Abstract
Study Design
Retrospective study.
Objective
To assess the learning curve of microendoscopic decompression surgery for lumbar spinal canal stenosis (LSCS).
Methods
Four hundred eighty LSCS cases involving 753 stenotic lesions limited to the intraspinal canal were treated with microendoscopic decompression by a single surgeon at an institution between November 2006 and January 2015. They were numbered chronologically, and the operating time, intraoperative blood loss, and perioperative complications were investigated. Surgical outcomes were evaluated using the Japanese Orthopedic Association (JOA) score for low back pain before and 1 year after the operation.
Results
The mean operating time per level was 66.1 minutes. There was a progressive reduction in the operating time through the case series, and the approximate curve seemed to be y = − 9.4Ln(x) + 115.0. The blood loss per level, which showed a mean value of 15.0 mL, was more than 50 mL in only 2.7% of the cases after case no. 30 and in 20% of the cases before it. There were 10 (2.1%) cases of perioperative complications, which occurred even after the surgeon had gained mastery of the procedure. The median JOA score improved significantly from 17 points preoperatively to 26 points postoperatively.
Conclusions
The learning curve of microendoscopic decompression surgery for LSCS has been defined with data for a single surgeon in an institution. The operating time seems to decrease along a natural logarithmic function. The intraoperative blood loss stabilizes after the first 30 cases, whereas perioperative complications can occur at any time even after mastery of the technique.
Keywords: learning curve, lumbar spinal canal stenosis, microendoscopic decompression, minimally invasive surgery, spinal surgeons
Introduction
The indications for microendoscopic spinal decompression for lumbar lesions, which was first reported in 1997 by Foley and Smith as diskectomy for lumbar disk herniation,1 have been expanded to include lumbar spinal canal stenosis (LSCS). This minimally invasive muscle-dilating technique provides wide visualization through oblique lenses and allows bilateral decompression via a unilateral approach through a partial resection of the base of the spinous process, thereby preserving the supraspinous and interspinous ligaments and the contralateral musculature.2 Minamide et al reported that microendoscopic laminotomy was a safe and very effective approach for the treatment of degenerative LSCS according to their 2-year follow-up results.3
In the cases of severe LSCS, however, the anatomy often shows considerable changes because of degeneration. The endoscope provides a view from close to the point of surgery, which can partly be responsible for disorientation in the operative field. Thus, video-assisted surgery performed through a tubular retractor naturally demands particular surgical skills different from those of traditional open surgeries. Surgeons require time to get skilled in such procedures, which results in a “learning curve.”
Although the learning curve of microendoscopic diskectomy (MED) has often been mentioned in the past,4 5 6 7 each report presented too few cases to discuss the learning curve as a “curve.” Moreover, the learning curves of the minimally invasive surgery for LSCS were seldom discussed in the literature.8 Understanding these learning curves is important because of their implications for surgical behavior and training and because of their role in assessments of the efficacy of the procedure. The present study therefore aims to assess the learning curve of microendoscopic decompression surgery for LSCS using data from the numerous cases in our institution.
Methods
The first author of this report had 10 years of experience as an orthopedic clinician, including 5 years as a spinal surgeon, before MED training in 2005. The indications of microendoscopic decompression surgery had been limited to lumbar disk herniation before November 2006 (35 cases) and were expanded to include LSCS thereafter. A total of 1,352 patients underwent microendoscopic decompression surgery performed by the first author for cervical, thoracic, and lumbar spine lesions between November 2006 and January 2015. Of these, 480 cases involved LSCS with lesions limited to the intraspinal canal (284 men and 196 women; mean age at the time of surgery, 68.2 years; range, 38 to 93 years). All the patients had been diagnosed as having LSCS based on clinical findings, magnetic resonance imaging, computed tomography, and additional examinations including radicular block and electromyography as needed. All of them complained of leg symptoms, for which conservative treatment had proven ineffective. Patients showing LSCS caused by spondylolisthesis with segmental instability proven by dynamic X-ray examination were included in this series unless the chief complaint was low back pain. Cases of diskectomy were not included in the present study. The number of decompressed intervertebral levels was one in 243 patients, two in 205 patients, three in 28 patients, and four in 4 patients. Thus, a total of 753 stenotic levels were decompressed. Typical comorbidities were degenerative spondylolisthesis, calcified/ossified ligament flavum, and adjacent segment disease after traditional posterolateral lumbar fusion (Table 1).
