Abstract
Study Design:
This study was a retrospective, multicenter cohort study.
Objectives:
Rare complications of cervical spine surgery are inherently difficult to investigate. Pseudomeningocoele (PMC), an abnormal collection of cerebrospinal fluid that communicates with the subarachnoid space, is one such complication. In order to evaluate and better understand the incidence, presentation, treatment, and outcome of PMC following cervical spine surgery, we conducted a multicenter study to pool our collective experience.
Methods:
This study was a retrospective, multicenter cohort study of patients who underwent cervical spine surgery at any level(s) from C2 to C7, inclusive; were over 18 years of age; and experienced a postoperative PMC.
Results:
Thirteen patients (0.08%) developed a postoperative PMC, 6 (46.2%) of whom were female. They had an average age of 48.2 years and stayed in hospital a mean of 11.2 days. Three patients were current smokers, 3 previous smokers, 5 had never smoked, and 2 had unknown smoking status. The majority, 10 (76.9%), were associated with posterior surgery, whereas 3 (23.1%) occurred after an anterior procedure. Myelopathy was the most common indication for operations that were complicated by PMC (46%). Seven patients (53%) required a surgical procedure to address the PMC, whereas the remaining 6 were treated conservatively. All PMCs ultimately resolved or were successfully treated with no residual effects.
Conclusions:
PMC is a rare complication of cervical surgery with an incidence of less than 0.1%. They prolong hospital stay. PMCs occurred more frequently in association with posterior approaches. Approximately half of PMCs required surgery and all ultimately resolved without residual neurologic or other long-term effects.
Keywords: pseudomeningocoele, cervical spine, retrospective, multicenter
Introduction
Pseudomeningocoele (PMC) is an abnormal collection of cerebrospinal fluid (CSF) that communicates with the subarachnoid space and extends from the spinal canal to the adjacent paraspinal soft tissue.1 Durotomies during spinal surgery are not uncommon. While the majority of durotomies heal after primary closure, in some cases the inability to obtain a watertight closure may lead to ongoing CSF egress. When the rate of CSF flow is high, a cutaneous CSF fistula may form with resultant leakage through the incision. Conversely, when the rate of CSF efflux is low, it may permit sufficient healing of the skin and subcutaneous tissue and instead lead to formation of a PMC in the paraspinal or prevertebral tissues. Due to the risk of a potentially life-threatening infection, CSF leakage through the skin necessitates prompt action.2,3 In contrast, PMCs, which tend to form slowly as encapsulated fluid-filled masses, may resolve gradually over time. Nevertheless, as they increase in size, PMC may result in pain, postural headaches, and, rarely, nerve root entrapment or spinal cord compression.4-13 Neurologic deficit may also result from herniation of neural structures through the dural defect, typically in a delayed fashion.8,11,14-16
The incidence of PMC after cervical spine surgery is currently unknown as the literature is limited to case reports and small series. Furthermore, the majority of cases are likely asymptomatic and, as such, go unreported. The rate of PMC after lumbar spine surgery appears to be less than 0.1%17 and that following cervical surgery is likely even lower. Due to the rare occurrence of PMC, its clinical presentation, management, and outcome are not well understood. While it is well accepted that most asymptomatic PMCs can be observed, the management of symptomatic cases is unclear and based on expert opinion. Some advocate for surgical repair for all symptomatic PMC,13 whereas others believe that many PMCs, especially when small, will sufficiently resolve spontaneously with conservative management.18 In order to evaluate and better understand the incidence, presentation, treatment, and outcome of PMC following cervical spine surgery, we conducted a multicenter study to pool a collective experience of this condition.
Methods
We have conducted a retrospective multicenter case series study involving 21 high-volume surgical centers from the AOSpine North America Clinical Research Network, selected for their excellence in spine care and clinical research infrastructure and experience. Medical records for 17 625 patients who received cervical spine surgery (levels from C2 to C7) between January 1, 2005, and December 31, 2011, inclusive, were reviewed to identify occurrence of 21 predefined rare treatment complications. The complications included reintubation requiring evacuation, esophageal perforation, epidural hematoma, C5 palsy, recurrent laryngeal nerve palsy, superior laryngeal nerve palsy, hypoglossal or glossopharyngeal nerve palsy, dural tear, brachial plexopathy, blindness, graft extrusion, misplaced screws requiring reoperation, anterior cervical infection, carotid artery injury or cerebrovascular accident, vertebral artery injuries, Horner’s syndrome, thoracic duct injury, tetraplegia, intraoperative death, revision of arthroplasty, and pseudomeningocele. Patients were excluded if the indication for surgery was to treat one of 21 rare complications being investigated. The present study reports on PMC specifically.
