Abstract
Study Design
In vitro disk explant culture.
Objective
Notochordal cells (NCs) have been shown to upregulate matrix production by nucleus pulposus (NP) cells in coculture. To examine the translation of these in vitro results to a nativelike setting, the regenerative potential of NCs injected into NP tissue was assessed in this study.
Methods
NP explants were cultured after injection with NCs in phosphate-buffered saline (PBS) or with PBS alone (sham). At days 0 and 42, cell viability and morphology, water, DNA, sulfated glycosaminoglycan and hydroxyproline content, and gene expression of anabolic markers were analyzed.
Results
NCs remained viable during culture, but their morphology changed. The biochemical content remained unchanged, except for the DNA content in the NC group. Overall ACAN expression remained unchanged, whereas COL2A1 decreased during culture.
Conclusions
No overall anabolic response was observed when NCs were injected into NP explants. NCs were found to survive but did not display the typical NC morphology by the end of the culture period.
Keywords: nucleus pulposus, explant, notochordal cell, intervertebral disk, regeneration
Introduction
The nucleus pulposus (NP) is a highly hydrated tissue, due to large amounts of proteoglycans (mainly aggrecan) incorporated in a loose collagen type II meshwork. Early in human life, this tissue is populated by two cell types: notochordal cells (NCs) and NP cells (NPCs). NCs naturally exist in clusters and contain large vesicles, whereas NPCs are small, nonclustered, and chondrocyte-like cells.1 Despite these morphologic differences, NCs and NPCs show similar gene expression profiles.2 However, NCs can be distinguished from NPCs by elevated brachyury (T) and cytokeratin 8, 18, and 19 expression.2 3 In humans, NCs disappear before adolescence, and thereafter the onset of intervertebral disk degeneration, starting in the NP, has been observed.4,5 Certain species keep their NCs throughout adulthood and are known to experience the incidence and onset of disk degeneration much later in life.6 Consequently, it has been proposed that NCs might be involved in the maintenance and regeneration of healthy NP tissue.7
It has been shown that NCs can indeed increase the proteoglycan production by NPCs in alginate coculture for up to 14 days.8,9 When NPCs were cultured in medium conditioned by NCs (for 4 days), increased proteoglycan production and aggrecan gene expression levels were observed as well.9–14
However, it is yet unknown if NCs can have a direct regenerative potential on NPCs in their native environment. This environment is characterized by low pH, oxygen, and glucose levels, as well as a high osmolarity. These factors, but also the composition of the extracellular matrix (proteoglycans, collagens), can affect the cell response to an exogenous stimulation. The aim of this study was to investigate the effects of direct NC therapy in NPC-populated bovine NP explants, using an NP culture model that was previously developed in our group.15 We hypothesized that NCs could stimulate extracellular matrix production in bovine NP tissue.
Methods
Porcine Notochordal Cell Isolation
Porcine spines (4 donors; 8 weeks old) were obtained from the slaughterhouse, in accordance with local regulations (no approval from an ethical board required), and stored overnight at 4°C. The thoracic and lumbar intervertebral disks were transversally opened to harvest the NP tissue under sterile conditions. The tissue was pooled per donor and digested in 0.1% pronase protease (Calbiochem, Darmstadt, Germany) in high glucose (4.5 g/L) Dulbecco's modified Eagle's medium (hgDMEM; Invitrogen, Bleiswijk, The Netherlands) + 1% penicillin/streptomycin (pen/strep; Lonza, Basel, Switzerland) for 1 hour at 37°C and in 0.025% collagenase P (Roche, Basel, Switzerland) in hgDMEM + 10% fetal bovine serum (FBS; Invitrogen) + 1% pen/strep overnight at 37°C. After filtration on a 40-µm cell strainer (BD Biosciences, Breda, The Netherlands), cells and cell clusters ≥ 40 µm were stained with 5 µM of carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) according to the manufacturer's protocol (Vybrant, Invitrogen) for cell tracking for the duration of the experiment.
