Abstract
Background:
The Balance Error Scoring System (BESS) shows that balance tends to recover within days after a concussion, whereas measures of the movement of the center of pressure (COP) show that balance deficits can persist up to 1 month after concussion. While approximately 30% of adolescents suffering concussion have functional consequences including balance deficits, evidence of the use of different balance assessments for concussion is limited within this population.
Purpose:
To compare performance on a series of balance assessments between adolescents with a diagnosed concussion at 1 month postinjury and noninjured control participants within the same age distribution.
Study Design:
Cross-sectional study; Level of evidence, 3.
Methods:
Thirty-three adolescents 1 month postconcussion and 33 control participants completed the BESS followed by two, 2-minute trials standing on a Nintendo Wii Balance Board (WBB), during which the COP under their feet was recorded using 2 testing protocols: (1) double-leg stance, eyes open (EO) and (2) double-leg stance, eyes closed (EC). Participants then completed a dual-task condition (DT) with eyes open combining a double-leg stance and a Stroop color and word test while standing on the WBB. Three commonly used COP variables, anterior-posterior (A/P) and mediolateral (M/L) velocity and 95% ellipse, were computed for each condition performed on the WBB.
Results:
Participants postconcussion swayed over a significantly larger ellipse area compared with the control group in the EO (P = .008), EC (P = .002), and DT (P = .003) conditions and also performed the DT condition with faster COP velocity in the M/L direction (P = .007). No significant group difference was identified for BESS total score.
Conclusion:
At 1 month postconcussion, participants continued to demonstrate balance deficits in COP control despite scoring similar to controls on the BESS. Simple COP measures of balance may identify subtle impairments not captured by the BESS.
Keywords: concussion, balance, adolescents, center of pressure, dual task
A concussion can result in a wide range of physical, cognitive, emotional, and sleep-related symptoms.15 While adolescents are among those most at risk for experiencing a concussion, most will recover from these acute symptoms within a couple of weeks.13 However, 30% of cases will experience symptoms that can last for months or even years postinjury.31 These persistent symptoms can include residual balance deficits, which may affect one’s ability to safely resume physical activity and further increase risk of reinjury.14,18
The Balance Error Scoring System (BESS) is the most commonly used balance assessment tool for concussion.9 The subject performing the test stands on 2 feet, 1 foot, and with feet in tandem for 20 seconds with eyes closed on firm and foam surfaces while an observer records errors such as opening the eyes, stepping, or stumbling.10 A modified version, with the firm surface only, is part of the third edition of the Sport Concussion Assessment Tool (SCAT3).15 Studies that have used the BESS with collegiate athletes who have sustained a concussion have shown that balance tends to recover within 3 to 5 days postinjury.10,24 However, the rapid recovery of balance identified with the BESS may simply be due the subjective scoring of the test. It has also been suggested that the same examiner score performance on the BESS during repeated testing due to the wide range of reliability reported for the BESS. Though this may be possible in a research environment, this is not a practical approach for sport and clinical settings.2
Objective assessments of balance are performed by measuring the movement of the center of pressure (COP) under the feet as an individual stands on a force plate. COP measures are considered to be the gold standard to evaluate static balance12 and may identify subtle changes not identified using subjective balance assessments.21 By incorporating COP measures, balance deficits have been identified in collegiate athletes who sustained concussions up to 15 days postinjury.27 Balance deficits have also been identified in a group of collegiate football players at the time that they obtained clearance to return to play at approximately 4 weeks postinjury.20 These periods of balance impairment extend beyond the 3- to 5-day recovery period typically identified with the BESS.
Although considered to the be the gold standard to assess static balance, the cost and lack of portability of force plates limits their widespread application in clinics and on sports sidelines. In contrast, the Nintendo Wii Balance Board (WBB) (Nintendo) is a low-cost and portable device instrumented with 4 pressure transducers from which the resultant movement of the COP can be computed. Studies that have compared COP values collected with a force plate and with a WBB have demonstrated that COP values recorded with a WBB are both valid and reliable.1,4,6,12,17 Recent studies have used the WBB to assess balance in children who have experienced a concussion.22,23
While objective measures including COP are valuable elements of a balance assessment, tasks and activities of everyday living including sports participation require the simultaneous integration of cognitive functions and balance abilities.3 The postural control system is not totally autonomous, and dual-task investigations strongly suggest that posture control and higher level cognition have common resource requirements. Therefore, completing conditions that involve balance only, such as standing on 2 feet with eyes open or closed, may not be sufficient to identify postural deficits associated with concussions. In a key study by Dorman et al,7 the movement of the COP was recorded while adolescents with concussion completed 2 simple balance conditions and 2 dual-task conditions (eg, completing a balance task while simultaneously completing a cognitive task). While the participants with concussion showed deficits on all 4 conditions within 10 days postinjury, at an average of 25 days postinjury, participants with concussion only showed deficits on dual-task conditions. This suggests that measures of balance recorded during dual tasks may identify more complex functional deficits reflecting the interaction between cognition and balance that would otherwise be overlooked by assessing balance alone.