Table 1.
Patient demographics (n = 480)
| Age (y) | |
| Average | 68.2 |
| Range | 38–93 |
| Sex (n) | |
| Male | 284 |
| Female | 196 |
| Number of operated levels (n) | |
| One | 243 |
| Two | 205 |
| Three | 28 |
| Four | 4 |
| Level of stenosis (n) | |
| L1–L2 | 4 |
| L2–L3 | 66 |
| L3–L4 | 218 |
| L4–L5 | 398 |
| L5–S1 | 67 |
| Comorbidity (n) | |
| Degenerative spondylolisthesis | 177 |
| Calcified ligamentum flavum | 51 |
| Ossified ligamentum flavum | 15 |
| Adjacent segment disease after PLF (revision after open surgery) | 24 |
Abbreviation: PLF, posterolateral lumbar fusion.
The cases were numbered in chronological order and the following items were investigated: (1) operating time per level (the time from skin incision to skin closure divided by the number of operated levels), (2) intraoperative blood loss per level (the blood loss collected via suction from skin incision to skin closure divided by the number of operated levels), and (3) perioperative complications. Over 1 year had passed since the operation in all 480 cases, and 390 cases could be followed at least 1 year after the operation. Surgical outcomes were evaluated using the Japanese Orthopedic Association (JOA) score for low back pain (29-point scale; Table 2) before and 1 year after the operation. The JOA scores were analyzed using the Mann-Whitney U test. Probability values of <0.05 were considered to be statistically significant. The data was collected and analyzed with a Microsoft Excel 2010 (Microsoft Corp., Seattle, Washington, United States) spreadsheet. The hospital ethical committee approved the study.
Table 2.
Japanese Orthopaedic Association score for low back pain (29-point scale)
| Item | Points |
|---|---|
| Subjective symptoms (9 points) | |
| Low back pain | 3, 2, 1, 0 |
| Leg pain and/or tingling | 3, 2, 1, 0 |
| Gait | 3, 2, 1, 0 |
| Clinical signs (6 points) | |
| Straight-leg-raising test | 2, 1, 0 |
| Sensory disturbance | 2, 1, 0 |
| Motor disturbance | 2, 1, 0 |
| Restriction of activities of daily living (14 points) | |
| Turning over while lying | 2, 1, 0 |
| Standing | 2, 1, 0 |
| Washing | 2, 1, 0 |
| Leaning forward | 2, 1, 0 |
| Sitting (1 h) | 2, 1, 0 |
| Lifting or holding heavy object | 2, 1, 0 |
| Walking | 2, 1, 0 |
| Urinary bladder function | 0, −3, −6 |
Surgical Procedures
The patient under general anesthesia was placed prone on a laminectomy frame. The operation levels were confirmed using an X-ray image intensifier and were marked on the skin with ink. The METRx system (Medtronic Sofamor Danek, Memphis, Tennessee, United States) was used for the operation. A 16-mm paramedian skin incision was usually sufficient for decompression at as many as three levels. An additional skin incision was made if the skin did not move sufficiently in the craniocaudal direction to allow decompression at three levels or more. The 16-mm skin incision was made ∼7 mm lateral to a spinous process. The muscle was sequentially dilated after fasciotomy, and a tubular retractor of 16-mm diameter was placed. The muscles covering the lamina and ligamentum flavum were carefully resected, and the bony structure was exposed. The surgical level was reconfirmed using an X-ray image intensifier.
