Abstract
Background:
Osteoarthritis (OA) is a degenerative joint disease affecting the knee joint of many middle-aged and older adults. As OA symptoms typically involve knee pain and stiffness, individuals with knee OA are often insufficiently physically active, have low levels of physical function, and are at increased risk of other comorbidities and reduced quality of life. While moderate-intensity continuous training (MICT) cycling is often recommended, little is known about the feasibility, safety, and benefits of high-intensity interval training (HIIT) cycling for this population, even though the feasibility, safety, and benefits of HIIT have been demonstrated in other chronic disease groups.
Purpose:
The primary objective of this pilot study was to examine the feasibility and safety of home-based HIIT and MICT cycling in middle-aged and older adults with knee OA. A secondary objective was to gain some insight into the relative efficacy of HIIT and MICT for improving health status (pain, stiffness, and disability), muscle function, and body composition in this population. This study protocol is being published separately to allow a detailed description of the research methods, explain the rationale for choosing the methodological details, and to stimulate consideration of the best means to simulate a research protocol that is relevant to a real-life treatment environment.
Study Design:
Randomized pilot study protocol.
Methods:
This trial sought to recruit 40 middle-aged and older adults with knee OA. Participants were randomly allocated to either continuous (MICT) or HIIT home-based cycle training programs, with both programs requiring the performance of 4 cycling sessions (approximately 25 minutes per session) each week. Participants were measured at baseline and postintervention (8 weeks). Feasibility and safety were assessed by adherence rate, dropout rate, and number of adverse events. The relative efficacy of the cycling programs was investigated by 2 knee OA health status questionnaires (Western Ontario and McMaster Universities Osteoarthritis Index scale[WOMAC] and the Lequesne Index) as well as the timed up and go, sit to stand, preferred gait speed, and body composition.
Discussion:
This pilot study appears to be the first study assessing the feasibility and safety of a home-based HIIT training program for middle-aged and older adults with knee OA. As HIIT has been demonstrated to be more effective than MICT for improving aspects of health status, body composition, and/or muscular function in other chronic disease groups, the current study has the potential to improve patient outcomes and inform the design of future randomized controlled trials.
Keywords: arthritis, cycling, exercise, function, pain, knee osteoarthritis
Osteoarthritis (OA) is a degenerative joint disease affecting many middle-aged and older adults. Recent global data indicate that of the 291 conditions analyzed, hip and knee OA was ranked as the 11th highest contributor to global disability and 38th highest in disability adjusted life years.8 One of the most common sites for OA is the knee, with symptoms including joint pain, tenderness, and reduced physical function and mobility.18 As a result of these symptoms, many middle-aged and older adults with knee OA are less physically active than those without OA.24 This reduction in physical activity may further accelerate the age-related loss of muscle mass (especially around the knee joint), muscle strength, and function seen in older adults, known as sarcopenia.9,10,44 These knee OA–related losses in physical activity levels, muscle mass, strength, and function may also increase the risk of obesity and metabolic syndrome.42 The development of obesity and metabolic syndrome may result in a vicious circle that further increases the progression and severity of OA and results in even more sedentary behavior, disability, and health complications.53
Systematic reviews and meta-analyses indicate that a variety of forms of exercise can improve aspects of body composition, physical function, metabolic health markers, and quality of life in middle-aged and older adults with OA.21,47,50 Along with aquatic exercise, cycling is perhaps the most commonly recommended and performed form of exercise for individuals with knee OA.12 The popularity of aquatic and cycling exercise for knee osteoarthritic patients may reflect the reduced joint loading during aquatic exercises and cycling compared with other land-based exercises, including sit to stand (STS), walking, and stair climbing.26,27 However, a systematic review indicates that the majority of exercise studies for knee OA patients used low- to moderate-intensity continuous training (MICT) protocols.12 Perhaps the only exception to this rule was the study by Mangione et al,34 who conducted a 10-week trial comparing the effects of high-intensity and low-intensity cycling for knee osteoarthritic patients. However, participants in both groups performed continuous rather than high-intensity interval training (HIIT) cycling, and even the high-intensity group only exercised at 70% of heart rate reserve.34
While MICT cycling may provide a range of cardiovascular health benefits and assist with weight maintenance for this population, it may not provide a sufficient stimulus to offset the potential sarcopenic changes in lower body muscle mass, strength, and function.6 Furthermore, MICT cycling may not provide a sufficient level of knee joint loading that is required to maintain and promote optimal articular cartilage composition and architecture.40,48,51
One possible exercise modality that may improve a range of cardiovascular, sarcopenic, and perhaps knee articular cartilage outcomes in knee OA patients could be HIIT cycling. HIIT involves alternating short periods of high-intensity activity (often 30-45 seconds) followed by a recovery period of 2 to 3 times the duration of the HIIT exercise bout at a lower intensity.45 The performance of HIIT may be more effective in improving muscle mass, strength, and function than MICT.6,43 Furthermore, HIIT also appears to be equally or significantly more effective in improving cardiorespiratory fitness, vascular function, cardiovascular disease risk factors, oxidative stress, and insulin sensitivity than MICT in a range of clinical populations.20,30,38,45
There is limited literature of the effect of HIIT exercise on measures of health status, muscle mass, strength, or function in musculoskeletal conditions like knee OA.43 One issue that may have contributed to this lack of research is the potential concerns that health professionals may have regarding the safety of this exercise for individuals with knee OA, especially if this exercise is performed unsupervised at home or in a fitness center. Such concerns are warranted, as the most common adverse events reported from any high-intensity exercise are likely to be musculoskeletal in nature, thereby potentially exacerbating knee OA–related symptoms such as joint pain.31 While HIIT appears to be well tolerated for older and middle-aged individuals with a variety of cardiovascular diseases,1,39 only 1 study appears to have assessed the safety of HIIT in arthritic populations, with this study involving young to middle-aged adults with rheumatoid arthritis and juvenile idiopathic arthritis.43
If it can be demonstrated that knee OA patients can perform HIIT at home with relative safety, such a result would allow orthopaedic surgeons and other allied health professionals to be more confident when prescribing home-based HIIT cycling to their knee OA patients. This is important as some of the largest barriers to group-based exercise for individuals with OA are time, convenience, cost, and comfort of the exercise environment (eg, cold temperatures, exercise instructor behavior).14,17 Therefore, the primary aim of this pilot study was to gain some insight into the feasibility and safety of a home-based HIIT cycling program in middle-aged and older knee OA patients. A secondary aim was to gain some preliminary insight into the relative efficacy of HIIT compared with MICT cycling for improving health status, physical function, and body composition in middle-aged and older knee OA patients.