Previous studies that have assessed balance in individuals postconcussion have included either COP measures from both single- and dual-task balance conditions7 or COP measures from single-task balance conditions and scores from the BESS.21 To date, no study has directly compared performance on the BESS and COP measures recorded during the performance of single- and dual-task balance assessments within the same group of individuals who have experienced a concussion. This makes it difficult to conclude whether one method is more sensitive than the others at identifying balance deficits after concussion.
The purpose of this study was to compare balance performance in adolescents diagnosed with a concussion at 1 month postinjury compared with a group of control participants within the same age distribution. We were specifically interested in scores on the BESS and COP measures recorded during 2 single-task balance conditions and a dual-task balance condition. It was hypothesized that there would be significant group differences in COP measures during the single-task balance conditions as well as during the dual-task balance condition but that there would be no significant group differences on the measures from the BESS.
Methods
Participants
Two groups of participants participated in this study. The first group, the concussion group, consisted of adolescents aged between 12 and 17 years who had been diagnosed with a concussion by a physician in the emergency department (ED) at a regional tertiary hospital. Participants were recruited through a larger study designed to derive a clinical prediction rule for postconcussion syndrome.30 Participants were diagnosed with a concussion if they met the criteria for concussion defined by the Zurich consensus statement,15 which includes a direct blow to the head, face, neck, or elsewhere on the body with an impulsive force transmitted to the head, resulting in 1 or more symptoms in 1 or more clinical domain (which may or may not have involved loss of consciousness):
Somatic symptoms (eg, headache, nausea, loss of balance, dizziness, sensitivity to light or noise, visual problems, and clumsiness)
Cognitive symptoms (eg, feeling like in a fog, difficulty concentrating or remembering, answering questions more slowly, and confused with directions/tasks)
Emotional/behavioral symptoms (eg, irritable, sad, nervous, and emotional lability)
Sleep disturbance (eg, sleeping more, fatigue, drowsiness, and insomnia)
Participants were excluded from the study if the ED Glasgow Coma Scale was less than 14, abnormalities were observed on neuroimaging (if performed), operative intervention or procedural sedation was required, or if they presented with multisystem injuries requiring admission.
The control group consisted of 33 noninjured adolescents within the same age distribution who reported no concussive symptoms and who had not suffered any head trauma within the past year. Participants in this group completed the protocol once. All participants and their parents provided written informed consent.
Experimental Protocol
Participants completed a series of balance conditions, during which they were given a rest of 30 to 60 seconds between each trial. Participants completed 2-minute trials with their feet shoulder width apart with eyes open (EO) and then with their eyes closed (EC) while standing on the WBB. Two-minute trials were chosen because the International Society for Posture and Gait Research recommends using trials of at least 30 seconds to obtain stable COP values.26 Participants also completed a single-leg condition with eyes open while standing on the WBB, but the data from this condition were removed from analysis since approximately one-third of participants in both the concussion (n = 11) and control (n = 9) groups were unable to successfully complete the trial. For the EO condition, participants were instructed to fixate on a dot on a board placed at eye level at a distance of 1 m and to focus on standing as still as possible. In the EC condition, participants fixated on the dot and then closed their eyes.
Participants completed a dual-task condition (DT) consisting of standing on the WBB with their feet shoulder width apart while simultaneously completing a Stroop color and word test.28 The Stroop color and word test was presented on a single board placed at eye level at a distance 1 m in front of the participant. The board contained a 10 × 10 matrix of the words “red,” “blue,” “yellow,” and “green” in a random order written in an incongruent ink color. Following the procedures of the classic Stroop test, the participants were instructed to name the color of the ink of each word as quickly and as accurately as possible, and the number of correct responses was recorded for each participant. Participants were also instructed to stand as still as possible while completing the DT condition.
