Abstract
The present study tested whether pet dogs have stress-buffering effects for children during a validated laboratory-based protocol, the Trier Social Stress Test for Children (TSST-C). Participants were 101 children aged 7–12 years with their primary caregivers and pet dogs. Children were randomly assigned in the TSST-C to a pet present condition or one of two comparison conditions: parent present or no support figure present. Baseline, response, and recovery indices of perceived stress and cortisol levels were computed based on children’s self-reported feelings of stress and salivary cortisol. Results indicated that in the alone (no social support) condition, children showed the expected rise for both perceived stress and cortisol response to stress. Pet dog presence significantly buffered the perceived stress response in comparison to children in the alone and parent present conditions. No main condition effect was observed for cortisol; however, for children experiencing the stressor with their pet present, lower cortisol response to stress was associated with more child-initiated petting and less dog proximity-seeking behavior. The results support the notion that pet dogs can provide socio-emotional benefits for children via stress buffering.
Keywords: pet dogs, Trier Social Stress Test, perceived stress, cortisol
Introduction
Pet dogs have a notable impact on the lives of children. More than 40 percent of American families with children have a pet dog (American Pet Products Association, 2014). Pet dogs have been proposed to benefit children by reducing anxiety and facilitating social interactions (e.g., Hoffmann et al., 2009; Kruger & Serpell, 2006). However, the research literature on potential benefits of child-dog interaction is scant. Dogs have been shown to promote positive affect and reduce problem behavior among typically developing and developmentally disordered children (e.g., Anderson & Olson, 2006; Kotrschal & Ortbauer, 2003). Other potential socio-emotional benefits of pet dogs for children include increased autonomy, self-concept, and empathy (Barker & Wolen, 2008). However, the bulk of this research has been descriptive, and relatively few experimental studies have been conducted to rigorously test commonly held beliefs about the benefits of pet ownership for children. Moreover, the mechanisms by which pet dogs may confer emotional health benefits for children are still unclear.
One potential mechanism may be via social buffering of stress responses. During childhood, emotional and physiological responses to stress are still developing (Eisenberg, Spinrad, & Eggum, 2010), and children possess less mature internal cognitive resources to self-regulate stress responses compared to adults (Cole, Michel, & Teti, 1994). As such, social regulation of stress responses provided by supportive figures, such as family members, is essential for children’s adaptive socio-emotional developmental outcomes (Gunnar & Donzella, 2002). The social buffering hypothesis has gained increasing traction over the past several decades as a means by which supportive social relationships may reduce responses to stressful or threatening events (Cohen & Wills, 1985; Hennessy, Kaiser, & Sachser, 2009; Hostinar, Sullivan, & Gunnar, 2014). In the developmental literature, social buffering has primarily focused on infancy and early childhood, with little research conducted in middle childhood (Hostinar et al., 2014).
We focused on typically developing children aged 7–12, based on social and emotional developmental changes in middle childhood. First, children of this age can reliably self-report feelings of stress (Achenbach, McConaughy, & Howell, 1987), owing partly to the development of metacognitive abilities (Grazzani & Ornaghi, 2012). Second, social stressors reliably elicit a cortisol response in this age group (Gunnar, Talge, & Herrera, 2009). Extensive research has shown that social evaluation is one of the most reliable elicitors of the cortisol stress response (Dickerson & Kemeny, 2004). Seven- to twelve-year old children, compared to younger children, more regularly engage in self-reflective perspective taking, viewing their behavior from another’s point of view (Cillessen & Bellmore, 1999; Selman, 1976). As a result, during middle childhood, self-concept more frequently involves social comparisons and is influenced by feedback from others (Cole et al., 2001; Harter, 1998). A consequence of these developmental changes relevant to stress research is that social evaluation becomes a salient stressor beginning in middle childhood.
Third, by middle childhood the amount of time children spend with parents declines dramatically compared to earlier ages (Lam, McHale, & Crouter, 2012). Simultaneously, parents typically engage in coregulation with children, exercising general oversight while shifting control of moment-to-moment decisions to children. Correspondingly, children start to utilize less parental support for stress coping (Kerns, Tomich, & Kim, 2006). Parental support is partially replaced by reliance on a broader network of social support figures compared to infancy and early childhood including pets (Bryant, 1985). This developmental change necessitates studies on social buffering resources other than parental figures for older children. This research is essential as there is solid evidence that the social environment during development has permanent, programming effects on the stress response system (Lupien, McEwen, Gunnar, & Heim, 2009). In addition, emotional and physiological responses to stress during childhood are known risk factors for internalizing and externalizing problems in childhood, as well as stress-related disorders in adulthood (Ingram & Price, 2010). An examination of stress buffering during childhood, therefore, is important to further the understanding of developmental trajectories of risk and resilience for stress-linked emotional, behavioral, and physical health problems.
