Abstract
Background
Fecal testing can only reduce colorectal cancer mortality if patients with an abnormal test result receive a follow-up colonoscopy. As part of the Strategies and Opportunities to STOP Colon Cancer in Priority Populations (STOP CRC) project, we examined factors associated with adherence to follow-up colonoscopy among patients with abnormal fecal test results.
Methods
As part of STOP CRC outreach, Virginia Garcia Memorial Health Center staff distributed 1,753 fecal immunochemical tests (FIT), of which 677 (39%) were completed, and 56 had an abnormal result (8%). Project staff used logistic regression analyses to examine factors associated with colonoscopy referral and completion.
Results
Of the 56 patients with abnormal FIT results; 45 (80%) had evidence of a referral for colonoscopy, 32 (57%) had evidence of a completed colonoscopy within 18 months, and 14 (25%) within 60 days of an abnormal fecal test result. In adjusted analysis, Hispanics had lower odds of completing follow-up colonoscopy within 60 days than non-Hispanic whites (adjusted OR = 0.20; 95% CI: 0.04, 0.92). Colonoscopy within 60 days trended lower for women than for men (adjusted OR = 0.25; 95% CI 0.06 – 1.04). Among the 24 patients lacking medical record evidence of a colonoscopy, 19 (79%) had a documented reason, including clinician did not pursue, patient refused, and colonoscopy not indicated. No reason was found for 21%.
Conclusion
Improvements are needed to increase rates of follow-up colonoscopy completion, especially among female and Hispanic patients.
Keywords: Colorectal cancer, Federally Qualified Health Center, Hispanic / Latino, Colonoscopy, Gastroenterology referral
Precis
Fecal testing can only reduce colorectal cancer mortality if patients with an abnormal test result receive a follow-up colonoscopy. We found lower follow-up colonoscopy adherence among Hispanics (compared to non-Hispanic whites) and women (compared to men).
Introduction
The United States Services Preventive Services Task Force (USPSTF) recommends that individuals between ages 50 and 75 undergo CRC screening with high-sensitivity guaiac fecal occult blood testing (FOBT) annually, sigmoidoscopy every 5 years with high-sensitivity FOBT every 3 years, or colonoscopy every 10 years [1]. In 2012, approximately 65% of the US adult population was up to date with USPTSF CRC screening recommendations [2].
In the 2012 Behavioral Risk Factor Surveillance System (BRFSS) survey, 66.4% of Non-Hispanics versus 53.1% of Hispanics were up to date with CRC screening. Forty-one percent of Hispanic respondents reported never being screened for CRC versus 26.3% of Non-Hispanic respondents [2]. Wang et al. [3] attribute lower CRC screening rates in minority populations to low literacy, influence of social groups, fatalism, and decreased confidence in health care providers. Cross-sectional studies also indicate that reduced language barriers, older age, discussion of CRC risk factors with a physician, not smoking, and encouraging family members or friends to be tested for CRC increase CRC screening rates in minority populations such as Hispanics [4, 5].
Fecal immunochemical tests (FIT) are a type of FOBT that use labeled antibodies to detect the globin protein of hemoglobin in stool. Another type is the guaiac FOBT (gFOBT), which detects peroxidase-like activity in stool; however, the FIT is considered superior because it can detect more advanced neoplasms and proximal colon lesions. Some FITs require only one stool sample with no dietary and medication restrictions, whereas the gFOBT requires three samples plus dietary and medication restrictions. In addition, the FIT is more specific for detecting lower GI bleeds than the gFOBT [6]. In a systematic review and meta-analysis conducted by Hernandez et al. [7], the calculated pooled sensitivity and specificity of FITs across 19 studies was 0.79 (95% CI: 0.69, 0.86) and 0.94 (95% CI: 0.92, 0.95), respectively. Compared to other CRC screening modalities such as colonoscopy or sigmoidoscopy, FITs are inexpensive, non-invasive, and convenient to use [6]. However, unlike colonoscopy or sigmoidoscopy, the FIT must be repeated yearly.
For the FIT to be effective, individuals with positive results must undergo timely follow-up colonoscopies. A large body of research has focused on understanding adherence and timeliness of follow-up colonoscopy in the VA population and in national CRC screening programs [8–13]. Research demonstrating racial, ethnic, and socioeconomic disparities in CRC screening, morbidity, and mortality highlight the need to understand factors associated with adherence to follow-up colonoscopy in specific populations and settings, including minority groups and patients served by community health centers.