The midline of the spinal canal was confirmed first by resecting the base of the spinous process with a high-speed drill. The base of the spinous process, which often obstructed the placement of the tubular retractor, was resected partially to secure a sufficiently large working space. The lamina was resected using a high-speed drill as far as the attachment of ligamentum flavum to the lamina. Once the ligament was detached from the bone, bleeding from the epidural space and dural pulsation through the ligament were seen. The ligamentum flavum was split from the midline like French doors, using a ball-tipped probe, and resected. Curved Kerrison rongeurs and a curved high-speed drill enlarged the lateral recess while keeping the facet joint intact. The end point of decompression was the outer edges of the nerve roots on both sides. After hemostasis and lavage, a drain was placed at every operated level, and the incision was closed in layers with 2–0 Vicryl Plus (Ethicon, Inc., Somerville, New Jersey, United States) and Steristrips (3M Health Care, St. Paul, Minnesota, United States).
Ambulation was allowed ∼5 hours after the surgery without a brace. Rehabilitation was started the day after the operation. Drains were removed 2 days after the operation. Most patients were discharged from the hospital between 6 and 8 days of the microendoscopic surgery. Every patient was funded by a public health insurance system.
Results
For the microendoscopic decompression in cases of LSCS, the mean operating time per level was 66.1 minutes (range, 23 to 165 minutes). There was a progressive reduction in the operating time over the span of the 480 cases (Fig. 1). The contribution rate (R 2) was 0.20 when the approximate curve was determined with the natural logarithmic function: y = − 9.4Ln(x) + 115.0.
Fig. 1.
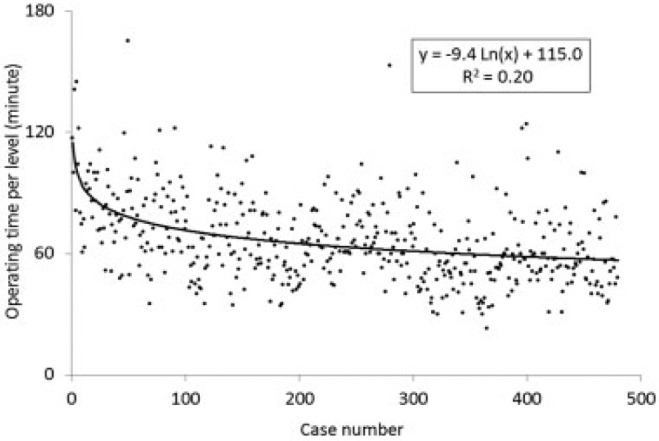
Scatterplot showing the learning curve of microendoscopic decompression surgery for lumbar spinal canal stenosis as depicted by operating time per level. When the approximate curve was determined with a natural logarithmic function, its contribution rate (R 2) was 0.20.
The mean intraoperative blood loss per level was 15.0 mL (range, 0.3 to 268 mL). The blood loss per level was more than 50 mL in only 2.7% of the cases after case no. 30 and in 20% of the cases before it (Fig. 2). Perioperative complications occurred in 10 cases (2.1%; Table 3). Nine cases involved dural tears, all of which were pinholes and were repaired with a patch technique without open conversion.9 These cases consisted of severe central canal stenosis, postoperative epidural fibrosis, and calcified/ossified ligamentum flavum. There was one episode of postoperative epidural hematoma, which required a microendoscopic hematoma removal because of the worsening pain. None of the patients showed postoperative deterioration in neurologic status or prolongation of the hospital stay. Perioperative complications occurred even though the surgeon gained experience from numerous cases.
Fig. 2.
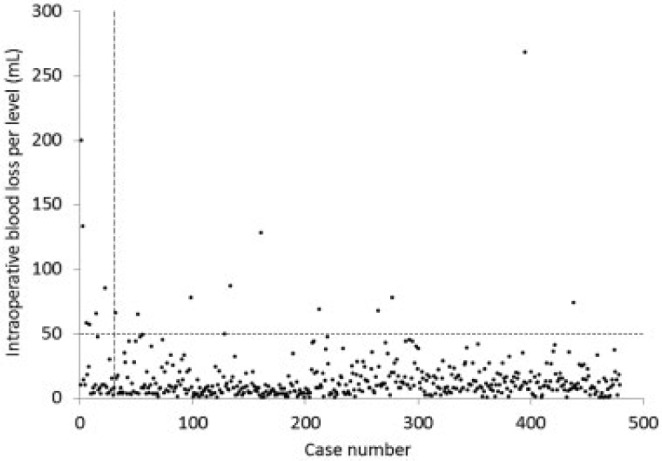
Scatterplot as depicted by intraoperative blood loss per level. The vertical broken line is drawn at case no. 30 and the horizontal one is at 50 mL of the intraoperative blood loss per level.
