Abstract
It is currently believed that reading disability (RD) should be defined by reading level without regard to broader aptitude (IQ). There is debate, however, about how to classify individuals who read in the typical range but less well than would be expected by their higher IQ. We used functional magnetic resonance imaging (fMRI) in 49 children to examine whether those with typical, but discrepantly low reading ability relative to IQ, show dyslexia-like activation patterns during reading. Children who were typical readers with high-IQ discrepancy showed reduced activation in left temporoparietal neocortex relative to two control groups of typical readers without IQ discrepancy. This pattern was consistent and spatially overlapping with results in children with RD compared to typically reading children. The results suggest a shared neurological atypicality in regions associated with phonological processing between children with dyslexia and children with typical reading ability that is substantially below their IQ.
Keywords: Dyslexia, neuroimaging, academic achievement
1. Introduction
Reading disability (RD) is the most common form of learning disability, affecting approximately 7% of school age children cross-culturally [1]. Historically, many definitions of RD have been based on a discrepancy between reading achievement and cognitive ability, commonly operationalized using IQ measures. This IQ-achievement discrepancy definition has been widely criticized on theoretical and methodological grounds [2–5] and on policy grounds [6–8]. Moreover, behavioral [9–11] and neuroimaging studies [12,13] indicate that individuals with low reading achievement show similar patterns of behavioral and neural deficits, regardless of IQ. Therefore, the requirement for an ability-achievement discrepancy in the diagnosis of RD has been dropped from some, but not all, definitions [14].
Eliminating the discrepancy requirement is consistent with empirical evidence supporting the use of a low-achievement definition of RD, but concerns remain that high- ability individuals with RD may not be identified under a low-achievement model [7,15,16]. These individuals may have reading achievement that, while substantially below their ability, is comparable to average reading according to standardized measures. The majority of the research on ability-achievement discrepancy in reading has been done in low achieving readers, where it has been established that poor readers with and without IQ discrepancy have similar cognitive [9–11] and neural [12,13] profiles. In one of the few studies to also study typical readers with an IQ-achievement discrepancy, Fletcher et al. [10] found that typically achieving, but discrepant, readers had reduced phonemic awareness relative to typically reading children who did not meet either discrepancy or low achievement criteria for RD. Consistent with the phonological deficit hypothesis, low achieving children also showed impaired phonemic awareness, although low-achieving children were also characterized by additional deficits in word finding relative to non-impaired readers. This finding suggests that children whose reading achievement is typical with respect to peers, but discrepantly low with respect to their IQ have at least some behavioral deficits in common with low-ability poor readers, but it is unknown if their unexpectedly low reading achievement has a distinct etiology.
From a neurobiological perspective, RD is frequently characterized by structural and functional abnormalities in left temporoparietal and occipitotemporal regions, including reduced blood oxygen level dependent (BOLD) activation during phonological and reading tasks [17–19]. Neurobiological measures provide an independent method of examining the etiology of what may be considered RD under different definitions, but these have not yet been used to investigate the basis for IQ-achievement discrepancy in young, typically achieving readers.
The present study examined the brain basis of IQ-achievement discrepancy in school-age children with typical reading achievement and discrepantly high IQ. Specifically, we investigated whether children whose single-word reading skill was within typical range, but discrepantly below their IQ (IQ-discrepant typical readers), would show neurological differences from two non-discrepant control groups: one reading-matched control group of typical readers matched on word identification skills and a second control group of typical readers matched on IQ. The critical comparison of interest between IQ-discrepant typical readers and non-discrepant reading-matched controls provides a direct comparison between equivalent reading achievement in the presence or absence of IQ discrepancy, i.e. any brain difference identified in IQ-discrepant typical readers in this contrast will not reflect group differences in reading skill. To further identify neurobiological differences between IQ-discrepant typical readers and non-discrepant typical readers that cannot be trivially attributed to the higher IQ of the discrepant group relative to non-discrepant reading-matched controls, we examined the conjunction of the contrast between IQ-discrepant and non-discrepant reading-matched typical readers with the contrast between discrepant typical readers and non-discrepant typical readers matched on IQ. This conjunctive analysis and three group design identifies neurobiological differences associated specifically IQ-reading discrepancy.
