Abstract
Study Design
Systematic review and meta-analysis.
Objective
Anterior cervical diskectomy and fusion (ACDF) is an effective surgical option for patients with cervical radiculopathy, myelopathy, or deformity. Although ACDF is generally safe, dysphagia is a common complication. Despite its high incidence, prolonged postoperative dysphagia is poorly understood; its etiology remains relatively unknown, and its risk factors are widely debated.
Methods
We searched MEDLINE, Scopus, Web of Science, and Embase for studies reporting complications for cervical diskectomy with plating. We recorded dysphagia events from all included studies and calculated effect summary values, 95% confidence intervals (CIs), Q values, and I 2 values.
Results
Of the 7,780 retrieved articles, 14 met inclusion criteria. The overall dysphagia rate was 8.5% (95% CI 5.7 to 11.3%). The rate of moderate or severe dysphagia was 4.4% (0.4 to 8.4%). Follow-up times of <12, 12 to 24, and >24 months reported rates of 19.9% (6.0 to 33.7%), 7.0% (5.2 to 8.7%), and 7.6% (1.4 to 13.8%), respectively. Studies utilizing the Bazaz Dysphagia Score resulted in an increase in dysphagia diagnosis relative to studies with no outlined criteria (19.8%, 5.9 to 33.7% and 6.9%, 3.7 to 10.0%, respectively), indicating that the criteria used for dysphagia identification are critical. There was no difference in dysphagia rate with the use of autograft versus allograft.
Conclusions
This review represents a comprehensive estimation of the actual incidence of dysphagia across a heterogeneous group of surgeons, patients, and criteria. The classification scheme for dysphagia varied significantly within the literature. To ensure its diagnosis and identification, we recommend the use of a standardized, well-outlined method for dysphagia diagnosis.
Keywords: anterior cervical diskectomy and fusion, ACDF, complications, dysphagia, systematic review, meta-analysis
Introduction
Anterior cervical diskectomy and fusion (ACDF) has been shown to yield positive clinical outcomes in patients with cervical radiculopathy, myelopathy, or deformity.1,2 Although ACDF is generally safe, dysphagia is a common complication.3,4 In fact, some authors consider some degree of dysphagia an inevitable outcome after ACDF rather than a complication.5,6 Despite its high incidence, prolonged postoperative dysphagia is poorly understood; its etiology remains relatively unknown, its treatment largely unexplored, and its risk factors widely debated.3,7–10 Several potential causes have been suggested, including the thickness of the cervical plate, prevertebral soft tissue swelling, and significant esophageal retraction, but the literature demonstrates limited evidence for each proposed etiology.11–17 Because of its multifactorial nature, few authors have explored ways of preventing or treating dysphagia.10,18–21 Furthermore, a wide array of patient and intraoperative factors—age, sex, body mass index, operative time, number of surgical levels, among others—have been studied as possible risk factors with inconsistent findings across multiple studies.14,15,17,19,21–25
Variation also exists in the incidence of dysphagia reported after ACDF, with various studies yielding estimates ranging from 1 to 79%.3,6,19,23–29 This variation, in part, may be attributed to the retrospective nature of many of these studies. As such, they are subject to surgeon recall and bias.20 Of note, Edwards et al found a poor correlation between surgeon records and patient surveys for dysphagia, indicating that its incidence may be underreported in retrospective studies.20 Variation in the reported incidence of dysphagia among different studies may also result from patient evaluations occurring at different times following surgery.30 Although dysphagia may persist for months or years, the symptoms are typically transient.29,31 Thus, the time of evaluation and follow-up influences the rate of diagnosis.
A variation in the incidence of postoperative dysphagia also arises in part from the lack of standard criteria for its diagnosis and measurement. Although there is no universally accepted instrument for objectively assessing dysphagia after ACDF, the most widely used method is the Bazaz Dysphagia Score.23,32 The Bazaz Score grades a patient's dysphagia as none, mild, moderate, or severe based on its frequency and the kinds of foods that precipitate the dysphagia.19 It was subsequently modified into a 10-point scale assessed over 4 nonconsecutive days. Dysphagia is then defined as an aggregate score greater than 12.14 However, neither of these two questionnaires has been validated, and their accuracy remains in question despite their use in multiple studies.14,19
Accurate knowledge of the adverse outcomes following ACDF is essential for both patients and surgeons. An analysis of the overall incidence of dysphagia after ACDF would be useful in educating patients and surgeons during the informed consent process and patient follow-up. We conducted a systematic literature review and meta-analysis to estimate the incidence of dysphagia following ACDF with plate fixation and to characterize significant differences in the rate of dysphagia associated with various graft materials, follow-up duration, and diagnostic criteria.
