Abstract
Study Design:
Multicenter retrospective case series and review of the literature.
Objective:
To determine the rate of esophageal perforations following anterior cervical spine surgery.
Methods:
As part of an AOSpine series on rare complications, a retrospective cohort study was conducted among 21 high-volume surgical centers to identify esophageal perforations following anterior cervical spine surgery. Staff at each center abstracted data from patients’ charts and created case report forms for each event identified. Case report forms were then sent to the AOSpine North America Clinical Research Network Methodological Core for data processing and analysis.
Results:
The records of 9591 patients who underwent anterior cervical spine surgery were reviewed. Two (0.02%) were found to have esophageal perforations following anterior cervical spine surgery. Both cases were detected and treated in the acute postoperative period. One patient was successfully treated with primary repair and debridement. One patient underwent multiple debridement attempts and expired.
Conclusions:
Esophageal perforation following anterior cervical spine surgery is a relatively rare occurrence. Prompt recognition and treatment of these injuries is critical to minimizing morbidity and mortality.
Keywords: esophageal perforation, anterior, cervical, spine, surgery
Introduction
The anterior approach to the cervical spine has been extensively used since its description in the late 1950s1 and is widely regarded as being safe and versatile with low rates of adverse events reported.2-6 Albeit very rare, esophageal perforation is a potentially fatal complication of anterior cervical spine surgery. A 5-year survey-based report of the Cervical Spine Research Society membership found an estimated prevalence of esophageal injuries during anterior cervical surgery to be 0.25%;7 other reports in the literature describe frequencies ranging from 0%8 to 1.62%,9 thus corroborating the low overall prevalence of this pathology.10-32 Prompt recognition of these injuries is critical, as perforations can lead to the formation of fistulae, abscesses, osteomyelitis, mediastinitis, sepsis, and death. Sealy described esophageal perforation as “the most rapidly fatal and serious perforation of the gastrointestinal tract.”33 Patients with esophageal perforations will often present with vague complaints, making the condition difficult to diagnose and potentially delaying the onset of treatment. Even with timely intervention, morbidity and mortality rates remain high—perforations detected and treated within 24 hours have seen mortality rates reported as high as 20%.6 Delays in treatment initiation, however, have seen mortality rates approaching 50%.34,35 Confounding the issue further is the lack of consensus on the management of these injuries—a number of different treatment options have been reported with varied results. Lately, urgent primary debridement and repair has begun to emerge as having the greatest rate of successful outcomes.6,36-40 In this article, esophageal anatomy, the prevalence of esophageal injury during anterior cervical surgery, clues to diagnose these injuries, and management strategies used in the treatment of esophageal injuries following anterior cervical spine surgery are addressed. Case reports and a review of the literature are presented here as part of AOSpine’s rare complication articles.
Methods
A retrospective, multicenter, case series was performed involving 21 high-volume surgical centers from the AOSpine North America Clinical Research Network. The charts of 17 625 patients who underwent cervical spine surgery (from C2 to C7) between January 1, 2005, and December 31, 2011, were examined for the occurrence of predefined surgical complications. The complications included reintubation, esophageal perforation, epidural hematoma, C5 nerve root palsy, recurrent laryngeal nerve palsy, superior laryngeal nerve palsy, hypoglossal or glossopharyngeal nerve palsy, durotomy, brachial plexopathy, blindness, graft extrusion, malpositioned screws requiring reoperation, anterior cervical infection, carotid artery injury or cerebrovascular accident, vertebral artery injuries, Horner’s syndrome, thoracic duct injury, tetraplegia, intraoperative death, revision of arthroplasty, and pseudomeningocele. Data were abstracted by trained research staff at each institution and then transcribed into study-specific case report forms. The case report forms were then transferred to the AOSpine North America Research Network Methodological Core for processing and data entry. Descriptive statistics were provided for baseline patient characteristics.
Results
Of the 17 625 patients who underwent cervical spine surgery during the assigned time period, 9591 were identified as having had an anterior cervical procedure. Charts, images, operative reports, notes, and narratives were then examined to identify esophageal perforations. Only 2 cases (0.02%) were noted—both occurred at the time of the index procedure and both were addressed in the acute postoperative period. Both patients were male. No cases of delayed presentation of esophageal perforation were noted.
Case 1
The first patient who sustained an esophageal perforation was a 52-year-old male who presented with right deltoid weakness. He underwent a corpectomy of C5; a small esophageal tear was noted intraoperatively and otolaryngology was called in to perform a repair. A swallow study was performed postoperatively, which revealed a persistent leak. A gastrotomy tube was placed and the patient continued with nothing by mouth. After 3 weeks, a liquid diet was started and was advanced to a full diet as tolerated. The patient healed uneventfully from that point forward; however, his deltoid weakness persisted.
Case 2
The second case occurred in a 61-year-old male with ankylosing spondylitis who sustained a fracture of C6 and C7. He was treated with a multilevel anterior discectomy and fusion with supplemental posterior fixation incorporating the upper thoracic spine. Preoperatively, the patient was noted to smoke cigarettes. Following surgery, he awoke paraplegic and was noted to have erythema and drainage of the anterior incision postoperatively. On postoperative day 14, an upper endoscopy was performed, which detected an esophageal injury. The patient was taken to the operating room where a primary repair and pectoralis flap were performed. Subsequently, he continued to deteriorate and the wound was noted to be colonized with a multidrug resistant Pseudomonas species; he went on to develop sepsis and multi-organ system failure. After 72 days in the hospital, the family elected to withdraw care and the patient expired.