Methods
Research Design
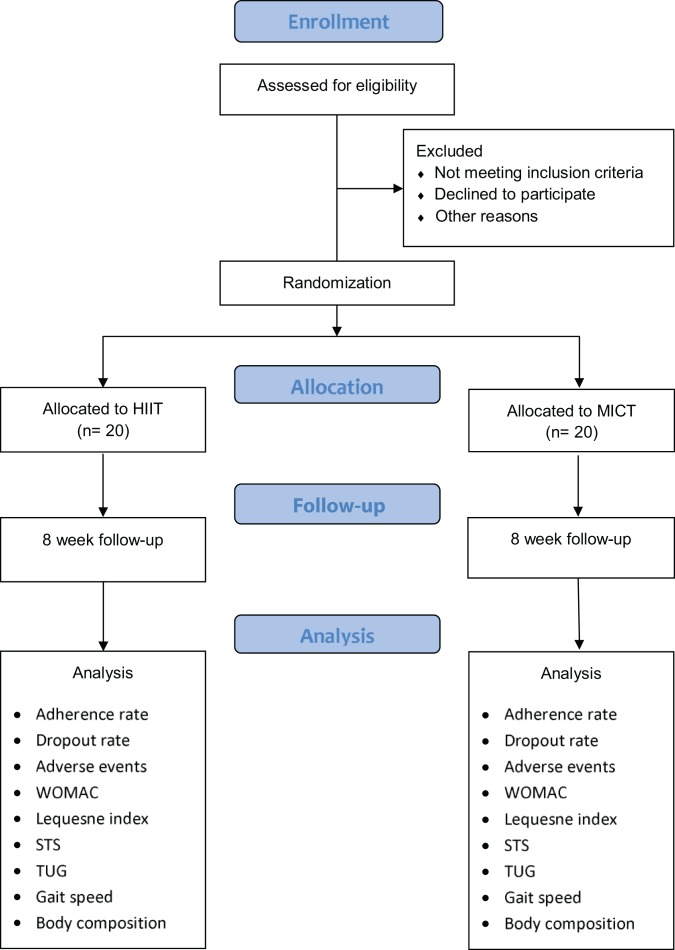
This study protocol is being published separately to allow a detailed description of the research methods, to explain the rationale for choosing the methodological details, and to stimulate consideration of the best means to simulate a research protocol that is relevant to a real-life treatment environment. This feasibility study was conducted using a randomized controlled trial (RCT) design, with a recent conceptual framework paper referring to such a study as a “randomized pilot study”.11 Participants were recruited through a variety of means, including physician and physical therapist referrals as well as responses to stories about the project that were published in a range of middle-aged and older adult-focused local magazines and newsletters. The lead investigator, who did not interact with the participants in relation to their assessments, used a computer-generated randomization sequence (https://www.random.org/) to determine participant allocation to either of the 2 cycling groups (HIIT or MICT). The results of this randomization sequence were concealed in an Excel spreadsheet (Microsoft) saved on a password-protected network drive that the research assistant only opened after completing the baseline assessment. At this point, the research assistant notified the participants about their allocation to either of the 2 cycling groups. The information provided to potential participants prior to their participation was that they would be randomly allocated to 1 of 2 cycling interventions. However, no additional information was provided to any potential participants on what forms of cycling were being evaluated in the project until they provided their consent to participate and were provided with their random allocation. A flowchart of the pilot study, including enrolment, allocation, follow-up, and analysis, is provided in Figure 1.
Figure 1.