Trained research staff at each site abstracted the data from medical records, surgical charts, radiology imaging, narratives, and other source documents for the patients who experienced one or more of the complications from the list. Data were transcribed into study-specific paper case report forms. Copies of case report forms were transferred to the AOSpine North America Clinical Research Network Methodological Core for processing, cleaning, and data entry.
Descriptive statistics were provided for baseline patient characteristics. Paired t test was used to analyze changes in clinical outcomes at follow-up compared to preoperative status where subgroup sizes permitted.
Results
Thirteen patients (0.08%) developed a postoperative PMC, 6 (46.2%) of whom were female. The incidence across the 21 participating sites ranged from 0.0% to 0.7%, with 11 sites reporting no events. Patients with PMC had an average age of 48.2 (± 14.8) years. Three patients were current smokers, 3 previous smokers, 5 had never smoked, and 2 had unknown smoking status.
The majority, 10 (76.9%), were associated with posterior surgery, whereas 3 (23.1%) occurred after an anterior procedure. Average blood loss was 1183.3 (± 1869.4) mL, and 2 of 13 patients required a blood transfusion. Some form of graft was placed in 10 of the 13 cases. Most of the procedures included the levels C4 (9/13), C5 (9/13), and/or C6 (12/13), with only 2 operations extending to C2 and T2. Myelopathy was the most common surgical indication for procedures that were complicated by PMC (46.2%). Only one procedure was performed primarily for cervical radiculopathy.
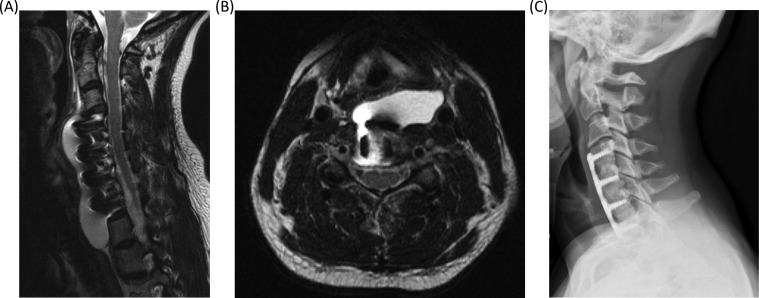
Symptoms of PMC occurred an average of 13.8 days (range 1-31) after the index procedure. Three patients complained of postural headaches, one of an enlarging anterior neck mass (Figure 1) and another developed transient paralysis of her lower extremities with pain radiating down her back on postoperative day 3. Clinical presentation was not known in 3 patients, and the remaining 5 were diagnosed based on physical examination and radiographic imaging.
Figure 1.
Images from a 37-year-old woman who underwent a C4-7 anterior discectomy and arthrodesis with plating to address spinal cord compression and myelopathy. A small durotomy occurred during decompression and this area was covered with a small piece of dural substitute and sealant. The patient did well following surgery until she represented 4 weeks later with a 5-day history of headache. T2-weighted sagittal (A) and axial (B) magnetic resonance imaging studies demonstrated a large pseudomeningocoele. The patient was admitted and treated with a course of dexamethasone with complete resolution of her headache. She was discharged from the hospital and had no further sequelae. At 8-month follow-up, the patient remained symptom-free and demonstrated solid arthrodesis (C).
Seven patients (53.8%) required a surgical procedure to address the PMC, 2 were managed with lumbar drains and bed rest, and 4 patients were managed conservatively. Conservative treatment included dexamethasone (1 patient), bed rest only (2 patients), and over-sewing the incision (1 patient). This last patient was diagnosed with a PMC based on physical exam and imaging and subsequently began to leak CSF through the skin. The majority of surgical procedures (5/7) entailed reopening of the wound and closure of the PMC through a variety of techniques including direct repair, direct repair with CSF diversion (lumbar drain), and muscle grafting. One patient underwent simple irrigation and debridement of a posterior cervical incision and one required emergent exploration and evacuation of CSF within a PMC that was compressing the spinal cord. The latter also required a second irrigation and debridement procedure. Patients with PMC stayed in the hospital a mean of 11.2 (± 12.5) days. All PMCs ultimately resolved or were successfully treated with no residual effects.
