Abstract
Study Design:
A multicenter retrospective case series.
Objective:
Horner’s syndrome is a known complication of anterior cervical spinal surgery, but it is rarely encountered in clinical practice. To better understand the incidence, risks, and neurologic outcomes associated with Horner’s syndrome, a multicenter study was performed to review a large collective experience with this rare complication.
Methods:
We conducted a retrospective multicenter case series study involving 21 high-volume surgical centers from the AOSpine North America Clinical Research Network. Medical records for 17 625 patients who received subaxial cervical spine surgery from 2005 to 2011 were reviewed to identify occurrence of 21 predefined treatment complications. Descriptive statistics were provided for baseline patient characteristics. Paired t test was used to analyze changes in clinical outcomes at follow-up compared to preoperative status.
Results:
In total, 8887 patients who underwent anterior cervical spine surgery at the participating institutions were screened. Postoperative Horner’s syndrome was identified in 5 (0.06%) patients. All patients experienced the complication following anterior cervical discectomy and fusion. The sympathetic trunk appeared to be more vulnerable when operating on midcervical levels (C5, C6), and most patients experienced at least a partial recovery without further treatment.
Conclusions:
This collective experience suggests that Horner’s syndrome is an exceedingly rare complication following anterior cervical spine surgery. Injury to the sympathetic trunk may be limited by maintaining a midline surgical trajectory when possible, and performing careful dissection and retraction of the longus colli muscle when lateral exposure is necessary, especially at caudal cervical levels.
Keywords: Horner’s syndrome, rare complications, cervical spine, anterior approach
Introduction
Horner’s syndrome is a well-recognized, but rare complication of anterior approaches to the subaxial cervical spine. It is characterized by ipsilateral pupillary miosis, facial anhydrosis, and ptosis, resulting from damage to cervical sympathetic trunk. Horner’s syndrome seldom leads to significant functional impairment, but the cosmetic effect may cause considerable distress. Damage to sympathetic fibers at any point along their route from the posteriolateral hypothalamus through the cervical sympathetic trunk can produce Horner’s syndrome.1 Accordingly, Horner’s serves as a clinical manifestation of a variety of underlying pathologies, from benign cluster headaches to malignant lung or thyroid cancers.
In anterior cervical spine surgery, the sympathetic trunk is thought to be most frequently damaged by sectioning or prolonged retraction of the longus colli muscle. The longus colli extends from the anterior tubercle of the atlas to the lateral vertebral bodies and transverse processes between C3 and T3. It is the longest and most medial of the prevertebral musculature. The sympathetic trunk courses over the anterior surface of the longus colli in a loose fascial layer lateral to the medial border of the muscle.1-5 Stretching sympathetic fibers while retracting the longus colli to expose the lateral vertebral bodies, uncovertebral joints, or transverse foramina may produce transient or irreversible Horner’s syndrome.1-5 The sympathetic trunk is particularly vulnerable to injury during surgery at middle and lower cervical levels where it lies in a relatively more medial position along the longus,1-5 or during anterolateral cervical approaches when it is directly encountered.6-9
Current estimates of the incidence of Horner’s syndrome following anterior cervical surgery are largely based on single institution, and in some instances single surgeon case series.10-16 It is best recognized as an infrequent complication of one of the most frequently performed procedures in spine surgery, anterior cervical discectomy and fusion (ACDF). The incidence of Horner’s syndrome in early ACDF series varied considerably, with reported rates ranging from 0.02% to 3.8%.10,12,13,16,17 In more recent ACDF series, the reported incidence has been more consistent (0.1% to 0.3%).11,14,15 Horner’s syndrome is far more common with anterolateral approaches to the cervical spine. Following oblique corpectomy, Horner’s syndrome occurs transiently in approximately 15.7% (73/465) of patients and permanently in 3.4% (16/465).7,8,18-21 Fortunately, most instances of postoperative Horner’s syndrome improve without treatment within 3 to 6 months.7,8,10,15,16,18-21
Due to its rarity, even experienced surgeons with large cervical spine practices may have limited experience with postoperative Horner’s syndrome. Indeed, in many of the series described above, incidence estimates are based on a single patient developing the complication.11,15,16 This multicenter study was conducted to better understand the incidence, risks, and neurologic outcomes of Horner’s syndrome following anterior cervical spinal surgery.