Flowchart of the pilot study: enrollment, allocation, follow-up, and analysis protocol. HIIT, high-intensity interval training; MICT, moderate-intensity continuous training; STS, sit to stand; TUG, timed up and go; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
This study was registered on the Australian New Zealand Clinical Trials Registry (trial registration number, ACTRN12616000273482), and did not receive any external funding. The trial started recruiting participants in May 2014, with data collection completed by January 2016.
Participants
Participants for this project were males and females between the ages of 40 and 80 years with a diagnosis of knee OA confirmed by an orthopaedic surgeon. To be eligible to participate, these potential participants also required their physician’s clearance, to agree to not change their OA management during the course of the study, and to have access to a stationary bicycle for the 8-week period of the study. Potential participants could have other comorbidities in addition to their knee OA as long as these did not pose contraindications to performing a home-based cycle exercise program. Patients with unstable cardiac conditions were specifically excluded, including a history of arrhythmia and cardiac ischemia.
Prior to enrolling in the study, all potential participants were screened for their suitability to participate in the exercise program by their physician, with this supplemented by the Australian Association of Exercise and Sports Science (ESSA) Adult Pre-exercise Screening System (APSS). The APSS is accepted as the industry standard preexercise screening system in Australia and was developed from a shared technical committee that included membership from ESSA, Fitness Australia, and Sport Medicine Australia.
Data Collection
Baseline data collection included 2 OA-specific health status questionnaires (Western Ontario and McMaster Universities Osteoarthritis Index [WOMAC] and the Lequesne Index), 3 objective muscle function tests (30 seconds STS, timed up and go [TUG], and habitual gait speed tests), and a body composition assessment (via the Tanita MC-980MA body composition analyzer). After completing the baseline assessments, participants were randomized to the appropriate intervention and provided with a written explanation and familiarization of how to perform their exercise program. Because of the nature of the intervention, the research assistant who informed the participants of their group allocation and conducted all of the assessments was not blinded to participant allocation.
The familiarization component of the baseline assessment focused on showing the participants how to set their bike to an appropriate seat height and how to maintain a cadence greater than 60 revolutions per minute (rpm) during the exercise program. Ensuring the participants understood the importance of seat height and cadence and were able to modify these parameters where appropriate was considered vital for the participant safety, as knee joint loading during cycling may be increased with low seat height and lower cadence.27 Therefore, at the end of the familiarization session, the participants completed an approximate 3-minute cycle at a seat height, cadence, and overall intensity comparable to what would be performed in their first training session.
To gain insight into the relative feasibility and safety of the HIIT compared with the MICT cycling exercise intervention, participants were also provided a training and adverse events diary. The training diary required participants to record the date for each of the training sessions performed over the 8 weeks of the intervention. Similarly, the adverse events calendar required the participants to note the date on which they suffered any adverse event and to provide a clear description of what symptoms they experienced. Participants were especially advised to take note of any muscle soreness, knee pain, or cardiovascular complications as adverse events they felt were caused by the cycling program. Participants were also encouraged to call the research assistant after experiencing any adverse event and to consult appropriate medical care if required.
Feasibility Outcomes
Consistent with previous studies examining the feasibility of novel exercise activities for a variety of at-risk populations,4,7,13 the feasibility and safety of the exercise program in the current study was assessed by quantifying the adherence rate, dropout rate, and number of adverse events. The adherence rate of each participant was calculated by dividing their number of completed training sessions by the requested number of training sessions (n = 32), with this value expressed as a percentage.13 The dropout rate (also expressed as a percentage) was calculated by dividing the number of participants who failed to complete posttesting by the number of participants who completed baseline testing.13 Adverse events were defined as “an exercise-induced change that worsens an aspect of your condition that is greater than expected day-to-day variation,” a definition similar to that used previously in a study of 1687 men and women undergoing exercise programs.5
Efficacy Outcomes
The efficacy outcomes chosen for this study were selected to obtain insight into whether the exercise programs could improve the participants’ self-reported health status (eg, pain, stiffness, and physical function/disability) as well as objective measures of physical function and body composition. These outcomes were chosen due to their widespread use in knee OA studies as well as their relative simplicity of measurement.
WOMAC
The WOMAC is a valid and reliable health status questionnaire containing 24 items that assesses the OA patient’s degree of pain (5 items), stiffness (2 items), and physical function/disability (17 items).2,3 Each of the 24 questions was graded on a 5-point Likert-type scale from none (0), mild (1), moderate (2), severe (3), and extreme (4) that quantifies the patient’s degree of pain, stiffness, and/or difficulty when performing a range of activities of daily living. The maximum obtainable score on the WOMAC was 96, with a score of 39 or greater set as the cut-point for severe arthritis.23
Lequesne Index of Severity for OA of the Knee
The Lequesne index is another commonly used knee OA health status questionnaire. It consists of 11 items that assess the patient’s perspective of their pain/discomfort (5 items), maximum walking distance with or without walking aids (2 items), and physical function/disability (4 items).28,29 Each of the 11 questions was graded using a Likert-type scale, with the majority of questions being graded from no difficulty (0) to impossible to perform (2). The score for each item was summed, with the maximum possible score being 24. Higher scores indicated decreased health status, with the severity of the knee OA-related disability being described as small (1-4), intermediate (5-7), serious (8-10), very serious (11-13), and extremely serious (≥14).28,29
STS
The STS test is a valid and reliable measure of lower extremity strength and function32 that has been used in a range of older adult populations, including those with knee OA.16 The STS was performed on a straight-backed, armless chair that was 43 cm in height. Participants were asked to sit on the chair and keep their arms crossed across the chest throughout the test. On the word “go,” participants stood up and sat down as many times as possible in 30 seconds. Participants were allowed 1 practice trial in which they performed 3 to 4 repetitions at a submaximal intensity. Because of the fatigue associated with this test, only 1 trial was performed.