Bovine Nucleus Pulposus Culture
Bovine tails (12 donors; 24 months old) were obtained from the slaughterhouse in accordance with local regulations (no approval from an ethical board required). Thirty-six NP samples (C2–C5) were harvested, as described previously by van Dijk et al.15 Samples of different levels were equally distributed among the native and culture groups (n = 12 per group). The NP samples from the culture groups were individually put in dialysis tubing (15-kDa molecular weight cut off, Spectra-Por, Rancho Dominquez, California, United States) and subsequently injected into the middle of the tissue with a 23-gauge needle (Hamilton, Bonaduz, Switzerland). The injection groups were: sham (10-μL injection of phosphate-buffered saline [PBS]) and NC (10-μL injection of 250,000 CFDA-SE-stained porcine NCs in PBS).
After the injection, the dialysis tubing was replaced and closed with a custom-made clip. The NP samples were cultured in hypertonic polyethylene glycol (PEG) medium (13.3% w/v 20 kDa PEG [Sigma, Zwijndrecht, The Netherlands]; 8.3 g/L DMEM + 3.7 g/L sodium bicarbonate + 50 mg/L ascorbic acid + 15.9 mg/L phenol red [all Sigma] + 1 g/L glucose + 10% FBS [both Invitrogen] + 2% L-glutamine + 1% sodium pyruvate + 1% pen/strep [all Lonza]; pH 7.4) under hypoxic conditions (37°C, 5% O2, 5% CO2). Samples were cultured for 42 days, and the medium was changed three times a week.
Analysis
Native samples (n = 12 donors) were harvested at day 0, and each injection group was analyzed after 42 days of culture (n = 12 donors for each group). Samples were cut in half, and for each of the four assays (cell viability, biochemical assays and water content, gene expression, and histology), six half samples were used (corresponding to six donors for each assay).
Cell Viability
NP samples were incubated in 10 µM Calcein Blue-AM and 10 µM propidium iodide (both Molecular Probes, Invitrogen) in PBS for 2 hours. The viable cells (λ = 730 nm), dead cells (λ = 535 nm), and porcine NC location and morphology (λ = 488 nm; CFDA-SE staining) were imaged with a confocal microscope (CLSM 510 Meta, Zeiss, München, Germany). The viability was assessed both near the center and the outer regions of the explant.
Biochemical Assays and Water Content
The NP samples were weighed (wet weight) and stored at −30°C until further use. After overnight lyophilization (Freezone 2.5, Labconco, Kansas City, Missouri, United States), the sample weights were measured (dry weight). The water content was calculated as (wet weight − dry weight)/wet weight. The lyophilized samples were digested overnight at 60°C in a papain solution (100 mM phosphate buffer, 5 mM L-cysteine, 5 mM ethylenediaminetetraacetic acid, and 140 μg/mL papain, all from Sigma). The DNA, sulfated glycosaminoglycans, and hydroxyproline contents were measured in each sample. The amount of DNA per sample was measured with a Hoechst 33528 assay as described by Cesarone et al,16 using a standard curve of double-stranded calf thymus DNA (Sigma). The amount of sulfated glycosaminoglycans per sample relates to the proteoglycan content and was measured with a 1,9-dimethylmethylene blue assay as described by Farndale et al,17 based on a standard curve of chondroitin-sulfate from shark cartilage (Sigma). The amount of hydroxyproline relates to the collagen content and was measured with a chloramine-T assay as described by Huszar et al,18 based on a standard curve of trans-4-hydroxyproline (Sigma).
Gene Expression
Bovine NP samples were snap-frozen in liquid N2 and stored at −80°C for RNA isolation. RNA was isolated as described by van Dijk et al.15 Briefly, samples were disrupted with a Mikro-Dismembrator (Sartorius, Goettingen, Germany). RNA was isolated from the disrupted samples using TRIzol (Invitrogen) and purified on a spin column (RNeasy mini kit, Qiagen, Venlo, The Netherlands). The RNA concentration and purity were measured on a spectrophotometer (ND-1000, Isogen, de Meern, The Netherlands). Of each sample, 75 ng of mRNA was reverse transcribed to cDNA (SuperScript VILO kit, Invitrogen), and gene expression was determined by quantitative polymerase chain reaction (CFX384, Biorad, Veenendaal, The Netherlands). 18s was selected as the endogenous reference gene because it was found to be more stable than GAPDH and RPL13a in all groups. The genes of interest were collagen type I (COL1A1), collagen type II (COL2A1), and aggrecan (ACAN; Table 1). All primer pairs could amplify both porcine and bovine cDNA. Relative quantification was determined with the comparative 2−ΔCt method, with the expression of the gene of interest relative to the expression of the reference gene 18s.