Methods
Study Search
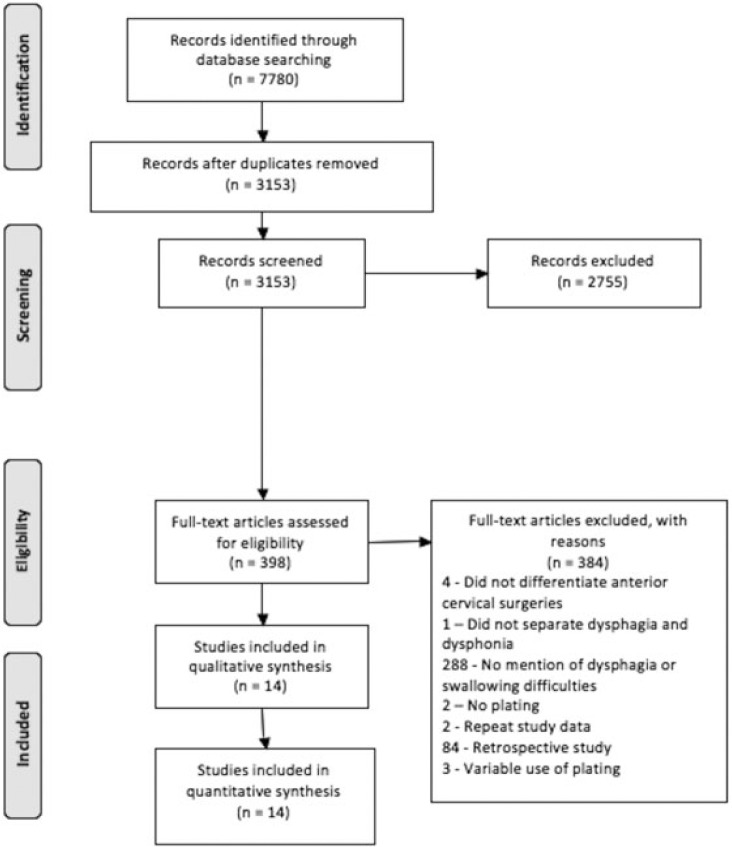
We searched MEDLINE, Scopus, Web of Science, and Embase databases with the search algorithm: (“Anterior Cervical Discectomy (ACDF) and Fusion Complication(s)”) OR (Anterior Cervical Discectomy and Fusion Complication(s) and Outcome(s)”) OR ((“Anterior” and “Cervical” and Discectomy”) AND (“fusion” or “arthrodesis”)). The search returned 7,780 citations (Fig. 1). The search period ended November 10, 2014.
Fig. 1.
PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow diagram for selection of studies based on inclusion criteria during systematic review.
Inclusion and Exclusion Criteria
Only prospective cohort studies and randomized controlled trials (RCTs) were included in this meta-analysis because of their superior evidence level compared with that of retrospective cohort studies.33 In particular, we felt that retrospective studies would more often underreport postoperative complications. Articles published prior to 1990 were excluded because anterior plate fixation became much more prevalent after 1990.34 To create a more homogenous patient cohort, studies only involving the following procedures were excluded: anterior cervical diskectomy without graft fusion, ACDF without plate fixation, anterior cervical corpectomy and fusion, arthroplasty, and combined anterior-posterior surgeries. Control arms meeting eligibility criteria were included in the analysis. We imposed no restrictions on the publication status. Animal, in vitro, biomechanical, and non-English studies were excluded. Studies using bone morphogenic protein-2 were excluded because it has been shown to increase complication rates and is not approved by the Food and Drug Administration for use in anterior cervical surgery. In fact, its use has plummeted since a 2008 Food and Drug Administration warning.35
Data Collection
Two reviewers (M.F.S., D.J.L.) independently conducted data extraction from the 14 included articles. The extracted data sets were compared to confirm accuracy. The level of evidence for each of the included articles was assessed using the Oxford Centre for Evidence-Based Medicine Level of Evidence 2 classification system.33 From the eligible articles, we obtained the following information: study type, publication year, graft type, follow-up time, number of reported dysphagia events through final follow-up, total study population, number of ACDF surgical levels, definition of dysphagia, and classification system used to rate the severity of dysphagia. We extracted the number of dysphagia events reported in each study, regardless of the study's method of diagnosis or severity of dysphagia. If a study reported dysphagia at multiple follow-up visits, the dysphagia rate was recorded from the last time.
To assess the risk of bias for each study, two reviewers independently investigated the individual studies and utilized The Cochrane Collaboration's tool for assessing risk of bias.36 Bias risk assessment was performed at the study level. Inconsistencies in bias risk assessment were reconciled through discussion.
Statistical Analysis
We analyzed study data using a random-effects model with inverse variance weighting. The calculations for the meta-analysis and construction of forest plots were completed using an established spreadsheet constructed by Neyeloff et al.37 The principal summary measures were the effect summary values and 95% confidence intervals (CIs). Because of the lack of consistent control groups across all included studies, we were unable to calculate relative risk ratios. We compared results among studies with 95% CIs and forest plots.
We completed meta-analysis calculations and constructed forest plots for all studies, including those with allograft or autograft and those with less than 12 months' follow-up, 12 to 24 months' follow-up, and greater than 24 months' follow-up. Additionally, we analyzed studies that used the Bazaz Dysphagia Score and no outlined criteria separately to identify differences in the rate of dysphagia identification. Three studies—Anderson et al,1 Coric et al,38 and Xie and Hurlbert39—were not grouped by their dysphagia classification system for analysis because of their use of different criteria compared with all other studies in our review (Table 1). The Bazaz Dysphagia Score was established by Bazaz et al in 2002 and utilizes a specific set of requirements to identify the presence and severity of dysphagia.19 Bazaz et al evaluated patients via telephone at different follow-up periods after ACDF.19 Patients were classified as having mild dysphagia if they stated upon questioning that they experienced rare events of difficulty swallowing with solids, but did not feel that this difficulty was a significant problem. Those with moderate dysphagia expressed occasional difficulty with swallowing only specific foods, and patients reporting frequent difficulty with a majority of solids were classified as having severe dysphagia. For all levels of dysphagia, there was either rare or no difficulty swallowing liquids.19
Table 1.