There was insufficient data to compare pre- and postoperative health-related quality of life, disability, or functional measures in patients with PMC.
Discussion
The incidence of PMC was expectedly low at 0.08% (range 0.0% to 0.7%), with over half of participating sites reporting no cases. Small numbers precluded identification of demographic factors associated with PMC. Typical of operations to treat cervical spondylosis, procedures in which PMC occurred were most commonly performed for myelopathy and the majority involved C5 and C6. The preponderance of cases (76.9%) occurred with posterior surgery, in keeping with the much greater dural exposure and bone removal involved in such procedures as compared to anterior cervical surgery. Although there is a paucity of reports of cervical PMC, the majority describe that occurring after posterior surgery,4,5,8-12 with fewer following anterior procedures.14,19,20
In our series, patients presented an average of 2 weeks after the index procedure. This is in keeping with the literature, which suggests that the majority of symptomatic cases present in a delayed fashion. The time from surgery to diagnosis of postoperative PMC varies greatly in previous reports from 1 week to 14 years, although most appear to occur within the first 4 months.4,5,8-12,14,19,20 Three patients in the present series developed symptoms in the first 3 days after surgery. One of these exhibited transient paralysis with associated pain radiating down her legs and required emergent exploration to evacuate a CSF collection that was exerting mass effect on the spinal cord. The other 2 patients that developed early symptoms also ultimately underwent surgery, though not on an emergent basis. No previous studies document symptomatic cervical PMC within the first postoperative week, although one report documented acute dysphagia, dyspnea, and neck swelling immediately following removal of a lumbar drain 1 week postoperatively.19 This may have presented earlier had a lumbar drain not been placed. Regardless, cervical PMC, in rare instances, appears to produce symptoms acutely and may require urgent intervention to address neural compromise or respiratory compromise.
Several factors may play a role in determining the size and progression of a PMC, including the size of the dural defect, CSF pressure, and the resistance of the surrounding soft tissues.14 It has also been suggested that development of a ball valve mechanism, whereby CSF can only pass in one direction from the subarachnoid space into the PMC, may underlie the formation of tense PMCs. Such a mechanism could explain the more acute presentation of some of the patients in our series as it may explain the rapid enlargement and mass effect of the PMCs in these cases.
The clinical presentation varied in our series, including asymptomatic swelling noted on physical examination or routine radiographic imaging, postural headaches, enlarging neck mass, and transient paralysis. Neurologic deficit secondary to cervical PMC has been reported and seems to occur through 1 of 2 mechanisms. Direct compression of the spinal cord by an enlarging PMC tends to occur early,6,9,21 whereas delayed myelopathy results from herniation of neural tissue through a dural defect, often several months to years later.4,5,8,9,11,12 The latter may result from chronic ischemia of an incarcerated spinal cord.9 The majority of these cases were treated with surgical exploration, reduction of the herniated nervous tissue, and direct repair of the dural defect. Interestingly, Horowitz et al14 report successful conservative management of a patient who presented with Brown-Séquard syndrome and neck swelling. Postmyelogram computed tomography showed a large anterior CSF collection without spinal cord compression and the patient was treated with bed rest. Follow-up computed tomography at 3 months demonstrated complete resolution of the PMC. Nevertheless, based on the present series and limited data in the literature, cervical PMC presenting with neurologic deficit resulting from spinal cord compression remains an absolute indication for operative management.
Management of PMC is determined by the size of the lesion, progressive enlargement, and, most important, the patient’s symptoms. Most previous reports of cervical PMC describe operative management4,5,8-12,19,20; however, it should be remembered that this likely represents publication bias. PMCs that are small, asymptomatic, and managed conservatively would probably not be reported. In the present series, 4 patients were successfully managed with conservative measures alone and another 2 with placement of a lumbar subarachnoid drain. In the absence of significant symptoms (neurologic deficit, respiratory compromise) or progressive enlargement of the PMC, it is reasonable to manage this condition with bed rest. Consideration can also be given to placement of a lumbar drain to relieve pressure on the defect and allow for satisfactory wound healing.