Discussion
Anatomy
The esophagus, lying directly anterior to the cervical spine, requires mobilization during anterior cervical spine surgery.1 Though its precarious position makes the esophagus somewhat vulnerable to injury, several anatomic layers must be disrupted in order to introduce the contents of the esophageal lumen to the retropharyngeal and prevertebral spaces.41 Adventitia overlies the outermost esophageal layer, thereby protecting the longitudinal and circular muscle underneath, as well as the submucosal and mucosal layers, respectively. Aggressive or improper retraction of the esophagus may result in injury of these layers during anterior cervical surgery.3 The area of the esophagus most vulnerable to injury is known as Killian’s Triangle, which is formed by the junction of the paired inferior constrictor pharyngeus muscles, and the cricopharyngeus. This region, which usually lies anterior to the C5/C6 disc but is occasionally found more caudally, is particularly susceptible to injury since the posterior esophageal mucosa lacks muscular protection. Here, only the thin buccopharyngeal fascia separates the esophagus from the retroesophageal space.40 A second area of esophageal weakness is located laterally at the level of the thyrohyoid membrane. Esophageal injuries are more likely to occur at these 2 specific locations.6 Esophageal perforations located in the cervical spine are generally considered less dangerous than esophageal injuries located in the thoracic region—cervical injuries tend to have a slower spread into the mediastinum, in part because thoracic injuries are subject to the negative pressures generated during inspiration.40,42
The majority of esophageal injuries are found to be iatrogenic, though lesions secondary to foreign bodies, trauma, and spontaneous perforations have all been reported in the literature.32,36,43 Inadvertent contact with a knife, high speed burr, and misuse of electrocautery have also been cited as potential sources of esophageal injury. Caution during the initial exposure coupled with judicious retractor placement has been suggested to help minimize esophageal injury.3 Furthermore, placing the retractor blades under the longus colli muscle can help prevent inadvertent esophageal “escape” during the procedure, thereby minimizing injury.44
Prevalence
In our series, only 2 cases of esophageal injury were reported in 9591 patients. This is somewhat lower (0.02%) than previously described in the literature but is consistent with others’ findings of an overall very low incidence. We attribute our series’ low prevalence to 2 factors. First, surgeons contributing to the AOSpine North America Clinical Research Network represent some of the most experienced surgeons in the country. Combining this experience with the advanced facilities available at the large academic institutions where these data were collected may have served to lower the prevalence of esophageal injury seen in our cohort. Second, our study design retrospectively identified these injuries based on medical record and chart review. Though these injuries are rare and likely to be remembered by most surgeons, collecting these data prospectively may have detected more cases.
The literature reveals that most cases of esophageal perforation are discovered at the time of surgery, or during the acute or subacute postoperative period (Table 1).17 Fountas et al reviewed 1015 primary anterior cervical surgeries performed at their institution and reported 3 esophageal perforations.4 Of the 3 perforations, 2 were recognized intraoperatively. The third patient was diagnosed on the second postoperative day and underwent primary repair along with mediastinal irrigation and debridement. Unfortunately, the patient expired 10 days after surgery, emphasizing the importance of early recognition. In the largest series described in the literature, Gaudinez’s group reported 44 esophageal perforations seen in 2946 patients treated at a single regional spinal cord injury referral center over a 25-year period; all patients had undergone surgery for cervical fractures at other institutions. They found that 77% of the esophageal injuries were at least in part related to patients’ anterior cervical spine surgery. Forty-two of the 44 patients (95%) underwent repair of the esophagus, with 4 patients requiring 2 or more procedures. They also noted that the length of hospital stay averaged 253 days in patients with esophageal perforations.16
Table 1.
Summary of Selected Case Series of Esophageal Injuries Following Anterior Cervical Spine Surgery.
| Author | Year | Cases (n) | Location at C5-C7 | Male | Time to Diagnosis | Treatment | Hospital Stay (Days) | Flaps | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Rueth et al | 2010 | 6 | — | 100% | <1 week (n = 2); delayed (n = 4) | Abx, NPO, surgical repair, and multiple debridements | 40 | 17% | 17% |
| Fountas et al | 2007 | 3 | — | — | Intraoperative (n = 2); day 2 (n = 1) | Abx, NPO, surgical repair, and debridement | — | 0% | 33% |
| Gaudinez et al | 2000 | 44 | — | 100% | — | Abx, NPO, surgical repair, and debridement (42/44) | 253 | — | 5% |
| Newhouse et al | 2009 | 22 | 11/16 | 70% (14/20); 2 unknown | Intraoperative (n = 7) | Abx, NPO, surgical repair, and debridement (20/22) | — | — | 5% |
| Patel et al | 2008 | 3 | 3/3 | 33% | <3 days (n = 2); 1 month (n = 1) | Abx, NPO, surgical repair, and debridement | — | 33% | 0% |
| Lu et al | 2012 | 6 | 6/6 | 66% | Intraoperative (n = 1); <3 weeks (n = 3); >3 years (n = 2) | Abx, NPO, surgical repair, and debridement | — | 0% | 0% |
| Zhong et al | 2013 | 6 | 5/6 | 100% | <1 week (n = 6) | Abx, NPO (6/6); surgical debridement (3/6); surgical repair (2/6) | — | 0% | 17% |
Abbreviations: Abx, antibiotics; NPO, nil per os (nothing by mouth).
Lu and colleagues reported their experience over a 10-year period—during that time, 6 esophageal perforations were discovered in 1045 anterior cervical surgeries; only one of those perforations was noted intraoperatively. Three of the cases were diagnosed between 7 and 18 days after surgery, and the remaining 2 cases presented years after the index procedure. Of note, 4 of the perforations occurred at C5/C6, and 2 at C6/C7, highlighting the aforementioned areas of esophageal anatomic vulnerability.22
With the help of the Cervical Spine Research Society, Newhouse’s group retrospectively collected data on 22 cases of esophageal perforation from multiple institutions. In that series, they noted that 6 of 22 cases of esophageal perforation were diagnosed at the time of surgery. An additional 6 cases were found during the acute postoperative period, and another 10 were discovered over a period of weeks to months. Only 1 of the 22 cases resulted in a fatality.7 The location of the tear was reported in 16 of the 22 cases—11 tears (68.8%) were found between C5 and C7. Also of note, less than a third of reported cases were noted to have occurred intraoperatively; more than two thirds of the cases presented in a delayed fashion and were felt to be due to prominence of metal, bone, or cement (Figures 1 and 2).