Dysphagia definitions and classification systems
| Study | Definition of dysphagia and classification system |
|---|---|
| Graham et al, 201548 | Not defined |
| Burkus et al, 201449 | Not defined |
| Hou et al, 201445 | Not defined |
| Kepler et al, 201212 | Bazaz Dysphagia Score, dysphagia numeric rating scalea |
| Coric et al, 201138 | Patient self-reported |
| Cheng et al, 201146 | Not defined |
| McAfee et al, 201018 | Bazaz Dysphagia Score |
| Anderson et al, 20081 | Patient self-reported |
| Fernández-Fairen et al, 200847 | Not defined |
| Xie and Hurlbert, 200739 | Questionnaire |
| Song and Lee, 200642 | Not defined |
| Bazaz et al, 200219 | Bazaz Dysphagia Score |
| Bruneau et al, 200143 | Not defined |
| Bolesta et al, 200044 | Not defined |
a0–10 scale similar to visual analogue scale.
To assess heterogeneity between individual studies, a Q statistic and I 2 value were calculated within each group's meta-analysis. Delong et al established an I 2 < 25% as low heterogeneity, 25 to 75% as moderate heterogeneity, and >75% as severe heterogeneity. These same values were used to assess heterogeneity in our meta-analysis.40
Results
Study Selection
The initial 7,780 retrieved citations were reviewed (Fig. 1). After removing 4,627 duplicates, the titles and abstracts of 3,153 publications were screened.41 At this stage, studies that did not mention ACDF and associated complications or that did not fulfill the inclusion criteria in any manner were excluded. After excluding 2,755 citations, the full text was assessed in the resulting 398 articles for eligibility criteria. Full-text assessment resulted in 14 eligible articles included in the final analysis. The definition and classification system for dysphagia varied among the studies and in some studies was not specified (Table 1).
Study Characteristics
Of the 14 studies evaluated in this review, the year of publication ranged from 2000 to 2015 (Table 2). Eight of the included studies were RCTs, and six were prospective cohort studies. Cohorts ranged in size from 15 to 265 patients. Follow-up time ranged from 1.5 to 84 months. Seven studies performed single-level ACDF exclusively, and all other studies performed ACDF over a variable number of levels. Unfortunately, most studies did not report dysphagia events based on the specific level or total number of surgical levels. As a result, we were unable to perform a meta-analysis to correlate dysphagia rates with the anatomic or number of operated levels. Bias risk assessment of the included studies identified a marked difference between RCTs and prospective cohort studies, with no studies demonstrating a high risk of incomplete outcome data or selective reporting of outcomes.
Table 2.
Study characteristics of 14 included studies
| Study | Study type | No. of patients | Follow-up (mo) | Graft type | Number of operated levels |
|---|---|---|---|---|---|
| Graham et al, 201548 | RCT | 86 | 6 | Allograft | One level, n = 26; two levels, n = 47; three levels, n = 25; four levels, n = 8 |
| Burkus et al, 201449 | RCT | 265 | 84 | Allograft | One level |
| Hou et al, 201445 | PC | 196 | 22.5a | Autograft | One level, n = 108; two levels, n = 88 |
| Kepler et al, 201212 | PC | 41 | 1.5 | Allograft or Autograft | One level, n = 15; two levels, n = 28 |
| Coric et al, 201138 | RCT | 115 | 24 | Allograft | One level |
| Cheng et al, 201146 | RCT | 42 | 36 | Autograft | One level, n = 21; two levels, n = 17; three levels, n = 4 |
| McAfee et al, 201018 | RCT | 100 | 24 | Allograft | One level |
| Anderson et al, 20081 | RCT | 221 | 24 | Allograft | One level |
| Fernández-Fairen et al, 200847 | RCT | 33 | 24 | Autograft | One level |
| Xie and Hurlbert, 200739 | RCT | 15 | 24 | Autograft | One level |
| Song and Lee, 200642 | PC | 39 | 24 | Autograft | One level |
| Bazaz et al, 200219 | PC | 114 | 6 | Autograft | Not defined |
| Bruneau et al, 200143 | PC | 54 | 24.6a | Hydroxyapatite | One level, n = 40; two levels, n = 14 |
| Bolesta et al, 200044 | PC | 15 | 42 | Autograft | Three levels, n = 12; four levels, n = 3 |
Abbreviations: PC, prospective cohort study; RCT, randomized controlled trial.
aMean follow-up period for patient cohort.