The few studies examining stress buffering effects of child–dog interaction have mostly been conducted in medical settings (Beetz, Uvnäs-Moberg, Julius, & Kotrschal, 2012; Hansen, Messinger, Baun, & Megel, 1999; Havener et al., 2001; Nagengast, Baun, Megel, & Leibowitz, 1997). These studies show inconsistent results, as some documented significant stress-reducing benefits of pet dogs (e.g., Nagengast et al., 1997), but others did not (e.g., Havener et al., 2001). These discrepancies are likely due in part to diverse, uncontrolled effects of a variety of medical procedures and hospital stays on emotional or physiological stress. Human-animal interaction (HAI) research with adults has provided some support for the idea that interaction with dogs has stress-reducing benefits (Barker & Wolen, 2008). Adults undergoing a laboratory stress task in the presence of a pet dog show reduced physiological response (heart rate, blood pressure, skin conductance) compared to adults undergoing the same task alone; in contrast, adults undergoing the task in the presence of a human friend showed elevated autonomic stress response compared to those performing the task alone (Allen, Blascovich, Tomaka, & Kelsey, 1991). Allen et al. (1991) have suggested that pet dogs provide non-evaluative companionship to owners that reduce stress responses compared to more evaluative human companions.
The bulk of the research on stress-buffering effects of HAI has focused on the autonomic component of the biological stress system, as indexed by heart rate, blood pressure, or skin conductance. Although autonomic activity plays a key role in mobilizing the body’s fast-acting response to threat, the emotional component of stress and the cortisol response to stress are arguably more relevant for long-term developmental consequences. Emotional and cortisol stress responses impact neural activity in developing prefrontal and limbic brain regions that control stress-related memory, emotion, and enduring changes in stress responsivity (McEwen, 2008). Moreover, perceived stress during childhood has been linked with emotional and behavioral problems as well as maladaptive coping styles (e.g., Dieleman, van der Ende, Verhulst, & Huizink, 2010; Hampel & Petermann, 2006). A proportional and time-limited cortisol response to stress can be adaptive in the face of an immediate danger or threat. However, disproportionate, frequent, or prolonged activation has been linked with multiple forms of psychopathology and related alterations in amygdalar and hypothalamic functioning (e.g., Dieleman et al., 2010; Vaisvaser et al., 2013).
Perceived stress and cortisol responses to stress are largely orthogonal dimensions of stress responding (Campbell & Ehlert, 2012). Whereas perceived stress reflects processes that are consciously accessed, cortisol responses to stress are not part of conscious awareness but also shape emotional and physiological responses to future stressors. Two studies with adults have examined both perceived stress (self-reported anxiety) and cortisol response to stress in the presence or absence of an unfamiliar friendly dog. However, these two studies yielded contradictory findings, with one study reporting a buffering effect of the unfamiliar dog’s presence for state anxiety but not cortisol, and the other showing the reverse pattern (Lass-Hennemann, Peyk, Streb, Holz, & Michael, 2014; Polheber & Matchock, 2014). There is only one published study reporting lower cortisol during the recovery phase of a stress protocol. However, that study compared the effects of an unfamiliar live dog to a toy dog and was conducted with boys recruited primarily from special education schools (Beetz, Julius, Turner, & Kotrschal, 2012). To date, the question of whether dogs buffer children’s cortisol responses has not been tested in an unselected sample.
There are no studies empirically testing whether dogs buffer children’s perceived stress responses. Among adults, social support figures buffer perceived stress via provision of emotional/companionship support as well as informational and instrumental support (Lakey & Cohen 2000). Children and adolescents report emotional/companionship support as more effective in reducing emotional distress compared to informational/instrumental support (e.g., Caserta, Punamäki, & Pirttilä-Backman, in press). Whereas guide, therapy, or rescue animals are trained to provide instrumental support, among typically developing children, pet dogs’ support is generally emotional/companionship in nature. With developmental changes in middle childhood leading to improved ability to engage in self-reflective thought (Achenbach et al., 1987), increased attention to self-evaluation (Cole et al., 2001; Harter, 1998), decreasing reliance on parents for stress coping (Kerns et al., 2006), and increased reliance on pets for emotional support (Bryant, 1985) it is plausible that dogs’ companionship during a stressor may buffer children’s perceived stress.