Figure 1.
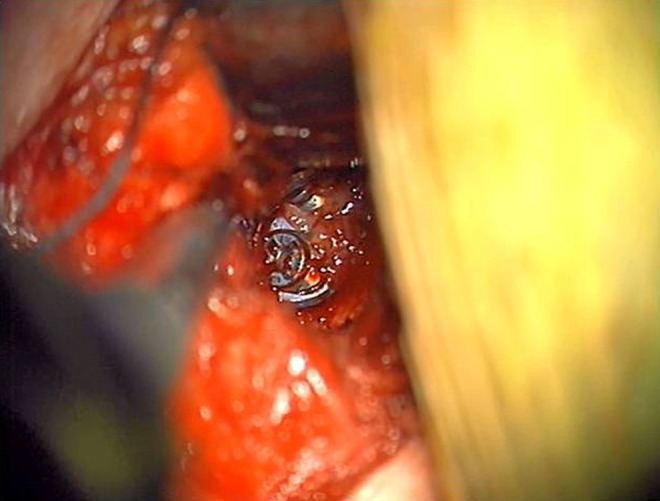
The esophagus is retracted medially exposing the cervical instrumentation.
Figure 2.
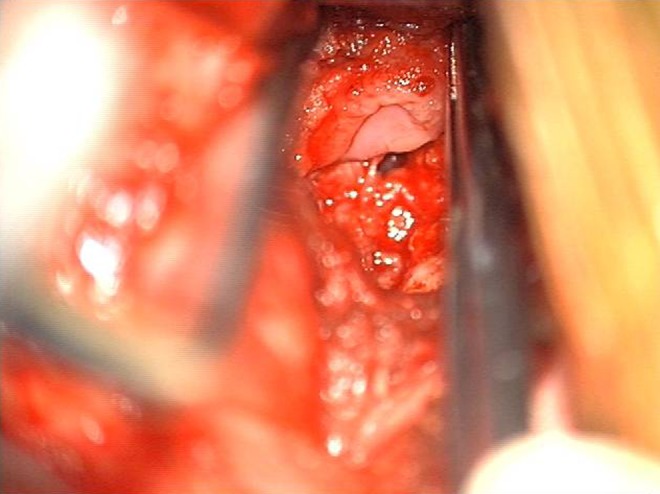
The cervical instrumentation has been removed. An esophageal perforation is identified adjacent to where the instrumentation had been.
Patel et al reviewed the prevalence of esophageal perforations in 3000 patients who had undergone anterior cervical surgery performed by 5 surgeons over a 30-year period. They found only 3 cases of perforation in their cohort (0.1%). All 3 cases were diagnosed during the acute postoperative period, and all 3 perforations occurred on the posterior portion of the esophagus at the C5/C6 interspace. Their report noted that 2 of the 3 patients had predisposing risk factors—antecedent cervical spine trauma and diverticulae.24
A 20-year retrospective cohort study detailing 1097 cases was conducted by Zhong et al.32 His group identified 5 patients with esophageal perforations that occurred at their institution, as well as one that occurred elsewhere but was managed at their facility. All the patients in their series were diagnosed in the early postoperative period, and all but one survived—the mortality was secondary to a postoperative pneumonia. The 6 patients were all treated with a nasogastric tube, intravenous antibiotics, enteral and parenteral nutrition, and surgical irrigation and debridement.32
Recently, a number of reports have highlighted the incidence of esophageal perforations presenting in a delayed fashion.* Hardware migration and irritation have been noted as causes of these injuries.† Many of these delayed presentations happen within the first 18 months after surgery‡; however some groups have reported perforations occurring many years following the index procedure. Gazzeri et al described the migration of a screw that caused perforation 11 years after the index procedure. In their report, they found initial screw pull out, followed by complete expulsion and entry into the digestive tract occurring over a period of just 6 days.46 Though uncommon, other reports of patients presenting after many years is not unheard of: Kim and colleagues reported an esophageal perforation that developed 8 years after the index procedure18; Lu, Tian, and Solerio each described a perforation at 7 years after the index procedure22,47,48; and Woolley reported a perforation seen 5 years postoperatively.27
Clinical Presentation and Diagnosis
The clinical presentation of patients with esophageal perforation is highly variable—patients may present with anything from no signs or symptoms at all, to florid sepsis and respiratory distress.§ Yee reported an asymptomatic individual who was found to have a screw missing from his anterior cervical construct on a postoperative radiograph 3 months after surgery; further imaging located the screw in the intestinal tract. The patient did not recall any symptoms related to dysphagia, odynophagia, neck pain, or cough.28 A similar case was reported by Pompili et al—his group described an asymptomatic patient who presented after 1 year with a screw seen backing out of the anterior cervical construct. The patient was followed with serial radiographs, which revealed the disappearance of the screw 6 months later. Further imaging failed to locate the hardware and it was presumed to have entered the gastrointestinal tract and exited the patient.34 This situation is rare however, as most patients typically complain of dysphagia, neck pain or fullness, pharyngeal pain, odynophagia, or present with fever or subcutaneous emphysema.16,17,22,52 A clinical triad consisting of vomiting, chest pain, and subcutaneous emphysema is seen in about 25% of patients with esophageal perforation—this is known as Mackler’s Triad; this triad is less commonly seen in patients with tears in the cervical esophagus than those occurring in the thoracic esophagus.53
Early diagnosis and intervention has been shown to reduce morbidity and mortality, so any intraoperative suspicion should warrant immediate further investigation.6,34,35 Taylor et al examined the use of methylene blue administered directly into the esophagus to detect perforations intraoperatively. This method was found to have an unacceptably high rate of false negatives when using just a single nasogastric tube; their group described a technique whereby one or more Foley catheters are inflated proximal and distal to the area in question in order to improve the detection rates. This technique improved the rates of detection but failed to identify many of the lesions, leading to the conclusion that a negative exam cannot rule out an esophageal perforation.44 If a tear is suspected intraoperatively, but not visualized using methylene blue, postoperative imaging and otolaryngology consultation is recommended. Postoperatively, a number of imaging modalities have been used to help determine the presence of an esophageal disruption. Computed tomography, magnetic resonance imaging, plain radiographs, esophagoscopy, contrast esophagram, endoscopy, and sinugram are just some of the tools that have been used to diagnose a perforated esophagus.