Analysis of All Studies
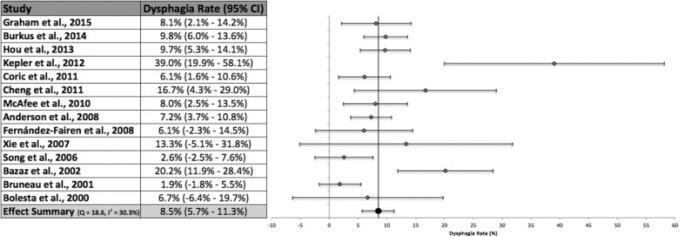
The mean follow-up time across all 14 included studies in our review was 26.2 months (Fig. 2). Across the combined interstudy population of 1,336 patients, studies reported dysphagia at any time during follow-up in 136 patients. Meta-analysis, calculated using a random-effects model with inverse variance weighting, of all included studies produced an overall dysphagia rate of 8.5% (95% CI 5.7 to 11.3%). Heterogeneity analysis indicated moderate heterogeneity.40 Only 4 of the 14 included studies did not contain the effect summary value within their respective 95% CIs.12,19,42,43 All studies reported at least one dysphagia event. Kepler et al reported a dysphagia rate of 39.0%, the highest of all included studies, and also produced the widest 95% CI due to its large standard error.12 Bruneau et al reported the lowest rate of dysphagia at 1.9%.43 Only six studies differentiated the severity of dysphagia.1,12,18,19,43,44 The rate of moderate or severe dysphagia reported by these studies was 4.4% (95% CI 0.4 to 8.4%).
Fig. 2.
Dysphagia rates, 95% confidence intervals (CIs), and forest plot for meta-analysis of all studies in our systematic review.
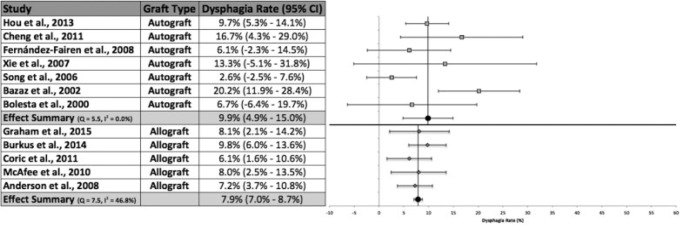
Graft Type
Seven studies used solely autograft fusion for their ACDF technique (Fig. 3).19,39,42,44–47 Fifty-five patients out of a total 454 patients across all studies utilizing autograft fusion were reported to have developed dysphagia. Meta-analysis calculations resulted in an effect summary value of 9.9% (95% CI 4.9 to 15.0%). Analysis of the studies indicated no apparent heterogeneity.40 Bazaz et al and Song and Lee were the only studies that did not contain the effect summary within their respective 95% CIs.19,42 The highest rate of dysphagia (20.2%) among studies using autograft fusion was reported by Bazaz et al.19 Conversely, five studies utilized allograft bone material for surgical fusion.1,18,38,48,49 Sixty-four patients developed dysphagia out of a total 787 interstudy combined patients. The meta-analysis produced a random effects overall rate of 7.9% (95% CI 7.0 to 8.7%). All studies contained the effect summary within their respective 95% CIs. The heterogeneity analysis identified moderate heterogeneity.40 The absolute difference between the two fusion materials was 2.0%, but this difference was not statistically significant.
Fig. 3.
Dysphagia rates, 95% confidence intervals (CIs), and forest plots for meta-analysis of studies using autograft or allograft fusion.
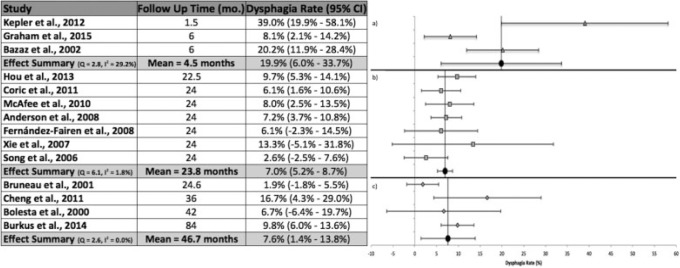
Follow-up Time < 12 Months
Three studies followed patients for less than 12 months (Fig. 4).12,19,48 The mean follow-up time for these studies was 4.5 months. Kepler et al followed patients for 1.5 months, the shortest time of all studies.12 This study also reported the highest rate of dysphagia at 39.0%.12 The calculated effect summary value was 19.9% (95% CI 6.0 to 33.7%). The heterogeneity analysis indicated moderate heterogeneity.40
Fig. 4.
Dysphagia rates, 95% confidence intervals (CIs), and forest plots for meta-analysis of studies with follow-up of (a) less than 12 months, (b) 12 to 24 months, and (c) greater than 24 months.