Table 1.
List of primers for real-time quantitative polymerase chain reaction
| Genes of interest | GenBank accession number | Oligonucleotide sequence (5′ → 3′) | Product size (bp) |
|---|---|---|---|
| 18s a | Not available | Not available | Not available |
| Collagen type Ib | NM_001034039 | 142 | |
| Forward | TGAGAGAGGGGTTGTTGGAC | ||
| Reverse | GGGAGACCATTGAGTCCATC | ||
| Collagen type IIb | NM_001001135 | 114 | |
| Forward | CCAGCGTCCCCAAGAAGA | ||
| Reverse | CCAGGTTGTCATCTCCATGC | ||
| Aggrecanb | NM_173981 | 107 | |
| Forward | CCAACGAAACCTATGACGTGTACT | ||
| Reverse | GCACTCGTTGGCTGCCTC |
aPrimer Design Ltd (Southampton, United Kingdom).
bDesigned with Beacon designer software (Premier Biosoft, Palo Alto, California, United States) and ordered from Sigma (Zwijndrecht, The Netherlands).
Histology
Bovine NP samples were embedded in Tissue-Tek O.C.T. compound (SakuraTek, Zoeterwoude, The Netherlands), slash frozen in isopentane at −80°C, and stored at −30°C until further use. From each sample, 10-µm thick cryosections were cut (Microm HM 550, Thermo Fisher Scientific, Kalamazoo, United Kingdom) and mounted on polysine adhesion slides (Thermo Scientific). The slides were stained with Weigert's hematoxylin for cell nuclei and eosin for eosinophilic matrix. Images were taken with a bright-field microscope (Observer, Zeiss).
Statistics
With R-project software (version 3.0.2),19 Levene test and Shapiro-Wilk test were used to test for homogeneity of variance and normality, respectively. The biochemical data, both homogeneous and normally distributed, was analyzed with a one-way analysis of variance followed by a Bonferroni adjusted post hoc independent test. The gene expression data was found to be nonnormally distributed and was analyzed with Kruskal-Wallis test followed by a Bonferroni corrected post hoc Mann-Whitney U test. Statistical significance was assumed for p < 0.05.
Results
Changes in the NC Morphology
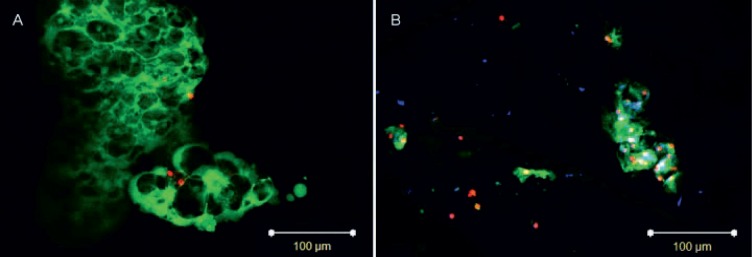
After 42 days of culture, the distribution and morphology of the NPCs in both culture groups were similar to native tissue (Fig. 1). Viable NCs were observed after culture (Figs. 1 and 2B), but the vacuolar structures were much smaller than before injection (Fig. 2A).
Fig. 1.
Staining of nucleus pulposus tissue on (A) day 0 and day 42 in the (B) sham or (C) notochordal cell–injected group. Cytoplasm and matrix are stained pink (eosin), cell nuclei black (hematoxylin). Scale bars = 100 μm. Representative of n = 6.
Fig. 2.
Notochordal cells (NCs) (A) prior to injection and (B) in nucleus pulposus tissue after 42 days of culture. Cytoplasm of NCs is stained in green (cell tracker carboxyfluorescein diacetate succinimidyl ester (CFDA-SE)), cytoplasm of all cells in blue (calcein blue-AM; only in B), and nuclei of dead cells in red (propidium iodide). Scale bars = 100 μm. Representative of n = 6.