Additional limitations of prior research are that most studies (in both adults and children) have utilized unfamiliar animals and participants comprised of both pet-and non-pet owners (e.g., Allen, Blascovich, & Mendes, 2002; Demello, 1999; Kingwell, Lomdahl, & Anderson, 2001). The use of unfamiliar animals as well as participants varying in pet ownership has likely contributed to the mixed findings in the literature. Indeed, research with adults has shown that presence of a dog lowers heart rate and blood pressure only among pet owners (Allen et al., 2002; Kingwell et al., 2001). Among non-owners, heart rate is higher in the presence of a dog (Kingwell et al., 2001). Dog owners may reap greater benefits from interaction with dogs based on their history of pet ownership. Moreover, a potential stress-buffering effect may be maximal in the presence of one’s own pet dog, as both children and adults report strong feelings of emotional attachment toward their pet dogs (Daly & Morton, 2006; Kurdek, 2008; Serpell, 1996).
The present study advances research on the potential stress-buffering effect of HAI in childhood by examining perceived stress and cortisol responses to stress among pet-owning children in a controlled laboratory experiment. Based on evidence that pet ownership impacts whether dogs can confer a stress-buffering effect (Allen et al., 2002; Kingwell et al., 2001), and that children form strong social bonds to pet dogs (Westgarth et al., 2013), we tested whether pet dogs would buffer stress responses among children. In contrast to most prior studies utilizing unfamiliar dogs (e.g., Beetz, Julius, et al., 2012; Friedmann, Katcher, Thomas, Lynch, & Messent, 1983), this study was conducted with children and their own pet dogs. To rigorously test the potential stress-buffering effect of pet dogs, we utilized a well-established laboratory-based paradigm, the Trier Social Stress Test for Children (TSST-C, Buske-Kirschbaum et al., 1997). Based on prior research using the TSST, we anticipated that results for perceived stress and cortisol stress responses may diverge (see for review Campbell & Ehlert, 2012). Nevertheless, as a direct test of the potential buffering effect of pet dogs we hypothesized that children experiencing the TSST-C in the presence of their pet dog would show lower perceived stress and cortisol response to stress in comparison to children experiencing the TSST-C without their pet dog present. As a robust test of the stress-buffering effect of pet dogs specifically, we also compared the stress-buffering effect of pet dog presence against a second group in which social support was provided by presence of a parent during the TSST-C.
A secondary aim specifically targeted the subset of children experiencing the stressor in the presence of their pet. Research has documented that forms of interaction between a child and pet dog can vary widely (Millot & Filiatre, 1986; Millot, Filiatre, Gagnon, Eckerlin, & Montagner, 1988). Anticipating that pet dog behavior and child-pet relationships would naturally show some variation even in an experimental paradigm such as the TSST-C, a secondary aim of this study was to test whether individual differences in child-dog interaction impacted the stress-buffering effect of pet dogs. Prior research suggests that dogs show differences in a variety of personality dimensions such as ‘aggression’, ‘playfulness’, and ‘sociability’ (Svartberg, Tapper, Temrin, Radesäter, & Thorman, 2005). Recent research in our lab has demonstrated that children report more positive emotional feelings toward pet dogs if the dogs are responsive to their communicative gestures (Hall, Liu, Kertes, & Wynne, 2016). Presumably, child-dog dyadic interaction during the TSST-C may impact the social buffering effect the dog may provide. However, no prior studies examining the stress-buffering effect of dog presence have assessed dog behaviors during the study and whether variation in these behaviors is related to stress responses. Prior research with adults demonstrates that petting and social interaction induces physiological changes in humans, including reductions in cortisol along with increases in β-endorphins, prolactin, β-phenylethylamine, oxytocin, and dopamine (Miller et al., 2009; Nagasawa et al., 2015; Odendaal & Meintjes, 2003). However, impacts of human–dog interaction with phenotypes similar to perceived stress (i.e., state anxiety) have been reported less consistently (Lass-Hennemann et al., 2014; Polheber & Matchock, 2014). The present study assessed child–dog interaction via behavioral coding of the child-dog dyad during the TSST-C. We hypothesized that for children in the pet present condition, lower perceived or cortisol stress response would be observed for dyads in which dogs sought out and remained near the child (dog initiated interactions) or dyads in which children actively engaged the dog (child initiated interactions).
Method
Participants
Participants were recruited from North Central Florida via locally distributed flyers, radio and TV advertisements, and direct mailings. Interested families contacted the research lab and a brief telephone screening survey was used to determine eligibility. Eligible children were between the ages of 7 and 12 years with no diagnosed medical conditions and not currently using steroid or hormonal medications. To be included in the study, the pet dog must have lived in the household for at least the past six months and have no history of aggression.