Follow-up Time 12 to 24 Months
Seven studies reported a follow-up time between 12 and 24 months with a mean of 23.8 months (Fig. 4).1,18,38,39,42,45,47 Six of the seven studies followed patients for 24 months, the most common follow-up length across all included studies. The meta-analysis calculations produced an effect summary of 7.0% (95% CI 5.2 to 8.7%). All studies produced 95% CIs that contained the effect summary value. Xie and Hurlbert reported the highest rate of dysphagia (13.3%) within this follow-up duration.39 Heterogeneity analysis resulted in low heterogeneity.40
Follow-up Time > 24 Months
A follow-up period of greater than 24 months was reported in four studies (Fig. 4).43,44,46,49 The mean follow-up time was 46.7 months. The overall rate of dysphagia was 7.6% (95% CI 1.4 to 13.8%). Cheng et al reported a 16.7% rate of dysphagia, the highest in this group.46 The longest follow-up time (84 months) was reported by Burkus et al,49 who had a 9.8% rate of dysphagia. No apparent heterogeneity was found upon analysis.40
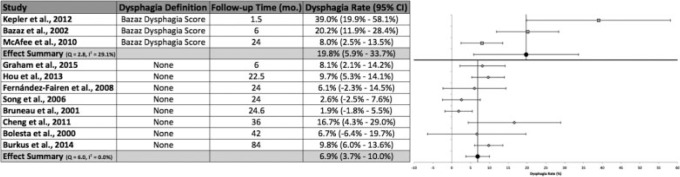
Dysphagia Definition or Classification Scheme
The Bazaz Dysphagia Score was utilized to define and classify dysphagia in three studies (Fig. 5).12,18,19 These studies identified dysphagia (mild, moderate, or severe) at a rate of 19.8% (95% CI 5.9 to 33.7%). These studies displayed moderate heterogeneity.40 Extended follow-up resulted in a progressively decreased dysphagia rate identified by the Bazaz Dysphagia score, with McAfee et al reporting a rate of 8.0% at 24 months.18 This rate suggested a statistically significant reduction in identified dysphagia with prolonged follow-up relative to 6 postoperative weeks, as documented by Kepler et al (39.0%).12 Additionally, although there was a 12.2% reduction in reported dysphagia between 6 months' follow-up by Bazaz et al and the prolonged follow-up reported by McAfee et al, this difference was not statistically significant.18,19
Fig. 5.
Dysphagia rates, 95% confidence intervals (CIs), and forest plots for meta-analysis of studies utilizing the Bazaz Dysphagia Score or no outlined criteria.
No defined method was reported for the diagnosis of dysphagia in eight studies.42 43 44 45 46 47 48 49 The overall dysphagia rate for these studies was 6.9% (95% CI 3.7 to 10.0%). Only one study did not contain the effect summary value within its 95% CI.43 The highest reported rate of dysphagia by any study without outlined criteria was 16.7% by Cheng et al.46 There was no apparent heterogeneity among the included studies.40 Although there was a decrease in the rate of dysphagia identified with prolonged follow-up utilizing the Bazaz Dysphagia Score, no apparent correlation was found among studies with no specific dysphagia criteria. As well, despite a 12.9% absolute difference in dysphagia rates between these two meta-analysis groups, this difference did not reach statistical significance.
Discussion
This study represents a comprehensive systematic literature review and meta-analysis of dysphagia rates associated with ACDF. The purpose of this study was to determine the rates of dysphagia associated with various factors. We analyzed the reported dysphagia rates and elucidated differences in the rate of dysphagia among distinct fusion materials, follow-up time, and classification systems.
Although the included studies did not separate dysphagia rates based on numbers of multilevel surgery, analysis of studies solely performing single-level ACDF resulted in an overall dysphagia rate of 6.5%. This rate was 2.0% less than the overall rate when all studies were included, but this difference was not statistically significant. Also, analysis of dysphagia rates reported by prospective cohort studies and RCTs did not demonstrate any statistical significance (data not shown). There was a 3.1% increase in dysphagia for prospective cohort studies compared with RCTs, but this difference was most likely due to the increased use of the Bazaz Dysphagia Score, a strong confounding factor.
Dysphagia Definition or Classification Scheme
Three studies—Kepler et al,12 McAfee et al,18 and Bazaz et al19—utilized the Bazaz Dysphagia Score for the identification of dysphagia. There was a large difference in the rate of dysphagia between studies using the Bazaz Dysphagia Score and those studies with no outlined dysphagia criteria or definition (Fig. 5). There was a 12.9% increase in dysphagia identification using the Bazaz Dysphagia Score. Additionally, there was a progressive decrease in the rate of dysphagia with longer follow-up among studies utilizing the Bazaz Dysphagia Score. Studies with no specific criteria did not elicit this same trend. Therefore, our review has identified an important difference in reported dysphagia rates that accompanies a lack of specific criteria. None of the studies defined dysphagia by objective assessment of swallowing ability, video fluoroscopy, or manometry.30 Objective assessments alone might produce a different rate of postoperative dysphagia. Kepler et al found no correlation between the amount of anterior soft tissue swelling on cervical radiographs at any anatomical level and the rate of dysphagia.12 In our review, the most common standardized method for reporting dysphagia was the Bazaz Dysphagia Score. Because there is currently large heterogeneity among surgeons in recognizing dysphagia, further studies are needed to compare dysphagia rates using various methods of identification. This study also highlights the need to establish a standardized and validated classification system for the identification of dysphagia.
Allograft versus Autograft Fusion
In our review, studies that performed ACDF with autograft fusion produced an overall dysphagia rate of 9.9%, whereas the use of allograft fusion resulted in a rate of 7.9%, but this 2.0% absolute difference was not significant. Our meta-analysis findings contradict those of a previously published systematic review by Miller and Block,50 who found a 4.3 and 0.0% dysphagia rate for allograft fusion and autograft fusion, respectively. However, the authors did not offer a reason for this finding, and there is no apparent reason for an increased risk of dysphagia with the use of allograft. This difference in dysphagia rates between autograft and allograft use may be confounded by factors such as number of surgical levels, smoking status, or surgical technique.