Additionally, we compare spatial overlap in dysfunction between a group of low-achieving children and the combined group of typical control readers (i.e., children matched to the discrepant typical readers on IQ or reading) to examine whether such differences correspond to dysfunction commonly seen in RD. Similar patterns of decreased BOLD responses during reading in both discrepant typical readers compared to their matched controls, and in poor readers compared to typically reading controls would suggest that discrepant, typically achieving readers have a similar neurobiological atypicality as low achieving readers. Specifically, convergent regions of reduced activation in both discrepant typical readers and low achieving readers are predicted in temporoparietal and/or occiptotemporal regions—regions commonly associated with RD. Such a finding would support the validity of an IQ-discrepancy definition of RD in typical achieving readers. Alternatively, discrepant children with typical reading achievement below their IQ may show distinct patterns of brain activation from low-achievers. Although different neural patterns may develop from a common etiology, distinct patterns of activity for discrepant and low-achieving readers could suggest that high IQ with typical achievement should be considered distinct from low achievement RD, whether due to distinct etiologies or differences in subsequent development.
2. Methods
2.1 Participants
Children in grades 3–5 were recruited from public schools near Pittsburg, PA, as part of a larger randomized reading intervention study [20] and functional magnetic resonance imaging (fMRI) data were obtained from 104 children. fMRI and behavioral data from 49 children were selected from this larger sample based on the availability and usability of fMRI data and test scores meeting the criteria described below. All participants were healthy, right-handed, native English speakers with no self-reported history of neurological or psychiatric disorders. All study procedures were approved by institutional review boards at the University of Pittsburg, Carnegie Mellon University and UCSF.
2.2 Group assignment
Groups of IQ-discrepant typical readers (TypReadHighIQ), non-discrepant, typical reader controls (ConIQ and ConRead) and poor readers (RD) were defined post hoc based on standard scores on the Peabody Picture Vocabulary Test (PPVT; [21]) and WID subtest of the Woodcock Reading Mastery Tests-Revised/Normative Update [22], a measure of single word reading ability. The PPVT is a receptive vocabulary measure that is highly correlated (r = .9) with full scale IQ [21] and used here as proxy for IQ, as in previous RD research [9,12,23,24]. Recommendations vary as to whether reading-IQ discrepancy should be evaluated with respect to performance or verbal IQ, but verbal IQ may be the most appropriate measure of potential language ability (see [10] for a discussion).
Discrepancy scores (PPVT-WID) were standardized by the standard difference error (SEdiff = 4.7) based on the published standard error of the mean (SEM) for WID (SEM = 3) and PPVT (average SEM = 3.6). The IQ-discrepant group (TypReadHighIQ; n = 11) was defined as typical readers (WID > 90) with a standardized discrepancy score > 1.96 (i.e. > 9.2 difference in standard scores). Typical, non-discrepant readers (standardized discrepancy score < 1) were matched to discrepant readers on PPVT (ConIQ; n = 11) or WID (ConRead; n = 11) from the two control groups. Sixteen poor readers (RD; WID ≤ 90) were also included in the analysis for comparison with discrepant readers. The comparison between this RD group and the two matched control groups (considered as a single group of typical non-discrepant controls [ConRead + ConIQ]) was used to identify activation patterns associated with low reading achievement. The Test of Word Reading Efficiency (TOWRE [25]) and the phonological awareness subtests of the Comprehensive Test of Phonological Processing (CTOPP [26]) were also administered. Characteristics of each group are given in Table 1.
Table 1.
Descriptive statistics by reading group
| TypReadHighIQ | ConRead | ConIQ | RD | |
|---|---|---|---|---|
| N (N male) | 11 (4) | 11 (2) | 11 (3) | 16 (11) |
| PPVT | 111.5 (5.6)r,p | 90.7 (8.8)i,p | 110.5 (6.0)r,p | 99.1 (8.6)r,i |
| WRMT WID | 96.5 (4.0)i,p | 96.5 (4.0)i,p | 113.1 (5.0)r,p | 82.8 (7.1)r,i |
| Discrepancy Score | 3.2 (1.0)r,i | −1.2 (1.9)p | −0.5 (1.0)p | 3.5 (2.1)r,i |
| CTOPP | 93.5 (16.9)i | 87.2 (8.3)i | 106.5 (11.0)r,p | 82.2 (9.5)i |
| TOWRE | 89.4 (6.9)i,p | 88.9 (9.2)i,p | 114.5 (18.1)r,p | 77.1 (9.0)r,i |
| Age range | 8.2015011.4 | 8.3–11.2 | 8.6–11.1 | 8.5–12.4 |
| Age mean (SD) | 10.0 (1.3) | 9.6 (1.1)p | 8.5–9.8 (0.9)p | 10.7 (1.0)r,i |
| Task accuracy | 0.89 (0.10)i | 0.86 (0.09)i | 0.98 (0.04)r,p | 0.78 (0.17)i |
Note: Means (standard deviations) are presented for each measure. Discrepancy score is the standardized difference score (PPVT – WID. TOWRE: Tests of Word Reading Efficiency; CTOPP: Comprehensive Test of Phonological Processing. Subscripts indicate significant (two-sample t-test, p < .05) differences from: (r) ConRead, (i) ConIQ, (p) RD.