The total sample included 101 typically developing children (M age =10.2 years, SD =1.31; 51 boys). Child ethnicity was reported by parents as follows: 11 percent Hispanic; 89 percent non-Hispanic. Child race was reported as follows: 85 percent White, 7 percent two or more races; 3 percent Latino; 2 percent Native American; 2 percent African American, and 1 percent Asian. Median parent education was college degree, ranging from partial high school to graduate or professional degree.
Procedure
Children participated in the study along with their primary caregivers (81 percent mother) and pet dogs at the research laboratory at the University of Florida. If multiple dogs resided in the home that met inclusion criteria, the family chose which dog they preferred to accompany the child based on the child-pet relationship. Children were assigned to one of three conditions in a stratified random sample (pet present, parent present, or alone), such that child sex was balanced across the three conditions. To control for the circadian rhythm in basal cortisol production, all lab visits were blocked to two start times, 10:00 am or 3:30 pm, balanced across the three conditions.
At the time of scheduling, parents were told that that the study was about children’s stress, and how parents and pets help children cope with stress. No hypotheses were revealed to parents or children prior to the study, and families were unaware prior to the study of the multiple conditions. As is customary for a TSST-C study, parents were told that the study would involve the child doing ‘school-like tasks’ in front of two adults and providing saliva samples but were asked not to provide details to the child ahead of time. Parents were asked to refrain from giving their child any dairy products at least 1 h prior to the study visit to avoid potential contamination in saliva. All families brought the pet dog to the laboratory.
Upon arrival to the research lab, parents and children provided informed consent and assent, respectively, in a waiting room. Regardless of which testing condition participants had been assigned to, the pet dog was brought by a trained dog handler to the experimental testing room to familiarize the dog to the testing space and study staff. The child remained with the experimenter in a ‘resting room’ equipped with child-friendly decorations, furniture, reading, and coloring materials. The experimenter subsequently escorted the child to the ‘testing room’, either with the pet dog, with the parent, or alone. Support figures not accompanying the child remained in the waiting room with a research assistant, the trained dog handler, or both. All three rooms (waiting, resting, and testing) were adjacent to one another. Children were aware of their support figures’ location at all times. In the two social support conditions, either a chair was available (for the parent) or a mat was available (for the dog) placed equidistant from the child and the judges. Dogs remained with their leash attached but were not restrained to the mat and could, therefore, move around or interact with the child. Parents typically remained seated but were not restricted from talking to the child. In the support conditions, the experimenter explicitly announced that the parent/dog was there to provide support to the child. The experimenter further stated that ‘some kids’ find that talking to/interacting with their parent/dog or just having them around helps them to stay calm, and indicated that it was OK for the child to talk to or interact with the parent/dog.
The TSST-C is a 15-min laboratory-based stressor and includes a 5-min speech preparation period, a 5-min speech delivery, and a 5-min mental arithmetic task. At the start of the speech preparation, the experimenter read a story stem to the child and instructed the child to make up an ending that would be better and more interesting than other children’s endings. After 5 min, two confederates, one male and one female, dressed in white lab coats and posing as judges, entered and were seated at a table at one end of the room with neutral facial expressions. The experimenter left the room and the male judge instructed the child to stand in a marked spot. The child faced the judges and a conspicuously placed video camera to deliver the speech. If the child stopped before reaching 5 min, the judge instructed the child to continue using a standard set of prompts. The female judge initiated the mental arithmetic task (serial subtraction). If child made a mistake, the judge instructed the child to start over; if no mistakes were made, the child was prompted to work faster.
The end of the stressor was marked by the re-entry of the experimenter into the testing room. The judges praised the child for his or her performance. The experimenter then escorted the child to the adjacent resting room. In the two social support conditions, the dog or parent accompanied the child in the resting room for the first 10 min, after which the support figure was escorted to the waiting room for an additional 25 min. During this time the child was with the experimenter and saliva was sampled at intervals as described below in Measures. The child and pet dog were subsequently reunited for additional assessments not described in this report.
Parents completed a questionnaire in the waiting room with a research assistant present. Parent report was used to obtain basic demographic information, as well as information on variables that have previously been shown to affect cortisol levels in children, including recent food intake, physical exercise, illness, and medication use, assessed for purposes of quality control checks (Gunnar et al., 2009; Hibel, Granger, Cicchetti, & Rogosch, 2007; Kertes & Gunnar, 2004). No associations with cortisol were observed for number of hours since last food intake, hours since exercise, same day physical complaints (headache, allergy, mild illness), or non-steroid medication use. Furthermore, excluding cases based on any of these potential confounds did not change the reported results. Parents also reported on breed of the dog, which was subsequently classified into breed group (Protopopova, Gilmour, Weiss, Shen, & Wynne, 2012). The breed groups included lap dogs (toy breeds such as Maltese and Chihuahua, n =32), sporting breeds (Labrador retrievers and golden retrievers, n =20), herders (e.g., German shepherds and Australian shepherds, n =19), terriers/ratters (e.g., Jack Russell terrier and rat terrier, n =13), bully/fighting breeds (e.g., Pit bulls, bulldogs, and boxers, n =11), and unknown mixes (n =5).