TUG
The TUG test is a valid and reliable measure of functional mobility36 that has been used in a range of older adult populations, including those with knee OA.16 Participants were asked to stand up from a 43-cm-high armless chair and then proceed to walk around a cone 3 meters away before sitting back on the chair.36 The following instructions were given: “Standup and walk around the flagpole and sit back down on the chair at a pace comfortable for you.” Participants were allowed 1 practice trial, with the best time from 3 timed trials used for analysis.
Habitual Gait Speed
Habitual gait speed was quantified using the GaitMat II pressure mat system (EQ Inc),which has been used previously in other older adult populations.41,49 All trials were initiated 2 m (6.56 ft) before the GaitMat II platform (3.66 m long) and finished 2 m after the GaitMat II to reduce the potential effect of acceleration and deceleration on the mean gait speed.25 Participants were provided with the following instructions prior to performing the task: “Walk toward the end of the room at a pace that is comfortable for you.”13 The average gait speed from 3 attempts was used for data analysis.
Body Composition
Body composition was assessed using the Tanita MC-980MA body composition analyzer. The Tanita MC-980MA indirectly assesses body composition (proportion of muscle, fat, and bone) via the bioelectric impedance assessment (BIA) method, with its relative validity compared with the dual-energy X-ray absorptionmetry (DEXA) established in a sample of 90 younger to older women.37 As the BIA method is sensitive to alterations in hydration, all participants were requested to be normally hydrated and to have not eaten or exercised for a period of 2 hours before the BIA assessment. To determine their body composition, participants were asked to stand motionless in bare feet on the Tanita MC-980MA platform while holding the handles for a period of 30 seconds, during which a small electric current was transmitted through their body. According to the manufacturer’s user manual, the sensitivity of this device was 0.1 kg for total body mass, muscle mass, fat-free mass, and fat mass.
Exercise Program
Participants in each group were requested to perform 4 stationary cycling sessions per week for 8 weeks, with each session involving approximately 25 minutes of exercise. Regardless of group allocation, all participants were encouraged to begin their exercise program somewhat conservatively with regard to the intensity of their initial sessions so as to minimize the chance of any exercise-related adverse events. After they felt comfortable performing their first few exercise sessions, they were requested to progressively increase the resistance (workload) and/or cycling cadence throughout the 8-week program.
The cycling program for the MICT group commenced with a 3-minute warm-up at a light intensity and finished with a 2-minute cool-down at a similar intensity. In between the warm-up and cool-down phases, the participants were requested to select a workload that they could cycle at a cadence of 60 to 80 rpm for 20 minutes at a moderate intensity. For the purposes of this home-based exercise program, moderate intensity was defined as “an intensity in which you are able to speak in complete sentences during the exercise. If you find yourself getting too puffed or out of breath, slow down a little.” For those in the HIIT group, their training sessions commenced with a 7-minute warm-up of progressively increasing intensity and concluded with a 6- to 7-minute cool-down of light to moderate cycling. The HIIT component of the training session involved 5 series of high- and low-intensity cycling, with each series commencing with 45 seconds at a moderately high to high intensity followed by 90 seconds at moderately low intensity. For the five, 45-second high-intensity bouts, the participants were requested to cycle at a higher cadence (up to 110 rpm) at a resistance similar or slightly higher than the low-intensity recovery bouts (performed at approximately 70 rpm). The intensity of the high-intensity bouts was described to the participants as “an intensity at which you felt it was quite difficult to complete sentences during the exercise.”
Statistical Analysis
If the data were normally distributed, centrality and dispersion of the continuous data will be reported as means and standard deviations, whereas categorical measures will be reported as a count and percentage. Within-group changes will be analyzed using a 2-tailed paired t test, with between-group changes analyzed using 2-tailed independent t tests with unequal variance. If the assumptions of normality are not met, within-group and between-group changes will be assessed using the Wilcoxon signed rank test and Mann-Whitney U test, respectively. All statistical analyses will be performed in Microsoft Excel 2010 (Microsoft) or SPSS version 23 (IBM Corp), with statistical significance set at P ≤ .05.