Table 3.
Perioperative complications
| Case no. | Complication | Cause | Salvage |
|---|---|---|---|
| 54 | Dural tear | Epidural adhesion | Patch technique |
| 64 | Dural tear | Calcified ligamentum flavum | Patch technique |
| 90 | Dural tear | Severe central canal stenosis | Patch technique |
| 216 | Epidural hematoma | Inadequate hemostasis | Hematoma removal |
| 274 | Dural tear | Severe central canal stenosis | Patch technique |
| 284 | Dural tear | Ossified ligamentum flavum | Patch technique |
| 390 | Dural tear | Severe central canal stenosis | Patch technique |
| 394 | Dural tear | Severe central canal stenosis | Patch technique |
| 400 | Dural tear | Postoperative epidural fibrosis | Patch technique |
| 441 | Dural tear | Ossified ligamentum flavum | Patch technique |
The postoperative follow-up rate at 1 year was 81.3%. Ten cases required additional operations within a year due to coexisting extraforaminal stenosis, postoperative facet cysts, and subsequent disk herniation, all of which were treated again with microendoscopic surgery. No cases required fixation within a year in the traceable patients. The median JOA scores for low back pain were 17 points before the operation and 26 points 1 year after surgery, and the improvement was statistically significant (p < 0.001).
Discussion
Where there is a procedure, there is a learning curve. Surgeons need time and patience to attain the skill to perform any operative procedure, and microendoscopic spinal surgery is no exception. Although the learning curve of MED has often been discussed previously,4 5 6 7 that of microendoscopic decompression surgery for LSCS has been seldom discussed.8 This study is unique in that it demonstrates the learning process for a surgeon in a single institution with 480 operative cases.
The asymptote of a specific operation is determined by the operating time, intraoperative blood loss, operation effectiveness, and complications as well as surgeons' preference for the procedure (microendoscopy) over the traditional one (open surgery).10 11 12 In the case of MED, Nowitzke reported that the asymptote of operating time was reached at ∼30 cases.4 In another report about the learning curve for MED, the operation time and blood loss tended to become steady after the first 20 cases, then they declined gradually.5 Both reports assumed an asymptote in describing the learning curve for MED.
In the present study on the learning curve for LSCS, objective indexes such as operating time, intraoperative blood loss, and perioperative complications were utilized for evaluation. The indexes appeared to show different trends. The operating time decreased gradually along a natural logarithmic function through the case series. Intraoperative blood loss stabilized after the first 30 cases, whereas perioperative complications occurred even after the operation was performed by a surgeon with expertise. Because the surgeon had been familiar with MED before the first case of the present study, the learning curve of LSCS decompression cases did not include the time to master the basic techniques of microendoscopy in common with MED. Therefore, the steep decline in the operating time during the early phase may be attributable to the accurate placement of a tubular retractor at the desired level and to the control of bleeding. Unlike MED cases, in LSCS cases, the interlaminar space often disappeared and the anatomy altered considerably because of degeneration. In particular, the surgeon required 20 minutes for tubular retractor placement in the early phase, but only required 3 to 5 minutes for the same procedure in the later phase. With the increase in the surgeon's experience, the procedure was applied to a wider variety of cases that were usually more technically demanding and complex, including severe central canal stenosis, calcified/ossified ligamentum flavum, and reoperation after open surgery. This change in the complexity of surgery needs to be considered during interpretation of the perioperative complications.