Measures
Perceived Stress
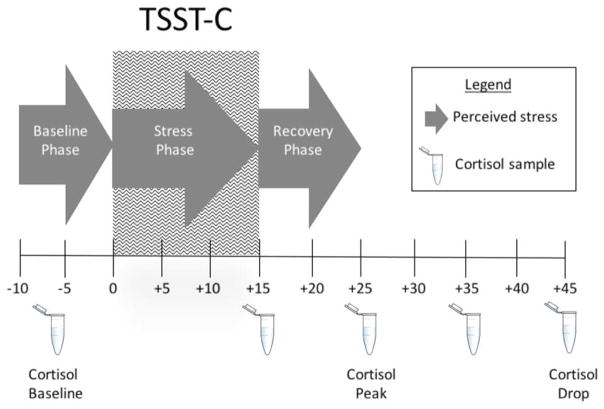
Children reported their perceived stress using a modified version of the Self-Assessment Manikin (SAM; Bradley & Lang, 1994), a pictorial scale for self-reports of emotion. The SAM has previously been reported to correlate with heart rate, respiratory sinus arrhythmia, as well as self-reported anxiety (Leen-Feldner, Zvolensky, & Feldner, 2004; Oldehinkel et al., 2010). Participants utilized the pictorial figures to indicate on a 9-point scale their perceived stress, ranged from 1 (totally relaxed) to 9 (totally stressed out). Children reported how stressed they felt during three phases of assessment: baseline (before the TSST-C), during the TSST-C, and during the recovery phase after the TSST-C (see Figure 1). The measure was administered following the conclusion of the TSST-C protocol. This was done so all children, regardless of testing condition, completed it while their social support figures were not present. Difference scores were computed to reflect 1) the change in perceived stress from baseline to end of TSST-C and 2) end of TSST-C to recovery phase to index perceived stress rise and recovery, respectively.
Figure 1.
Experimental Protocol.
Salivary Cortisol
Salivary cortisol samples were collected to assess baseline, stress response, and recovery. The baseline sample was collected immediately prior to the start of the TSST-C, which occurred 30 min after arrival to the lab to allow for acclimation to the lab. As shown in Figure 1, additional saliva samples were collected at +15 min, +25 min, +35 min, and +45 min, where 0 min indicates the onset of the TSST-C stressor. Based on an extensive literature documenting the typical peak salivary cortisol response time and typical recovery time to the TSST protocol, we anticipated that the +25 min sample would indicate cortisol peak and +45 min would indicate cortisol recovery (Kirschbaum & Hellhammer, 1989; Kudielka, Hellhammer, & Kirschbaum, 2007). Raw cortisol values at each time point were Mbaseline = .11 μg/dl (SD =.09), M+15 = .14 μg/dl (SD =.09), M+25 = .18 μg/dl (SD =.16), M+35 μg/dl =.16 (SD =.14), M+45 = .12 μg/dl (SD =.09). As expected, peak cortisol response occurred at +25 min and the lowest poststress value was observed at +45 min in the present sample. Thus we considered the change from baseline to +25 min as the cortisol response and the change from +25 min to +45 min as the cortisol recovery.
Salivary cortisol samples were collected via passive drool through a short straw into a 2.0 ml centrifuge tube. Saliva samples were stored at −80°C until assay. Cortisol concentrations were determined using enzyme immunoassay kits (Salimetrics, LLC, State College, PA). Blind controls generated from pooled saliva were inserted in each assay batch. Two participants were dropped from the study because of recent steroid medication use. An additional five participants’ samples consistently had insufficient saliva volume for assay, yielding a final sample of 94 children in the cortisol analyses. No outliers (defined as cortisol level >3 SD above mean or >2.0 μg/dl) were observed. Intra-assay and interassay coefficients of variation were 3.2 and 6.1, respectively.
Behavioral Observation of Child–Dog Interaction
Because of anticipated variation in owned dogs’ behaviors during the pet present condition, video recordings of child–pet dyadic interaction during the pet present condition were coded by two experts in dog behavior analysis. Video recordings were available for 29 of 35 participants, with the others lost due to technical errors. Videos were coded from the time the participant entered the testing room until the end of the TSST-C. Video coding was conducted using J-WatcherR software. Five key behaviors were scored, including: (1) the number of times the dog approached the child by placing its head or body in contact with the child; (2) the duration of time the dog remained within arm’s reach of the child (.5 m); (3) the duration of time the dyad was in non-petting contact (e.g., child picking up or holding the dog); (4) the duration of time the child spent petting the dog; and (5) the number of times the child gave the dog a verbal solicitation (e.g., ‘come’ or ‘sit’).