Methods
We conducted a retrospective multicenter case series study involving 21 high-volume surgical centers from the AOSpine North America Clinical Research Network, selected for their excellence in spine care and clinical research infrastructure and experience. Medical records for 17 625 patients who received subaxial cervical spine surgery from January 1, 2005, to December 31, 2011, were reviewed to identify an occurrence of 21 predefined treatment complications. The complications included reintubation requiring evacuation, esophageal perforation, epidural hematoma, C5 palsy, recurrent laryngeal nerve palsy, superior laryngeal nerve palsy, hypoglossal or glossopharyngeal nerve palsy, dural tear, brachial plexopathy, blindness, graft extrusion, misplaced screws requiring reoperation, anterior cervical infection, carotid artery injury or cerebrovascular accident, vertebral artery injuries, thoracic duct injury, tetraplegia, intraoperative death, revision of arthroplasty, pseudomeningocele, and Horner’s syndrome.
Trained research staff at each site abstracted the data from medical records, surgical charts, radiology imaging, narratives, and other source documents for the patients who experienced one or more of the complications from the list. Data was transcribed into study-specific paper case report forms. Copies of case report forms were transferred to the AOSpine North America Clinical Research Network Methodological Core for processing, cleaning, and data entry. Descriptive statistics were provided for baseline patient characteristics. Paired t test was used to analyze changes in clinical outcomes at follow-up compared to preoperative status.
Results
Patient Characteristics
In total, 8887 patients who received cervical spine surgery via an anterior approach were screened. Postoperative Horner’s syndrome was identified as a surgical complication in 5 patients (0.06%; Table 1). The incidence of Horner’s at individual participating institutions ranged from 0.0% to 1.28%. The average age of patients who developed Horner’s syndrome was 51 (±2.6) years, and the majority (4/5) were female. Average weight was 78.76 (±2.6) kg, and average height was 168 cm. Of these 5 patients, 2 (40%) were treated for axial neck pain, 2 (40%) for myelopathy, and 1 (20%) for radiculopathy. None of the patients had spinal instability, infection, fracture, or metastatic disease.
Table 1.
Characteristics of Patients Experiencing Horner’s Syndrome Following Anterior Approaches to the Cervical Spine.
| Baseline Characteristics | Patients With Postoperative Horner’s Syndrome (n = 5) |
|---|---|
| Age (years) | 51 ± 2.6 |
| Females/males (ratio) | 4:1 |
| Height (cm) | 167.7 ± 7.3 |
| Weight (kg) | 78.8 ± 15.0 |
| Operative time (minutes) | 157.2 ± 39.2 |
| Smoker/nonsmoker (ratio) | 0:5 |
| Blood loss (mL) | 157.5 ± 167.0 |
| Presentation | |
| Degenerative disk disease | 2 (40%) |
| Radiculopathy | 1 (20%) |
| Myelopathy | 2 (40%) |
| Surgery | |
| ACDF | 5 (100%) |
| Corpectomy | 0 (0.0%) |
| Laterality of approach | 2 left, 1 right, 2 NA |
| Levels included | |
| C3 | 1 (20%) |
| C4 | 2 (40%) |
| C5 | 5 (100%) |
| C6 | 3 (60%) |
| C7 | 2 (40%) |
| T1 | 0 (0.0%) |
Abbreviation: ACDF, anterior cervical discectomy and fusion.