2.3 Rhyme task
A word rhyming task was used in the scanner in which there were two conditions: rhyme and fixation. During the rhyme condition, participants judged whether two visually presented words rhymed (e.g., bait—gate) or not (e.g., price—miss), and indicated each response with a right- or left-handed button press, respectively. Word pairs were selected so that the visual appearance of the last letters of the two words could not be used to determine whether they rhymed. Stimuli were balanced for frequency of occurrence, number of letters, and syllables between the rhyme and nonrhyme trials and across blocks [27]. Each trial lasted a total of 6 s, consisting of a 4 s period where the two words were presented simultaneously followed by a 2 s fixation cross. Each task block consisted of a 2 s cue period followed by five trials (32 s total). During the fixation block, subjects saw a fixation cross on the screen for 16 s. The entire scan was 234 s long, including two practice trials at the beginning, and consisted of four rhyme blocks and five fixation blocks.
2.4 Image acquisition
The fMRI imaging was performed at the Brain Imaging Research Center (Carnegie Mellon University and University of Pittsburgh) with a 3.0 Tesla (T) Allegra scanner (Siemens Medical, Malvern, PA). A T2*-weighted gradient echo, resonant echo planar pulse sequence sensitive to blood oxygen level-dependent contrast was used with the following acquisition parameters: TR (repetition time) 1,000ms, TE (time to echo) 30ms, flip-angle 60°, field of view (FOV) 20×20cm, matrix size 64×64, axial-oblique plane with 16 slices, and slice- thickness of 6mm with a 1-mm gap. The number of slices did not provide consistent coverage of the sensorimotor cortex and cerebellum. T1-weighted anatomical volumes were acquired using a spoiled gradient echo sequence (TE 2ms; TR 9000ms; flip angle 15º; FOV 24x24cm; 2 excitations).
2.5 fMRI data analysis
Preprocessing and statistical analysis were performed using the FMRIB Software Library (FSL; www.fmrib.ox.ac.uk/fsl). Functional runs from each subject were corrected for slice acquisition time, realigned to the middle volume of the series, spatially smoothed with a Gaussian kernel (7 mm FWHM) and highpass filtered using Gaussian-weighted least-squares (σ = 60s). Functional data were linearly aligned to an MNI template using a two-stage alignment from the functional volume to the individual T1 volume and from the individual T1 to MNI template. Single subject data were analyzed using a fixed effects model with task and fixation blocks modeled as boxcar functions convolved with a canonical hemodynamic response. Confound regressors for volumes displaced more than 1 mm from the previous volume and 6 motion parameter estimates were also included in the model. Contrasts between task and fixation blocks were analyzed for group differences using FMRIB’s Local Analysis of Mixed Effects (FLAME). A minimum statistic conjunction [28] of ConRead > TypReadHighIQ and ConIQ > TypReadHighIQ was used to identify common regions where activation in the Discrepant group differed significantly from both control groups. A three-way conjunction between ConRead > TypReadHighIQ, ConIQ > TypReadHighIQ and ConRead + ConIQ > RD was used to identify regions of decreased activation common to both discrepant and poor readers. Statistical maps were thresholded at p < .01 and cluster significance of p < .05.