Each participant then completed the BESS, as outlined by Guskiewicz el al.10 Participants completed six, 20-second balance conditions with their eyes closed, consisting of 3 stances on both firm and foam surfaces: double-leg stance with feet together, a single-leg stance on the nondominant leg, and a tandem stance with the nondominant leg in the back. The number of errors defined as moving the hands off the iliac crest(s); opening the eyes; stepping, stumbling, or falling; abducting or flexing the hip beyond 30°; lifting the foot or heel off the testing surface; and remaining out of the testing position for more than 5 seconds were recorded, with a maximum number of 10 errors per condition. A total BESS score was obtained by adding the number of errors during each of the 6 conditions.
Participants in the concussion group completed the validated Post-Concussion Symptoms Inventory (PCSI)25 at the time of ED presentation and at 1 month postinjury. The PCSI consists of a list of concussion-related symptoms for which participants are asked to rate their preinjury as well as postinjury degree of symptoms to calculate a direct index of difference of a patient’s perceived difference from their own norms. The 12-year old participants completed the 8- to 12-year-old age version (17 symptoms, 3-point graded scale) and participants aged 13 years or older completed the adolescent version (20 symptoms, 7-point graded scale).
Material and Data Processing
The WBB raw pressure data were recorded at a sampling frequency of 30 Hz. The WBB was connected to a laptop computer via a Bluetooth device. The raw pressure data were transformed to the COP using Matlab (The Mathworks Inc). The COP data were filtered using a second-order low-pass Butterworth filter with a cutoff frequency of 12 Hz. The velocity of the COP in the mediolateral (M/L) and anterior-posterior (A/P) directions and the COP 95% ellipse were calculated for each of the 3 balance tasks (EO, EC, DT) for each participant. The velocity was characterized as the mean absolute speed of the COP displacements and was calculated using the following equations8:
The 95% ellipse consisted of an ellipse that covered 95% of the participant’s COP trajectory and was calculated using the following equation8:
An example of an ellipse of a participant in the concussion group and a participant in the control group is shown in Figure 1.
Figure 1.
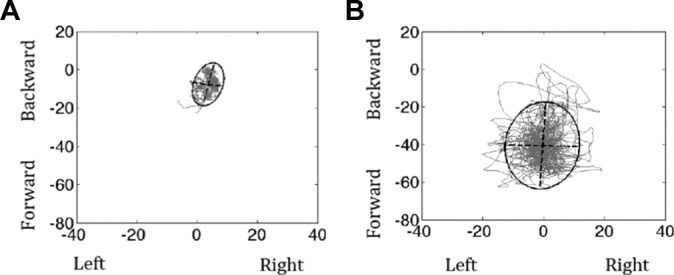
Example of a 95% ellipse for a participant in the (A) control group and (B) concussion group.
Statistical Analysis
Separate 2-way (condition × group) repeated-measures analyses of variance were used to determine differences between conditions (within-subjects factor) and groups (between-subjects factor) for each COP variable. Post hoc comparisons were performed using independent-samples t tests to identify the conditions for which there was a significant difference between the concussion and control groups. For the independent-samples t tests, the level of significance was adjusted to .017 to correct for multiple comparisons.
A Mann-Whitney U test was used to determine whether there was a significant difference for the number of correct responses on the Stroop color and word test between the concussion and control groups. A Mann-Whitney U test was also used to determine whether there was a significant difference for BESS total score between the concussion and control groups.
Finally, correlations between the 3 dependent variables with 95% CIs were calculated to determine whether any of the variables were correlated within each condition. All analyses were completed using IBM SPSS Statistics version 23 (IBM Corp).
Results
Thirty-three participants with concussion (mean age, 14.2 ± 1.5 years; 21 females, 12 males) completed the protocol between 28 and 40 days postinjury (mean, 32 ± 3 days). Thirty-three noninjured participants within the same age distribution (mean age, 15.0 ± 1.5 years; 24 females, 9 males) completed testing once. Baseline characteristics for participants in the concussion group are summarized in Table 1. The incidence for specific postconcussion symptoms at the time of the ED visit and at 1 month are summarized in Table 2.
TABLE 1.
