Abstract
Study Design:
Retrospective cohort study of prospectively collected data.
Objective:
To examine the incidence of iatrogenic spinal cord injury following elective cervical spine surgery.
Methods:
A retrospective multicenter case series study involving 21 high-volume surgical centers from the AOSpine North America Clinical Research Network was conducted. Medical records for 17 625 patients who received cervical spine surgery (levels from C2 to C7) between January 1, 2005, and December 31, 2011, were reviewed to identify occurrence of iatrogenic spinal cord injury.
Results:
In total, 3 cases of iatrogenic spinal cord injury following cervical spine surgery were identified. Institutional incidence rates ranged from 0.0% to 0.24%. Of the 3 patients with quadriplegia, one underwent anterior-only surgery with 2-level cervical corpectomy, one underwent anterior surgery with corpectomy in addition to posterior surgery, and one underwent posterior decompression and fusion surgery alone. One patient had complete neurologic recovery, one partially recovered, and one did not recover motor function.
Conclusion:
Iatrogenic spinal cord injury following cervical spine surgery is a rare and devastating adverse event. No standard protocol exists that can guarantee prevention of this complication, and there is a lack of consensus regarding evaluation and treatment when it does occur. Emergent imaging with magnetic resonance imaging or computed tomography myelography to evaluate for compressive etiology or malpositioned instrumentation and avoidance of hypotension should be performed in cases of intraoperative and postoperative spinal cord injury.
Keywords: cervical spine surgery, complication, quadriplegia, iatrogenic spinal cord injury
Study Rationale and Context
Iatrogenic spinal cord injury resulting from elective cervical spine surgery is a rare and devastating adverse event. The incidence of iatrogenic spinal cord injury following cervical spine operations is challenging to determine. Flynn reviewed 82 114 anterior cervical spine operations and documented a postoperative neurological injury rate of 0.3%.1 Lee et al examined 1445 anterior cervical spine surgery patients and reported a rate of 0.1% spinal cord injury with neurological deficit.2
Some instances of postoperative neurological deficit following cervical spine surgery can be predicted by neuromonitoring changes during the procedure, or due to an obvious intraoperative event leading to injury of the spinal cord, while others may only be recognized when the patient emerges from anesthesia. Each of these scenarios requires a unique response from the surgeon.
Given the low incidence, it is not surprising that clearly defined protocols to manage interoperative spinal cord injury during elective cervical spine surgery have not been developed. Most spine surgeons will encounter this adverse event once or twice, if at all, in an entire career. Given these small numbers, appropriate practices must be determined more based on consensus rather than data. A review of cases of iatrogenic spinal cord injury might serve to inform development of a plan for response to such events.
Although the incidence is rare, the impact of iatrogenic spinal cord injury resulting from cervical spine surgery is substantial and has potential for serious patient, surgeon, institutional, and medicolegal ramifications. The purpose of this investigation is to examine the rate of iatrogenic spinal cord injury associated with cervical spine surgery and to report patient and surgical factors associated with these injuries.
Objective or Clinical Question
This study aimed to evaluate the incidence and factors associated with iatrogenic spinal cord injury during elective cervical spine surgery.
Methods
We conducted a retrospective multicenter case series study involving 21 high-volume spine surgical centers from the AOSpine North America Clinical Research Network, selected for their clinical research infrastructure and experience. Medical records for 17 625 patients who received cervical spine surgery (levels from C2 to C7) between January 1, 2005, and December 31, 2011, were reviewed to identify occurrence of 21 predefined treatment adverse events.
Adverse events examined included reintubation requiring evacuation, esophageal perforation, epidural hematoma, C5 palsy, recurrent laryngeal nerve palsy, superior laryngeal nerve palsy, hypoglossal or glossopharyngeal nerve palsy, dural tear, brachial plexopathy, blindness, graft extrusion, misplaced screws requiring reoperation, anterior cervical infection, carotid artery injury or cerebrovascular accident, vertebral artery injuries, Horner’s syndrome, thoracic duct injury, quadriplegia, intraoperative death, revision of arthroplasty, and pseudomeningocele. This investigation examined only patients with quadriplegia following surgery.
Trained research staff at each site abstracted the data from medical records, surgical charts, radiologic imaging, narratives, and other source documents for the patients who experienced one or more of the adverse events from the list. Data were transcribed into study-specific paper case report forms. Copies of case report forms were transferred to the AOSpine North America Clinical Research Network Methodological Core for processing, cleaning, and data entry.