3 Results
3.1 Behavioral measures
The TypReadHighIQ group had significantly lower word reading (WID) scores compared to ConIQ (t(20) = −8.49, p < .001) and significantly higher IQ scores than the ConRead group (t(20) = 6.59, p < .001), as expected given that the control groups were selected to have low IQ-reading discrepancy, but matched on IQ or reading, respectively. A similar pattern of differences was found for phonological awareness (CTOPP) and timed-reading (TOWRE) scores, i.e., TypReadHighIQ had significantly lower CTOPP (t(20) = −2.14, p = .05) and TOWRE (t(20) = −4.29, p < .001) scores compared to ConIQ, but TypReadHighIQ and ConRead did not differ significantly on either measure (p >.25). TypReadHighIQ had significantly higher IQ, WID, and TOWRE scores than the RD group (p < .001) and a trend for higher CTOPP scores (t(25) = 1.99, p = .06).
With regards to in-scanner fMRI task performance, RD individuals had significantly lower accuracy than the collective (ConIQ + ConRead) control group (t(36) = 3.12, p = .003). TypReadHighIQ performance was significantly lower than ConIQ (t(20) = −2.87, p = .013) but not ConRead (p > .25). Male:female proportions did not differ significantly across TypReadHighIQ, ConRead and ConIQ (χ2(2) = .92, p = .63), although there were significantly more males in the RD group (χ2(3) = 8.43, p = .04). Age did not differ significantly (p > .44) across the three groups of typical readers (TypReadHighIQ, ConRead, ConIQ) or between the TypReadHighIQ and RD group (t(25) = −1.43, p = .17), but the RD group was significantly older (t(36) = 2.99, p = .005) than the combined ConRead+ConIQ control group.
3.2 fMRI results
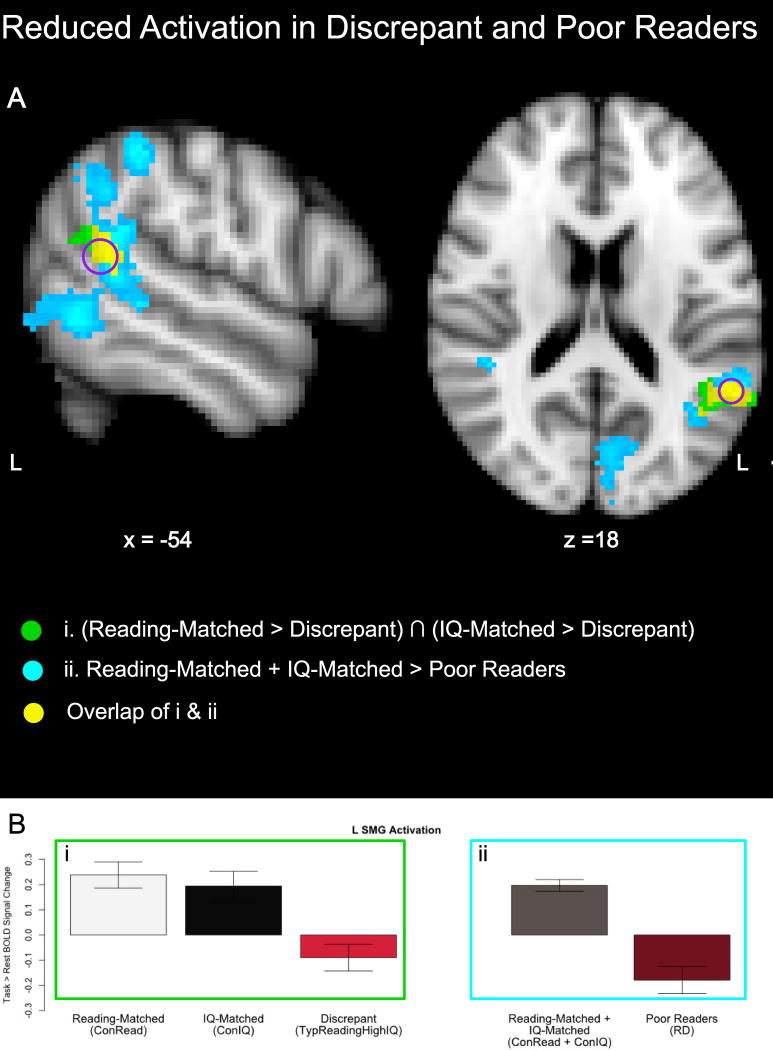
The conjunctive comparison between ConIQ > TypReadHighIQ and ConRead > TypReadHighIQ revealed a single suprathreshold cluster of decreased activation in discrepant readers (TypReadHighIQ) relative to both control groups in the left supramarginal (SMG) and angular (AG) gyrus (MNI coordinates: (−56, −52, 16); Figure 1). The ConRead + ConIQ > RD comparison also showed decreased activation in this region in RD, in addition to more extensive reductions in activation in RD (Table 2).