The Strategies and Opportunities to STOP Colon Cancer in Priority Populations (STOP CRC) project, funded by the National Cancer Institute, is testing an intervention to improve rates of CRC screening in community health centers. The purpose of the current study was to understand the patient and clinical factors associated with adherence to follow-up colonoscopy after a positive FIT in community health center populations; the current study was part of the larger STOP CRC study that also explored patients’ CRC screening experiences.
We hypothesized that 1) adherence to follow-up colonoscopy after a positive FIT would vary by specific patient characteristics (i.e., age, gender, ethnicity, race, language, insurance status, and co-morbidities), 2) time to referral for colonoscopy after a positive fecal test result would be longer among Hispanics than Non-Hispanics, and 3) time to referral would mediate the associations between predictor variables and time to colonoscopy.
Methods
The cohort for this study came from the Virginia Garcia Memorial Health Center (VGMHC), a federally qualified health center that serves approximately 36,000 patients annually in Oregon’s Washington and Yamhill counties. Sixty percent of VGMHC patients are Hispanic, 57% live in poverty, and 80% live below 200% of the federal poverty level. Uninsured patients comprise about 25% of patients, while Medicare, Medicaid, and individual/private subscribers represent about 5%, 62%, and 7% of patients, respectively [14].
Recruitment
There were two main recruitment phases, both involving VGMHC patients at the Cornelius and Hillsboro clinics for whom FIT kits (InSure FIT ®) were ordered between September 2013 and October 2014. The first phase was part of STOP CRC. FIT kits were mailed to eligible participants ages 50–74 who were identified through an electronic health record registry. Participants then used pre-paid envelopes to mail their completed FITs to a laboratory for analysis. The second phase was launched when VGMHC attained additional grant funding to increase CRC screening. Eligible patients were offered FIT kits in clinic and counseled on the importance of CRC screening by either a medical assistant or provider. Interested patients were given the FIT kit along with instructions on submitting their stool sample for analysis. All study procedures were reviewed and approved by the institutional review board at Kaiser Permanente Northwest.
Data Collection
Retrospective chart reviews were conducted to ascertain demographic information, status of FIT results and colonoscopy adherence, date of colonoscopy, referral information, and pathology results. Chart reviews were conducted by three individuals. A senior data analyst at VGMHC completed chart abstractions for participants who received FIT kits as part of the STOP CRC program. A research intern completed chart reviews for individuals who received FIT kits in clinic. A physician at VGMC completed further chart abstractions as needed to obtain other information (e.g., pathology reports and referral information).
A FIT result was considered positive based on the manufacturer’s guidelines for the detection of hemoglobin in stool. Time to colonoscopy (TTC) was calculated as the time elapsed between the date of a completed FIT kit and the date of the follow-up colonoscopy procedure. Likewise, time to referral (TTR) was calculated as the time elapsed between the date of a completed FIT kit and the date the referral was made. Complete colonoscopy adherence was defined as having received a colonoscopy within our study period of 18 months. We also considered colonoscopy adherence within 60 days, selecting that time period for two reasons: 1) Several previous studies, especially those involving the VA medical system, used 60 days as the standard time to complete follow-up colonoscopy, and 2) the median time to follow-up colonoscopy in our study was 62 days.
We worked with an experienced clinician to risk-stratify patients in terms of colorectal malignancy based on colonoscopy findings and pathological diagnoses. We used a modified pathological classification scheme presented by Lieberman et al. [15] to create the risk categories based on the presence/absence of polyps, type of polyps (hyperplastic, tubular adenomas, villous adenomas, sessile serrated), and polyp size.
Statistical Analysis
All analyses were conducted using STATA version 13 for Windows (StataCorp, College Station, TX). The two primary patient outcomes of interest were 1) overall adherence to follow-up colonoscopy after a positive FIT result and 2) adherence to follow-up colonoscopy within 60 days of a positive FIT result. Our secondary outcome of interest was receipt of referral for colonoscopy. We additionally examined whether differences in adherence to follow-up colonoscopy were mediated by differences in referral.