Follow-up Time
The studies included in our review were assigned to one of three meta-analysis groups based on length of follow-up time: less than 12 months, 12 to 24 months, or greater than 24 months. These groups produced dysphagia rates of 19.9, 7.0, and 7.6%, respectively. This data indicates that there is a higher rate of dysphagia diagnosed and reported during the first year following ACDF, which reduces significantly in the second year and plateaus in subsequent years. Thus, a portion of dysphagia identified in the postoperative period appears to be transient, although some patients continue to show symptoms for extended periods. In the study by Rihn et al,3 which was excluded from our meta-analysis, 71% of patients presented with dysphagia at 2 weeks of follow-up. However, by weeks 6 and 7, only 26 and 8% of patients, respectively, still presented with some degree of dysphagia. Bazaz et al reported a similar pattern of postoperative dysphagia.19 In their study, 47.3% of patients reported dysphagia at 1 month following surgery, but by months 2 and 6, only 30.8 and 20.2% of patients still reported dysphagia, respectively.19 The rates of permanent dysphagia after ACDF need to be further studied and defined based on time-dependent progression of symptoms during the postoperative period.
Limitations
Most abstracts and studies screened for dysphagia rates in this review were retrospective case series, which limited the number of studies we were able to include based on our criteria. As a result, a small number of studies were included, which restricted our ability to compare the surgical techniques. Because of this limitation and the concomitant lack of studies with a consistent control group, we were unable to calculate the relative risk values for various factors impacting the development of dysphagia. In addition, although stratification of dysphagia rates by age, cervical levels, number of surgical levels, and extent of retraction would decrease heterogeneity among studies and reveal inherent differences associated with these factors, the primary literature is varied and does not routinely discuss surgical levels in reporting complications. Because of inconsistent reporting, we were unable to analyze the rate of dysphagia associated with these factors. Finally, this meta-analysis solely investigated the dysphagia rates for ACDF with plate fixation. Further research should be performed to explore dysphagia rates across all forms of anterior cervical surgery, allowing for comparative effectiveness investigation.
Conclusions
The resultant dysphagia rates from this systematic review and meta-analysis represent a comprehensive estimation of the actual incidence of dysphagia following cervical diskectomy with plating across a heterogeneous group of surgeons, patients, and techniques. Dysphagia rates decreased drastically between follow-up times of less than 12 months (19.9%) and 12 to 24 months (7.0%), and then stabilized with further follow-up. Additionally, a higher proportion of dysphagia was reported in studies utilizing the Bazaz Dysphagia Score relative to studies with no specified definition. There was no difference in dysphagia rate with the use of autograft versus allograft. To ensure the proper diagnosis of dysphagia, we recommend surgeons use a standardized system for dysphagia identification in the postoperative period. Our investigation should serve as a framework for individual surgeons to understand the impact of various surgical techniques and diagnostic criteria on the rate of dysphagia in their patient populations and permit a more accurate preoperative discussion with patients.
Footnotes
Disclosures: Michael F. Shriver: none
Daniel J. Lewis: none
Varun R. Kshettry: none
Benjamin P. Rosenbaum: none
Edward C. Benzel: Consultant (Axiomed); Stock ownership (Axiomed, Depuy, Orthomens, Turning Point); Grant (OREF, Rawlings); Royalties (Elsevier, Thieme)
Thomas E. Mroz: Stock ownership (PearlDiver); Personal fees (Globus Medical, AOSpine, CeramTec)
References
- 1. Anderson PA, Sasso RC, Riew KD. Comparison of adverse events between the Bryan artificial cervical disc and anterior cervical arthrodesis Spine (Phila Pa 1976) 2008; 33 (12): 1305–1312 [DOI] [PubMed] [Google Scholar]
- 2. Cho DY, Lee WY, Sheu PC. Treatment of multilevel cervical fusion with cages Surg Neurol 2004; 62 (5): 378–385 discussion 385–386 [DOI] [PubMed] [Google Scholar]
- 3. Rihn JA, Kane J, Albert TJ, Vaccaro AR, Hilibrand AS. What is the incidence and severity of dysphagia after anterior cervical surgery? Clin Orthop Relat Res 2011; 469 (3): 658–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Razfar A, Sadr-Hosseini SM, Rosen CA, et al. Prevention and management of dysphonia during anterior cervical spine surgery Laryngoscope 2012; 122 (10): 2179–2183 [DOI] [PubMed] [Google Scholar]