Figure 1. Reduced activation in discrepant and poor readers during rhyming.
(A) Regions of reduced activation during rhyme judgment in IQ-discrepant typical readers (TypReadHighIQ) relative to both reading-matched (ConRead) and IQ-matched (ConIQ) controls without discrepancy (green); in poor readers (RD) relative to the combined group of typically reading, non-discrepant controls (ConRead + ConIQ; cyan); and common to both comparisons (yellow). Results are height thresholded at p < .01 with a cluster significance of p < .05. The purple circle shows a region of interest (5 mm radius sphere) in the L SMG (−54, −50, 14) from a meta-analysis of RD studies [18]. (B) Parameter estimates from the L SMG ROI for each group entering into the (i) discrepancy conjunction and (ii) ConRead + ConIQ > RD contrast. Error bars show 95% confidence intervals.
Table 2.
Group differences during rhyme judgment.
| Region | Voxels | Max Z | x | y | z |
|---|---|---|---|---|---|
| Non-discrepant typical > Discrepant typical readers | |||||
| (ConIQ > TypReadHighIQ) | |||||
| ∩ (ConRead > TypReadHighIQ) | |||||
| L SMG/AG | 266 | 3.45 | −56 | −52 | 16 |
| Non-discrepant typical > Poor readers | |||||
| ConRead + ConIQ > RD | |||||
| L SMG/AG | 1808 | 4.13 | −54 | −48 | 22 |
| R Lingual gyrus | 1155 | 3.79 | 16 | −78 | 6 |
| R MTG | 598 | 3.64 | 44 | −56 | 0 |
| R LOC | 424 | 3.62 | 28 | −80 | 36 |
| L MFG | 338 | 3.23 | −42 | 12 | 26 |
Abbreviations: supramarginal gyrus (SMG); angular gyrus (AG); middle temporal gyrus (MTG); lateral occipital cortex (LOC); middle frontal gyrus (MFG). Coordinates are reported in MNI space. Clusters were determined with a height threshold of p < .01 and cluster p < .05. ConIQ and ConRead groups were collapsed for the ConRead + ConIQ > RD comparison.
A direct comparison between discrepant and poor readers (RD group) revealed a significant cluster of greater activation in the bilateral occipital pole for TypReadHighIQ > RD. At an uncorrected threshold of p < .01, small clusters of greater activation in discrepant readers were found in the left inferior frontal gyrus (IFG), AG and SMG (Table 3).
Table 3.
Comparisons between Discrepant and Poor Readers.
| Region | Voxels | Peak Z | X | Y | Z | |||
|---|---|---|---|---|---|---|---|---|
| TypReadHighIQ > RD | ||||||||
| L Occipital pole | 433* | 3.52 | −14 | −94 | 12 | |||
| R Occipital pole | 340* | 4.58 | 8 | −90 | 2 | |||
| L SFG | 253 | 3.36 | −8 | 26 | 42 | |||
| L Parietal operculum | 232 | 3.85 | −46 | −40 | 26 | |||
| R MFG | 161 | 3.13 | 48 | 30 | 20 | |||
| L MFG | 144 | 3.58 | −36 | 6 | 36 | |||
| L SMG | 94 | 3.46 | −54 | −40 | 50 | |||
| L LOC | 83 | 3.97 | −54 | −76 | −8 | |||
| R LOC | 64 | 2.81 | 40 | −58 | 40 | |||
| RD > TypReadHighIQ | ||||||||
| R Pallidum | 173 | 3.05 | 24 | −14 | −10 | |||
| L Precuneous | 166 | 3.17 | −22 | −58 | 20 | |||
| R PCC | 137 | 2.99 | 8 | −42 | 4 | |||
| R MTG | 121 | 3.18 | 54 | −2 | −20 | |||
| L Parahippocampal gyrus | 74 | 2.81 | −20 | −40 | −22 | |||
| L MTG | 63 | 2.97 | −60 | −4 | −22 | |||
Note: Results are reported at an uncorrected threshold of p < .01. Only clusters > 50 voxels are reported.
Significant cluster (p < .05).