NP Tissue Is Maintained during Culture, but Not Gene Expression
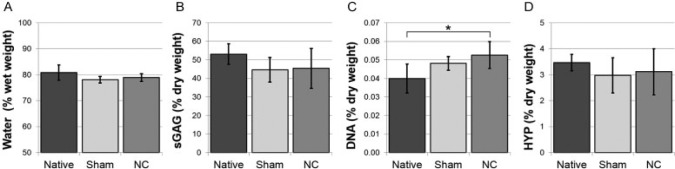
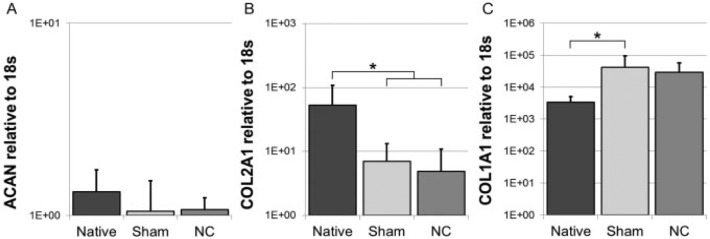
As expected, DNA content in the NC group was larger than in the native samples at day 0 due to the injection of NCs (p = 0.016), but that of the sham-injected group was not significantly different (Fig. 3C). For both culture groups, the water and proteoglycan contents remained statistically unchanged over time (Fig. 3A, B), which was corroborated by the ACAN expression (Fig. 4A). The collagen content remained unchanged, but gene expression data indicates that there was a shift in the type of collagen: COL2A1 decreased in both groups (p = 0.003 NC, p = 0.004 sham), whereas COL1A1 increased statistically only in the sham group (p = 0.01; Fig. 4B, C).
Fig. 3.
The water content (A) per wet weight, and (B) sulfated glycosaminoglycans (sGAG), (C) DNA, and (D) hydroxyproline (HYP) content per dry weight on day 0 (native) and day 42 (sham or notochordal cell [NC] injection). Values are mean ± standard deviation; n = 6 (sham group: n = 5). *p < 0.05.
Fig. 4.
Gene expression of (A) aggrecan (ACAN), (B) collagen type II (COL2A1), and (C) collagen type I (COL1A1) relative to 18s on day 0 (native) and day 42 (sham or notochordal cell [NC] injection). Values are mean ± standard deviation; n = 6. *p < 0.05. Please note the logarithmic y-axes and error bars.
Discussion
This study is the first to show that translating the results of in vitro coculture of NCs/NPCs to in situ repair in tissue is not straightforward. Whereas previous studies have shown that NCs were able to stimulate extracellular matrix production and related gene expression by isolated NPCs,9–13 this effect was not observed when NCs were injected in NP explants.
This discrepancy may be explained by two mechanisms: the different ratio of NC to NPC used or the initial presence of a proteoglycan-rich extracellular matrix. First, the NC-to-NPC ratio in the current study was approximately 20:80, whereas this ratio was 50:50 in previous direct cell cocultures.8,9 In a therapeutic perspective, however, a ratio of 50:50 is not realistic: due to the nutrient limitations of the intervertebral disk in vivo, multiplying the local cell density by 2 may lead to the death of both endogenous and exogenous cell populations. Moreover, a ratio of 30:70, close to what was used here, was also reported to have a positive, even if milder, effect on NPCs.8 Second, in the in vitro studies, the NPCs were isolated from their extracellular matrix prior to coculture in alginate. Because the matrix produced during culture is compared with the initial amount, which is small (or even nonexistent) in alginate cultures after cell isolation, production of proteoglycans will appear as large increases in matrix production. Contrarily, the initial amount of matrix in the explants is high, and the matrix production per cell has to increase to a great extent to change the total matrix content. Because of this difference, it is unclear how the matrix production rates by NPCs in alginate cocultures relate to those in tissue. However, it has been observed that the expression of ACAN and COL2A1 decreased significantly after NPC isolation, but gradually increased in three-dimensional culture (agarose) until reaching native gene expression levels by day 25 of culture.20 This result indicates that the isolation procedure and three-dimensional culture of NPCs have an effect on their phenotype and metabolism. The proteoglycan production by NPCs in three-dimensional culture (alginate and agarose) also increased in a time-dependent manner,20,21 but was not compared with the native proteoglycan production rate.