‖ Plain radiographs may reveal subcutaneous emphysema or prevertebral air; however, this finding is not ubiquitous and is therefore not reliable. Contrast swallow studies have been utilized with some success24,32,40; others, however, have failed to demonstrate consistent detection of esophageal defects.16 Gaudinez’s group used a variety of imaging modalities to diagnose an esophageal tear. They noted that a tear was visualized on at least one modality in only 32 of the 44 patients (72.7%) and that 10 of the 44 patients (22.7%) had imaging studies that were read as negative for perforation. In their series, endoscopic exams were performed on 40 of the 44 patients—a firm diagnosis of esophageal perforation was made in only 28 of the 40 (63.6%). Eight of the 44 patients were diagnosed only during surgical exploration of the neck.16 Though often recommended and performed, esophagoscopy remains controversial as it can exacerbate a small perforation and may miss perforations hidden in mucosal folds.59 Computed tomography and magnetic resonance imaging may reveal brooding infections or subcutaneous air but will often fail to detect acute injuries. Lu’s group recommended contrast swallow studies, noting that 4 of the 6 patients in their cohort were diagnosed using contrast esophagrams; the 2 other patients were seen to have food residue leaking from the surgical incision, obviating the need for further diagnostic workup.22
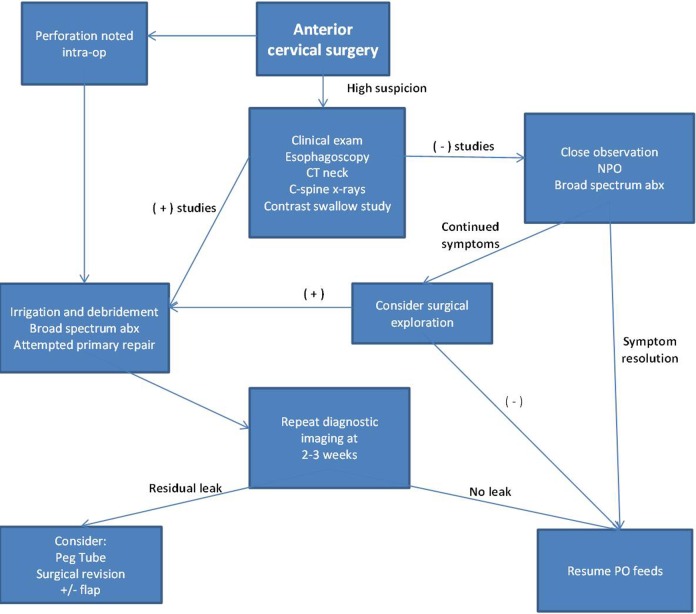
Management
Many different treatment modalities have been utilized in the management of esophageal perforations occurring during anterior cervical surgery. Nonoperative management with antibiotics, nasogastric placement, and esophageal diversion has a very limited role; the general consensus is that surgical debridement and an attempt at closure is warranted.7,24,38,54 Rueth and colleagues described their experience treating 6 esophageal perforations resulting from anterior cervical spine surgery. Their group proposed an algorithm beginning with early neck exploration and wide surgical debridement (see Figure 3). They stated that an attempt should be made at a primary closure, but did not find this to be critical. They reported a high rate of resolution when leaving the wound open to facilitate drainage, whereas closed wound management led to recurrence. None of their 6 patients required flaps, though one patient expired secondary to respiratory failure which was present upon initial transfer to their institution.38
Figure 3.
Proposed algorithm in the management of esophageal injuries following anterior cervical spine surgery.
In addition to primary closure, multiple flap and coverage options exist.¶ Benazzo et al reported the results of using a sternocleidomastoid flap for esophageal repair following injuries incurred during anterior cervical spine surgery. Three patients sustained an intraoperative esophageal perforation in their series. The mean time to diagnosis was 4 days, and all patients underwent subsequent irrigation and debridement, and treatment with antibiotics. A plan for definitive reconstruction with a sternocleidomastoid muscle flap was made, and the mean time from diagnosis to definitive treatment was 44.3 days. Oral feeding resumed at an average of 17.6 days after flap reconstruction, and the mean hospital stay was 19 days. No recurrences were seen.45
Despite the successful outcomes with sternocleidomastoid flaps noted in some studies, there has been some concern regarding the flaps’ vascular reliability.# For this reason and others, multiple other flap options have been explored. Recently, Hanwright et al examined their experience over an 18-year period performing flap reconstruction for patients with esophageal injuries secondary to anterior cervical spine surgery. Five different types of flaps were attempted; altogether 13 flaps were performed in 11 patients. They concluded that using a free omentum flap was associated with a significantly faster functional recovery in comparison to several other types of flaps; resumption to oral feeding averaged 22.5 days in the omental flap group versus 268 days in the group receiving other types of flaps (P < .05).62
Bhatia et al reported their management of esophageal perforations due to varying etiologies over a 27-year period. Of 119 patients with esophageal tears, 15 were found in the cervical region, and 14 of the 15 were iatrogenic (this includes those caused by endoscopy as well as anterior cervical surgery). Contrary to previous reports, their group found that the overall mortality rate was more closely related to the preoperative morbidity of the patient rather than the time to diagnosis or the time to treatment of the perforation. In their report, the average time from diagnosis of the esophageal tear to treatment was 37 hours in the cervical group; 2 of the 15 patients in that group died. They found the average length of hospital stay was 25.1 days, and 7 of those days were spent in the intensive care unit. They concluded that patients with preoperative sepsis, ventilator dependency, and multiple medical comorbidities (especially pulmonary) were found to have significantly worse outcomes.55
Conclusion
Esophageal perforations following anterior cervical spine surgery are a rare but potentially devastating complication. Most esophageal perforations occur at portions of the esophagus that are known to be structurally vulnerable. Meticulous surgical dissection, judicious retractor placement, and cautious use of electrocautery and high-speed drills can minimize intraoperative esophageal injury. A small percentage of esophageal perforations will present months or even years after anterior cervical surgery, and the surgeon must remain aware of this possibility. The majority of patients with esophageal perforations will present with symptoms of dysphagia, neck pain, odynophagia, or drainage. Imaging studies such as contrast esophagraphy can be helpful but are often unreliable—a high clinical suspicion despite negative imaging studies may warrant surgical exploration. The presence of subcutaneous emphysema is concerning for an esophageal perforation.