Abbreviations: supramarginal gyrus (SMG); superior frontal gyrus (SFG); middle temporal gyrus (MTG); middle frontal gyrus (MFG); lateral occipital cortex (LOC); posterior cingulate cortex (PCC). Coordinates are reported in MNI space.
4 Discussion
Children with reading scores within the typical range but substantially below their verbal IQ scores (discrepant typical readers) exhibited a critical brain difference that resembled that seen in low achieving readers. During rhyme judgment, discrepant typical readers showed reduced activation relative to non-discrepant typical reading controls in the left SMG. This reduced activation in the discrepant typical readers occurred in comparison to both non-discrepant reading-matched typical children (who had lower IQ scores) and non-discrepant IQ-matched typical children (who had higher reading scores), so the reductions could not simply be secondary to reading or IQ levels. This region also showed reduced activation in our sample of poor readers and overlapped with regions previously implicated in RD [18]. Further, despite the higher reading scores of the discrepant typical readers, activation patterns in the language/reading network did not differ significantly between discrepant typical readers and poor readers. These findings suggest a shared neural basis for RD and unexpectedly low reading achievement in IQ discrepant typical readers.
These results, while limited by the small sample, provide neural evidence in favor of an IQ-discrepancy definition of RD for individuals having typical reading achievement. While it would be ill-advised to broadly apply a discrepancy criterion when identifying RD—considering evidence that IQ-discrepancy is largely irrelevant at low achievement levels [12,13]—our results suggest that considering discrepancy as a diagnostic criterion for typical achievers may be biologically justified.
One limitation of this interpretation is that group differences were identified on the basis of comparisons between task and rest conditions, making it difficult to determine if group differences are specific to the linguistic processes of interest. In addition, the print-based task used in this study may recruit multiple reading-related processes, beyond phonological processing. We also employ a less stringent criterion for defining discrepancy (a standard score difference of >9.2) than some researchers (e.g. Fletcher et al. [10] adopt a standard score difference >22.5). The use of large gaps in defining discrepancy is motivated in practice as a way of reducing false positive identifications. While our more liberal definition of discrepancy potentially misclassifies some children as discrepant, we stress that relaxed group separations introduce bias against identifying neurological differences. Since the discrepant typical group has reading achievement scores falling at the lower end of the normal range, it is also possible that, due to measurement error, this group contains children with RD. However, children with RD also have tend to have lower PPVT scores [10,21], whereas the discrepant typical readers have slightly elevated PPVT. Thus our use of PPVT as an IQ measure decreases the likelihood that the discrepant typical group contains children with RD.
The finding of neurological differences in discrepant typical readers in regions associated with phonological processing and RD-related deficits, even under a relatively liberal definition of discrepancy, suggests that IQ discrepancy in typical readers has an associated neural basis, even when the discrepancy is small. The choice of IQ measure may affect the sensitivity of the design. Among studies that have compared readers classified as RD based on low achievement and IQ-discrepancy, those that have used full scale IQ as an ability measure have reported larger effect sizes for behavioral differences than those studies that have used a verbal IQ measure [9]. This suggests that using verbal IQ to identify discrepancy, as we did, is more consistent with the consensus view that IQ-reading discrepancy is not necessary for identifying RD. These prior behavioral results suggest that our use of a verbal IQ measure of ability may, if anything, bias our results against finding converging neural patterns in both discrepant and RD groups.
The comparison of discrepancy-related and RD-related patterns of reduced temporoparietal activation is limited by differences in the sex distribution across groups. As is typical, the RD group is predominately male, while all three groups of typical readers are predominately female. Although our key comparisons across groups of typical readers are not confounded by sex differences, it is possible that males could show a different neurological pattern associated with IQ-reading discrepancy. The sex differences between the RD group and typical reading groups may also limit the comparability of RD-related and discrepancy-related reductions in temporoparietal activation. However, the region of reduced activation found in both RD relative to controls and in IQ discrepant typical readers is consistent with prior meta-analyses of RD. Replication in larger samples is needed to examine possible sex differences in the neural correlates of IQ-reading discrepancy.
The finding that relatively low levels of ability-achievement discrepancy, within typical readers, are associated with neural differences may appear to conflict with past studies that have found no neurological distinction between discrepant and non-discrepant readers at low levels of achievement [12]., i.e. the presence of a discrepancy does not appear to exaggerate deficits beyond those associated with low achievement. One explanation for this apparent inconsistency is that discrepant low-achieving readers in previous studies [12] have had average IQ scores, whereas the typical readers with IQ-reading discrepancy in the present study have a slightly elevated IQ. This increased IQ, relative to that of discrepant low-achieving readers, could reflect a greater capacity for successful compensation for reduced temporoparietal function shared with poor readers.