Similarly, the isolation of NCs from their native environment affects the cell phenotype. The native expression of brachyury (T), cytokeratins 8 and 18, ACAN, and COL2A1 by NCs decreased significantly after 1 day of culture in alginate beads.22 In addition, NCs are sensitive to the culture conditions: ascorbic acid, physiologic osmolarity, medium type, and hypoxia contribute to maintenance of their phenotype, whereas a pH < 7.2,24 both very low and high glucose concentrations, and FBS in the culture medium (unpublished data from our group) are detrimental to the phenotype.22,24,26,27 The presence of FBS in the medium during the 42 days of culture could explain the disappearance of NC vesicles by the end of the culture. However, the morphologic changes can also be attributed to the isolation of NCs from their natural environment and culture in a mature NP.
The change in morphology is probably coupled to a change in functionality of the NCs. If the morphology and gene expression profiles of NCs change gradually during culture, this result could explain that the regulatory effect of NCs is stronger in short-term (3 days) than in long-term (>14 days) coculture.8,9,12 In this respect, the use of NC-conditioned medium may be a solution for long-term in situ cultures. Medium conditioned by NCs for 4 days has indeed been shown to increase the proteoglycan production by NPCs in in vitro cultures,9,11 in levels even higher than in cocultures with direct cell–cell contact.9 These results indicate that the NCs are stimulating NPCs via soluble molecular factors. The administration of soluble factors in fact could be more attractive than exogenous cell injection as smaller-gauge needles can be used. Needle punctures of 25 gauge (required for cell injection) have been shown to have a detrimental effect on disk biomechanics.28 This effect may be diminished by the use of smaller needles.
The NP explants were cultured in hypertonic PEG, as described by van Dijk et al.29 This culture system previously showed a stable biochemical content and COL2A1 gene expression during long-term culture (42 days), but native ACAN and COL1A1 expression were not maintained.29 In the current study, the expression of the latter genes did not decrease, which may be due to two differences in culture conditions: (1) injection of PBS prior to culture, or (2) the pH of the culture medium, which was not adjusted to 7.1 in the current study. It is possible that the injection of PBS in the tissue triggered a change in gene expression, but an anabolic response would not be expected. Furthermore, it has been described that the proteoglycan synthesis by NPCs is higher at pH 7.1 than at 7.4,30 which is not reflected in the proteoglycan content and related ACAN gene expression in the current study compared with the previous one.15 Improved NP culture systems are currently available for future experiments.29,31
Although the results from this study show that direct injections of NCs are not effective for NP regeneration, this finding should be interpreted with care for translation to the human situation. First of all, the NP tissue used in this study corresponded to human grade II on the Thompson grading scale.32 Although the mechanical loading of bovine caudal tissue may be more similar to that in the human cervical rather than lumbar spine, the biochemical composition and cell population of bovine and human grade II NP tissue have been shown to be similar.33 Although early stage regeneration would theoretically be ideal, the regenerative potential of these cells may be less than for cells of a higher grade of degeneration.34 Therefore, human tissues of more advanced degeneration may be more physiologically relevant. Second, although the issue of interspecies size differences was avoided by using an NP explant culture,35 such a system also introduced drawbacks. Interplay with the surrounding tissues was not possible, and certain physiologic factors (i.e., dynamic loading, limited nutrient supply) could not be simulated in the current system.36
Molecular-based regenerative therapies are aimed for treatment of NPs with moderate degeneration. In this stage, the resident cells are still chondrocytic in nature, able to produce matrix, and the degenerative changes are not yet insurmountable. Cell-based regenerative therapies are considered applicable for more advanced degeneration, where the depleted, senescent, or dedifferentiated resident cell population would need additional help to replenish the extracellular matrix.37 However, the sensitivity of detection methods for moderate disk degeneration is currently insufficient, and more importantly, our inability to provide accurate prognosis for aggressive symptomatic disk degeneration is actually a challenge to introduce early preventive treatments. Certainly, as more sensitive diagnostic tools will be developed, the selection of patients for disk regeneration will become clearer.