Descriptive statistics were computed for age, gender, ethnicity, race, language, insurance type, clinic, and comorbidities. We collapsed age into three ordinal categories. We also calculated time to referral (TTR) and time to colonoscopy (TTC) within days.
We used Fisher’s exact text to assess the association between each predictor variable and each primary dichotomous outcome. We used logistic regression models to assess the association between patient characteristics and adherence to colonoscopy. Unadjusted and adjusted analyses were performed; the former included each variable individually, and the latter included potential confounders. Based on an extensive review of the literature, potential confounders included age, race, ethnicity, sex, and insurance. All associations with p < 0.05 were considered statistically significant.
We tested TTR as a potential mediator (predictor variable → TTR → TTC) using methods developed by Baron and Kenny [16]. We followed the procedures by Kleinbaum et al. [17] to complete simple linear regression using the least-squares method. Because TTC and TTR were not normally distributed, we log transformed these variables. After transformation, all assumptions of linear regression were met.
Results
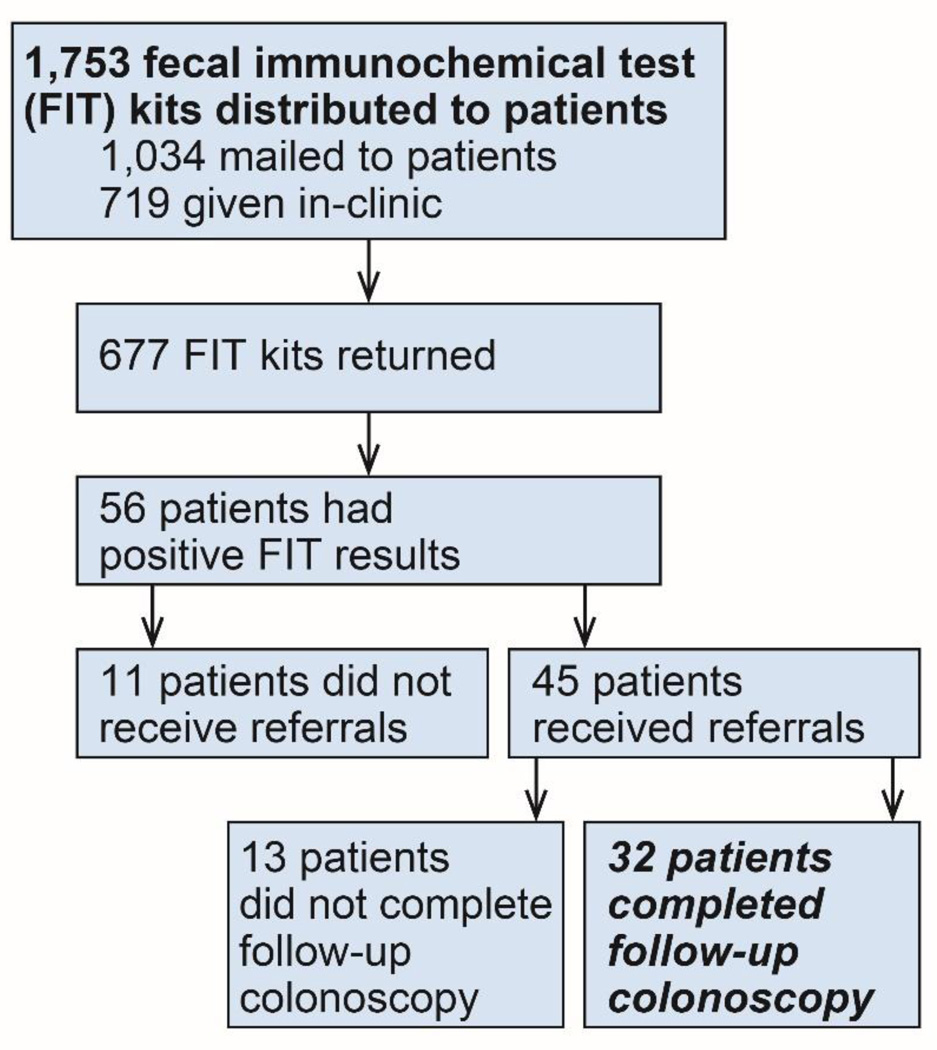
Between September 2013 and October 2014, there were 56 positive FIT results among patients 50–75 years old, half each at VGMHC’s Cornelius and Hillsboro clinics (Figure 1). Over half of these patients were female (55%) or white (91%), and 52% of the patients self-identified as being of Hispanic origin (Table 1). Over one-half of these patients received public insurance (21% Medicaid; 34% Medicare); others were either uninsured (30%) or had employer-sponsored insurance (17%). Of the 56 individuals with positive FIT results, 32 (57%) completed colonoscopy, 14 of them (44%) within 60 days.