The shared pattern of reduced temporoparietal brain activation in discrepant typical readers and poor readers raises the possibility that typically achieving discrepant readers could benefit from targeted interventions that can improve reading and phonological processing in poor readers (e.g. [20]) In low achieving readers, IQ is positively associated with treatment outcomes [29], which could suggest that interventions would be particularly effective in discrepant typical readers with higher IQ. Future research is needed to evaluate the effectiveness of intervention in discrepant, typical readers.
The ability of higher-IQ discrepant readers to reach typical levels of reading achievement, despite atypical brain activation similar to that seen in RD children, suggests compensation processes, potentially associated with higher cognitive abilities, that were not revealed in this study. These findings motivate further studies of discrepant typical readers to identify potential natural compensation processes, e.g. compensatory recruitment of additional networks, that might be facilitated in low-achieving readers.
Acknowledgments
Funding
This work was supported by grants from National Institute of Child Health and Human Development (Grants K23HD054720, R01HD078351) to F.H. F.H. was also supported in part by NICHD R01HD078351, R01HD044073 (principal investigator: L. Cutting, Vanderbilt University, Nashville, TN), R01HD065794 (principal investigator: K. Pugh, Haskins Laboratories, Yale University, New Haven, CT), and P01HD001994 (principal investigator: J. Rueckl, Haskins Laboratories), National Institute of Mental Health Grants R01MH104438 (principal investigator: C. Wu Nordahl, University of California Davis MIND Institute, Sacramento, CA) and R01MH103371 (principal investigator: D. Amaral, University of California Davis MIND Institute), National Science Foundation Grant NSF1540854 SL-CN (principal investigator: A. Gazzaley), the UCSF Dyslexia Center, the UCSF Academic Senate Award, a UCSF – Center for Childhood Creativity Neuroscience Fellowship (and Liebe Patterson), the Dennis and Shannon Wong DSEA ‘88 Family Foundation and the Potter Family Foundation.
Footnotes
Declaration of Conflicting Interests
The authors declared no conflicts of interest with respect to the authorship or the publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foresight Mental Capital and Wellbeing Project, Mental Capital and Wellbeing: Final Project Report. London: 2008. [Google Scholar]
- 2.Van den Broeck W. Will the real discrepant learning disability please stand up? J Learn Disabil. 2002;35:209–13. doi: 10.1177/002221940203500303. [DOI] [PubMed] [Google Scholar]
- 3.Stanovich KE. Dysrationalia: a new specific learning disability. J Learn Disabil. 1993;26:501–15. doi: 10.1177/002221949302600803. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher JM, Denton C, Francis DJ. Validity of alternative approaches for the identification of learning disabilities. J Learn Disabil. 2005;38:545–552. doi: 10.1177/00222194050380061101. [DOI] [PubMed] [Google Scholar]
- 5.Meyer MS. The ability–achievement discrepancy: Does it contribute to an understanding of learning disabilities? Educ Psychol Rev. 2000;12:315–337. doi: 10.1023/A:1009070006373. [DOI] [Google Scholar]
- 6.Sternberg RJ, Grigorenko EL. Difference scores in the identification of children with learning disabilities: It’s time to use a different method. Psychology. 2002;40:65–83. [Google Scholar]
- 7.Hale AJ, Alfonso V, Berninger V, Bracken B, Christo C, Clark E, et al. Critical issues in response-to-intervention, comprehensive evaluation, and specific learning disabilities identification and intervention: An expert white paper consensus. Learn Disabil Q. 2010;33:223–236. [Google Scholar]
- 8.Machek GR, Nelson JM. How should reading disabilities be operationalized? a survey of practicing school psychologists. Learn Disabil Res Pract. 2007;22:147–157. doi: 10.1111/j.1540-5826.2007.00239.x. [DOI] [Google Scholar]
- 9.Stuebing KK, Fletcher JM, LeDoux JM, Lyon GR, Shaywitz SE, Shaywitz BA. Validity of IQ-Discrepancy Classifications of Reading Disabilities: A Meta-Analysis. Am Educ Res J. 2002;39:469–518. doi: 10.3102/00028312039002469. [DOI] [Google Scholar]