Indications for surgical management of cervical PMC include neurologic deficit, respiratory compromise, progressive enlargement, and failure of conservative management. As with most cases of cervical PMC reported in the literature, several patients (7; 53.8%) in the present series underwent some form of operative management. For all but one, this included repair of the defect either directly or with grafting of a local muscle flap. Similarly, previous reports describe exploration and repair of the dural defect through a variety of techniques including direct repair,5 with and without adjuvant fibrin glue8,20 or dural substitute,9,15 fascial or muscle graft.19 Closure was supplemented by intraoperative placement of a lumbar drain in 1 of 7 patients treated surgically in our series. Likewise, Varma et al9 employed a lumbar drain following repair of a PMC that resulted from a dural vent over the C6 nerve root for which primary repair was difficult. Andrew and Sidhu utilized a cervical-peritoneal shunt to divert CSF from an anterior PMC after failure of direct repair and lumbar-peritoneal shunt.19 The main goal of surgical management is to identify the dural opening and obtain a watertight closure thereof. This can often be achieved through direct repair but, in complex cases, may require alternate strategies such as muscle grafting. In such cases, consideration may be given to CSF diversion either through lumbar drainage or by placement of a shunt.
This study’s most significant limitation is its retrospective design. It likely resulted in underreporting of cases and, consequently, a falsely low estimate of the true incidence of cervical PMC. This would be particularly true for smaller, asymptomatic lesions as routine imaging was not performed for all cases. The incidence may also have been artificially low due to loss to follow-up of patients who presented to other medical centers for treatment of their postoperative complication. The fact that over half of the patients in our series underwent surgery to repair their PMC may represent reporting bias of more severe cases. Another limitation of this study was the small case number—inherent to studying rare complications such as PMC—which precluded comparison of risk factors and outcomes with patients that did not have this complication. Finally, because of incomplete surgical data, we were not able to determine the suspected cause of PMC in the cases we report. For 7 of the 13 patients who underwent surgery for treatment of myelopathy or radiculopathy, PMCs resulted from unintentional durotomies or unrecognized dural defects. For the remaining 6 patients, the cause was unknown.
Conclusions
Pseudomeningocoele is a rare complication of cervical surgery with an incidence of less than 0.1%. They occur more frequently after posterior than anterior approaches. The hospital stay tends to be prolonged relative to the expected duration after cervical surgery, and PMC may be associated with significant symptoms such as neurologic decline and respiratory compromise. In the absence of such symptoms, many cases can be successfully managed with conservative measures or placement of a lumbar drain. A variety of surgical techniques can be employed to treat PMC with the common goal of obliterating the dural opening.
Footnotes
Authors’ Note: This study was ethically approved by the institutional ethics committees at all participating sites.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Tamir Ailon reports grants from AOSpine North America, during the conduct of the study; Justin S. Smith reports grants from AOSpine North America during the conduct of the study, personal fees from Biomet, personal fees from Nuvasive, personal fees from Cerapedics, personal fees from K2M, personal fees and other from DePuy, personal fees from Medtronic, outside the submitted work; Zachary A. Smith reports grants from AOSpine North America during the conduct of the study; Wellington K. Hsu reports grants from AOSpine North America during the conduct of the study, personal fees from Medtronic, personal fees from Stryker, personal fees from Bacterin, personal fees from Graftys, personal fees from Ceramtec, personal fees from Relievant, personal fees from Bioventus, personal fees from Globus, personal fees from SpineSmith, outside