Many surgeons seem to have limited the indications for spinal microendoscopy to lumbar disk herniation and avoid the procedure for LSCS due to the unfamiliar environment. Initial case selection is important to continue the procedure without major complications. In addition, many pitfalls that beginners encounter in this procedure can be avoided easily with guidance from an instructor. Thus, the best method of learning microendoscopic decompression surgery for LSCS seems to be surgical training under clinical supervision of an instructor in a teaching environment at the early phase of the learning curve.
Conclusions
The learning curve of microendoscopic decompression surgery for LSCS has been defined with data for a single surgeon in an institution. The operating time seemed to decrease along a natural logarithmic function. The intraoperative blood loss stabilized after the first 30 cases, whereas perioperative complications occurred at any time even after mastery of the technique.
Acknowledgments
We thank Masaki Kawai, Motohiro Okada, Shin-ichi Nakao, Yosuke Nakamura, Kenichi Yawatari, Tomowaki Nakagawa, Takao Minami, and Akikazu Sumiya for their assistance in the operations.
Footnotes
Disclosures: Kazunori Nomura: none
Munehito Yoshida: none
References
- 1. Foley KT, Smith MM. Microendoscopic discectomy Tech Neurosurg 1997; 3: 301– 307 [Google Scholar]
- 2. Nomura K, Yoshida M. Microendoscopic decompression surgery for lumbar spinal canal stenosis via the paramedian approach: preliminary results Global Spine J 2012; 2 (2): 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Minamide A, Yoshida M, Yamada H, et al. Endoscope-assisted spinal decompression surgery for lumbar spinal stenosis J Neurosurg Spine 2013; 19 (6): 664–671 [DOI] [PubMed] [Google Scholar]
- 4. Nowitzke AM. Assessment of the learning curve for lumbar microendoscopic discectomy Neurosurgery 2005; 56 (4): 755–762, discussion 755–762 [DOI] [PubMed] [Google Scholar]
- 5. Rong LM, Xie PG, Shi DH, et al. Spinal surgeons' learning curve for lumbar microendoscopic discectomy: a prospective study of our first 50 and latest 10 cases Chin Med J (Engl) 2008; 121 (21): 2148–2151 [PubMed] [Google Scholar]
- 6. Nomura K, Yoshida M, Kawai M, et al. Microendoscopic discectomy as a minimally invasive surgery for lumbar disc herniation: technical training and learning curve The Journal of the Japanese Society for Spine Surgery and Related Research 2009; 20: 649–652 [Google Scholar]
- 7. Ohashi M, Yamazaki A, Watanabe K, et al. Learning curve for microendoscopic lumbar discectomy: a comparative study among 3 spinal surgeons J Spine Res 2011; 2: 1342–1345 [Google Scholar]
- 8. Mannion RJ, Guilfoyle MR, Efendy J, Nowitzke AM, Laing RJ, Wood MJ. Minimally invasive lumbar decompression: long-term outcome, morbidity, and the learning curve from the first 50 cases J Spinal Disord Tech 2012; 25 (1): 47–51 [DOI] [PubMed] [Google Scholar]
- 9. Shibayama M, Mizutani J, Takahashi I, Nagao S, Ohta H, Otsuka T. Patch technique for repair of a dural tear in microendoscopic spinal surgery J Bone Joint Surg Br 2008; 90 (8): 1066–1067 [DOI] [PubMed] [Google Scholar]
- 10. Cook JA, Ramsay CR, Fayers P. Statistical evaluation of learning curve effects in surgical trials Clin Trials 2004; 1 (5): 421–427 [DOI] [PubMed] [Google Scholar]
- 11. Righesso O, Falavigna A, Avanzi O. Comparison of open discectomy with microendoscopic discectomy in lumbar disc herniations: results of a randomized controlled trial Neurosurgery 2007; 61 (3): 545–549, discussion 549 [DOI] [PubMed] [Google Scholar]
- 12. Palmer S, Turner R, Palmer R. Bilateral decompression of lumbar spinal stenosis involving a unilateral approach with microscope and tubular retractor system J Neurosurg 2002; 97 (2, Suppl): 213–217 [DOI] [PubMed] [Google Scholar]