Baseline Characteristics for Participants in the Concussion Groupa
| Characteristic | n (%) |
|---|---|
| Age, y, median (IQR) | 14.09 (13.36-16.05) |
| 8-12 | 2 (6.1) |
| 13-18 | 31 (93.9) |
| Female | 20 (60.6) |
| History | |
| Hours between ED visit and injury, median (IQR) | 15.15 (2.92 – 23.39) |
| Previous number of concussions | |
| 0 | 22 (66.7) |
| 1 | 8 (24.2) |
| 2 | 2 (6.1) |
| 3+ | 1 (3.0) |
| Longest symptom duration of previous concussion | |
| <1 wk | 6 (18.2) |
| 1-4 wk | 2 (6.1) |
| 5+ wk | 3 (9.1) |
| Migraine | 7 (21.2) |
| Learning disabilities | 3 (9.1) |
| ADD/ADHD | 3 (9.1) |
| Other developmental disorder | 3 (9.1) |
| Anxiety | 4 (12.1) |
| Depression | 2 (6.1) |
| Loss of consciousness | 4 (12.1) |
| Duration, min, mean (SD) | 0.30 (0.39) |
| Seizure | 1 (3.0) |
| Mechanism of injury | |
| Sports/recreational play | 22 (66.7) |
| Soccer | 4 (12.1) |
| Recreational play (gym, recess) | 4 (12.1) |
| Hockey | 3 (9.1) |
| Football | 1 (3.0) |
| Ski/snowboarding | 1 (3.0) |
| Bicycling | 1 (3.0) |
| Trampoline | 1 (3.0) |
| Nonsport-related injury/fall | 8 (24.2) |
| Motor vehicle collision | 1 (3.0) |
| Assault | 2 (6.1) |
| Helmet use | 5 (15.2) |
| Mouth guard use | 6 (18.2) |
aADD, attention deficit disorder; ADHD, attention deficit hyperactivity disorder; ED, emergency department; IQR, interquartile range.
TABLE 2.
Percentage of Participants in the Concussion Group Reporting Specific Symptoms at the Time of Injury and 1 Month Postinjury
| Symptom | Time of Injury, n (%) | 1 Month Postinjury, n (%) |
|---|---|---|
| Headache | 32 (96.7) | 16 (48.5) |
| Nausea | 25 (75.8) | 12 (36.4) |
| Balance problems | 26 (78.8) | 12 (36.4) |
| Dizziness | 30 (90.9) | 11 (33.3) |
| Fatigue | 32 (97.0) | 17 (51.5) |
| Drowsiness | 29 (87.9) | 11 (33.3) |
| Sensitivity to light | 28 (84.8) | 12 (36.4) |
| Sensitivity to noise | 20 (60.6) | 11 (33.3) |
| Irritability | 18 (54.5) | 7 (21.2) |
| Sadness | 11 (33.3) | 4 (12.1) |
| Nervousness | 12 (36.4) | 8 (24.2) |
| Feeling slowed down | 30 (90.9) | 10 (30.3) |
| Difficulty concentrating | 23 (69.7) | 11 (33.3) |
| Difficulty remembering | 15 (45.5) | 7 (21.2) |
| Visual problems | 19 (57.6) | 3 (9.09) |
Significant group effects were obtained for the 95% ellipse (F = 13.0, P = .001), A/P velocity (F = 5.83, P = .02), and M/L velocity (F = 9.67, P = .003). These significant group effects show that, regardless of the balance condition, the concussion group swayed over a larger area and also swayed faster in both A/P and M/L directions compared with the control group. Significant group × condition interactions were also obtained for the 95% ellipse (F = 5.74, P = .005) and M/L velocity (F = 4.27, P = .02) (Figure 2). For the 95% ellipse, post hoc comparisons showed that the participants in the concussion group swayed over a larger area compared with the control group for the EO (P = .008), EC (P = .002), and DT (P = .003) conditions (Figure 2A). For M/L velocity, post hoc comparisons showed that the concussion group completed the DT condition with greater M/L velocity compared with the control group (P = .007) (Figure 2B).
Figure 2.