If a perforation is detected, aggressive management should be taken and many strategies have been employed. Broad spectrum antibiotics, esophageal diversion (with consideration for percutaneous endoscopic gastrotomy tube placement), and surgical exploration with irrigation and debridement are all considerations in the acute management of an esophageal tear. Primary repair has been successful, and multiple flap options exist to aid in the closure of a defect. With prompt, aggressive management, long-term morbidity and mortality from these injuries can be reduced.
Footnotes
Authors’ Note: This study was ethically approved by the institutional ethics committees at all participating sites.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Stuart H. Hershman reports grants from AOSpine North America during the conduct of the study; William A. Kunkle reports grants from AOSpine North America during the conduct of the study; Michael P. Kelly reports grants from AOSpine North America during the conduct of the study; Wilson Z. Ray reports grants from NIH/NINDs, grants from Department of Defense, grants from DARPA, other from Depuy/Synthes, other from Ulrich, other from Harvest Technologies, outside the submitted work; David B. Bumpass reports grants from AOSpine North America during the conduct of the study, grants from North American Spine Society, personal fees from Doximity, personal fees from Gerson Lehrman Group, outside the submitted work; Jeffrey L. Gum reports grants from AOSpine North America during the conduct of the study, personal fees from Medtronic, Alphatec, Stryker, LifeSpine, Acuity, Pacira, PAKmed, Gerson Lehrman Group, personal fees from OREF, AOSpine, personal fees from Acuity, other from Medtronic, personal fees from MiMedx, Pacira Pharmaceuticals, Alphatec, grants from Fischer Owen Fund, nonfinancial support from American Journal of Orthopaedics, nonfinancial support from American Journal of Orthopaedics, The Spine Journal, outside the submitted work; Colleen M. Peters reports grants from AOSpine North America during the conduct of the study; Wellington K. Hsu reports grants from AOSpine North America during the conduct of the study, personal fees from Medtronic, personal fees from Stryker, personal fees from Bacterin, personal fees from Graftys, personal fees from Ceramtec, personal fees from Relievant, personal fees from Bioventus, personal fees from Globus, personal fees from SpineSmith, outside the submitted work; Bradford L. Currier reports grants from AOSpine North America during the conduct of the study, personal fees from DePuy Spine, personal fees from Stryker Spine, personal fees from Zimmer Spine, other from Zimmer Spine, other from Tenex, other from Spinology, other from LSRS, other from AOSNA, outside the submitted work; Robert E. Isaacs reports grants from AOSpine North America during the conduct of the study, grants and personal fees from NuVasive, Inc., personal fees from Association for Collaborative Spine research, outside the submitted work; Justin S. Smith reports grants from AOSpine North America during the conduct of the study, personal fees from Biomet, personal fees from Nuvasive, personal fees from Cerapedics, personal fees from K2M, personal fees and other from DePuy, personal fees from Medtronic, outside the submitted work; Christopher Shaffrey reports grants from AOSpine North America during the conduct of the study, personal fees from Biomet, personal fees from Medtronic, from Nuvasive, personal fees from K2M, personal fees from Stryker, outside the submitted work; and Editorial Board Spine, Spinal Deformity and Neurosurgery; Sara E. Thompson reports grants from AOSpine North America during the conduct of the study; Jeffrey C. Wang reports grants from AOSpine North America during the conduct of the study; Elizabeth L. Lord reports grants from AOSpine North America during the conduct of the study; Zorica Buser reports grants from AOSpine North America during the conduct of the study; Michael G. Fehlings reports grants from AOSpine North America during the conduct of the study; Thomas E. Mroz reports other from AOSpine during the conduct of the study, personal fees from Stryker, personal fees from Ceramtec, other from Pearl Diver, outside the submitted work; and K. Daniel Riew reports personal fees from AOSpine International, other from Global Spine Journal, other from Spine Journal, other from Neurosurgery, personal fees from Multiple Entities for defense, plaintiff, grants from AOSpine, grants from Cerapedics, grants from Medtronic, personal fees from AOSpine, personal fees from NASS, personal fees from Biomet, personal fees from Medtronic, nonfinancial support from Broadwater, outside the submitted work; Jacob M. Buchowski reports grants from AOSpine North America during the conduct of the study; personal fees from Advance Medical, personal fees from DePuy, personal fees from CoreLink, Inc., personal fees from Globus Medical, Inc., personal fees from K2M, Inc., personal fees from Medtronic, Inc., personal fees from Stryker, Inc., personal fees from Broadwater/Vertical Health, personal fees from DePuy Synthes, personal fees from Globus Medical, Inc., personal fees from Orthofix, personal fees from Stryker, Inc., personal fees from Wolters Kluwer Health, Inc., personal fees from Globus Medical, Inc., outside the submitted work; and AO Foundation (parent organization to AO Spine). AO FOUNDATION is a non for profit organization. “Other”, “Teaching”, “Not for Profit Organization” Weerasak Singhatanadgige reports grants from AOSpine North America during the conduct of the study; Jin Young Kim Dr. Kim reports grants from AOSpine North America, during the conduct of the study; Zachary A. Smith Dr. Smith reports grants from AOSpine North America, during the conduct of the study; Ahmad Nassr reports grants from AOSpine North America during the conduct of the study; Ra'Kerry K. Rahman reports grants from AOSpine North America during the conduct of the study; in addition, Dr. Rahman has a patent Deformity System & Pedicle Screws pending; Paul M. Arnold reports grants from AOSpine North America during the conduct of the study; other from Z-Plasty, other from Medtronic Sofamore Danek, other from Stryker Spine, other from FzioMed, other from AOSpine North America, other from Life Spine, other from Integra Life, other from Spine Wave, other from MIEMS, other from Cerapedics, other from AOSpine North America, outside the submitted work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by AOSpine North America Inc, a 501(c)3 nonprofit corporation.