the submitted work; Michael G. Fehlings reports grants from AOSpine North America during the conduct of the study; David E. Fish reports grants from AOSpine North America during the conduct of the study; Jeffrey C. Wang reports grants from AOSpine North America during the conduct of the study; Alan S. Hilibrand reports grants from AOSpine North America during the conduct of the study, other from Amedica, Vertiflex, Benvenue, Lifespine, Paradigm Spine, PSD, Spinal Ventures, outside the submitted work, and in addition, Dr. Hilibrand has a patent Aesculap, Amedica, Biomet, Stryker, Alphatec, with royalties paid; Praveen V. Mummaneni reports grants from AOSpine North America during the conduct of the study, other from Depuy Spine, grants and other from AOSpine, other from Globus, other from Springer Publishers, other from Thieme Publishers, other from Taylor and Francis Publishers, other from Spincity/ISD, outside the submitted work; Dean Chou reports grants from AOSpine North America during the conduct of the study, other from Globus, other from Medtronic, other from Orthofix, outside the submitted work; Vincent C. Traynelis reports grants from AOSpine North America during the conduct of the study, and Medtronic - Royalties and Consultant Globus - Institutional Fellowship Support; Thomas E. Mroz reports other from AO Spine, grants from AOSpine North America during the conduct of the study, personal fees from Stryker, personal fees from Ceramtec, other from Pearl Diver, outside the submitted work; Zorica Buser reports grants from AOSpine North America during the conduct of the study; Elizabeth L. Lord reports grants from AOSpine North America during the conduct of the study; Eric M. Massicotte reports grants from AOSpine North America during the conduct of the study, grants from Medtronic, Depuy-Synthes Spine Canada, personal fees from Watermark consulting, grants from AOSpine North America, nonfinancial support from AOSpine North America, outside the submitted work; Arjun S. Sebastian reports grants from AOSpine North America during the conduct of the study; Khoi D. Than reports grants from AOSpine North America during the conduct of the study; Michael P. Steinmetz reports grants from AOSpine North America during the conduct of the study; Gabriel A. Smith reports grants from AOSpine North America during the conduct of the study; Jonathan Pace reports grants from AOSpine North America during the conduct of the study; K. Daniel Riew reports personal fees from AOSpine International, other from Global Spine Journal, other from Spine Journal, other from Neurosurgery, personal fees from Multiple Entities for defense, plantiff, grants from AOSpine, grants from Cerapedics, grants from Medtronic, personal fees from AOSpine, personal fees from NASS, personal fees from Biomet, personal fees from Medtronic, nonfinancial support from Broadwater, outside the submitted work; and Christopher Shaffrey reports grants from AOSpine North America during the conduct of the study, personal fees from Biomet, personal fees from Medtronic, from Nuvasive, personal fees from K2M, personal fees from Stryker, outside the submitted work, and Editorial Board Spine, Spinal Deformity and Neurosurgery; Hani R. Malone reports grants from AOSpine North America during the conduct of the study; Adam S. Kanter reports grants from AOSpine North America during the conduct of the study; Samuel K. Cho reports grants from AOSpine North America during the conduct of the study; grants from OREF, personal fees from Stryker, personal fees from Medtronic, personal fees from DePuy Synthes, outside the submitted work; Ra'Kerry K. Rahman reports grants from AOSpine North America during the conduct of the study; in addition, Dr. Rahman has a patent Deformity System & Pedicle Screws pending. Paul M. Arnold reports grants from AOSpine North America during the conduct of the study; other from Z-Plasty, other from Medtronic Sofamore Danek, other from Stryker Spine, other from FzioMed, other from AOSpine North America, other from Life Spine, other from Integra Life, other from Spine Wave, other from MIEMS, other from Cerapedics, other from AOSpine North America, outside the submitted work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by AOSpine North America Inc, a 501(c)3 nonprofit corporation.