Surgical Characteristics
Surgery lasted an average of 157.2 (±39.2) minutes with an average of 157.50 mL of blood loss. None of the patients who developed Horner’s syndrome required a blood transfusion and none smoked. The cervical levels most often involved with Horner’s syndrome were C5 (5/5; 100%), C6 (3/5; 60%), C7 (2/5; 40%), C4 (2/5; 40%), and C3 (1/5; 20%). No instances of Horner’s syndrome occurred in cases that involved spinal levels rostral to C3 or caudal to C7. All 5 cases occurred after cervical discectomy with grafting. Laterality of approach was denoted in 3 of the 5 cases, with 2 occurring on the left and 1 on the right. Cervical traction was used in one of the cases (20%).
Recovery
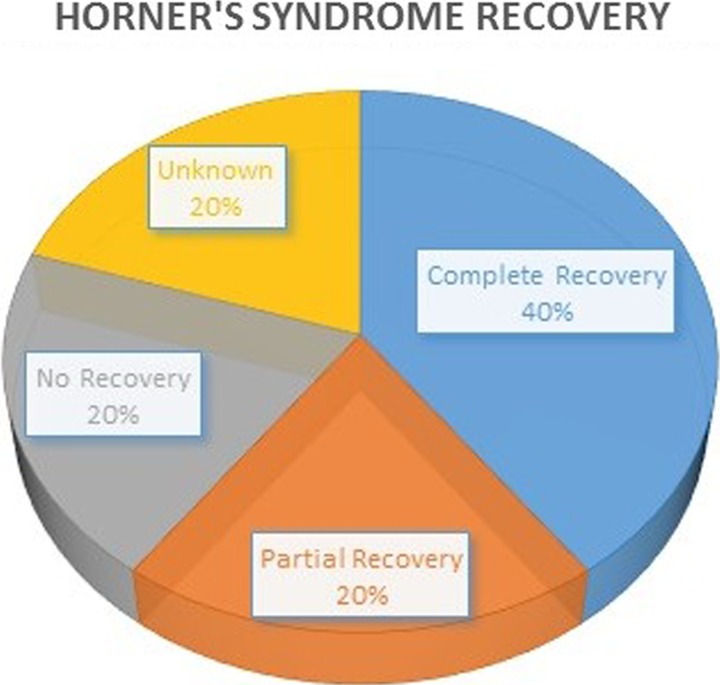
Symptoms completely resolved in 2 patients (40%), while 1 patient experienced an incomplete resolution (20%) and another experienced no improvement (20%; Figure 1). Long-term follow-up was unavailable for one patient. Data regarding the specific measures taken to promote recovery, such as steroids, are not available. However, the complication did not lead to any hospital readmissions or subsequent surgical procedures.
Figure 1.
Symptomatic recovery from Horner’s syndrome caused by anterior surgical approaches to the cervical spine.
Discussion
We screened 8887 patients who received anterior cervical spine surgery at 21 different institutions for the presence of postoperative Horner’s syndrome. The complication was identified in 5 patients, all of whom received ACDF. Consistent with previous studies, the sympathetic trunk appeared to be more vulnerable at midcervical levels (C5, C6), and most patients experienced at least a partial recovery without further treatment. However, the incidence of Horner’s syndrome found in this collaborative effort (5/8887, 0.06%) is well below that of most previous reports (Table 2).
Table 2.
Incidence of Horner’s Syndrome Following Anterior Cervical Discectomy (With or Without Fusion).
| Year | Author | Patients | All Complications | Horner’s Syndrome |
|---|---|---|---|---|
| 1975 | Hankinson12 ,a | 52 | — | 3.8% |
| 1976 | Tew and Mayfield16 ,a | 500 | 4.4% | 0.2% |
| 1982 | Flynn17,b | 69 590 | 0.45%c | 0.02% |
| 1989 | Bertalanffy10 ,a | 450 | 14.7% | 1.1% |
| 1995 | Johnston13 | 50 | — | 2.0% |
| 2006 | Fountas11 | 1015 | 19.6% | 0.1% |
| 2008 | Lied14 | 390 | 9.5% | 0.3% |
| 2014 | Nanda15 | 1576 | 8.4% | 0.1% |
| 2015 | AOSpine/Traynelis (current study)b | 8887 | — | 0.06% |
aAnterior cervical discectomy without fusion.
bStudy based on survey data.
cLimited to “major neurologic” complications.