References
- 1. Robinson RA, Smith GW. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull Johns Hopkins Hosp. 1955;(96):223–224. [Google Scholar]
- 2. Cho SK, Lu Y, Lee D. Dysphagia following anterior cervical spinal surgery: a systematic review. Bone Joint J. 2013;95:868–873. doi:10.1302/0301-620X.95B7.31029. [DOI] [PubMed] [Google Scholar]
- 3. Daniels AH, Riew KD, Yoo JU, et al. Adverse events associated with anterior cervical spine surgery. J Am Acad Orthop Surg. 2008;16:729–738. [DOI] [PubMed] [Google Scholar]
- 4. Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976). 2007;32:2310–2317. doi:10.1097/BRS.0b013e318154c57e. [DOI] [PubMed] [Google Scholar]
- 5. Okubadejo GO, Hohl JB, Donaldson WF. Dysphonia, dysphagia, and esophageal injuries after anterior cervical spine surgery. Instr Course Lect. 2009;58:729–736. [PubMed] [Google Scholar]
- 6. Orlando ER, Caroli E, Ferrante L. Management of the cervical esophagus and hypofarinx perforations complicating anterior cervical spine surgery. Spine (Phila Pa 1976). 2003;28:E290–E295. doi:10.1097/01.BRS.0000087093.89889.0A. [DOI] [PubMed] [Google Scholar]
- 7. Newhouse KE, Lindsey RW, Clark CR, Lieponis J, Murphy MJ. Esophageal perforation following anterior cervical spine surgery. Spine (Phila Pa 1976). 1989;14:1051–1053. [DOI] [PubMed] [Google Scholar]
- 8. Cloward RB. Complications of anterior cervical disk operation and their treatment. Surgery. 1971;(69):175–182. [PubMed] [Google Scholar]
- 9. Eleraky MA, Llanos C, Sonntag VK. Cervical corpectomy: report of 185 cases and review of the literature. J Neurosurg. 1999;90(1 suppl):35–41. [DOI] [PubMed] [Google Scholar]
- 10. Capen DA, Garland DE, Waters RL. Surgical stabilization of the cervical spine. A comparative analysis of anterior and posterior spine fusions. Clin Orthop Relat Res. 1985;(196):229–237. [PubMed] [Google Scholar]
- 11. van Berge Henegouwen DP, Roukema JA, de Nie JC, van den Werken C. Esophageal perforation during surgery on the cervical spine. Neurosurgery. 1991;29:766–768. [DOI] [PubMed] [Google Scholar]
- 12. Graham JJ. Complications of cervical spine surgery. A five-year report on a survey of the membership of the Cervical Spine Research Society by the Morbidity and Mortality Committee. Spine (Phila Pa 1976). 1989;14:1046–1050. [PubMed] [Google Scholar]
- 13. Dabija MG, Iliescu BF, Andronic D, Popescu C, Ianovici N. Rare complication of the cervical spine trauma—traumatic esophageal fistula: case report and review of the literature. Rev Med Chir Soc Med Nat Iasi. 2014;118:683–687. [PubMed] [Google Scholar]
- 14. Dakwar E, Uribe JS, Padhya TA, Vale FL. Management of delayed esophageal perforations after anterior cervical spinal surgery. J Neurosurg Spine. 2009;11:320–325. doi:10.3171/2009.3.SPINE08522. [DOI] [PubMed] [Google Scholar]
- 15. Dray TG, Pyle PB. Delayed pharyngoesophageal perforation following anterior spine surgery. Ear Nose Throat J. 1997;76:442–444. [PubMed] [Google Scholar]
- 16. Gaudinez RF, English GM, Gebhard JS, Brugman JL, Donaldson DH, Brown CW. Esophageal perforations after anterior cervical surgery. J Spinal Disord. 2000;13:77–84. [DOI] [PubMed] [Google Scholar]
- 17. Kelly MF, Spiegel J, Rizzo KA, Zwillenberg D. Delayed pharyngoesophageal perforation: a complication of anterior spine surgery. Ann Otol Rhinol Laryngol. 1991;100:201–205. [DOI] [PubMed] [Google Scholar]
- 18. Kim SJ, Ju CI, Kim DM, Kim SW. Delayed esophageal perforation after cervical spine plating. Korean J Spine. 2013;10:174 doi:10.14245/kjs.2013.10.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leaver N, Colby A, Appleton N, Vimalachandran D. Oesophageal perforation caused by screw displacement 16 months following anterior cervical spine fixation. BMJ Case Rep. 2015;2015. doi:10.1136/bcr-2014-207738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee S, Kim K, Suk K, Park K, Oh K. Effect of retropharyngeal steroid on prevertebral soft tissue swelling following anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2011;36:2286–2292. doi:10.1097/BRS.0b013e318237e5d0. [DOI] [PubMed] [Google Scholar]
- 21. Lee S, Mesfin A, Riew KD. Delayed esophageal perforation following anterior cervical fusion and retropharyngeal steroid use: a report of two cases. Spine J. 2015;15:e75–e80. doi:10.1016/j.spinee.2015.06.058. [DOI] [PubMed] [Google Scholar]
- 22. Lu X, Guo Q, Ni B. Esophagus perforation complicating anterior cervical spine surgery. Eur Spine J. 2012;21:172–177. doi:10.1007/s00586-011-1982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Orlewicz M, Amhaz H, Kuo R, Vaidya R. Esophageal perforation following cervical spine surgery: a review with considerations in airway management. Int J Crit Illn Inj Sci. 2013;3:276–278. doi:10.4103/2229-5151.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel NP, Wolcott WP, Johnson JP, et al. Esophageal injury associated with anterior cervical spine surgery. Surg Neurol. 2008;69:20–24. doi:10.1016/j.surneu.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 25. Shenoy SN, Raja A. Delayed pharyngo-esophageal perforation: rare complication of anterior cervical spine surgery. Neurol India. 2003;51:534–536. [PubMed] [Google Scholar]