Results
Three cases of quadriplegia were reported from 12 903 patients. Incidence rates of the participating centers ranged from 0.0% to 0.24%.
Of the 3 patients suffering iatrogenic spinal cord injury, 2 were male and 1 was female. The mean age was 57.3 years, with an average hospital length of stay of 12 days. One injury occurred in 2007 and 2 occurred in 2011. All 3 were nonsmokers. The diagnosis and reason for surgery was myelopathy for 2 patients and degenerative disk disease for 1 patient.
One patient underwent anterior surgery only with 2-level cervical corpectomy (C5, C6), one underwent posterior surgery only, and one underwent circumferential surgery (anterior and posterior) including cervical corpectomy. Two patients underwent surgery from C3 to C7, while one patient underwent surgery from C4 to C7. All 3 patients had interoperative neuromonitoring (IONM) utilized during the procedure. Poor baseline neuromonitoring signals were noted in one patient, no baseline motor response was noted in another, with data unknown from the third patient.
In patient 1, a 67-year-old patient who underwent 2-level anterior corpectomy of C5 and C6, a dural defect was identified during resection of ossification of the posterior longitudinal ligament (PLL) with subsequent neuromonitoring change following its removal. The dural defect was covered then with a Duragen patch, followed by graft placement. The patient underwent the remaining portion of the surgical procedure prior to closure. The patient had a partial recovery but had residual upper and lower extremity weakness at follow-up.
Patient 2 was a 36-year-old patient who underwent both transcranial motor evoked potential and somatosensory evoked potential monitoring. At the outset of the case, the patient had no motor response on monitoring. The patient underwent anterior corpectomy surgery, and on awaking from anesthesia was found to be quadriplegic. The patient was taken for emergent magnetic resonance imaging (MRI), following which the posterior component of the surgery was completed. No additional details regarding the surgery or MRI results are available. The patient recovered to baseline motor function at the time of hospital discharge.
Patient 3 was a 69-year-old patient who underwent C3-7 posterior decompression and fusion. The patient awoke from anesthesia with quadriplegia, and did not recover function at the time of discharge. No neuromonitoring or follow-up data are available for this patient.
Discussion
Iatrogenic spinal cord injury is a devastating and rare adverse event following cervical spine surgery, with previously reported rates between 0.1% and 0.3%.1-3 In this series, incidence rates of the participating centers ranged from 0.0% to 0.24% of cases. Injury to the spinal cord may result in a range of clinical severity from incomplete injury with mild motor or sensory deficit to complete quadriplegia with loss of sensation and bowel/bladder function (American Spinal Injury Association [ASIA] A spinal cord injury). This study only examined quadriplegia, and thus may not include patients that had less severe spinal cord or nerve root injuries.
There are multiple potential etiologies of spinal cord injury during cervical spine surgery, including aggravation of preexisting spinal stenosis during positioning or surgical approach, malpositioned instrumentation or bone graft penetrating or compressing the cord, mechanical blunt trauma to the spinal cord, and vascular injury due to hypotension or arterial interruption. It is also possible that some cases of iatrogenic spinal cord injury occur due to a combination of these factors.
Several iatrogenic cervical spinal cord injuries have been reported previously.1,3-7 This adverse event is too rare to accurately calculate a formal incidence, although a rate between 0.1% and 0.3% appears to be a reasonable estimate across all types and indications for cervical spine surgery. It is expected that this rate will depend on the nature and severity of the spinal pathology being addressed. For example, surgical correction of complex cervical deformity would be expected to have higher rates of spinal cord injury than anterior cervical discectomy and fusion or posterior foraminotomy, with neurological deficits reported in up to 13.5% of deformity patients.8
Response to Interoperative Neuromonitoring Alerts
Neuromonitoring utilizing somatosensory evoked potentials and transcranial motor evoked potentials (tcMEPs) is frequently used in cervical spine surgery and may help surgeons intervene to reverse the immediate cause of intraoperative spinal cord injury.9,10 For procedures performed in the prone position, obtaining potentials with the neck in a neutral posture (prior to prone positioning) may be beneficial in some cases to provide baseline neurophysiologic data. Potentials can then be repeated in the prone surgical position to help identify cervical positioning related neuromonitoring alterations.