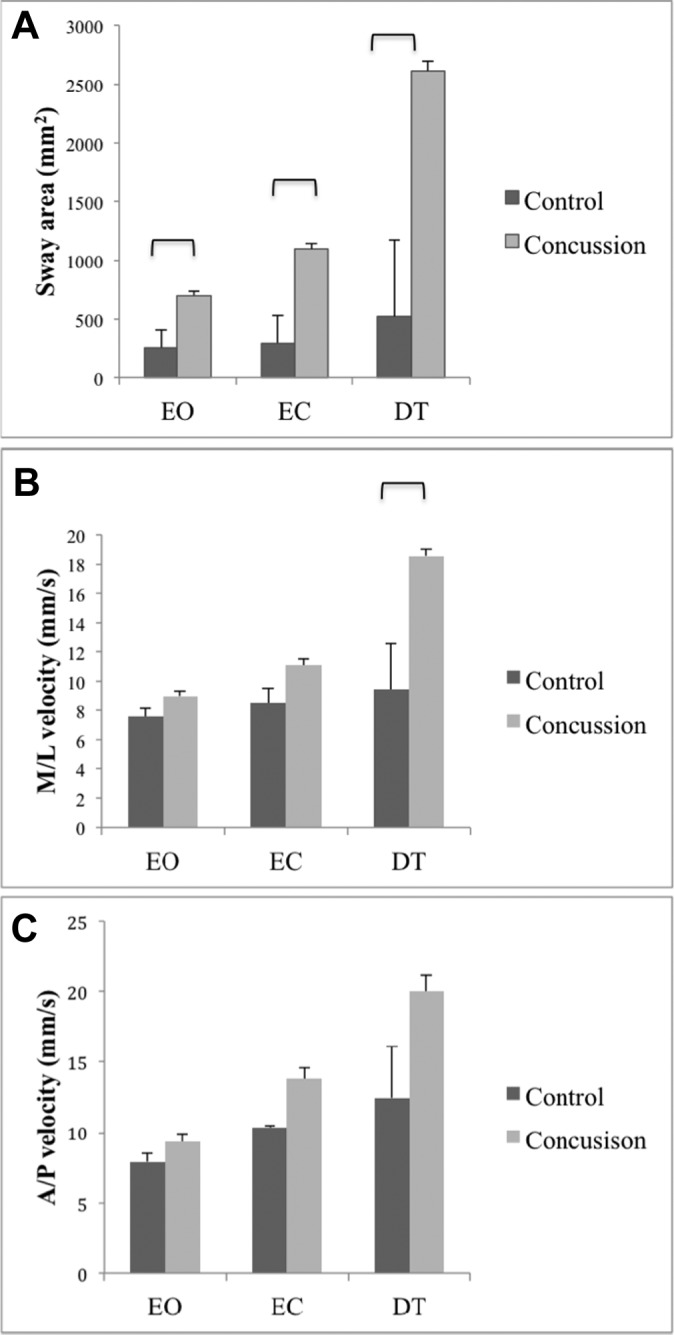
Data (mean + standard error) regarding the 3 balance conditions for the control and concussion groups: (A) 95% ellipse, (B) mediolateral (M/L) velocity, and (C) anterior-posterior (A/P) velocity. Significant differences (P < .017) between groups are identified with brackets. DT, dual-task; EC, eyes closed; EO, eyes open.
No significant group differences were obtained for any of the conditions for A/P velocity (Figure 2C), the number of correct responses on the Stroop color and word test (P = .5; data not shown), and BESS total score (P = 0.2). The median number of errors and the interquartile ranges for each condition of the BESS and BESS total score are shown in Table 3.
TABLE 3.
Median Number of Errors and Interquartile Ranges for Each Condition of the BESS and BESS Total Score for Control and Concussion Groupsa
| Double-Leg Firm | Tandem Firm | Single Firm | Double-Leg Foam | Tandem Foam | Single Foam | BESS Total | |
|---|---|---|---|---|---|---|---|
| Control | 0 (0-0) | 1 (0-1) | 2 (1-3.5) | 0 (0-0) | 2 (1-2.5) | 4 (2.5-6) | 8 (6-12.5) |
| Concussion | 0 (0-0) | 1 (0-2) | 2 (1-4) | 0 (0-0) | 3 (1-4) | 4 (3-7) | 11 (6.5-15) |
aBESS, Balance Error Scoring System.
Correlations between the dependent variables are shown in Table 4. The correlations between A/P velocity and the 95% ellipse were high for the EO (r = 0.81) and EC (r = 0.82) conditions but lower for the DT (r = 0.63) condition. Similar trends were obtained for the correlations between A/P and M/L velocity: correlations were high for the EO (r = 0.78) and EC (r = 0.90) conditions and again lower for the DT condition (r = 0.45). In contrast, the correlation between M/L velocity and the 95% ellipse was high for the DT condition (r = 0.87) and lower for the EC (r = 0.79) and EO conditions (r = 0.69). These results demonstrate that the participants’ 95% ellipses were related to A/P sway velocity for the EO and EC conditions but related to M/L velocity for the DT condition.
TABLE 4.