- 26. Vrouenraets BC, Been HD, Brouwer-Mladin R, Bruno M, van Lanschot JJB. Esophageal perforation associated with cervical spine surgery: report of two cases and review of the literature. Dig Surg. 2004;21:246–249. doi:10.1159/000079495. [DOI] [PubMed] [Google Scholar]
- 27. Woolley SL, Smith DRK. Pharyngeal perforation: a late complication of cervical spine surgery. J Laryngol Otol. 2005;119:913–916. doi:10.1258/002221505774783386. [DOI] [PubMed] [Google Scholar]
- 28. Yee GK, Terry AF. Esophageal penetration by an anterior cervical fixation device. A case report. Spine (Phila Pa 1976). 1993;18:522–527. [PubMed] [Google Scholar]
- 29. Zairi F, Tetard M, Thines L, Assaker R. Management of delayed oesophagus perforation and osteomyelitis after cervical spine surgery: review of the literature. Br J Neurosurg. 2012;26:185–188. doi:10.3109/02688697.2011.609604. [DOI] [PubMed] [Google Scholar]
- 30. Zdichavsky M, Blauth M, Bosch U, Rosenthal H, Knop C, Bastian L. Late esophageal perforation complicating anterior cervical plate fixation in ankylosing spondylitis: a case report and review of the literature. Arch Orthop Trauma Surg. 2004;124:349–353. doi:10.1007/s00402-004-0654-9. [DOI] [PubMed] [Google Scholar]
- 31. Zenga J, Kreisel D, Kushnir VM, Rich JT. Management of cervical esophageal and hypopharyngeal perforations. Am J Otolaryngol. 2015;36:678–685. doi:10.1016/j.amjoto.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 32. Zhong Z, Jiang J, Qu D, et al. Esophageal perforation related to anterior cervical spinal surgery. J Clin Neurosci. 2013;20:1402–1405. doi:10.1016/j.jocn.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 33. Sealy WC. Rupture of the esophagus. Am J Surg. 1963;105:505–510. [DOI] [PubMed] [Google Scholar]
- 34. Pompili A, Canitano S, Caroli F, et al. Asymptomatic esophageal perforation caused by late screw migration after anterior cervical plating: report of a case and review of relevant literature. Spine (Phila Pa 1976). 2002;27:E499–E502. doi:10.1097/01.BRS.0000035309.04502.52. [DOI] [PubMed] [Google Scholar]
- 35. Sharma RR, Sethu AU, Lad SD, Turel KE, Pawar SJ. Pharyngeal perforation and spontaneous extrusion of the cervical graft with its fixation device: a late complication of C2-C3 fusion via anterior approach. J Clin Neurosci. 2001;8:464–468. doi:10.1054/jocn.2000.0826. [DOI] [PubMed] [Google Scholar]
- 36. Eroglu A, Turkyilmaz A, Aydin Y, Yekeler E, Karaoglanoglu N. Current management of esophageal perforation: 20 years experience. Dis Esophagus. 2009;22:374–380. doi:10.1111/j.1442-2050.2008.00918.x. [DOI] [PubMed] [Google Scholar]
- 37. Ji H, Liu D, You W, Zhou F, Liu Z. Success in esophageal perforation repair with open-wound management after revision cervical spine surgery: a case report. Spine (Phila Pa 1976). 2015;40:E183–E185. doi:10.1097/BRS.0000000000000712. [DOI] [PubMed] [Google Scholar]
- 38. Rueth N, Shaw D, Groth S, et al. Management of cervical esophageal injury after spinal surgery. Ann Thorac Surg. 2010;90:1128–1133. doi:10.1016/j.athoracsur.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 39. Navarro R, Javahery R, Eismont F, et al. The role of the sternocleidomastoid muscle flap for esophageal fistula repair in anterior cervical spine surgery. Spine (Phila Pa 1976). 2005;30:E617–E622. [DOI] [PubMed] [Google Scholar]
- 40. Brinster CJ, Singhal S, Lee L, Marshall MB, Kaiser LR, Kucharczuk JC. Evolving options in the management of esophageal perforation. Ann Thorac Surg. 2004;77:1475–1483. doi:10.1016/j.athoracsur.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 41. von Rahden BHA, Stein HJ, Scherer MA. Late hypopharyngo-esophageal perforation after cervical spine surgery: proposal of a therapeutic strategy. Eur Spine J. 2005;14:880–886. doi:10.1007/s00586-005-1006-3. [DOI] [PubMed] [Google Scholar]
- 42. Gupta NM, Kaman L. Personal management of 57 consecutive patients with esophageal perforation. Am J Surg. 2004;187:58–63. [DOI] [PubMed] [Google Scholar]
- 43. Eroglu A, Can Kurkcuoglu I, Karaoglanoglu N, Tekinbas C, Yimaz O, Basoglu M. Esophageal perforation: the importance of early diagnosis and primary repair. Dis Esophagus. 2004;17:91–94. doi:10.1111/j.1442-2050.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- 44. Taylor B, Patel AA, Okubadejo GO, Albert T, Riew KD. Detection of esophageal perforation using intraesophageal dye injection. J Spinal Disord Tech. 2006;19:191–193. doi:10.1097/01.bsd.0000190559.20872.2a. [DOI] [PubMed] [Google Scholar]