Some instances of intraoperative neuromonitoring changes occur due to spinal cord hypoperfusion.2,3,8,11,12 Spinal cord oxygenation and perfusion are known to correlate with neuromonitoring alerts. Direct correlation between cerebrospinal fluid (CSF) oxygenation and TcMEPs has been shown in a pig model with clamping of spinal radicular arteries, with reversal of these neuromonitoring changes following unclamping of the vessels.13 In a canine study, multiple bilateral spinal radicular vessel ligation was required to create irreversible neurological deficit.14 In human studies examining neuromonitoring changes during scoliosis surgery, neuromonitoring changes associated with hypotension are often reversible with mean arterial pressure (MAP) elevation, and do not lead to permanent postoperative neurological deficit in the majority of situations.15 In cases of neuromonitoring changes without an obvious reversible surgical explanation, evaluation of blood pressure and correction of hypotension if possible should be undertaken.
Literature regarding the utility of neuromonitoring during cervical spine surgery is relatively limited. An investigation by Clark et al16 retrospectively reviewed 140 patients with cervicothoracic spondylotic myelopathy undergoing spine surgery, of which 16 (11%) had intraoperative deceases in tcMEPs. In total, there were 8 patients from this group who awoke with neurological deficits: 5 with C5 palsy and 2 with paraparesis. A significant correlation (P < .001) was found between persistent tcMEP changes and postoperative neurological deficits, with a sensitivity of 75%, specificity of 98%, positive predictive value of 75%, and a negative predictive value of 98%. In patients with vascular disease, the sensitivity of tcMEPs decreased to 60%.
Although neuromonitoring may be able to predict some cases of postoperative neurological deficit, the appropriate response by the surgeon, anesthesia staff, and neurophysiologist is not clear in many cases. Ziewacz et al designed a checklist for responding to neuromonitoring changes during spinal myelopathy and deformity spine surgery in 2012.17 They utilized expert consensus and aviation and surgical literature to create their algorithm (Figure 1), which highlights initial logical responses to MEP changes as well as additional considerations if the MEPs do not respond to initial interventions. Surgeon responses recommended include stopping the current manipulation, assessing the field for structural spinal cord compression, and consideration for further spinal cord decompression and stenosis is present.
Figure 1.
Checklist for neuromonitoring (MEP) alerts in patients with myelopathy or spinal deformity. From Ziewacz et al.17
Although there is relatively little literature specific to neuromonitoring changes during cervical spine surgery, there is a large body of work regarding thoracolumbar spinal deformity surgery, which may be informative to cervical spine surgery.10 The incidence of spinal cord injury has been reported to occur in 0.26% to 1.75% of thoracolumbar deformity operations.11,18
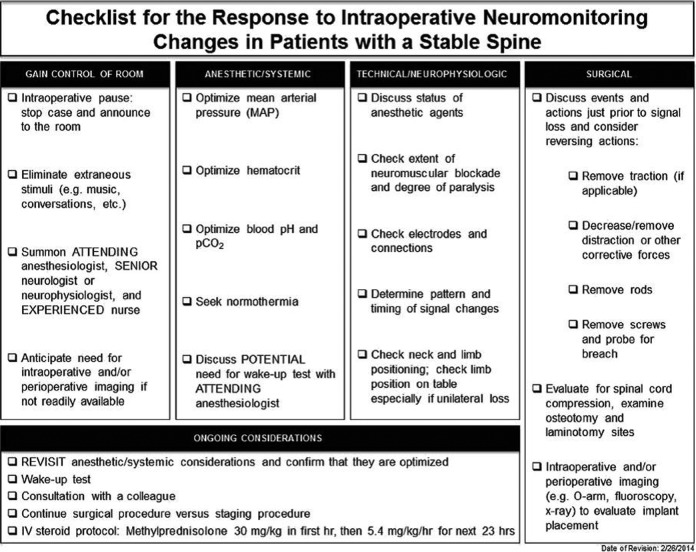
The surgeon and surgical team response to neuromonitoring changes in spinal deformity surgery have been thoroughly evaluated in a Delphi Consensus Report by Vitale et al.10 In this investigation, they separated the “mechanically stable spine” from the unstable spine following spinal osteotomy as appropriate response in these 2 situations differs substantially. This delineation may similarly be useful for cervical spine surgery, in which cervical spine trauma or spinal osteotomy may require a specific surgeon response to neuromonitoring changes. Although designed for thoracolumbar deformity surgery, the results of this Delphi Report provide a valuable guide for response to neuromonitoring alerts during cervical spine surgery. Recommended responses to neuromonitoring changes include an intraoperative pause, summoning the attending anesthesiologist and senior neurophysiologist, determination of the need for intraoperative imaging, optimization of patient MAPs, hematocrit, pCO2, and temperature, consideration of a wakeup test, checking technical neuromonitoring factors, and evaluation and correction of surgical factors (ie, remove traction, remove instrumentation or bone graft, etc; Figure 2).