Figure 1.
Flowchart of patients who were offered fecal immunochemical testing (FIT) and obtained a follow-up colonoscopy. Follow-up colonoscopy
Table 1.
Demographic and healthcare characteristics of patients with a positive fecal immunochemical test (FIT)
| Characteristic | N = 56 N (%) |
|---|---|
| Age (years) | |
| < 65 | 40 (71.4) |
| 65+ | 16 (28.6) |
| Gender | |
| Female | 31 (55.4) |
| Ethnicity | |
| Non-Hispanic/Unknown | 27 (48.2) |
| Hispanic | 29 (51.8) |
| Race | |
| White | 51 (91.1) |
| Non-White | 5 (8.9) |
| Language | |
| English/Other | 28 (50) |
| Spanish | 28 (50) |
| Insurance Status | |
| Medicaid | 12 (21.4) |
| Medicare | 19 (33.9) |
| Uninsured | 17 (30.4) |
| Other | 8 (14.3) |
| Primary clinic | |
| Clinic 1 | 28 (50.0) |
| Clinic 2 | 28 (50.0) |
| Co-morbidities and behaviors | |
| Diabetes | 21 (37.5) |
| Hypertension | 33 (58.9) |
| Smoker (former or current) | 19 (33.9) |
| Colonoscopy referral and receipt | |
| Referred for colonoscopy | 45 (80.4) |
| Obtained colonoscopy within 18 months | 32 (57.1) |
| Obtained colonoscopy within 60 days | 14 (25.0) |
Patient Characteristics Associated with Timely Adherence
In unadjusted and adjusted calculations, age, gender, language, insurance status, and presence of co-morbidities (e.g. diabetes, hypertension, and smoking status) were not significantly associated with completion of follow-up colonoscopy at either 18 months or 60 days (Table 2). In adjusted calculations, Hispanics were less likely than non-Hispanics to complete follow-up colonoscopy within 60 days of an abnormal fecal test result (adjusted OR = 0.20; 95% CI: 0.04, 0.92). Similarly, Hispanic colonoscopy completion at 18 months was lower than for non-Hispanics, but the associations did not reach significance. There was also a non-significant trend suggesting that women were less likely than men to complete follow up colonoscopy within 60 days (adjusted OR = 0.25; 95% CI 0.06 – 1.04).
Table 2.
Associations between patient characteristics and follow-up colonoscopy completion (within 18 months & within 60 days)
| Completed Colonoscopy within 18 months (n = 32) | Completed Colonoscopy within 60 days (n = 14) | |||||
|---|---|---|---|---|---|---|
| Characteristic | Completed colonoscopy N (%) |
Unadjusted OR (95% CI) |
Adjusted ORa (95% CI) |
Completed colonoscopy N (%) |
Unadjusted OR (95% CI) |
Adjusted ORa (95% CI) |
| Age (years) | ||||||
| < 65 | 24 (60.0) | 10 (25.0) | ||||
| 65+ | 8 (50.0) | 0.87 (0.21–2.14) | 0.85 (0.22–3.37) | 4 (25.0) | 1.00 (0.26–3.82) | 0.94 (0.19–4.60) |
| Gender | ||||||
| Male Female |
14 (56.0) 18 (58.1) |
1.09 (0.38 – 3.15) | 0.88 (0.29 – 2.75) | 9 (37.0) 5 (17.2) |
0.34 (0.10–1.20) |
0.25 (0.06–1.04)b |
| Ethnicity | ||||||
| Non-Hispanic | 19 (70.4) | 10 (37.0) | ||||
| Hispanic | 13 (44.8) | 0.34 (0.11 – 1.03)b | 0.36 (0.11 – 1.25) | 4 (13.8) | 0.27 (0.07 – 1.01)b | 0.20 (0.04–0.92)c |
| Language | ||||||
| English/Other | 18 (64.3) | 10 (35.7) | ||||
| Spanish | 14 (50.0) | 0.56 (0.19 – 1.62) | 2.44 (0.21 –27.80) | 4 (14.3) | 0.30 (0.08 – 1.11) | 0.97 (0.04 – 21.23) |
| Insurance | ||||||
| No | 10 (58.8) | 2 (11.8) | ||||
| Yes | 22 (56.4) | 0.91 (0.29 –2.88) | 0.80 (0.20 – 3.22) | 12 (30.8) | 3.33 (0.66 – 16.92) | 2.66 (0.42-16.95) |
| Co-morbidities | ||||||
| Diabetes | ||||||
| No | 20 (57.1) | 11 (31.4) | ||||
| Yes | 12 (57.1) | 1.00 (0.34 – 2.98) | 1.03 (0.31 – 3.44) | 3 (14.3) | 0.36 (0.09 – 1.50) | 0.49 (0.10 – 2.44) |