There are several possible explanations for this discrepancy. The first is primarily statistical. Given the rarity of postoperative Horner’s, even a single incidence may exaggerate its true incidence in smaller case series. This trend can be easily appreciated in the existing ACDF literature. In small series described by Johnston and Crockard13 (n = 50) and Hankinson and Wilson12 (n = 52), 1 and 2 instances on Horner’s syndrome led to incidence rates of 2.0% and 3.8%, respectively. A single occurrence of postoperative Horner’s syndrome led to a much smaller perceived incidence (<0.1%) in larger series by Fountas et al11 (n = 1105) and Nanda et al15 (n = 1576). In the largest previously reported ACDF survey (n = 69 590), Flynn found 13 cases of postoperative Horner’s syndrome, yielding a still smaller incidence of approximately 0.02%, which is more consistent with the 0.06% found in our study.17
It is important to note, that like the work of Flynn, our data were derived from a multi-institutional survey, which could be prone to underreporting or differences in the propensity to report. Importantly, in our study complication reporting was anonymous to both operating surgeon and institution, in an effort to mitigate reporting bias. Nevertheless, the retrospective nature of our data collection represents an important limitation in our study. Prospective data collection will always yield a higher rate of reported complications due to the special attention paid to adverse events. Horner’s syndrome is also a primarily clinical diagnosis. When it occurs as a complication of cervical spine surgery, there is little need for diagnostic imaging22-24 or clinical testing25 (such as cocaine or apraclonidine eye drops) to more objectively document the phenomena, perhaps making it less salient in retrospective review.
Regardless of estimated incidence, recommendations to mitigate the risk of Horner’s syndrome are fairly consistent in the literature. In general, risk of injury to the sympathetic trunk is minimized by staying midline whenever possible. Extensive retraction or lateral sectioning of the longus colli increases risk. When lateral exposure is needed, it is important that dissection is carried out in the subperiosteal plane.11,26 This will allow for proper placement of retractors beneath, rather than on the surface of, the longus colli. There is some debate as to whether sharp27 or blunt2 tip lateral retractors are optimal, but periodic release after retractor placement has been proposed as a strategy that might mitigate the risk of both Horner’s syndrome and recurrent laryngeal palsy.28
A sound understanding of the regional anatomy of the cervical sympathetic trunk is key to avoiding iatrogenic Horner’s syndrome. To this end, several cadaveric studies delineating the anatomy of the sympathetic cervical trunk and its ganglia have been published in recent years (Table 3).2,3,5,9 There are 3 ganglion in the cervical sympathetic trunk: the superior cervical ganglion positioned between the carotid sheath and longus capitus muscle at the level of C2/C3, the small cervical ganglion at C6, and the stellate ganglion that lies inferiorly along the longus colli between the transverse process of C7 and the neck of the first rib.1,29 From these cadaveric studies, we know that the superior cervical and stellate ganglia are consistently present, but the middle cervical ganglia is only appreciable in 33% to 48% of individuals.3,5 Also, the distance between the medial border of the longus colli and the cervical sympathetic trunk laterally decreases considerable in the caudal cervical spine (C6: 10.6-16.8 mm; C7: 12.4 mm), compared to more rostral levels (C2: 21.1 mm; C3: 17.2 mm).2,3,5,9 The mean diameter of the cervical sympathetic trunk at the C6 level ranges between 2.7 and 3.3 mm.2,9
Table 3.