Variables scored as a duration (duration of dog in close proximity, duration of dog in physical contact, duration of child petting dog) were then recomputed as the duration of the behavior divided by the total duration of interaction in minutes. For variables scored as a count (number of dog approaches, number of child verbal solicitations), the score was computed as the number of events divided by the duration of interaction in minutes. To assess interobserver agreement, approximately 40 percent of the videos were double-coded. Intraclass correlations were very good to excellent (range .71–.96).
To reduce the number of behavior dimensions, the variables were subject to principal components analysis with varimax rotation to generate a set of dyadic interaction factors. The five quantitatively measured variables were well represented by two underlying factors with eigenvalues >1 with low cross-loadings. Table 1 shows the measured variables and their factor loadings. One factor included the proportion of time the dog stayed within child’s reach, frequency of the dog placing its head in physical contact with the child, and proportion of time the dog stayed in non-petting physical contact, which we conceptually labeled Dog Proximity-Seeking. The second factor included the frequency of the child giving the dog a solicitation command (such as ‘come’ or ‘sit’) and the duration the child pet the dog, which was conceptually labeled Child-Solicited Pet.
Table 1.
Principal Components and Factor Loadings of the Dyadic Interaction Variables
|
|
|||
|---|---|---|---|
| Components | Coded items | 1 | 2 |
| 1. Dog proximity-seeking | Frequency of dog placing head in direct contact with child | .617 | .244 |
| Duration of non-petting contact | .768 | −.251 | |
| Duration of dog staying within arm’s reach of child | .838 | .063 | |
| 2. Child-solicited petting | Frequency of child giving dog command | .280 | .855 |
| Duration of child petting dog | −.183 | .849 | |
Behavioral Observation of Child Performance
As a quality control check for unanticipated performance differences between conditions that might have influenced stress responses, child performance in the TSST-C was scored from video recordings by two trained raters. For the speech task, the number of verbal prompts required to sustain the speech was coded. For the math task, the lowest correct number reached by the child was recorded. Thirteen percent of the videos were double-coded. Intraclass correlations were .95–.99.
Results
All statistical analyses were performed using SPSS 22.0. Cortisol data were positively skewed; therefore, conventional log10 transformations were applied to the cortisol data to restore normality of the distributions. Preliminary analyses conducted for quality control purposes checked that children assigned to the three testing conditions did not differ on key demographic variables. Children in the three experimental conditions did not differ by age or sex, F(2, 98) =.30–1.13, ps =.33–.74. There were also no significant differences in dog breed group by condition, χ2(10) =4.31, p =.93.
Next, analyses tested for any potential demographic effects on the key outcome measures for perceived stress and cortisol levels. There were no significant effects for child age (rs =−.09−.13, ps =.21–.64), sex (ts =.32–1.32, ps =.19–.97), and dog breed group (Fs =.33–1.88, ps =.12–.89) and these variables were, therefore, not considered further. Because of the diurnal rhythm in cortisol, we tested for time of day at testing (late morning vs. afternoon time blocks). Mean cortisol rise/drop during the AM block was .042/.040 μg/dl (SD =.10/.05) and during the PM block was .083/.065 (SD =.17/.10). Although time of day was not significantly associated with cortisol [ts(92) =−1.1–1.0, ps =.28–.89] or perceived stress [ts(97) =.99–1.32, ps =.18–.32], upon a reviewer’s request we retained this variable in all analyses as a control variable.
Children assigned to the three experimental conditions did not differ on the verbal and math performance indicators in the TSST-C. No group differences were observed for either the number of verbal prompts required to sustain the speech task, F(2, 77) =.034, p =.97, or for the lowest number reached during the serial subtraction task, F(2, 77) =1.126, p =.33.
Children in the three experimental conditions did not differ in their baseline perceived stress levels, F(2, 96) =1.66, p =.20, or baseline cortisol levels, F(2, 91) =.06, p =.93. These null findings are important as they lend confidence that any significant effects observed for changes in perceived stress or cortisol levels following the TSST-C are not due to differences prior to the start of the stressor.
Given known challenges in eliciting stress responses in children (Gunnar et al., 2009) we next tested whether the TSST-C successfully elicited a significant rise in perceived stress and the cortisol response to stress. Among children in the standard TSST-C protocol, that is, those who completed the TSST-C alone without a social support figure, there was a significant rise in perceived stress from baseline to stress phases [t(30)= 7.01, p <.001]. Likewise, children in the alone condition showed a significant rise in cortisol [from 0 to +25 min following the start of the TSST-C; t(28) =2.37, p <.05].