Figure 2.
Checklist for responses to neuromonitoring changes in the stable spine. From Vitale et al.10
While it is not clear that a separate checklist for IONM alerts during cervical spine surgery is needed, the work done by Ziewacz et al17 and Vitale et al10 would serve as an excellent starting point. The review of cases here suggests that a standardized approach to use of IONM, as well as response to alerts, is not part of current practice among cervical spine surgeons as the monitoring strategy differed in each of the 3 cases presented in this investigation.
Response to Postoperative Motor Deficit
A separate but related issue is how the operating surgeon should respond when a patient awakens from anesthesia with a new motor deficit of clinical significance. The optimal postoperative management following iatrogenic cervical spinal cord injury should generally include emergent MRI or computed tomography (CT) myelogram to evaluate for spinal cord compression from hematoma, bone graft, vertebral displacement, or malpositioned instrumentation. If a compressive etiology is discovered, return to the operating room for alleviation of the cause of neural compression is indicated at the earliest possible opportunity that the patient can safely tolerate.
Additionally, avoidance of hypotension with induced hypertension is recommended in cases of acute spinal cord injury of any etiology. Keeping MAPs >85 mm Hg has been shown to improve motor function and bowel/bladder recovery following traumatic spinal cord injury,19 and may be performed for up to 7 days, although some centers perform only 48 to 72 hours of MAP elevation. Optimizing spinal cord oxygenation and avoiding hypotension are important interventions in optimizing outcomes following iatrogenic spinal cord injury.
The neurological sequelae of traumatic spinal cord injury occurs due to an initial traumatic mechanical injury followed by secondary insult stemming from ischemia, reperfusion, ionic dysregulation, cellular excitotoxicity, swelling, and free-radical–mediated peroxidation.20 Numerous prospective human studies have been performed to investigate pharmacologic interventions to reverse the deleterious inflammatory response and neurological deficits from traumatic spinal cord injury, although unfortunately none have proven dramatically successful thus far. Therefore at this time, no strong recommendations regarding steroids or other investigational medications can be made to provide to patients who suffer iatrogenic spinal cord injury resulting from cervical spine surgery.20 Other strategies to mitigate spinal cord injury may exist. Placing a CSF drain is commonly performed to decrease CSF pressure in an attempt to prevent spinal cord injury during thoracoabdominal aorta surgery21; no data currently exist to examine whether this may be beneficial in cases of iatrogenic spinal cord injury during cervical spine surgery.
This review demonstrates a similar lack of a protocol-based approach to discovery of a new postoperative neurological deficit. As case numbers will be too small to develop such a protocol based on data, a consensus-based approach appears appropriate. Postoperative institutional safety improvement review of protocols and procedures are imperative following serious adverse events such as iatrogenic spinal cord injury and were likely performed in each of the cases presented in this investigation. Unfortunately, details of individual institution safety improvement initiatives were not included in our data set.
Conclusion
Iatrogenic spinal cord injury following elective cervical spine surgery is a rare and devastating adverse event occurring in up to 0.24% of cases in this multicenter cohort. This study was limited in its ability thoroughly assess risk factors and outcomes of this adverse event due to the rarity of the event and the small number of cases encountered. No standard protocol exists that can guarantee prevention of this complication, and there is a lack of consensus regarding evaluation and treatment when it does occur. Utilization of IONM and response to interoperative alerts should be standardized based on surgeon consensus. Similarly, response to postoperative motor deficits is not yet protocolized. Emergent imaging with MRI or CT myelography to evaluate for compressive etiology or malpositioned instrumentation, appropriate surgical correction when appropriate, and maintenance of adequate mean arterial blood pressure should generally be performed in cases of postoperative spinal cord injury.