Conclusions
Contrary to alginate coculture, no anabolic response was observed when NCs were injected into NP explants, which may be due to the observed changed morphology of the NCs during the 42 days of culture where they became more similar in appearance to resident NPCs. Therefore, considering the results with NC-conditioned medium, it may be a more promising alternative for in situ stimulation of NPCs.
Footnotes
Disclosures: Irene T. M. Arkesteijn: none
Esther Potier: none
Keita Ito: none
References
- 1.Trout JJ, Buckwalter JA, Moore KC, Landas SK. Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell 1982;14(2):359–369 [DOI] [PubMed] [Google Scholar]
- 2.Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther 2010;12(1):R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilson A, Dreger M, Urban JP. Differential expression level of cytokeratin 8 in cells of the bovine nucleus pulposus complicates the search for specific intervertebral disc cell markers. Arthritis Res Ther 2010;12(1):R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao C-Q, Wang L-M, Jiang L-S, Dai L-Y. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev 2007;6(3):247–261 [DOI] [PubMed] [Google Scholar]
- 5.Liebscher T, Haefeli M, Wuertz K, Nerlich AG, Boos N. Age-related variation in cell density of human lumbar intervertebral disc. Spine (Phila Pa 1976) 2011;36(2):153–159 [DOI] [PubMed] [Google Scholar]
- 6.Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng 2003;9(4):667–677 [DOI] [PubMed] [Google Scholar]
- 7.Hunter CJ, Bianchi S, Cheng P, Muldrew K. Osmoregulatory function of large vacuoles found in notochordal cells of the intervertebral disc running title: an osmoregulatory vacuole. Mol Cell Biomech 2007;4(4):227–237 [PMC free article] [PubMed] [Google Scholar]
- 8.Gantenbein-Ritter B, Chan SCW. The evolutionary importance of cell ratio between notochordal and nucleus pulposus cells: an experimental 3-D co-culture study. Eur Spine J 2012;21(Suppl 6):S819–S825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res 1999;246(1):129–137 [DOI] [PubMed] [Google Scholar]
- 10.Abbott RD, Purmessur D, Monsey RD, Iatridis JC. Regenerative potential of TGFβ3 + Dex and notochordal cell conditioned media on degenerated human intervertebral disc cells. J Orthop Res 2012;30(3):482–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erwin WM, Inman RD. Notochord cells regulate intervertebral disc chondrocyte proteoglycan production and cell proliferation. Spine (Phila Pa 1976) 2006;31(10):1094–1099 [DOI] [PubMed] [Google Scholar]
- 12.Potier E, de Vries S, van Doeselaar M, Ito K. Potential application of notochordal cells for intervertebral disc regeneration: an in vitro assessment. Eur Cell Mater 2014;28:68–80, discussion 80–81 [DOI] [PubMed] [Google Scholar]
- 13.de Vries SA, Potier E, van Doeselaar M, Meij BP, Tryfonidou MA, Ito K. Conditioned medium derived from notochordal cell-rich nucleus pulposus tissue stimulates matrix production by canine nucleus pulposus cells and bone marrow-derived stromal cells. Tissue Eng Part A 2015;21(5–6):1077–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erwin WM, Ashman K, O'Donnel P, Inman RD. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum 2006;54(12):3859–3867 [DOI] [PubMed] [Google Scholar]
- 15.van Dijk B, Potier E, Ito K. Culturing bovine nucleus pulposus explants by balancing medium osmolarity. Tissue Eng Part C Methods 2011;17(11):1089–1096 [DOI] [PubMed] [Google Scholar]
- 16.Cesarone CF, Bolognesi C, Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem 1979;100(1):188–197 [DOI] [PubMed] [Google Scholar]
- 17.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 1986;883(2):173–177 [DOI] [PubMed] [Google Scholar]
- 18.Huszar G, Maiocco J, Naftolin F. Monitoring of collagen and collagen fragments in chromatography of protein mixtures. Anal Biochem 1980;105(2):424–429 [DOI] [PubMed] [Google Scholar]