- 5. Campbell PG, Yadla S, Malone J, et al. Early complications related to approach in cervical spine surgery: single-center prospective study World Neurosurg 2010; 74 (2–3): 363–368 [DOI] [PubMed] [Google Scholar]
- 6. Danto J, DiCapua J, Nardi D, et al. Multiple cervical levels: increased risk of dysphagia and dysphonia during anterior cervical discectomy J Neurosurg Anesthesiol 2012; 24 (4): 350–355 [DOI] [PubMed] [Google Scholar]
- 7. Kirshblum S, Johnston MV, Brown J, O'Connor KC, Jarosz P. Predictors of dysphagia after spinal cord injury Arch Phys Med Rehabil 1999; 80 (9): 1101–1105 [DOI] [PubMed] [Google Scholar]
- 8. Siska PA, Ponnappan RK, Hohl JB, Lee JY, Kang JD, Donaldson WF., III Dysphagia after anterior cervical spine surgery: a prospective study using the swallowing-quality of life questionnaire and analysis of patient comorbidities Spine (Phila Pa 1976) 2011; 36 (17): 1387–1391 [DOI] [PubMed] [Google Scholar]
- 9. Cho SK, Lu Y, Lee DH. Dysphagia following anterior cervical spinal surgery: a systematic review Bone Joint J 2013; 95-B (7): 868–873 [DOI] [PubMed] [Google Scholar]
- 10. Segebarth B, Datta JC, Darden B, et al. Incidence of dysphagia comparing cervical arthroplasty and ACDF SAS J 2010; 4 (1): 3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang SH, Kim DK, Seo KM, Kim KT, Kim YB. Multi-level spinal fusion and postoperative prevertebral thickness increase the risk of dysphagia after anterior cervical spine surgery J Clin Neurosci 2011; 18 (10): 1369–1373 [DOI] [PubMed] [Google Scholar]
- 12. Kepler CK, Rihn JA, Bennett JD, et al. Dysphagia and soft-tissue swelling after anterior cervical surgery: a radiographic analysis Spine J 2012; 12 (8): 639–644 [DOI] [PubMed] [Google Scholar]
- 13. Mendoza-Lattes S, Clifford K, Bartelt R, Stewart J, Clark CR, Boezaart AP. Dysphagia following anterior cervical arthrodesis is associated with continuous, strong retraction of the esophagus J Bone Joint Surg Am 2008; 90 (2): 256–263 [DOI] [PubMed] [Google Scholar]
- 14. Papavero L, Heese O, Klotz-Regener V, Buchalla R, Schröder F, Westphal M. The impact of esophagus retraction on early dysphagia after anterior cervical surgery: does a correlation exist? Spine (Phila Pa 1976) 2007; 32 (10): 1089–1093 [DOI] [PubMed] [Google Scholar]
- 15. Lee MJ, Bazaz R, Furey CG, Yoo J. Influence of anterior cervical plate design on dysphagia: a 2-year prospective longitudinal follow-up study J Spinal Disord Tech 2005; 18 (5): 406–409 [DOI] [PubMed] [Google Scholar]
- 16. Scholz M, Schnake KJ, Pingel A, Hoffmann R, Kandziora F. A new zero-profile implant for stand-alone anterior cervical interbody fusion Clin Orthop Relat Res 2011; 469 (3): 666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chin KR, Eiszner JR, Adams SB., Jr Role of plate thickness as a cause of dysphagia after anterior cervical fusion Spine (Phila Pa 1976) 2007; 32 (23): 2585–2590 [DOI] [PubMed] [Google Scholar]
- 18. McAfee PC, Cappuccino A, Cunningham BW, et al. Lower incidence of dysphagia with cervical arthroplasty compared with ACDF in a prospective randomized clinical trial J Spinal Disord Tech 2010; 23 (1): 1–8 [DOI] [PubMed] [Google Scholar]
- 19. Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study Spine (Phila Pa 1976) 2002; 27 (22): 2453–2458 [DOI] [PubMed] [Google Scholar]
- 20. Edwards CC, II, Karpitskaya Y, Cha C, et al. Accurate identification of adverse outcomes after cervical spine surgery J Bone Joint Surg Am 2004; 86-A (2): 251–256 [DOI] [PubMed] [Google Scholar]
- 21. Lee MJ, Bazaz R, Furey CG, Yoo J. Risk factors for dysphagia after anterior cervical spine surgery: a two-year prospective cohort study Spine J 2007; 7 (2): 141–147 [DOI] [PubMed] [Google Scholar]
- 22. Kalb S, Reis MT, Cowperthwaite MC, et al. Dysphagia after anterior cervical spine surgery: incidence and risk factors World Neurosurg 2012; 77 (1): 183–187 [DOI] [PubMed] [Google Scholar]
- 23. Riley LH, III, Vaccaro AR, Dettori JR, Hashimoto R. Postoperative dysphagia in anterior cervical spine surgery Spine (Phila Pa 1976) 2010; 35 (9, Suppl): S76–S85 [DOI] [PubMed] [Google Scholar]
- 24. Smith-Hammond CA, New KC, Pietrobon R, Curtis DJ, Scharver CH, Turner DA. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: comparison of anterior cervical, posterior cervical, and lumbar procedures Spine (Phila Pa 1976) 2004; 29 (13): 1441–1446 [DOI] [PubMed] [Google Scholar]
- 25. Tervonen H, Niemelä M, Lauri ER, et al. Dysphonia and dysphagia after anterior cervical decompression J Neurosurg Spine 2007; 7 (2): 124–130 [DOI] [PubMed] [Google Scholar]
- 26. Winslow CP, Winslow TJ, Wax MK. Dysphonia and dysphagia following the anterior approach to the cervical spine Arch Otolaryngol Head Neck Surg 2001; 127 (1): 51–55 [DOI] [PubMed] [Google Scholar]
- 27. Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications Spine (Phila Pa 1976) 2007; 32 (21): 2310–2317 [DOI] [PubMed] [Google Scholar]