- 45. Benazzo M, Spasiano R, Bertino G, Occhini A, Gatti P. Sternocleidomastoid muscle flap in esophageal perforation repair after cervical spine surgery. J Spinal Disord Tech. 2008;21:597–605. doi:10.1097/BSD.0b013e31815c5f96. [DOI] [PubMed] [Google Scholar]
- 46. Gazzeri R, Tamorri M, Faiola A, Gazzeri G. Delayed migration of a screw into the gastrointestinal tract after anterior cervical spine plating. Spine (Phila Pa 1976). 2008;33:E268–E271. doi:10.1097/BRS.0b013e31816b8831. [DOI] [PubMed] [Google Scholar]
- 47. Solerio D, Ruffini E, Gargiulo G, et al. Successful surgical management of a delayed pharyngo-esophageal perforation after anterior cervical spine plating. Eur Spine J. 2008;17(suppl 2):280–284. doi:10.1007/s00586-007-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tian H, Yuan W, Johnson JS, Chen H, Chen D. Pharyngoesophageal diverticulum: a delayed complication of anterior cervical spine surgery. Eur Spine J. 2011;20(suppl 2):211–216. doi:10.1007/s00586-010-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duransoy YK, Mete M, Zengel B, Selçukı M. Missing screw as a rare complication of anterior cervical instrumentation. Case Rep Orthop. 2013;2013:593905 doi:10.1155/2013/593905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fujibayashi S, Shikata J, Kamiya N, Tanaka C. Missing anterior cervical plate and screws: a case report. Spine (Phila Pa 1976). 2000;25:2258–2261. [DOI] [PubMed] [Google Scholar]
- 51. Nérot C, Jeanneret B, Lardenois T, Lépousé C. Esophageal perforation after fracture of the cervical spine: case report and review of the literature. J Spinal Disord Tech. 2002;15:513–518. [DOI] [PubMed] [Google Scholar]
- 52. Loop FD, Groves LK. Esophageal perforations. Ann Thorac Surg. 1970;10:571–587. doi:10.1016/S0003-4975(10)65400-8. [DOI] [PubMed] [Google Scholar]
- 53. Søreide JA, Viste A. Esophageal perforation: diagnostic work-up and clinical decision-making in the first 24 hours. Scand J Trauma Resusc Emerg Med. 2011;19:66 doi:10.1186/1757-7241-19-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ardon H, van Calenbergh F, van Raemdonck D, et al. Oesophageal perforation after anterior cervical surgery: management in four patients. Acta Neurochir (Wien). 2009;151:297–302. doi:10.1007/s00701-009-0241-5. [DOI] [PubMed] [Google Scholar]
- 55. Bhatia P, Fortin D, Inculet RI, Malthaner RA. Current concepts in the management of esophageal perforations: a twenty-seven year Canadian experience. Ann Thorac Surg. 2011;92:209–215. doi:10.1016/j.athoracsur.2011.03.131. [DOI] [PubMed] [Google Scholar]
- 56. Grabowski G, Cornett CA, Kang JD. Esophageal and vertebral artery injuries during complex cervical spine surgery—avoidance and management. Orthop Clin North Am. 2012;43:63–74. doi:10.1016/j.ocl.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 57. de Moor V, de Witte O, Zalcman M, Gelin M, Le Moine O, El Nakadi I. Oesophageal perforation by an anterior cervical fixation device: management in debilitated patients. Acta Gastroenterol Belg. 2005;68:267–269. [PubMed] [Google Scholar]
- 58. Wu JT, Mattox KL, Wall MJ. Esophageal perforations: new perspectives and treatment paradigms. J Trauma. 2007;63:1173–1184. doi:10.1097/TA.0b013e31805c0dd4. [DOI] [PubMed] [Google Scholar]
- 59. Pasricha PJ, Fleischer DE, Kalloo AN. Endoscopic perforations of the upper digestive tract: a review of their pathogenesis, prevention, and management. Gastroenterology. 1994;106:787–802. [DOI] [PubMed] [Google Scholar]
- 60. Ahn S, Lee S, Kim ES, Eoh W. Successful repair of esophageal perforation after anterior cervical fusion for cervical spine fracture. J Clin Neurosci. 2011;18:1374–1380. doi:10.1016/j.jocn.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 61. Haku T, Okuda S, Kanematsu F, et al. Repair of cervical esophageal perforation using longus colli muscle flap: a case report of a patient with cervical spinal cord injury. Spine J. 2008;8:831–835. doi:10.1016/j.spinee.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 62. Hanwright PJ, Purnell CA, Dumanian GA. Flap reconstruction for esophageal perforation complicating anterior cervical spinal fusion: an 18-year experience. Plast Reconstr Surg Glob Open. 2015;3(5):e400 doi:10.1097/GOX.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kau RL, Kim N, Hinni ML, Patel NP. Repair of esophageal perforation due to anterior cervical spine instrumentation. Laryngoscope. 2010;120:739–742. doi:10.1002/lary.20842. [DOI] [PubMed] [Google Scholar]
- 64. Phommachanh V, Patil YJ, McCaffrey TV, Vale F, Freeman TB, Padhya TA. Otolaryngologic management of delayed pharyngoesophageal perforation following anterior cervical spine surgery. Laryngoscope. 2010;120:930–936. doi:10.1002/lary.20747. [DOI] [PubMed] [Google Scholar]
- 65. Fuji T, Kuratsu S, Shirasaki N, et al. Esophagocutaneous fistula after anterior cervical spine surgery and successful treatment using a sternocleidomastoid muscle flap. A case report. Clin Orthop Relat Res. 1991;(267):8–13. [PubMed] [Google Scholar]