| Hypertension | ||||||
| No | 11 (47.8) | 6 (26.1) | ||||
| Yes | 21 (63.6) | 1.91 (0.65 – 5.64) | 2.05 (0.65 – 6.43) | 8 (24.2) | 0.91 (0.27 – 3.09) | 0.97 (0.25 – 3.79) |
| Smoker | ||||||
| No | 21 (56.7) | 8 (21.6) | ||||
| Yes | 11 (57.8) | 1.05 (0.34 – 3.21) | 0.67 (0.18 – 2.39) | 6 (31.6) | 1.67 (0.48 – 5.81) | 0.90 (0.21 – 3.92) |
Adjusted for age, sex, race, ethnicity, insurance status;
P-value trends toward significance of 0.05 < 0.06;
significant at p < 0.05
Patient Ethnicity and Referral to Colonoscopy
In terms of referral for colonoscopy, 85% of Non-Hispanics (n = 23) and 76% of Hispanics (n = 22) received referrals (p = 0.45) (data not shown). Among patients who received referrals, the median time to referral was 1 day for Non-Hispanics and 3 days for Hispanics (p = 0.18; data not shown). In mediation analysis, TTR did not mediate the association between ethnicity and TTC.
Reasons for Lack of Adherence to Follow-up Colonoscopy
There are a variety of reasons, documented in VGMHC medical records, why 24 (48%) of the 56 FIT positive individuals did not adhere to follow-up colonoscopy (Table 3). Twenty-five percent of these patients either refused colonoscopy or cancelled their colonoscopy appointment. For 4 (17%) of the 24 individuals who did not adhere to follow-up colonoscopy, colonoscopy was not indicated because the procedure had been performed within 10 years prior to their positive FIT result. For 38% of non-adherent patients, charts indicated miscellaneous reasons that colonoscopy was not performed. For example, several patients were leaving the country for an extended period and therefore would not be available to complete a colonoscopy. Finally, for 5 (21%) of the 24 non-adhering patients, there were no documented reasons in the medical records for non-adherence.
Table 3.
Reasons for lack of follow-up colonoscopy within 18 months (N = 24)
| Reason | N (%) |
|---|---|
| Clinician documented reasons that complete colon evaluation was not pursued (e.g. hemorrhoids, medical complications, patient plans to obtain colonoscopy in Mexico) |
9 (37.5) |
| Patient refused colonoscopy or cancelled colonoscopy appointment | 6 (25.0) |
| No reason found in health record | 5 (20.8) |
| Colonoscopy not indicated (e.g. patient had a normal colonoscopy within 10 years) | 4 (16.7) |
Discussion
Compared with other CRC screening programs that used either FOBT or FIT as a primary CRC screening tool, our study’s follow-up colonoscopy adherence rate of 57% after a positive FIT result was moderate [12, 18–21]. Compared with community health centers that have used FITs, our follow-up colonoscopy adherence rate was lower than that found by Hillyer et al.[18] (84.2% over 4 years in northern Manhattan) and LoConte et al.[19] (91% over 2 years in Wisconsin). However, a similar adherence rate was found in a study by Levy et al.[20] (61% in Iowa; time frame not stated). Such comparisons suggest that CRC screening program evaluations with longer durations had higher rates of adherence to follow-up colonoscopy. Longer-running programs could create more efficient systems and processes that transition patients from positive FIT results to follow-up colonoscopy. For example, in the Loconte et al. study [19], adjustments such as increasing the number of interpreters were made after an initial evaluation of the program.