- 28. Frempong-Boadu A, Houten JK, Osborn B, et al. Swallowing and speech dysfunction in patients undergoing anterior cervical discectomy and fusion: a prospective, objective preoperative and postoperative assessment J Spinal Disord Tech 2002; 15 (5): 362–368 [DOI] [PubMed] [Google Scholar]
- 29. Yue WM, Brodner W, Highland TR. Persistent swallowing and voice problems after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year follow-up study Eur Spine J 2005; 14 (7): 677–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skeppholm M, Ingebro C, Engström T, Olerud C. The Dysphagia Short Questionnaire: an instrument for evaluation of dysphagia: a validation study with 12 months' follow-up after anterior cervical spine surgery Spine (Phila Pa 1976) 2012; 37 (11): 996–1002 [DOI] [PubMed] [Google Scholar]
- 31. Lee SK, Lee GY, Wong GT. Prolonged and severe dysphagia following anterior cervical surgery J Clin Neurosci 2004; 11 (4): 424–427 [DOI] [PubMed] [Google Scholar]
- 32. Anderson KK, Arnold PM. Oropharyngeal dysphagia after anterior cervical spine surgery: a review Global Spine J 2013; 3 (4): 273–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. OCEBM Levels of Evidence Working Group The Oxford Levels of Evidence 2. Oxford Centre for Evidence-Based Medicine Available at: http://www.cebm.net/index.aspx?o=5653
- 34. Moftakhar R, Trost GR. Anterior cervical plates: a historical perspective Neurosurg Focus 2004; 16 (1): E8 [DOI] [PubMed] [Google Scholar]
- 35. Lu DC, Tumialán LM, Chou D. Multilevel anterior cervical discectomy and fusion with and without rhBMP-2: a comparison of dysphagia rates and outcomes in 150 patients J Neurosurg Spine 2013; 18 (1): 43–49 [DOI] [PubMed] [Google Scholar]
- 36. Higgins JPT Green S , eds. . Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 Available at: www.cochrane-handbook.org. Accessed December 16, 2015
- 37. Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and forest plots using a Microsoft Excel spreadsheet: step-by-step guide focusing on descriptive data analysis BMC Res Notes 2012; 5: 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coric D, Nunley PD, Guyer RD, et al. Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex|C artificial disc investigational device exemption study with a minimum 2-year follow-up: clinical article J Neurosurg Spine 2011; 15 (4): 348–358 [DOI] [PubMed] [Google Scholar]
- 39. Xie JC, Hurlbert RJ. Discectomy versus discectomy with fusion versus discectomy with fusion and instrumentation: a prospective randomized study Neurosurgery 2007; 61 (1): 107–116 discussion 116–117 [DOI] [PubMed] [Google Scholar]
- 40. DeLong WB, Polissar N, Neradilek B. Timing of surgery in cauda equina syndrome with urinary retention: meta-analysis of observational studies J Neurosurg Spine 2008; 8 (4): 305–320 [DOI] [PubMed] [Google Scholar]
- 41. Moher D Liberati A Tetzlaff J Altman DG ; PRISMA Group. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement BMJ 2009; 339: b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song KJ, Lee KB. A preliminary study of the use of cage and plating for single-segment fusion in degenerative cervical spine disease J Clin Neurosci 2006; 13 (2): 181–187 [DOI] [PubMed] [Google Scholar]
- 43. Bruneau M, Nisolle JF, Gilliard C, Gustin T. Anterior cervical interbody fusion with hydroxyapatite graft and plate system Neurosurg Focus 2001; 10 (4): E8 [DOI] [PubMed] [Google Scholar]
- 44. Bolesta MJ, Rechtine GR, II, Chrin AM. Three- and four-level anterior cervical discectomy and fusion with plate fixation: a prospective study Spine (Phila Pa 1976) 2000; 25 (16): 2040–2044 discussion 2045–2046 [DOI] [PubMed] [Google Scholar]
- 45. Hou Y, Liu Y, Yuan W, et al. Cervical kinematics and radiological changes after Discover artificial disc replacement versus fusion Spine J 2014; 14 (6): 867–877 [DOI] [PubMed] [Google Scholar]
- 46. Cheng L, Nie L, Li M, Huo Y, Pan X. Superiority of the Bryan(®) disc prosthesis for cervical myelopathy: a randomized study with 3-year followup Clin Orthop Relat Res 2011; 469 (12): 3408–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernández-Fairen M, Sala P, Dufoo M, Jr, Ballester J, Murcia A, Merzthal L. Anterior cervical fusion with tantalum implant: a prospective randomized controlled study Spine (Phila Pa 1976) 2008; 33 (5): 465–472 [DOI] [PubMed] [Google Scholar]
- 48. Graham RS, Samsell BJ, Proffer A, et al. Evaluation of glycerol-preserved bone allografts in cervical spine fusion: a prospective, randomized controlled trial J Neurosurg Spine 2015; 22 (1): 1–10 [DOI] [PubMed] [Google Scholar]
- 49. Burkus JK, Traynelis VC, Haid RW, Jr, Mummaneni PV. Clinical and radiographic analysis of an artificial cervical disc: 7-year follow-up from the Prestige prospective randomized controlled clinical trial: Clinical article J Neurosurg Spine 2014; 21 (4): 516–528 [DOI] [PubMed] [Google Scholar]
- 50. Miller LE, Block JE. Safety and effectiveness of bone allografts in anterior cervical discectomy and fusion surgery Spine (Phila Pa 1976) 2011; 36 (24): 2045–2050 [DOI] [PubMed] [Google Scholar]