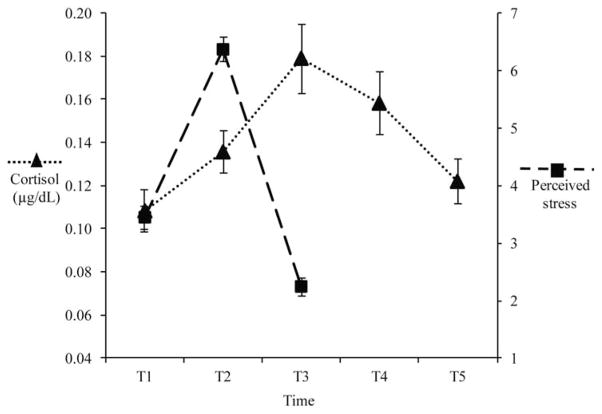
Repeated measures ANOVA (RMANOVA) was performed to analyze the change in perceived stress between baseline, end of stress, and recovery phases. Condition was examined as a between-subject factor, and a condition x time interaction was used to examine differences between groups in the change over time. Within-subjects contrasts were used to compare perceived stress from baseline to end of stress phase (i.e., stress response) and from end of stress to recovery (i.e., stress recovery). Greenhouse-Geisser corrections were used to correct for non-sphericity. The within-subject main effect of time was significant, F(1.84, 173.58) =156.89, p <.001. Change over time in perceived stress is shown in Figure 2. Within-subjects contrasts showed the change from time baseline to stress was significant, Mbaseline-stress = 2.9, SD = 2.67, F(1, 94) = 130.25, p < .001. The change between stress and recovery was also significant, Mstress-recovery =−4.2, SD = 2.56, F(1, 94) =193.38, p <.001. The between-subject main effect of experimental condition was not significant, F(2, 94) =.77, p =.46; however, the time x condition interaction was significant, F(3.69, 173.58) =3.48, p <.05. Within-subjects contrasts show that change between baseline and stress significantly differed by condition, F(2, 94) =5.30, p <.01; whereas change from stress to recovery did not differ by condition, F(2, 94) =.99, p =.38.
Figure 2.
Mean Change Over Time in Perceived Stress and Cortisol During the Experimental Protocol.
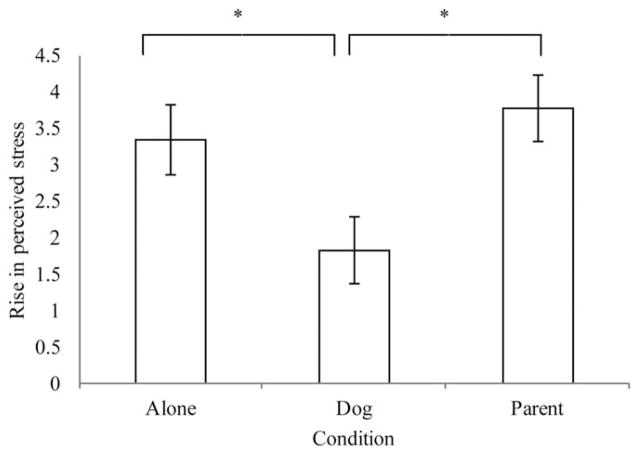
To clarify the nature of the time x condition interaction, ANOVAs were conducted to evaluate the effect of testing condition on rise in perceived stress (baseline to stress). As in the RMANOVA, results showed that the rise in perceived stress from baseline to end of TSST-C differed significantly across experimental conditions, F(2, 94) =5.30, p <.01. Post hoc comparisons with Tukey’s HSD to correct for multiple comparisons showed that children in the pet present condition had a lower rise in perceived stress (see Figure 3) compared to children in the parent condition (Mdiff =−1.96, p <.01) and alone condition (Mdiff =−1.53, p <.05), whereas children in the parent present and alone condition did not significantly differ in perceived stress (Mdiff =−.42, p = .79).
Figure 3.
Rise in Perceived Stress from Baseline to End of TSST-C by Testing Condition. Pet Dog Presence is Associated with a Lower Rise in Perceived Stress. Error Bars Indicate Standard Error. *p <.05 After Correction for Multiple Comparisons.