Discussion
In this protocol article, we have described the methods of a RCT that, to the authors’ knowledge, is the first to examine the feasibility, safety, and/or efficacy of a home-based HIIT cycling program for middle-aged and older adults with knee OA. We believe the data to be obtained in this study have the potential to improve usual care practice for individuals with knee OA, as MICT cycling is currently one of the most recommended and commonly performed types of exercise performed by this population. This belief is based on the fact that although MICT cycling has a range of cardiovascular health benefits and may assist with weight maintenance for this population, it may not provide a sufficient stimulus to arrest the OA-related decline in lower body muscle mass, muscle strength, and articular cartilage composition and architecture that contribute to the pain, stiffness, and functional limitations seen with knee OA.23,40,48,51 Therefore, the primary aim of this study was to examine the feasibility and safety of an alternative form of exercise (HIIT cycling) that can be feasibly and safely performed at home by individuals with knee OA without any medical or allied health supervision.6,43 A secondary aim of this study was to gain some preliminary insight into whether HIIT provides comparable or greater improvements in self-reported health status, muscle function, and body composition to MICT. The results of this study may therefore improve outcomes for knee OA patients and be used to power larger RCTs that would be better able to determine the relative efficacy of HIIT compared with MICT cycling for this population. As the design of exercise trials for clinical populations is always challenging and can result in substantial debate between researchers and practitioners, the following sections of this discussion provide a rationale for the methods described in this protocol and acknowledge some of its limitations.
Internal and External Validity
One of the primary issues of health research is the sometimes diverging requirements to maximize the level of internal and external validity.22,46 Internal validity, also referred to as methodological rigor, is typically maximized by using an RCT design to control for potential covariates that may influence the outcome of the trial. We therefore decided to utilize an RCT design to ensure a high degree of internal validity for this pilot study, even though such a design is not always used in feasibility exercise trials involving various older adult populations with chronic disease and/or complex care needs.4,7,13
We also wished to conduct a trial that had a high degree of external validity (generalizability). We therefore designed this study so that the participants would be asked to perform their cycling program at home in an unsupervised capacity rather than attend a university or hospital exercise clinic for supervised sessions. We chose this home-based design as it better allows us to quantify the true feasibility and safety of this form of exercise in the way that it is likely to be performed by a high proportion of OA patients in the real world. We do, however, acknowledge that this research design reduces aspects of its internal validity in that we are unable to objectively record the frequency, duration, and intensity of exercise performed by the participants. This means we are unable to directly quantify the dose-response to these 2 forms of cycling exercise.
Translational Ability
While MICT cycling is one of the more highly recommended and popular forms of exercise for individuals with knee OA, it may not be as highly recommended by clinicians, allied health professionals, and support groups as aquatic exercise. This may reflect the finding that the aquatic environment can reduce knee joint loading, thereby resulting in immediate and long-term reductions in joint pain.12,52 However, there remain a number of issues that may affect the external validity of this recommendation, that is, the likelihood that individuals with knee OA would obtain benefits from regularly performing aquatic exercise in the real world. For example, the majority of studies examining the potential benefits of aquatic exercise for individuals with OA have utilized hydrotherapy sessions, which are quite expensive as they are typically supervised by a physical therapist and involve only a single or small number of patients.19,33 Group-based aquatic exercise classes (often referred to as aqua fitness classes) are routinely held in community-based swimming pools. These classes can be quite varied but may be typically categorized as deep water aquatic exercise activities whereby floatation devices are used to remain in a vertical position or shallow water aquatic training whereby the exerciser stands upright in water that reaches a depth no higher than the chest, meaning that their feet can still touch the pool’s bottom surface. These aquatic fitness exercise options are considerably less expensive than hydrotherapy but less is known about the feasibility, safety, and efficacy of such aqua fitness programs for individuals with knee OA.14–17 Furthermore, to access any of these aquatic exercise options, individuals with knee OA would need to travel to a nearby pool, with this costing additional time and money compared with a home-based exercise program. Aquatic exercise may also not be available throughout the year due to cool weather and/or a closing down of public swimming pools. We would therefore argue that the selection of cycling as the form of exercise in this study may increase the translational ability of these findings if we can demonstrate the feasibility and safety of home-based HIIT and MICT cycling for individuals with knee OA.
Blinding of Participants and Research Staff
One of the primary issues affecting the scientific rigor of exercise trials is the challenge in blinding participants to their allocation, especially when 1 group is allocated to a nonexercising control group. The design adopted in this study protocol seeks to minimize this effect by randomizing the participants into 1 of 2 cycling programs. This approach was chosen to reduce the potential for selection bias and a range of accidental biases that may occur when comparing the results of the 2 groups.
Blinding of research staff to the allocation of participants can also be an issue in many studies, including those involving exercise. It would have been preferable to have had a blinded research assistant perform the baseline and follow-up assessments of the participants to reduce the potential for any bias in the data collection process. However, as this study was not funded, we were unable to procure sufficient resources to pay for a blinded research assistant.
Outcome Assessment and Statistical Analysis
According to the CONSORT (Consolidated Standards of Reporting Trials) statement,35 researchers should select outcomes of the greatest importance to the relevant stakeholders, in this case, middle-aged and older adults with knee OA and their clinicians. The CONSORT statement also requires researchers to specify the study’s primary and secondary outcomes and the time points at which these outcomes would be obtained so as to minimize the risk of selective reporting of data.