Anatomic Variations of the Course of the Cervical Sympathetic Trunk Based on Human Cadaveric Studies, Demonstrating the Prevalence Of Middle Cervical Sympathetic Ganglion, the Mean Distance Between the Sympathetic Trunk (ST) and the Medial Border of the Longus Colli Muscle (mLCM), and Cervical Sympathetic Trunk Diameter (CSTd) at the C6 Level.
Conclusion
Horner’s syndrome is a rare but understood complication of anterior approaches to the cervical spine. A survey of 8887 anterior cervical spine surgeries from 21 different institutions suggests that the incidence of Horner’s syndrome is very low (n = 5, 0.06%). Common causes of Horner’s include lateral dissection of the longus colli with monopolar cautery and placement of retractor blades above or upon the longus. Accordingly, injury to the sympathetic trunk may be limited by maintaining a midline surgical trajectory when possible, and performing a subperiosteal dissection that facilitates retractor placement under the longus colli when lateral exposure is necessary. Sound knowledge of the regional anatomy of the cervical sympathetic trunk is critical to limiting iatrogenic Horner’s syndrome. Prospective data collection from a large multi-institution study might help further clarify the true incidence and risks associated with Horner’s syndrome following anterior cervical spine surgery.
Footnotes
Authors’ Note: This study was ethically approved by the institutional ethics committees at all participating sites.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Vincent C. Traynelis reports grants from AOSpine North America during the conduct of the study, and Medtronic - Royalties, Consultant Globus - Institutional Fellowship Support; Zachary A. Smith reports grants from AOSpine North America during the conduct of the study; Wellington K. Hsu reports grants from AOSpine North America during the conduct of the study, personal fees from Medtronic, personal fees from Stryker, personal fees from Bacterin, personal fees from Graftys, personal fees from Ceramtec, personal fees from Relievant, personal fees from Bioventus, personal fees from Globus, personal fees from SpineSmith, outside the submitted work; Sheeraz A. Qureshi reports grants from AOSpine North America during the conduct of the study, and is a consultant and receives royalties from Stryker Spine, Biomet Spine, and RTI; Evan O. Baird reports grants from AOSpine North America during the conduct of the study; Robert E. Isaacs reports grants from AOSpine North America during the conduct of the study, grants and personal fees from NuVasive, Inc., personal fees from Association for Collaborative Spine research, outside the submitted work; Galina Polevaya reports grants from AOSpine North America during the conduct of the study; Justin S. Smith reports grants from AOSpine North America during the conduct of the study, personal fees from Biomet, personal fees from Nuvasive, personal fees from Cerapedics, personal fees from K2M, personal fees and other from DePuy, personal fees from Medtronic, outside the submitted work; Christopher Shaffrey reports grants from AOSpine North America during the conduct of the study, personal fees from Biomet, personal fees from Medtronic, from Nuvasive, personal fees from K2M, personal fees from Stryker, outside the submitted work, and Editorial Board Spine, Spinal Deformity and Neurosurgery; P. Justin Tortolani reports grants from AOSpine North America during the conduct of the study, other from Globus Medical, grants from Spineology, other from Innovasis, outside the submitted work, and in addition, Dr. Tortolani has a patent Globus with royalties paid; D. Alex Stroh reports grants from AOSpine North America during the conduct of the study; Michael G. Fehlings reports grants from AOSpine North America during the conduct of the study; Thomas E. Mroz reports other from AOSpine, grants from AOSpine North America, during the conduct of the study, personal fees from Stryker, personal fees from Ceramtec, other from Pearl Diver, outside the submitted work; and K. Daniel Riew reports personal fees from AOSpine International, other from Global Spine Journal, other from Spine Journal, other from Neurosurgery, personal fees from Multiple Entities for defense, plantiff , grants from AOSpine, grants from Cerapedics, grants from Medtronic, personal fees from AOSpine, personal fees from NASS, personal fees from Biomet, personal fees from Medtronic, nonfinancial support from Broadwater, outside the submitted work; Hani R. Malone reports grants from AOSpine North America during the conduct of the study; Adam S. Kanter reports grants from AOSpine North America during the conduct of the study; Samuel K. Cho reports grants from AOSpine North America during the conduct of the study; grants from OREF, personal fees from Stryker, personal fees from Medtronic, personal fees from DePuy Synthes, outside the submitted work; Ra'Kerry K. Rahman reports grants from AOSpine North America during the conduct of the study; in addition, Dr. Rahman has a patent Deformity System & Pedicle Screws pending. Paul M. Arnold reports grants from AOSpine North America during the conduct of the study; other from Z-Plasty, other from Medtronic Sofamore Danek, other from Stryker Spine, other from FzioMed, other from AOSpine North America, other from Life Spine, other from Integra Life, other from Spine Wave, other from MIEMS, other from Cerapedics, other from AOSpine North America, outside the submitted work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by AOSpine North America Inc, a 501(c)3 nonprofit corporation.