Footnotes
Authors’ Note: This study was ethically approved by the institutional ethics committees at all participating sites.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Alan H. Daniels reports grants from AOSpine North America during the conduct of the study, personal fees from Stryker, personal fees from Depuy, other from Globus, nonfinancial support from Medtronic, outside the submitted work; Robert A. Hart reports grants from AOSpine North America during the conduct of the study, other from CSRS Board, other from ISSLS, other from ISSG Executive Commitee, personal fees from DepuySynthes, personal fees from Globus, personal fees from Medtronic, other from Evans, Craven & Lackie, other from Benson, Bertoldo, Baker, & Carter, grants from Medtronic, grants from ISSGF, personal fees from Seaspine, personal fees from DepuySynthes, other from Spine Connect, personal fees from DepuySynthes, outside the submitted work; Alan S. Hilibrand reports grants from AOSpine North America during the conduct of the study, other from Amedica, Vertiflex, Benvenue, Lifespine, Paradigm Spine, PSD, Spinal Ventures, outside the submitted work, and in addition, Dr. Hilibrand has a patent Aesculap, Amedica, Biomet, Stryker, Alphatec, with royalties paid; David E. Fish reports grants from AOSpine North America during the conduct of the study; Jeffrey C. Wang reports grants from AOSpine North America during the conduct of the study; Elizabeth L. Lord reports grants from AOSpine North America during the conduct of the study; Zorica Buser reports grants from AOSpine North America during the conduct of the study; P. Justin Tortolani reports grants from AOSpine North America during the conduct of the study, other from Globus Medical, grants from Spineology, other from Innovasis, outside the submitted work, and in addition, Dr. Tortolani has a patent Globus with royalties paid; D. Alex Stroh reports grants from AOSpine North America during the conduct of the study; Ahmad Nassr reports grants from AOSpine North America during the conduct of the study; Bradford L. Currier reports grants from AOSpine North America during the conduct of the study, personal fees from DePuy Spine, personal fees from Stryker Spine, personal fees from Zimmer Spine, other from Zimmer Spine, other from Tenex, other from Spinology, other from LSRS, other from AOSNA, outside the submitted work; Arjun S. Sebastian reports grants from AOSpine North America during the conduct of the study; Michael G. Fehlings reports grants from AOSpine North America during the conduct of the study; Thomas E. Mroz reports other from AOSpine, grants from AOSpine North America during the conduct of the study, personal fees from Stryker, personal fees from Ceramtec, other from Pearl Diver, outside the submitted work; K. Daniel Riew reports personal fees from AOSpine International, other from Global Spine Journal, other from Spine Journal, other from Neurosurgery, personal fees from Multiple Entities for defense, plantiff, grants from AOSpine, grants from Cerapedics, grants from Medtronic, personal fees from AOSpine, personal fees from NASS, personal fees from Biomet, personal fees from Medtronic, nonfinancial support from Broadwater, outside the submitted workPaul M. Arnold reports grants from AOSpine North America during the conduct of the study; other from Z-Plasty, other from Medtronic Sofamore Danek, other from Stryker Spine, other from FzioMed, other from AOSpine North America, other from Life Spine, other from Integra Life, other from Spine Wave, other from MIEMS, other from Cerapedics, other from AOSpine North America, outside the submitted work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by AOSpine North America Inc, a 501(c)3 nonprofit corporation.