The primary variables of interest to this pilot study were the adherence rate, dropout rate, and number and type of adverse events. These outcomes were very similar to other exercise feasibility trials/pilot studies that have been performed with older adults with chronic disease and complex care needs.4,7,13 The rationale for the selection of these feasibility outcomes was that in order for an exercise intervention to affect public health (in this case, middle-aged and older adults with knee OA), the likely participants need to adhere to the required exercise prescription (ie, frequency, duration, and intensity of exercise). It is also vital that the participants do not discontinue (dropout) the study for any reasons, as the rate of dropouts may be indicative of a high number of barriers and/or adverse events or a lack of perceived benefits from the exercise program. Quantification of the number and severity of adverse events was also deemed vital for a number of reasons, as the severity and number of adverse events in exercise studies involving populations with chronic disease are often underreported due to a lack of consensus on the definition.31 We felt that recording the adverse events was an example of our duty of care to the participants in this study, as we did not wish to exacerbate any of their OA-related symptoms or cause them any other form of harm. Compared with cardiovascular exercise studies where the relative safety of HIIT has been well demonstrated in supervised trials involving approximately 5000 cardiac rehabilitation patients39 and 90 unsupervised home-based coronary artery disease patients,1 very little of the HIIT research has focused on recruiting individuals with musculoskeletal conditions.43 While no significant changes in disease activity or pain were reported after 10 weeks of HIIT cycling in young to middle-aged adults with rheumatoid arthritis (n = 7) or juvenile idiopathic arthritis (n = 11),43 our study appears to be the first to utilize HIIT in a group of middle-aged and older adults with knee OA. We therefore felt it was very important to monitor the relative safety of this form of activity in this population, as most exercise-related adverse events are likely to be musculoskeletal in nature, with muscle strain or joint pain most commonly reported.31 The number of adverse events has also been shown to increase in trials similar to our study (ie, involving participants with a variety of chronic diseases, functional limitations, and/or low levels of physical activity), especially when the exercise was of a high intensity.31 The second reason we wished to monitor the number of adverse events is that increased exercise adherence and reduced dropout rates may be associated with the number and severity of adverse events.31 Therefore, if we could minimize the number of adverse events it was also likely that we could increase the adherence rate and reduce the dropout rate for this trial. If this can be achieved, the efficacy of the trial as indicated by the change in the secondary outcomes may also be maximized.
Considerable discussion occurred with respect to which aspects of physical function should be included in the efficacy outcomes assessed in this pilot study. Aspects of physical function that may be relevant to individuals with knee OA but were not included in the assessment protocol included cardiovascular fitness and knee joint range of motion. Such decisions were not taken lightly but reflect the following 2 reasons. The first reason was that the trial was primarily focused on the feasibility outcomes, and as a result, did not want to burden the participants with a large number of secondary efficacy outcomes. Second, the rationale for the development of the HIIT cycle protocol reflected the clinician’s observations that while MICT cycling provided some benefits, it did not optimally address the loss of muscle mass, strength, and function of the affected lower limb. We would, however, suggest that larger RCTs look to include such measures in the future to get a better idea of the relative efficacy of HIIT compared with MICT cycling in this population.
Limitations
Overall, we feel there are 3 main limitations within the study, with these reflecting the potential heterogeneity of the sample to be recruited, a lack of blinding, and the lack of objective data regarding what was performed in the 2 exercise programs. We acknowledge that even with the number of inclusion and exclusion criteria used in this trial, it is likely that the participants would be somewhat heterogeneous in relation to their demographic and knee OA–related treatment history, symptoms, and overall health status. Such heterogeneity in our sample has the potential to impact on the primary outcomes (adherence rate, dropout rate, and number of adverse events) along with the secondary efficacy measures. Specifically, it is possible that independent variables such as age, sex, severity of OA, and number and type of other comorbidities could all influence the feasibility, safety, and efficacy of these 2 cycling protocols. While the results of this study may not be able to be applied to any particular subpopulation of knee OA patients, we felt that this approach was best to demonstrate the general feasibility and safety of this approach and to power larger RCTs in this area.
With respect to blinding, the research assistant was not blinded to the allocation of participants, and all exercise participants knew they would get to perform a form of cycling exercise. The lack of blinding of the assessors obviously introduces the potential for measurement bias, particularly when performing the posttest assessments. We have also acknowledged that the home-based exercise design of our study limits our ability to objectively quantify the frequency, duration, and intensity of exercise performed by participants in both groups. The lack of such data does not allow us to quantify the dose-response relationship with respect to the feasibility and efficacy outcomes assessed in the study. However, as this was an unfunded pilot study, the primary emphasis was on gaining insight into the relative feasibility and safety of these forms of home-based cycling exercise rather than determining the dose-response relationship of the efficacy outcomes.
We therefore hope that this study protocol will stimulate further research into the potential application of HIIT for individuals with musculoskeletal conditions such as knee OA. Such studies could further increase the level of external validity by supervising the exercise sessions or providing the home-based participants with heart rate monitors and/or cycle ergometer instrumentation that records the power outputs (or other measures of frequency, duration, and intensity) of the exercise performed. Such research should provide the necessary scientific basis for orthopaedic surgeons and allied health professionals to better understand which of their patients are most likely to adhere to and benefit from HIIT cycling programs.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
Ethical approval for this study was obtained from the Bond University Research Ethics Committee (RO1776).