In the current study, patient-level factors such as age, language, insurance status, and comorbidities were not associated with adherence to follow-up colonoscopy within 60 days or with overall completion of colonoscopy after a positive FIT result (i.e. within 18 months). Forty-five percent of Hispanic, compared to 70% of non-Hispanic whites, completed a colonoscopy within 18 months. However, the association between ethnicity and colonoscopy adherence only reached significance for colonoscopy completion within 60 days (14% vs. 37%, p<0.05). Similar studies have found no association between patient-level factors such as age or comorbidity and follow-up colonoscopy completion [21–25]. However, in studies by Choi et al.[26] in Korea and Morris et al.[27] in England, insurance status and socioeconomic factors were associated with follow-up colonoscopy among those with positive FOBT results. We did not see a statistically significant association between insurance status and colonoscopy adherence, possibly because implementation of the Affordable Care Act has increased access to health insurance and care for many underserved individuals in Oregon, and occurred right at the time of this study. In addition, VGMHC can refer patients to specialty providers through the Project Access NOW program, which connects low-income and uninsured individuals in the Portland metropolitan area with donated health care services. Further investigation of this relationship in a larger patient sample is warranted.
Moreover, in our study, 37% of men and 17% of women obtained a colonoscopy within 60 days (37% vs. 17%), but the association did not reach significance. In the study by Ferrat et al.,[23] gender was not associated (p = 0.41) with colonoscopy completion within 58 days of a positive FOBT result. Fisher et al. [25] and Dupont-Lucas et al. [22] also found no association between gender and colonoscopy completion within 1 year and 2 years of a positive FOBT, respectively. However, Miglorietti et al. [28] did find an association between gender and follow-up colonoscopy within 1 year of a positive FOBT, with higher rates observed among men.
Using the Health Belief Model, potential explanations for these findings are that women: 1) initially may be more concerned with other forms of cancer like breast cancer but over time their concern spans all types of cancers, and 2) may be more concerned and have more responsibilities regarding the well-being of other household members, therefore placing less importance on getting the follow-up colonoscopy. Also, because the incidence of CRC and CRC-related mortality is higher in men, health care providers may encourage men more often than women to complete follow-up colonoscopy.
Another important point to note is that 4 out of the 24 patients who did not adhere to colonoscopy, had obtained a colonoscopy and received a normal finding within the 10 years prior to completing their FIT kit. Because these individuals received FIT kits, it suggests that improvement might be made to the documentation of colonoscopy in the electronic health record and selection of patients for screening with FIT.
In addition to examining the association between patient-level factors and adherence to colonoscopy, we examined the relationship between time to referral and time to colonoscopy. Through mediation analysis, our results indicate that time to referral is not a mediator in the pathway from patient-level factors to timeliness of colonoscopy. A potential explanation for this observation is that VGMHC uses an electronic health system that allows providers to easily input information and submit referrals in a timely fashion. Moreover, in nearly 80% of cases, a reason for lack of referral was documented in the chart (e.g. medical complication, hemorrhoids (37%), patient refusal (25%), and contraindications (17%).
Limitations
There are several limitations to this study. First, the small sample size led to a non-normal distribution of data, thus requiring the data to be transformed for linear regression analysis. Second, the study focused on two clinics in one geographic region; therefore, generalizability may be limited. We attempted to overcome shortcomings in this study by using objective markers to verify information (e.g., completion of colonoscopy) we obtained. Finally, there may have been incomplete ascertainment of colonoscopy completion if procedures were done outside the VGMHC system and not recorded in clinic records.
Implications for Public Health
Findings from this study have several implications for public health. First, underserved communities are receptive to using FIT kits as a CRC screening method. However, patients should be taught at the onset that CRC screening via FIT kits will involve several subsequent steps if the result is positive. One evidence-based approach to reducing time to resolution of abnormal cancer screening tests is culturally tailored patient navigation programs [29, 30]. Patient navigator may facilitate a seamless transition to gastroenterology facilities by offering tailored motivational messages, patient education, and resources to overcome barriers. Furthermore, specific public health interventions should focus on reducing barriers associated with follow-up colonoscopy. For example, in our study population, Spanish-speaking participants had more difficulty than did English-speaking participants in understanding written instructions and test results. Many Hispanic populations have an oral and pictorial tradition; thus, it may be useful to combine written instructions with pictorial or wordless instructions [31]. By the same token, patient-provider discussions and health education materials addressing fecal testing as first-line screening might emphasize the importance of obtaining a follow-up colonoscopy if the test result is abnormal.
