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol 8: 987–997. [DOI] [PubMed] [Google Scholar]
- Kappos L., Kuhle J., Multanen J., Kremenchutzky M., di Cantogno E., Cornelisse P., et al. (2015) Factors influencing long-term outcomes in relapsing-remitting multiple sclerosis: PRISMS-15. J Neurol Neurosurg Psychiatry 86: 1202–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaver R., De Vries H., Schenk G., Geurts J. (2013) Grey matter damage in multiple sclerosis: a pathology perspective. Prion 7: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp L., LaRocca N., Muir-Nash J., Steinbery A. (1989) The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46: 1121–1123. [DOI] [PubMed] [Google Scholar]
- Krupp L., Serafin D., Christodoulou C. (2010) Multiple sclerosis-associated fatigue. Expert Rev Neurother 10: 1437–1447. [DOI] [PubMed] [Google Scholar]
- Kurtzke J. (1983) Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- Langdon D. (2011) Cognition in multiple sclerosis. Curr Opin Neurol 24: 244–249. [DOI] [PubMed] [Google Scholar]
- Lerdal A., Celius E., Krupp L., Dahl A. (2007) A prospective study of patterns of fatigue in multiple sclerosis. Eur J Neurol 14: 1338–1343. [DOI] [PubMed] [Google Scholar]
- Lincoln N., Dent A., Harding J., Weyman N., Nicholl C., Blumhardt L., et al. (2002) Evaluation of cognitive assessment and cognitive intervention for people with multiple sclerosis. J Neurol Neurosurg Psychiatry 72: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobentanz I., Asenbaum S., Vass K., Sauter C., Klösch G., Kollegger H., et al. (2004) Factors influencing quality of life in multiple sclerosis patients: disability, depressive mood, fatigue and sleep quality. Acta Neurol Scand 110: 6–13. [DOI] [PubMed] [Google Scholar]
- McDonald W., Compston A., Edan G., Goodkin D., Hartung H., Lublin F., et al. (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 50: 121–127. [DOI] [PubMed] [Google Scholar]
- MacLullich A., Ferguson K., Deary I., Seckl J., Starr J., Wardlaw J. (2002) Intracranial capacity and brain volumes are associated with cognition in healthy elderly men. Neurology 59: 169–174. [DOI] [PubMed] [Google Scholar]
- Marrie R., Goldman M. (2007) Validity of performance scales for disability assessment in multiple sclerosis. Mult Scler 13: 1176–1182. [DOI] [PubMed] [Google Scholar]
- Masri S., Blodau A., Zessack N., Lang M.; High Dose, High Frequency Study Group. (2010) Optimizing immunomodulatory therapy with interferon beta in patients with multiple sclerosis. A prospective study. Int J MS Care 12: 147–154. [Google Scholar]
- Melanson M., Grossberndt A., Klowak M., Leong C., Frost E., Prout M., et al. (2010) Fatigue and cognition in patients with relapsing multiple sclerosis treated with interferon β. Int J Neurosci 120: 631–640. [DOI] [PubMed] [Google Scholar]
- Merkelbach S., Sittinger H., Koenig J. (2002) Is there a differential impact of fatigue and physical disability on quality of life in multiple sclerosis? J Nerv Ment Dis 190: 388–393. [DOI] [PubMed] [Google Scholar]
- Meyer-Moock S., Feng Y., Maeurer M., Dippel F., Kohlmann T. (2014) Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol 14: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modica C., Bergsland N., Dwyer M., Ramasamy D., Carl E., Zivadinov R., et al. (2016) Cognitive reserve moderates the impact of subcortical gray matter atrophy on neuropsychological status in multiple sclerosis. Mult Scler 22: 36–42. [DOI] [PubMed] [Google Scholar]
- Niino M., Mifune N., Kohriyama T., Mori M., Ohashi T., Kawachi I., et al. (2014). Apathy/depression, but not subjective fatigue, is related with cognitive dysfunction in patients with multiple sclerosis. BMC Neurol 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozenci V., Kouwenhoven M., Teleshova N., Pashenkov M., Fredrikson S., Link H. (2000) Multiple sclerosis: pro- and anti-inflammatory cytokines and metalloproteinases are affected differentially by treatment with IFN-β. J Neuroimmunol 108: 236–243. [DOI] [PubMed] [Google Scholar]