References
- 1. Lu J, Ebraheim NA, Nadim Y, Huntoon M. Anterior approach to the cervical spine: surgical anatomy. Orthopedics. 2000;23:841–845. [DOI] [PubMed] [Google Scholar]
- 2. Ebraheim NA, Lu J, Yang H, Heck BE, Yeasting RA. Vulnerability of the sympathetic trunk during the anterior approach to the lower cervical spine. Spine (Phila Pa 1976). 2000;25:1603–1606. [DOI] [PubMed] [Google Scholar]
- 3. Kiray A, Arman C, Naderi S, Guvencer M, Korman E. Surgical anatomy of the cervical sympathetic trunk. Clin Anat. 2005;18:179–185. [DOI] [PubMed] [Google Scholar]
- 4. Pait TG, Killefer JA, Arnautovic KI. Surgical anatomy of the anterior cervical spine: the disc space, vertebral artery, and associated bony structures. Neurosurgery. 1996;39:769–776. [DOI] [PubMed] [Google Scholar]
- 5. Saylam CY, Ozgiray E, Orhan M, Cagli S, Zileli M. Neuroanatomy of cervical sympathetic trunk: a cadaveric study. Clin Anat. 2009;22:324–330. [DOI] [PubMed] [Google Scholar]
- 6. Bruneau M, Cornelius JF, George B. Microsurgical cervical nerve root decompression by anterolateral approach. Neurosurgery. 2006;58:ONS108–113. [DOI] [PubMed] [Google Scholar]
- 7. Traynelis VC, Arnold PM, Fourney DR, Bransford RJ, Fischer DJ, Skelly AC. Alternative procedures for the treatment of cervical spondylotic myelopathy: arthroplasty, oblique corpectomy, skip laminectomy: evaluation of comparative effectiveness and safety. Spine (Phila Pa 1976). 2013;38:S210–S231. [DOI] [PubMed] [Google Scholar]
- 8. Chacko AG, Turel MK, Sarkar S, Prabhu K, Daniel RT. Clinical and radiological outcomes in 153 patients undergoing oblique corpectomy for cervical spondylotic myelopathy. Br J Neurosurg. 2014;28:49–55. [DOI] [PubMed] [Google Scholar]
- 9. Civelek E, Karasu A, Cansever T, et al. Surgical anatomy of the cervical sympathetic trunk during anterolateral approach to cervical spine. Eur Spine J. 2008;17:991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bertalanffy H, Eggert HR. Complications of anterior cervical discectomy without fusion in 450 consecutive patients. Acta Neurochir (Wien). 1989;99:41–50. [DOI] [PubMed] [Google Scholar]
- 11. Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976). 2007;32:2310–2317. [DOI] [PubMed] [Google Scholar]
- 12. Hankinson HL, Wilson CB. Use of the operating microscope in anterior cervical discectomy without fusion. J Neurosurg. 1975;43:452–456. [DOI] [PubMed] [Google Scholar]
- 13. Johnston FG, Crockard HA. One-stage internal fixation and anterior fusion in complex cervical spinal disorders. J Neurosurg. 1995;82:234–238. [DOI] [PubMed] [Google Scholar]
- 14. Lied B, Sundseth J, Helseth E. Immediate (0-6 h), early (6-72 h) and late (>72 h) complications after anterior cervical discectomy with fusion for cervical disc degeneration; discharge six hours after operation is feasible. Acta Neurochir (Wien). 2008;150:111–118. [DOI] [PubMed] [Google Scholar]