Correlations Between All 3 Dependent Variables Within Each Condition Performed on the WBB With 95% CIsa
| Eyes Open | Eyes Closed | Dual Task | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Lower 95% | Upper 95% | Estimate | Lower 95% | Upper 95% | Estimate | Lower 95% | Upper 95% | |
| 95% ellipse and A/P velocity | 0.81 | 0.70 | 0.88 | 0.82 | 0.72 | 0.88 | 0.63 | 0.46 | 0.76 |
| 95% ellipse and M/L velocity | 0.69 | 0.54 | 0.79 | 0.79 | 0.68 | 0.87 | 0.87 | 0.80 | 0.92 |
| A/P velocity and M/L velocity | 0.78 | 0.67 | 0.86 | 0.90 | 0.84 | 0.94 | 0.45 | 0.23 | 0.62 |
aA/P, anterior-posterior; M/L, mediolateral; WBB, Nintendo Wii Balance Board.
Discussion
In this study, we compared balance characteristics between adolescents at 1 month postconcussion and control participants within the same age distribution. The concussed group demonstrated increased area of sway compared with the control group while standing on 2 feet with eyes open, eyes closed, and while completing the dual-task condition. Participants with concussion also completed the dual-task condition with greater M/L velocity compared with control participants. Both groups made a similar number of errors on the Stroop color and word test. Therefore, the differences in balance characteristics seen in the concussed participants are likely due to impaired balance rather than to prioritizing the cognitive task. In contrast to COP measures, the BESS was not sensitive in detecting residual balance deficits in the concussed adolescents as both groups performed similarly.
To our knowledge, this is the first study to have measured both COP measures during single- and dual-task static balance conditions and scores from the BESS in the same group of concussed individuals and to have compared their performance with a group of control participants. Previous studies that have used only the BESS have identified that balance recovers within 3 to 5 days postconcussion,10,24 and although it is likely that a subset of these individuals would have continued to show balance deficits based on COP measures, this assumption could not be made without using both methods to assess balance in the same group of individuals. This study also differs from previous studies that have measured the COP in individuals who have experienced a concussion in the sense that the COP was recorded using a WBB as opposed to a standard force plate. Since the WBB is a low-cost and portable device, it would be much easier to implement the protocol used in this study in clinical and sport settings.
Dorman et al7 conducted a study for which 18 adolescents with concussion and 26 adolescents without concussion completed 4 conditions during which the movement of their COP was recorded. The conditions included a double-leg stance performed with eyes open and with eyes closed and a dual-task performed with eyes open and eyes closed that involved maintaining balance in a double-leg stance while simultaneously reciting the months of the year in reverse. Two COP variables were measured: 95% ellipse and the velocity combining both A/P and M/L directions into 1 resultant measure. During the first session, which took place within 10 days postinjury, participants with concussion showed significant differences compared with control participants for both variables on all 4 conditions. However, during the second session, which took place at an average 15 days after the first session, the concussed group had significantly greater 95% ellipses than the control group in the 2 DT conditions only. In contrast to the study by Dorman et al,7 the current study also showed significant group differences for the 95% ellipse for the double-leg stance with eyes open and eyes closed at approximately 1 month postinjury. These inconsistencies may be due to differences in the duration of the trials. In the current study, the COP was recorded for a total of 2 minutes for the EO and EC conditions, whereas in Dorman’s study, the COP was recorded for 20 seconds.7 The International Society for Posture and Gait Research recommends using trials of at least 30 seconds to obtain stable COP values.26
Unexpected observations were obtained while some participants in the concussion group performed the DT condition. Specifically, we observed that a subgroup of participants appeared to stabilize their head on their trunk and to shift their body from side to side while completing this condition. This side-to-side motion is also mirrored in the COP data since the correlations demonstrated that the participants’ 95% ellipses were related to A/P sway velocity for the EO and EC conditions and related to M/L velocity for the DT condition. This inverse relationship obtained for the DT condition could be due to the participants shifting their body from side to side while completing this condition. It is possible that certain participants in the concussion group adopted this type of motion while completing the DT condition due to difficulties with eye tracking. The board used for the Stroop color and word test was 50 cm wide and therefore required that the participant track the words in each row laterally. Studies have shown that when individuals with concussion are instructed to change their point of gaze toward a new target position, they tend to undershoot the position of the new target5,11 and also perform a greater number of saccades than necessary to successfully shift their point of gaze toward the new target position.29 Thus, it is possible that some of the participants in the concussion group struggled to track the words of the Stoop color and word test with their eye movements, forcing them to shift their body from side to side to successfully track the words. These observations require further examination by measuring both saccadic eye movement parameters and COP parameters while participants with concussion complete the DT condition.