- 19.R Core Team. R: A Language and Environment for Statistical Computing. 2014. Available at: http://www.r-project.org. Accessed August 15, 2015 [Google Scholar]
- 20.Iwata M, Ochi H, Asou Y, et al. Variations in gene and protein expression in canine chondrodystrophic nucleus pulposus cells following long-term three-dimensional culture. PLoS ONE 2013;8(5):e63120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melrose J, Smith S, Ghosh P, Taylor TKF. Differential expression of proteoglycan epitopes and growth characteristics of intervertebral disc cells grown in alginate bead culture. Cells Tissues Organs 2001;168(3):137–146 [DOI] [PubMed] [Google Scholar]
- 22.Spillekom S, Smolders LA, Grinwis GCM, et al. Increased osmolarity and cell clustering preserve canine notochordal cell phenotype in culture. Tissue Eng Part C Methods 2014;20(8):652–662 [DOI] [PubMed] [Google Scholar]
- 23.Rastogi A, Thakore P, Leung A, et al. Environmental regulation of notochordal gene expression in nucleus pulposus cells. J Cell Physiol 2009;220(3):698–705 [DOI] [PubMed] [Google Scholar]
- 24.Guehring T, Wilde G, Sumner M, et al. Notochordal intervertebral disc cells: sensitivity to nutrient deprivation. Arthritis Rheum 2009;60(4):1026–1034 [DOI] [PubMed] [Google Scholar]
- 25.Erwin WM, Las Heras F, Islam D, Fehlings MG, Inman RD. The regenerative capacity of the notochordal cell: tissue constructs generated in vitro under hypoxic conditions. J Neurosurg Spine 2009;10(6):513–521 [DOI] [PubMed] [Google Scholar]
- 26.Park E-Y, Park J-B. Dose- and time-dependent effect of high glucose concentration on viability of notochordal cells and expression of matrix degrading and fibrotic enzymes. Int Orthop 2013;37(6):1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Won H-Y, Park J-B, Park E-Y, Riew KD. Effect of hyperglycemia on apoptosis of notochordal cells and intervertebral disc degeneration in diabetic rats. J Neurosurg Spine 2009;11(6):741–748 [DOI] [PubMed] [Google Scholar]
- 28.Korecki CL, Costi JJ, Iatridis JC. Needle puncture injury affects intervertebral disc mechanics and biology in an organ culture model. Spine (Phila Pa 1976) 2008;33(3):235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Dijk BGM, Potier E, Ito K. Long-term culture of bovine nucleus pulposus explants in a native environment. Spine J 2013;13(4):454–463 [DOI] [PubMed] [Google Scholar]
- 30.Ohshima H, Urban JP. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine (Phila Pa 1976) 1992;17(9):1079–1082 [DOI] [PubMed] [Google Scholar]
- 31.Arkesteijn ITM, Mouser VHM, Mwale F, van Dijk BGM, Ito K. A well-controlled nucleus pulposus tissue culture system with injection port for evaluating regenerative therapies. Ann Biomed Eng 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine (Phila Pa 1976) 1990;15(5):411–415 [DOI] [PubMed] [Google Scholar]
- 33.Demers CN, Antoniou J, Mwale F. Value and limitations of using the bovine tail as a model for the human lumbar spine. Spine (Phila Pa 1976) 2004;29(24):2793–2799 [DOI] [PubMed] [Google Scholar]
- 34.Yang S-H, Lin C-C, Hu M-H, Shih TT-F, Sun Y-H, Lin F-H. Influence of age-related degeneration on regenerative potential of human nucleus pulposus cells. J Orthop Res 2010;28(3):379–383 [DOI] [PubMed] [Google Scholar]
- 35.Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration?. Eur Spine J 2008;17(1):2–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urban JPG, Smith S, Fairbank JCT. Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 2004;29(23):2700–2709 [DOI] [PubMed] [Google Scholar]
- 37.Ho G, Leung VYL, Cheung KMC, Chan D. Effect of severity of intervertebral disc injury on mesenchymal stem cell-based regeneration. Connect Tissue Res 2008;49(1):15–21 [DOI] [PubMed] [Google Scholar]