References
- 1. Flynn TB. Neurologic complications of anterior cervical interbody fusion. Spine (Phila Pa 1976). 1982;7:536–539. [DOI] [PubMed] [Google Scholar]
- 2. Lee JY, Hilibrand AS, Lim MR, et al. Characterization of neurophysiologic alerts during anterior cervical spine surgery. Spine (Phila Pa 1976). 2006;31:1916–1922. [DOI] [PubMed] [Google Scholar]
- 3. Daniels AH, Riew KD, Yoo JU, et al. Adverse events associated with anterior cervical spine surgery. J Am Acad Orthop Surg. 2008;16:729–738. [DOI] [PubMed] [Google Scholar]
- 4. Baker R, Dreyfuss P, Mercer S, Bogduk N. Cervical transforaminal injection of corticosteroids into a radicular artery: a possible mechanism for spinal cord injury. Pain. 2003;103:211–215. [DOI] [PubMed] [Google Scholar]
- 5. Graham JJ. Complications of cervical spine surgery. A five-year report on a survey of the membership of the Cervical Spine Research Society by the Morbidity and Mortality Committee. Spine (Phila Pa 1976). 1989;14:1046–1050. [PubMed] [Google Scholar]
- 6. Muckart DJ, Bhagwanjee S, van der Merwe R. Spinal cord injury as a result of endotracheal intubation in patients with undiagnosed cervical spine fractures. Anesthesiology. 1997;87:418–420. [DOI] [PubMed] [Google Scholar]
- 7. Rhee KJ, Green W, Holcroft JW, Mangili JA. Oral intubation in the multiply injured patient: the risk of exacerbating spinal cord damage. Ann Emerg Med. 1990;19:511–514. [DOI] [PubMed] [Google Scholar]
- 8. Etame AB, Wang AC, Than KD, La Marca F, Park P. Outcomes after surgery for cervical spine deformity: review of the literature. Neurosurg Focus. 2010;28(3):E14. [DOI] [PubMed] [Google Scholar]
- 9. Vitale MG, Moore DW, Matsumoto H, et al. Risk factors for spinal cord injury during surgery for spinal deformity. J Bone Joint Surg Am. 2010;92:64–71. [DOI] [PubMed] [Google Scholar]
- 10. Vitale MG, Skaggs DL, Pace GI, et al. Best practices in intraoperative neuromonitoring in spine deformity surgery: development of an intraoperative checklist to optimize response. Spine Deform. 2014;2:333–339. [DOI] [PubMed] [Google Scholar]
- 11. MacEwen GD, Bunnell WP, Sriram K. Acute neurological complications in the treatment of scoliosis. A report of the Scoliosis Research Society. J Bone Joint Surg Am. 1975;57:404–408. [PubMed] [Google Scholar]
- 12. Schossberger P. Vasculature of the spinal cord: a review. I. Anatomy and physiology. Bull Los Angeles Neurol Soc. 1974;39:71–85. [PubMed] [Google Scholar]
- 13. Lips J, de Haan P, Bouma GJ, Holman R, van Dongen E, Kalkman CJ. Continuous monitoring of cerebrospinal fluid oxygen tension in relation to motor evoked potentials during spinal cord ischemia in pigs. Anesthesiology. 2005;102:340–345. [DOI] [PubMed] [Google Scholar]
- 14. Fujimaki Y, Kawahara N, Tomita K, Murakami H, Ueda Y. How many ligations of bilateral segmental arteries cause ischemic spinal cord dysfunction? An experimental study using a dog model. Spine (Phila Pa 1976). 2006;31:E781–E789. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz DM, Auerbach JD, Dormans JP, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89:2440–2449. [DOI] [PubMed] [Google Scholar]
- 16. Clark AJ, Ziewacz JE, Safaee M, et al. Intraoperative neuromonitoring with MEPs and prediction of postoperative neurological deficits in patients undergoing surgery for cervical and cervicothoracic myelopathy. Neurosurg Focus. 2013;35(1):E7. [DOI] [PubMed] [Google Scholar]
- 17. Ziewacz JE, Berven SH, Mummaneni VP, et al. The design, development, and implementation of a checklist for intraoperative neuromonitoring changes. Neurosurg Focus. 2012;33(5):E11. [DOI] [PubMed] [Google Scholar]
- 18. Coe JD, Arlet V, Donaldson W, et al. Complications in spinal fusion for adolescent idiopathic scoliosis in the new millennium. A report of the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976). 2006;31:345–349. [DOI] [PubMed] [Google Scholar]
- 19. Vale FL, Burns J, Jackson AB, Hadley MN. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87:239–246. [DOI] [PubMed] [Google Scholar]
- 20. Witiw CD, Fehlings MG. Acute spinal cord injury. J Spinal Disord Tech. 2015;28:202–210. [DOI] [PubMed] [Google Scholar]
- 21. Wong CS, Healy D, Canning C, Coffey JC, Boyle JR, Walsh SR. A systematic review of spinal cord injury and cerebrospinal fluid drainage after thoracic aortic endografting. J Vasc Surg. 2012;56:1438–1447. [DOI] [PubMed] [Google Scholar]