- 10.Fletcher JM, Shaywitz SE, Shankweiler DP, Katz L, Liberman IY, Stuebing KK, et al. Cognitive profiles of reading disability: Comparisons of discrepancy and low achievement definitions. J Educ Psychol. 1994;86:6–23. [Google Scholar]
- 11.Shankweiler DP, Crain S, Katz L, Fowler AE, Liberman AM, Brady SA, et al. Cognitive profiles of reading disability: Comparison of language skills in phonology, morphology, and syntax. Psychol Sci. 1995;6:149–156. [Google Scholar]
- 12.Tanaka H, Black JM, Hulme C, Stanley LM, Kesler SR, Whitfield-Gabrieli S, et al. The brain basis of the phonological deficit in dyslexia is independent of IQ. Psychol Sci a J Am Psychol Soc / APS. 2011;22:1442–1451. doi: 10.1177/0956797611419521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simos PG, Rezaie R, Papanicolaou AC, Fletcher JM. Does IQ affect the functional brain network involved in pseudoword reading in students with reading disability? A magnetoencephalography study. Front Hum Neurosci. 2014;7:932. doi: 10.3389/fnhum.2013.00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mather N, Schneider D. The Use of Intelligence Tests in the Diagnosis of Specific Reading Disability Nancy. In: Goldstein S, editor. Handb Intell Evol Theory, Hist Perspect Curr Concepts. Springer; New York: 2015. pp. 415–433. [DOI] [Google Scholar]
- 15.Mather N, Gerner ME. Postsecondary students with high abilities and reading disabilities: case analyses and commentary. Learn Disabil A Multidiscip J. 2008;15:121–129. [Google Scholar]
- 16.Crepeau-Hobson F, Bianco M. Identification of gifted students with learning disabilities in a Response-to-Intervention era. Psychol Sch. 2011;48:102–109. doi: 10.1002/pits.20528. [DOI] [Google Scholar]
- 17.Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Ann N Y Acad Sci. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- 18.Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp. 2009;30:3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linkersdörfer J, Lonnemann J, Lindberg S, Hasselhorn M, Fiebach CJ. Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: An ALE meta-analysis. PLoS One. 2012;7:e43122. doi: 10.1371/journal.pone.0043122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torgesen JK, Myers D, Schirm A, Stuart E, Vartivarian S, Mansfield W, et al. National Assessment of Title I : Interim Report Volume II: Closing the Reading Gap: First Year Findings from a Randomized Trial of Four Reading Interventions for Striving Readers. Washington, D.C: 2006. [Google Scholar]
- 21.Dunn LM, Dunn DM. Peabody Picture Vocabulary Test. 4. Pearson; 2007. [Google Scholar]
- 22.Woodcock RW. Woodcock Reading Mastery Tests-Revised/Normative Update. American Guidance Service; Circle Pines, MN: 1998. [Google Scholar]
- 23.Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, et al. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci. 2006;26:10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurford DP, Schauf JD, Bunce L, Blaich T, Moore K. Early identification of children at risk for reading disabilities. [accessed October 14, 2015];J Learn Disabil. 1994 27:371–82. doi: 10.1177/002221949402700604. http://www.ncbi.nlm.nih.gov/pubmed/8051510. [DOI] [PubMed] [Google Scholar]
- 25.Torgesen J, Wagner RK, Rashotte CA. Test of Word Reading Efficiency 2. Pro-Ed Publishing; Austin, TX: 2012. [Google Scholar]
- 26.Wagner RK, Torgesen JK, Rashotte CA. Comprehensive Test of Phonological Processes. Pro-Ed Publishing; Austin, TX: 1999. [Google Scholar]
- 27.Zeno SM, Ivens SH, Millard RT, Duvvuri R. The educator’s word frequency guide. Touchstone Applied Science Associates; New York, New York, USA: 1995. [Google Scholar]
- 28.Nichols T, Brett M, Andersson J, Wager T, Poline J-B. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Nelson R, Benner GJ, Gonzalez J. Learner characteristics that influence the treatment effectiveness of early literacy interventions: a meta-analytic review. Learn Disabil Res Pract. 2003;18:255–267. doi: 10.1111/1540-5826.00080. [DOI] [Google Scholar]