In terms of clinical factors, public health interventions might focus on improving clinical information systems like electronic medical records. Clinical information systems in community health settings can be improved by designing 1) reminders that alert providers or screening program managers about patients who have yet to complete a follow-up colonoscopy within a defined amount of time after a positive FIT and 2) procedures to generate automatic phone calls and/or reminder emails and letters that can be sent to these patients.
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health through the National Center for Complementary &Alternative Medicine under Award Number UH2AT007782 and the National Cancer Institute under Award Number 4UH3CA188640-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary & Alternative Medicine or the National Cancer Institute. Dr. Saha’s contribution to this work was supported with resources and the use of facilities at the VA Portland Health Care System.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.US Preventive Services Task Force. Screening for Colorectal Cancer, Topic Page. 2008. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Awareness of prediabetes--United States, 2005–2010. MMWR Morb Mortal Wkly Rep. 2013;62(11):209–212. [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Moehring J, Stuhr S, Krug M. Barriers to colorectal cancer screening in Hispanics in the United States: an integrative review. Applied nursing research. 2013;26(4):218–224. doi: 10.1016/j.apnr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Otiniano ME, Wood RC, Poursani RS, Katerndahl DA, Siddiqui S, Nadeau MT. Association of knowledge, attitudes, and behaviors for colon cancer screening in Hispanic patients. Ethn Dis. 2013;23(3):343–348. [PubMed] [Google Scholar]
- 5.Johnson-Kozlow M, Roussos S, Rovniak L, Hovell M. Colorectal cancer test use among Californians of Mexican origin: influence of language barriers. Ethn Dis. 2009;19(3):315–322. [PMC free article] [PubMed] [Google Scholar]
- 6.Day LW, Bhuket T, Allison J. FIT testing: an overview. Current gastroenterology reports. 2013;15(11):357. doi: 10.1007/s11894-013-0357-x. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez V, Cubiella J, Gonzalez-Mao MC, Iglesias F, Rivera C, Iglesias MB, et al. Fecal immunochemical test accuracy in average-risk colorectal cancer screening. World J Gastroenterol. 2014;20(4):1038–1047. doi: 10.3748/wjg.v20.i4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh H, Petersen LA, Daci K, Collins C, Khan M, El-Serag HB. Reducing referral delays in colorectal cancer diagnosis: is it about how you ask? Qual Saf Health Care. 2010;19(5):e27. doi: 10.1136/qshc.2009.033712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell AA, Nugent S, Ordin DL, Noorbaloochi S, Partin MR. Evaluation of a VHA collaborative to improve follow-up after a positive colorectal cancer screening test. Med Care. 2011;49(10):897–903. doi: 10.1097/MLR.0b013e3182204944. [DOI] [PubMed] [Google Scholar]
- 10.Etzioni DA, Yano EM, Rubenstein LV, Lee ML, Ko CY, Brook RH, et al. Measuring the quality of colorectal cancer screening: the importance of follow-up. Dis Colon Rectum. 2006;49(7):1002–1010. doi: 10.1007/s10350-006-0533-2. [DOI] [PubMed] [Google Scholar]
- 11.Powell AA, Gravely AA, Ordin DL, Schlosser JE, Partin MR. Timely follow-up of positive fecal occult blood tests strategies associated with improvement. Am J Prev Med. 2009;37(2):87–93. doi: 10.1016/j.amepre.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Partin MR, Burgess DJ, Burgess JF, Jr, Gravely A, Haggstrom D, Lillie SE, et al. Organizational predictors of colonoscopy follow-up for positive fecal occult blood test results: an observational study. Cancer Epidemiol Biomarkers Prev. 2015;24(2):422–434. doi: 10.1158/1055-9965.EPI-14-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partin MR, Gravely A, Gellad ZF, Nugent S, Burgess JF, Jr, Shaukat A, et al. Factors Associated With Missed and Cancelled Colonoscopy Appointments at Veterans Health Administration Facilities. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.07.051. [DOI] [PubMed] [Google Scholar]