References
- 1. Jain NK, Dao K, Ortiz AO. Radiologic evaluation and management of postoperative spine paraspinal fluid collections. Neuroimaging Clin N Am. 2014;24:375–389. doi:10.1016/j.nic.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 2. Shapiro SA, Scully T. Closed continuous drainage of cerebrospinal fluid via a lumbar subarachnoid catheter for treatment or prevention of cranial/spinal cerebrospinal fluid fistula. Neurosurgery. 1992;30:241–245. [DOI] [PubMed] [Google Scholar]
- 3. Findler G, Sahar A, Beller AJ. Continuous lumbar drainage of cerebrospinal fluid in neurosurgical patients. Surg Neurol. 1977;8:455–457. [PubMed] [Google Scholar]
- 4. Hanakita J, Kinuta Y, Suzuki T. Spinal cord compression due to postoperative cervical pseudomeningocele. Neurosurgery. 1985;17:317–319. [DOI] [PubMed] [Google Scholar]
- 5. Helle TL, Conley FK. Postoperative cervical pseudomeningocele as a cause of delayed myelopathy. Neurosurgery. 1981;9:314–316. [PubMed] [Google Scholar]
- 6. Horn EM, Bristol RE, Feiz-Erfan I, Beres EJ, Bambakidis NC, Theodore N. Spinal cord compression from traumatic anterior cervical pseudomeningoceles. Report of three cases. J Neurosurg Spine. 2006;5:254–258. doi:10.3171/spi.2006.5.3.254. [DOI] [PubMed] [Google Scholar]
- 7. Macki M, Lo SF, Bydon M, Kaloostian P, Bydon A. Post-surgical thoracic pseudomeningocele causing spinal cord compression. J Clin Neurosci. 2014;21:367–372. doi:10.1016/j.jocn.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 8. Hosono N, Yonenobu K, Ono K. Postoperative cervical pseudomeningocele with herniation of the spinal cord. Spine (Phila Pa 1976). 1995;20:2147–2150. [DOI] [PubMed] [Google Scholar]
- 9. Varma A, Morgan S, Krishna V. Cervical pseudomeningocele as a cause of neurological decline after posterior cervical spine surgery. Neurol India. 2012;60:256–253. doi:10.4103/0028-3886.96434. [DOI] [PubMed] [Google Scholar]
- 10. Goodman SJ, Gregorius FK. Cervical pseudomeningocele after laminectomy as a cause of progressive myelopathy. Bull Los Angeles Neurol Soc. 1974;39:121–127. [PubMed] [Google Scholar]
- 11. Cobb C, 3rd, Ehni G. Herniation of the spinal cord into an iatrogenic meningocele. Case report. J Neurosurg. 1973;39:533–536. doi:10.3171/jns.1973.39.4.0533. [DOI] [PubMed] [Google Scholar]
- 12. Burres KP, Conley FK. Progressive neurological dysfunction secondary to postoperative cervical pseudomeningocele in a C-4 quadriplegic. Case report. J Neurosurg. 1978;48:289–291. doi:10.3171/jns.1978.48.2.0289. [DOI] [PubMed] [Google Scholar]
- 13. Maiuri F, Corriero G, Giamundo A, Donati P, Gambardella A. Postoperative cervical pseudomeningocele. Neurochirurgia (Stuttg). 1988;31:29–31. doi:10.1055/s-2008-1053895. [DOI] [PubMed] [Google Scholar]
- 14. Horowitz SW, Azar-Kia B, Fine M. Postoperative cervical pseudomeningocele. AJNR Am J Neuroradiol. 1990;11:784. [PMC free article] [PubMed] [Google Scholar]
- 15. Moriyama T, Tachibana T, Maruo K, Inoue S, Okada F, Yoshiya S. Postoperative spinal cord herniation with pseudomeningocele in the cervical spine: a case report. Spine J. 2013;13:e43–e45. doi:10.1016/j.spinee.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 16. Mizuno J, Nakagawa H, Iwata K. Postoperative spinal cord herniation diagnosed by metrizamide CT: a case report [in Japanese]. No Shinkei Geka. 1986;14:681–685. [PubMed] [Google Scholar]
- 17. Schumacher HW, Wassmann H, Podlinski C. Pseudomeningocele of the lumbar spine. Surg Neurol. 1988;29:77–78. [DOI] [PubMed] [Google Scholar]
- 18. Hawk MW, Kim KD. Review of spinal pseudomeningoceles and cerebrospinal fluid fistulas. Neurosurg Focus. 2000;9:e5. [DOI] [PubMed] [Google Scholar]
- 19. Andrew SA, Sidhu KS. Cervical-peritoneal shunt placement for postoperative cervical pseudomeningocele. J Spinal Disord Tech. 2005;18:290–292. [PubMed] [Google Scholar]
- 20. Nair SB, Flood LM, Nath F. An unusual complication of Cloward’s procedure presenting to the otolaryngologist. J Laryngol Otol. 1998;112:1087–1089. [DOI] [PubMed] [Google Scholar]
- 21. Vaccaro AR, Lehman RA, Jr, Hurlbert RJ, et al. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine (Phila Pa 1976). 2005;30:2325–2333. [DOI] [PubMed] [Google Scholar]