- Pacheco R., Contreras F., Zouali M. (2014) The dopaminergic system in autoimmune diseases. Front Immunol 5: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitch H., Goodin D., Francis G., Chang P., Coyle P., O’Connor P., et al. (2002) Randomized, comparative study of interferon beta-1a treatment regimens in MS: the EVIDENCE Trial. Neurology 59: 1496–1506. [DOI] [PubMed] [Google Scholar]
- Patti F. (2009) Cognitive impairment in multiple sclerosis. Mult Scler 15: 2–8. [DOI] [PubMed] [Google Scholar]
- Patti F., Amato M., Bastianello S., Caniatti L., Di Monte E., Ferrazza P., et al. (2010) Effects of immunomodulatory treatment with subcutaneous interferon beta-1a on cognitive decline in mildly disabled patients with relapsing–remitting multiple sclerosis. Mult Scler 16: 68–77. [DOI] [PubMed] [Google Scholar]
- Patti F., Morra V., Amato M., Trojano M., Bastianello S., Tola M., et al. (2013) Subcutaneous interferon β-1a May protect against cognitive impairment in patients with relapsing–remitting multiple sclerosis: 5-year follow-up of the COGIMUS study. PLoS ONE 8: e74111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter D., Sumowski J., DeLuca J., Fazekas F., Pichler A., Khalil M., et al. (2014) Higher education moderates the effect of T2 lesion load and third ventricle width on cognition in multiple sclerosis. PLoS ONE 9: e87567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman C., Rudick R. (2010) The multiple sclerosis functional composite: a clinically meaningful measure of disability. Neurology 74: S8–S15. [DOI] [PubMed] [Google Scholar]
- Rahn K., Watkins C., Alt J., Rais R., Stathis M., Grishkan I., et al. (2012) Inhibition of glutamate carboxypeptidase II (GCPII) activity as a treatment for cognitive impairment in multiple sclerosis. Proc Natl Acad Sci U S A 109: 20101–20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Leo G., Bernardin L., Unverzagt F. (1991) Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns and prediction. Neurology 41: 685–691. [DOI] [PubMed] [Google Scholar]
- Rao S.M. (1995) Neuropsychology of multiple sclerosis. Curr Opin Neurol 8: 216–220. [DOI] [PubMed] [Google Scholar]
- Reuter F., Zaaraoui W., Crespy L., Faivre A., Rico A., Malikova I., et al. (2011) Frequency of cognitive impairment dramatically increases during the first 5 years of multiple sclerosis. J Neurol Neurosurg Psychiatry 82: 1157–1159. [DOI] [PubMed] [Google Scholar]
- Rogers J., Panegyres P. (2007) Cognitive impairment in multiple sclerosis: evidence-based analysis and recommendations. J Clin Neurosci 14: 919–927. [DOI] [PubMed] [Google Scholar]
- Roosendaal S., Moraal B., Vrenken H., Castelijns. J., Pouwels P., Barkhof F., et al. (2008) In vivo MR imaging of hippocampal lesions in multiple sclerosis. J Magn Reson Imaging 27: 726–731. [DOI] [PubMed] [Google Scholar]
- Schwartz C., Snook E., Quaranto B., Benedict R., Vollmer T. (2013) Cognitive reserve and patient-reported outcomes in multiple sclerosis. Mult Scler 19: 87–105. [DOI] [PubMed] [Google Scholar]
- Sumowski J. (2015) Cognitive reserve as a useful concept for early intervention research in multiple sclerosis. Front Neurol 6: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumowski J., Rocca M., Leavitt V., Riccitelli G., Sandry J., DeLuca J., et al. (2016) Searching for the neural basis of reserve against memory decline: intellectual enrichment linked to larger hippocampal volume in multiple sclerosis. Eur J Neurol 23(1): 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kessel K., Moss-Morris R. (2006) Understanding multiple sclerosis fatigue: a synthesis of biological and psychological factors. J Psychosom Res 61: 583–585. [DOI] [PubMed] [Google Scholar]
- Zaffaroni M., Marino F., Bombelli R., Rasini E., Monti M., Ferrari M., et al. (2008) Therapy with interferon-beta modulates endogenous catecholamines in lymphocytes of patients with multiple sclerosis. Exp Neurol 214: 315–321. [DOI] [PubMed] [Google Scholar]