References
- 1. Aamot IL, Forbord SH, Gustad K, et al. Home-based versus hospital-based high-intensity interval training in cardiac rehabilitation: a randomized study. Eur J Prev Cardiol. 2014;21:1070–1078. [DOI] [PubMed] [Google Scholar]
- 2. Bellamy N. Pain assessment in osteoarthritis: experience with the WOMAC osteoarthritis index. Semin Arthritis Rheum. 1989;18(4 suppl 2):14–17. [DOI] [PubMed] [Google Scholar]
- 3. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 4. Bossers WJ, Scherder EJ, Boersma F, Hortobágyi T, van der Woude LH, van Heuvelen MJ. Feasibility of a combined aerobic and strength training program and its effects on cognitive and physical function in institutionalized dementia patients. a pilot study. PLoS One. 2014;9:e97577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouchard C, Blair SN, Church TS, et al. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One. 2012;7:e37887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buckley S, Knapp K, Lackie A, et al. Multimodal high-intensity interval training increases muscle function and metabolic performance in females. Appl Physiol Nutr Metab. 2015;40:1157–1162. [DOI] [PubMed] [Google Scholar]
- 7. Cheema BS, Davies TB, Stewart M, Papalia S, Atlantis E. The feasibility and effectiveness of high-intensity boxing training versus moderate-intensity brisk walking in adults with abdominal obesity: a pilot study. BMC Sports Sci Med Rehabil. 2015;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:1323–1330. [DOI] [PubMed] [Google Scholar]
- 9. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunlop DD, Song J, Semanik PA, et al. Objective physical activity measurement in the osteoarthritis initiative: are guidelines being met? Arthritis Rheum. 2011;63:3372–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eldridge SM, Lancaster GA, Campbell MJ, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLos One. 2016;11:e0150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Escalante Y, García-Hermoso A, Saavedra JM. Effects of exercise on functional aerobic capacity in lower limb osteoarthritis: a systematic review. J Sci Med Sport. 2011;14:190–198. [DOI] [PubMed] [Google Scholar]
- 13. Fien S, Henwood T, Climstein M, Keogh JW. Feasibility and benefits of group-based exercise in residential aged care adults: a pilot study for the GrACE programme. PeerJ. 2016;4:e2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fisken A, Keogh JWL, Waters D, Hing W. Perceived benefits, motives, and barriers to aqua-based exercise among older adults with and without osteoarthritis. J Appl Gerontol. 2015;34:377–396. [DOI] [PubMed] [Google Scholar]
- 15. Fisken A, Waters D, Hing W, Keogh JW. Perception and responses to different forms of aqua-based exercise among older adults with osteoarthritis. Int J Aquatic Res Educ. 2014;8:32–52. [Google Scholar]
- 16. Fisken A, Waters D, Hing W, Steele M, Keogh JW. Comparative effects of 2 aqua-exercise programs on physical function, balance and perceived quality-of-life in older adults with osteoarthritis. J Geriaric Phys Ther. 2015;38:17–27. [DOI] [PubMed] [Google Scholar]
- 17. Fisken AL, Waters DL, Hing WA, Keogh JW. Perceptions towards aqua-based exercise among older adults with osteoarthritis who have discontinued participation in this exercise mode. Aust J Ageing. 2016;35:12–17. [DOI] [PubMed] [Google Scholar]
- 18. Flores RH, Hochberg MC. Definition and classification of osteoarthritis In: Brandt KD, Doherty M, Lohmander LS, eds. Osteoarthritis. 2nd ed Oxford, England: Oxford University Press; 2003:1–8. [Google Scholar]
- 19. Foley A, Halber J, Hewitt T, Crotty M. Does hydrotherapy improve strength and physical function in patients with osteoarthritiss—a randomised controlled trial comparing a gym based and a hydrotherapy strengthening programme. Ann Rheum Dis. 2003;62:1162–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Francois ME, Little JP. Effectiveness and safety of high-intensity interval training in patients with type 2 diabetes. Diabetes Spectr. 2015;28:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:Cd004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Godwin M, Ruhland L, Casson I, et al. Pragmatic controlled clinical trials in primary care: the struggle between external and internal validity. BMC Med Res Methodol. 2003;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hawker GA, Wright JG, Coyte PC, et al. Differences between men and women in the rate of use of hip and knee arthroplasty. N Eng J Med. 2000;342:1016–1022. [DOI] [PubMed] [Google Scholar]
- 24. Hootman JM, Macera CA, Ham SA, Helmick CG, Sniezek JE. Physical activity levels among the general US adult population and in adults with and without arthritis. Arthritis Rheum. 2003;49:129–135. [DOI] [PubMed] [Google Scholar]
- 25. Kressig RW, Beauchet O. Guidelines for clinical applications of spatio-temporal gait analysis in older adults. Aging Clin Exp Res. 2006;18:174–176. [DOI] [PubMed] [Google Scholar]