One limitation of this study is that not all participants in the concussion group were tested within the same number of days postinjury. Since the purpose of this study was to compare balance characteristics between adolescents at 1 month postconcussion and noninjured control participants, it was essential that each participant in the concussion group complete the protocol as close as possible to 28 days postinjury. However, due to time constraints, some participants completed the protocol after 28 days postinjury. Yet, to keep the sample of participants as uniform as possible with regard to the time at which they completed the protocol, we ensured that all participants completed the protocol by 40 days postinjury at the latest. A second limitation is that no preinjury data were obtained for participants in the concussion group. Although it is possible that some participants in the concussion group performed poorly on the balance tasks due to preexisting balance issues related to factors other than their head injury, including control participants within the same age distribution suggests this was not the case.
The results from this study clearly highlight the importance of assessing balance with more objective measures since the BESS failed to differentiate balance performance between the concussion and control groups whereas measures of the COP identified several significant differences between the 2 groups. The BESS involves 20-second trials, and it is possible that these trials are not long enough to identify balance impairments in individuals who have experienced a concussion. However, the BESS may also only be sensitive enough to identify balance deficits at the time of injury when individuals show more severe balance impairments. Yet, as individuals recover and show more subtle deficits, the BESS no longer identifies these deficits. Although no studies have explicitly shown that subtle balance deficits increase the risk for reinjury in individuals who have experienced a concussion, studies have shown that increased COP displacement is associated with an increased risk for falls in older adults19 and associated with a risk for ankle sprains in young basketball players.16 As a result, it is likely that subtle balance deficits could also lead to functional consequences in adolescents who have suffered a concussion and could place them at risk for a second concussion if not fully recovered.
Conclusion
We demonstrated that adolescents continued to show balance deficits for both easy (EO, EC) and more difficult (DT) balance tasks at 1 month postconcussion despite scoring similar to control participants on the BESS. Measures of the COP recorded with a WBB may identify subtle postural impairments not captured by the BESS. Future research should focus on developing objective and sensitive balance assessments for concussion that can be used in clinics and on the sidelines in sport.
Acknowledgment
The authors would like to thank the parents and children for their participation. They would also like to thank all undergraduate student volunteers for their help with data collection.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: Funding for the parent 5P study was provided by the Canadian Institutes of Health Research (CIHR) Operating Grant (126197), CIHR-Ontario Neurotrauma Foundation Mild Traumatic Brain Injury Team Grant (127047), and CIHR planning grant (119829).
Ethical approval for this study was obtained from the Children’s Hospital of Eastern Ontario Research Ethics Board.
References
- 1. Bartlett HL, Ting LH, Bingham JT. Accuracy of force and center of pressure measures of the Wii balance board. Gait Posture. 2014;39:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bell DR, Guskiewicz KM, Clark MA, Padua DA. Systematic review of the Balance Error Scoring System. Sports Health. 2011;3:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Broglio SP, Tomporowski PD, Ferrara MS. Balance performance with a cognitive task: a dual-task testing paradigm. Med Sci Sports Exerc. 2005;37:689–695. [DOI] [PubMed] [Google Scholar]
- 4. Chang JO, Levy SS, Seay SW, Goble DJ. An alternative to the Balance Error Scoring System: using a low-cost balance board to improve the validity/reliability of sports-related concussion balance testing. Clin J Sport Med. 2014;24:256–262. [DOI] [PubMed] [Google Scholar]
- 5. Cifu DX, Wares JR, Hoke KW, Wetzel PA, Gitchel G, Carne W. Differential eye movements in mild traumatic brain injury versus normal controls. J Head Trauma Rehabil. 2015;30:21–28. [DOI] [PubMed] [Google Scholar]
- 6. Clark RA, Bryant AL, Pua Y, McCrory P, Bennell K, Hunt M. Validity and reliability of the Nintendo Wii balance board for assessment of standing balance. Gait Posture. 2010;31:307–310. [DOI] [PubMed] [Google Scholar]