- 15. Nanda A, Sharma M, Sonig A, Ambekar S, Bollam P. Surgical complications of anterior cervical diskectomy and fusion for cervical degenerative disk disease: a single surgeon’s experience of 1,576 patients. World Neurosurg. 2014;82:1380–1387. [DOI] [PubMed] [Google Scholar]
- 16. Tew JM, Jr, Mayfield FH. Complications of surgery of the anterior cervical spine. Clin Neurosurg. 1976;23:424–434. [DOI] [PubMed] [Google Scholar]
- 17. Flynn TB. Neurologic complications of anterior cervical interbody fusion. Spine (Phila Pa 1976). 1982;7:536–539. [DOI] [PubMed] [Google Scholar]
- 18. Chacko AG, Joseph M, Turel MK, Prabhu K, Daniel RT, Jacob KS. Multilevel oblique corpectomy for cervical spondylotic myelopathy preserves segmental motion. Eur Spine J. 2012;21:1360–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chibbaro S, Mirone G, Makiese O, George B. Multilevel oblique corpectomy without fusion in managing cervical myelopathy: long-term outcome and stability evaluation in 268 patients. J Neurosurg Spine. 2009;10:458–465. [DOI] [PubMed] [Google Scholar]
- 20. Kiris T, Kilincer C. Cervical spondylotic myelopathy treated by oblique corpectomy: a prospective study. Neurosurgery. 2008;62:674–682. [DOI] [PubMed] [Google Scholar]
- 21. Rocchi G, Caroli E, Salvati M, Delfini R. Multilevel oblique corpectomy without fusion: our experience in 48 patients. Spine (Phila Pa 1976). 2005;30:1963–1969. [DOI] [PubMed] [Google Scholar]
- 22. Almog Y, Gepstein R, Kesler A. Diagnostic value of imaging in Horner syndrome in adults. J Neuroophthalmol. 2010;30:7–11. [DOI] [PubMed] [Google Scholar]
- 23. Digre KB, Smoker WR, Johnston P, et al. Selective MR imaging approach for evaluation of patients with Horner’s syndrome. AJNR Am J Neuroradiol. 1992;13:223–227. [PMC free article] [PubMed] [Google Scholar]
- 24. George A, Haydar AA, Adams WM. Imaging of Horner’s syndrome. Clin Radiol. 2008;63:499–505. [DOI] [PubMed] [Google Scholar]
- 25. Allen AY, Meyer DR. Neck procedures resulting in Horner syndrome. Ophthal Plast Reconstr Surg. 2009;25:16–18. [DOI] [PubMed] [Google Scholar]
- 26. Albert TJ, Balderstone RA, Northrup BE. Surgical Approaches to the Spine. Philadelphia, PA: Saunders; 1997. [Google Scholar]
- 27. Perez-Cruet MJ, Samartzis D, Fessler RG. Anterior cervical discectomy and corpectomy. Neurosurgery. 2006;58:ONS–355–359. [DOI] [PubMed] [Google Scholar]
- 28. Apfelbaum RI, Kriskovich MD, Haller JR. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine (Phila Pa 1976). 2000;25:2906–2912. [DOI] [PubMed] [Google Scholar]
- 29. Zhang B, Li Z, Yang X, et al. Anatomical variations of the upper thoracic sympathetic chain. Clin Anat. 2009;22:595–600. [DOI] [PubMed] [Google Scholar]