- 26. Kutzner I, Heinlein B, Graichen F, et al. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J Biomech. 2010;43:2164–2173. [DOI] [PubMed] [Google Scholar]
- 27. Kutzner I, Heinlein B, Graichen F, et al. Loading of the knee joint during ergometer cycling: telemetric in vivo data. J Orthop Sports Phys Ther. 2012;42:1032–1038. [DOI] [PubMed] [Google Scholar]
- 28. Lequesne M. Indices of severity and disease activity for osteoarthritis. Semin Arthritis Rheum. 1991;20(6 suppl 2):48–54. [DOI] [PubMed] [Google Scholar]
- 29. Lequesne MG. The algofunctional indices for hip and knee osteoarthritis. J Rheumatol. 1997;24:779–781. [PubMed] [Google Scholar]
- 30. Liou K, Ho S, Fildes J, Ooi SY. High intensity interval versus moderate intensity continuous training in patients with coronary artery disease: a meta-analysis of physiological and clinical parameters. Heart Lung Circ. 2016;25:166–174. [DOI] [PubMed] [Google Scholar]
- 31. Liu C-J, Latham N. Adverse events reported in progressive resistance strength training trials in older adults: 2 sides of a coin. Arch Phys Med Rehabil. 2010;91:1471–1473. [DOI] [PubMed] [Google Scholar]
- 32. Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci. 2002;57:M539–M543. [DOI] [PubMed] [Google Scholar]
- 33. Lund H, Weile U, Christensen R, et al. A randomized controlled trial of aquatic and land-based exercise in patients with knee osteoarthritis. J Rehabil Med. 2008;40:137–144. [DOI] [PubMed] [Google Scholar]
- 34. Mangione KK, McCully K, Gloviak A, Lefebvre I, Hofmann M, Craik R. The effects of high-intensity and low-intensity cycle ergometry in older adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci. 1999;54:M184–190. [DOI] [PubMed] [Google Scholar]
- 35. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 37. Ragini B, Aishwarya SR, Tamil Selvan M, Pillai A, Anburajan M. Prediction of body fat using segmental body composition by bioelectrical impedance in the evaluation of obesity. J Eng Appl Sci. 2015;10:3627–3632. [Google Scholar]
- 38. Ramos JS, Dalleck LC, Tjonna AE, Beetham KS, Coombes JS. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med. 2015;45:679–692. [DOI] [PubMed] [Google Scholar]
- 39. Rognmo Ø, Moholdt T, Bakken H, et al. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation. 2012;126:1436–1440. [DOI] [PubMed] [Google Scholar]
- 40. Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005;52:3507–3514. [DOI] [PubMed] [Google Scholar]
- 41. Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roubenoff R. Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci. 2000;904:553–557. [DOI] [PubMed] [Google Scholar]
- 43. Sandstad J, Stensvold D, Hoff M, Nes BM, Arbo I, Bye A. The effects of high intensity interval training in women with rheumatic disease: a pilot study. Eur J Appl Physiol. 2015;115:2081–2089. [DOI] [PubMed] [Google Scholar]
- 44. Senior HE, Henwood TR, Beller EM, Mitchell GK, Keogh JW. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas. 2015;82:418–423. [DOI] [PubMed] [Google Scholar]
- 45. Shiraev T, Barclay G. Evidence based exercise. Clinical benefits of high intensity interval training. Aust Fam Physician. 2012;41:960–962. [PubMed] [Google Scholar]
- 46. Slack MK, Draugalis JR. Establishing the internal and external validity of experimental studies. Am J Health Syst Pharm. 2001;58:2173–2181. [PubMed] [Google Scholar]
- 47. Tanaka R, Ozawa J, Kito N, Moriyama H. Does exercise therapy improve the health-related quality of life of people with knee osteoarthritis? A systematic review and meta-analysis of randomized controlled trials. J Phys Ther Sci. 2015;27:3309–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tiderius CJ, Svensson J, Leander P, Ola T, Dahlberg L. dGEMRIC (delayed gadolinium-enhanced MRI of cartilage) indicates adaptive capacity of human knee cartilage. Magn Reson Med. 2004;51:286–290. [DOI] [PubMed] [Google Scholar]
- 49. Trehan SK, Wolff AL, Gibbons M, Hillstrom HJ, Daluiski A. The effect of simulated elbow contracture on temporal and distance gait parameters. Gait Posture. 2015;41:791–794. [DOI] [PubMed] [Google Scholar]
- 50. Uthman OA, van der Windt DA, Jordan JL, et al. Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. BMJ. 2013;347:f5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Ginckel A, Baelde N, Almqvist KF, Roosen P, McNair P, Witvrouw E. Functional adaptation of knee cartilage in asymptomatic female novice runners compared to sedentary controls. A longitudinal analysis using delayed gadolinium enhanced magnetic resonance imaging of cartilage (dGEMRIC). Osteoarthritis Cartilage. 2010;18:1564–1569. [DOI] [PubMed] [Google Scholar]
- 52. Westby MD. A health professional’s guide to exercise prescription for people with arthritis: a review of aerobic fitness activities. Arthritis Care Res. 2001;45:501–511. [DOI] [PubMed] [Google Scholar]
- 53. Yoshimura N, Muraki S, Oka H, et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage. 2012;20:1217–1226. [DOI] [PubMed] [Google Scholar]