- 7. Dorman JC, Valentine VD, Munce TA, Tjarks BJ, Thompson PA, Bergeron MF. Tracking postural stability of young concussion patients using dual-task interference. J Sci Med Sport. 2015;18:2–7. [DOI] [PubMed] [Google Scholar]
- 8. Duarte M, Freitas SM. Revision of posturography based on force plate for balance evaluation. Braz J Phys Ther. 2010;14:183–192. [PubMed] [Google Scholar]
- 9. Furman GR, Lin CC, Bellanca JL, Marchetti GF, Collins MW, Whitney SL. Comparison of the balance accelerometer measure and Balance Error Scoring System in adolescent concussions in sports. Am J Sports Med. 2013;41:1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36:263–273. [PMC free article] [PubMed] [Google Scholar]
- 11. Heitger MH, Anderson TJ, Jones RD. Saccade sequences as markers for cerebral dysfunction following mild closed head injury. Prog Brain Res. 2002;140:433–448. [DOI] [PubMed] [Google Scholar]
- 12. Huurnink A, Fransz DP, Kingma I, van Dieen JH. Comparison of a laboratory grade force platform with a Nintendo Wii balance board on measurement of postural control in single-leg stance balance tasks. J Biomech. 2013;46:1392–1395. [DOI] [PubMed] [Google Scholar]
- 13. Kirkwood MW, Yeates KO, Wilson PE. Pediatric sport-related concussion: a review of the clinical management of an oft-neglected population. Pediatrics. 2006;117:1359–1371. [DOI] [PubMed] [Google Scholar]
- 14. Lynal RC, Mauntel TC, Padua DA, Mihalik JP. Acute lower extremity injury rates increase after concussion in college athletes. Med Sci Sports Exerc. 2015;47:2487–2492. [DOI] [PubMed] [Google Scholar]
- 15. McCrory P, Meeuwisse W, Aubry M, et al. Consensus statement on concussion in sport—the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Phys Ther Sport. 2013;14:e1–e13. [DOI] [PubMed] [Google Scholar]
- 16. McGuine TA, Greene JJ, Best T, Leverson G. Balance as a predictor of ankle injuries in high school basketball players. Clin J Sport Med. 2000;10:239–244. [DOI] [PubMed] [Google Scholar]
- 17. Pavan P, Cardaioli M, Ferri I, Gobbi E, Carraro A. A contribution to the validation of the Wii balance board for the assessment of standing balance. Eur J Sport Sci. 2015;15(7):1–6. [DOI] [PubMed] [Google Scholar]
- 18. Pietrosimone B, Golightly YM, Mihalik JP, Guskiewicz KM. Concussion frequency associates with musculoskeletal injury in retired NFL players. Med Sci Sports Exerc. 2015;47:2366–2372. [DOI] [PubMed] [Google Scholar]
- 19. Piirtola M, Era P. Force platform measurements as predictors of falls among older people—a review. Gerontology. 2006;52:1–16. [DOI] [PubMed] [Google Scholar]
- 20. Powers KC, Kalmar JM, Cinelli ME. Recovery of static stability following a concussion. Gait Posture. 2014;39:611–614. [DOI] [PubMed] [Google Scholar]
- 21. Quatman-Yates CC, Bonnette S, Hugentobler JA, et al. Postconcussion postural sway variability changes in youth: the benefit of structural variability analyses. Pediatr Phys Ther. 2015;27:316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rhine T, Quatman-Yates C, Clark RA. A longitudinal examination of postural impairments in children with mild traumatic brain injury: implications for acute testing [published online November 17, 2015]. J Head Trauma Rehabil. doi:10.1097/HTR.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 23. Rhine TD, Byczkowski TL, Clark RA, Babcock L. Investigating the feasibility and utility of bedside balance technology acutely after pediatric concussion: a pilot study. Clin J Sport Med. 2016;26:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35:19–25. [PMC free article] [PubMed] [Google Scholar]
- 25. Sady MD, Vaughan CG, Gioia GA. Psychometric characteristics of the postconcussion symptom inventory in children and adolescents. Arch Clin Neuropsychol. 2014;20:348–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scoppa F, Capra R, Gallamini M, Shiffer R. Clinical stabilometry standardization: basic definitions–acquisition interval–sampling frequency. Gait Posture. 2013;37:290–292. [DOI] [PubMed] [Google Scholar]
- 27. Slobounov S, Sebastianelli W, Hallett M. Residual brain dysfunction observed one year post-mild traumatic brain injury: combined EEG and balance study. Clin Neurophysiol. 2012;123:1755–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 29. Thiagarajan P, Ciuffreda KJ. Versional eye tracking in mild traumatic brain injury (mTBI): effects of oculomotor training (OMT). Brain Inj. 2014;28:930–943. [DOI] [PubMed] [Google Scholar]
- 30. Zemek R, Barrowman N, Freedman SB, et al. Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA. 2016;315:1014–1025. [DOI] [PubMed] [Google Scholar]
- 31. Zemek RL, Farion KJ, Sampson M, McGahern C. Prognosticators of persistent symptoms following pediatric concussion: a systematic review. JAMA Pediatr. 2013;167:259–265. [DOI] [PubMed] [Google Scholar]