- 14.Virginia Garcia Memorial Health Center. Funding & Patient Demographics. 2014. [Google Scholar]
- 15.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of personality and social psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 17.Kleinbaum D, Lawrence K, Nizam A, Muller K. Applied Regression Analysis and other Applied Multivariable Analysis. 4. Australia, Belmont, CA: Brooks/Cole; 2008. [Google Scholar]
- 18.Hillyer GC, Schmitt KM, Freedberg DE, Kramer RA, Su Y, Rosenberg RM, et al. Fecal-based colorectal cancer screening among the uninsured in northern Manhattan. Am J Prev Med. 2014;47(2):182–187. doi: 10.1016/j.amepre.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LoConte NK, Weeth-Feinstein L, Conlon A, Scott S. Engaging health systems to increase colorectal cancer screening: community-clinical outreach in underserved areas of Wisconsin. Prev Chronic Dis. 2013;10:E192. doi: 10.5888/pcd10.130180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy BT, Daly JM, Luxon B, Merchant ML, Xu Y, Levitz CE, et al. The "Iowa get screened" colon cancer screening program. J. 2010;1(1):43–49. doi: 10.1177/2150131909352191. [DOI] [PubMed] [Google Scholar]
- 21.Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up? Arch Intern Med. 2011;171(3):249–256. doi: 10.1001/archinternmed.2010.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupont-Lucas C, Dejardin O, Dancourt V, Launay L, Launoy G, Guittet L. Socio-geographical determinants of colonoscopy uptake after faecal occult blood test. Dig Liver Dis. 2011;43(9):714–720. doi: 10.1016/j.dld.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Ferrat E, Le Breton J, Veerabudun K, Bercier S, Brixi Z, Khoshnood B, et al. Colorectal cancer screening: factors associated with colonoscopy after a positive faecal occult blood test. Br J Cancer. 2013;109(6):1437–1444. doi: 10.1038/bjc.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansouri D, McMillan DC, Grant Y, Crighton EM, Horgan PG. The impact of age, sex and socioeconomic deprivation on outcomes in a colorectal cancer screening programme. PLoS ONE. 2013;8(6):e66063. doi: 10.1371/journal.pone.0066063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher DA, Jeffreys A, Coffman CJ, Fasanella K. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1232–1235. doi: 10.1158/1055-9965.EPI-05-0916. [DOI] [PubMed] [Google Scholar]
- 26.Choi KS, Lee HY, Jun JK, Shin A, Park EC. Adherence to follow-up after a positive fecal occult blood test in an organized colorectal cancer screening program in Korea, 2004–2008. J Gastroenterol Hepatol. 2012;27(6):1070–1077. doi: 10.1111/j.1440-1746.2011.06944.x. [DOI] [PubMed] [Google Scholar]
- 27.Morris S, Baio G, Kendall E, von Wagner C, Wardle J, Atkin W, et al. Socioeconomic variation in uptake of colonoscopy following a positive faecal occult blood test result: a retrospective analysis of the NHS Bowel Cancer Screening Programme. Br J Cancer. 2012;107(5):765–771. doi: 10.1038/bjc.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miglioretti DL, Rutter CM, Bradford SC, Zauber AG, Kessler LG, Feuer EJ, et al. Improvement in the diagnostic evaluation of a positive fecal occult blood test in an integrated health care organization. Med Care. 2008;46(9 Suppl 1):S91–S96. doi: 10.1097/MLR.0b013e31817946c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Percac-Lima S, Grant RW, Green AR, Ashburner JM, Gamba G, Oo S, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009;24(2):211–217. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Percac-Lima S, Benner CS, Lui R, Aldrich LS, Oo SA, Regan N, et al. The impact of a culturally tailored patient navigator program on cervical cancer prevention in Latina women. J Womens Health (Larchmt) 2013;22(5):426–431. doi: 10.1089/jwh.2012.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coronado GD, Schneider JL, Sanchez JJ, Petrik AF, Green B. Reasons for non-response to a direct-mailed FIT kit program: lessons learned from a pragmatic colorectal-cancer screening study in a federally sponsored health center. Transl Behav Med. 2015;5(1):60–67. doi: 10.1007/s13142-014-0276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]