A RMANOVA was similarly performed to analyze the change of cortisol from 0, +25, and +45 min to correspond to baseline, stress response, and recovery. The within-subject main effect of time was significant, F(1.37, 123.18) =17.59, p <.001. Change over time in cortisol levels are shown in Figure 2. Within-subject contrasts showed that the change from baseline to stress was significant Mbaseline-stress = .07μg/dl, SD =.16, F(1, 90) =21.97, p <.001. The change between stress and recovery was also significant, Mstress-recovery = .10μg/dl, SD =.15, F(1, 90) =8.45, p <.01. The between-subject main effect of experimental condition was a non-significant trend, F(2, 90) =2.85, p =.06 and the time x condition interaction was a non-significant trend, F(2.73, 123.18) =2.22, p =.10. Table 2 indicates the mean rise in perceived stress and cortisol levels in response to the TSST-C by experimental condition.
Table 2.
Perceived Stress and Cortisol Rise to TSST-C by Experimental Condition
| Mean perceived stress response (SD) | Mean cortisol response in μg/dl (SD) | |
|---|---|---|
| Total | 2.93 (2.68) | .07 (.16) |
| Alone condition | 3.33 (2.72) | .05 (.10) |
| Dog condition | 1.80 (2.54) | .11 (.21) |
| Parent condition | 3.76 (2.45) | .04 (.12) |
Note: Cortisol response, reflecting the rise from baseline to +25 min after the onset of the stressor, are shown as raw values for ease of interpretation; however, cortisol analyses were conducted on log10 transformed data as per standard conventions.
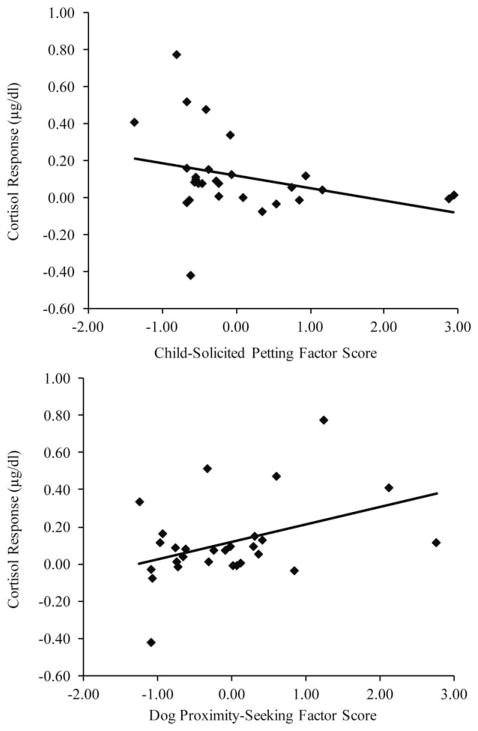
Finally, we tested whether the variation in interaction between the child and dog was related to the rise in perceived stress or cortisol levels in the dyads assigned to the pet present condition. Hierarchical regressions were used to test the associations of Dog Proximity-Seeking and Child-Solicited Pet with cortisol and perceived stress response in two separate analyses. Time of day was entered as a control variable on the first step, and Dog Proximity-Seeking and Child-Solicited Pet were entered on the second step. Variation in dyadic interaction was unrelated to children’s perceived stress response, F(3, 24) =.76, p =.53. However, dyadic interaction was significantly associated with cortisol response F(3, 24) =3.91, p <.05 (see Figure 4). Smaller cortisol responses were associated with both higher Child-Solicited Pet (t =−2.41, β =−.41, p <.05) and with lower Dog Proximity-Seeking (t =2.39, β =.40, p <.05). Dyadic interaction factors during the TSST-C were unrelated to perceived stress or cortisol assessed for baseline or recovery, indicating that dyadic interaction was linked only with the rise in cortisol during the TSST-C.
Figure 4.
When a Pet Dog was Present During the TSST-C, Lower Cortisol Response to Stress was Associated with Higher Child-Initiated Petting and Lower Dog Proximity-Seeking Behavior.
Discussion
The primary aim of this study was to test whether the presence of a child’s pet dog buffered perceived or cortisol stress responses during a standardized laboratory stressor, in comparison to a traditional ‘alone’ condition or an alternate social support condition provided by parental presence. A secondary aim was to examine whether natural variation in child-pet interaction impacted the stress response in the pet present condition. Results supported the notion that pet dogs buffer children’s perceived stress response; however, the results with children’s cortisol response were more complex.
The divergent finding for perceived stress and cortisol stress responses is not entirely unexpected, as perceived stress and neuroendocrine responses to stress are believed to be mediated by different cognitive and neurobiological pathways (Folkman, Lazarus, Dunkel-Schetter, DeLongis, & Gruen, 1986; Gross, 2002; Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005). Even for the TSST, widely considered the most robust laboratory based psychosocial stress protocol, dissociations among perceived stress and cortisol stress responses are observed in ~75 percent of published studies incorporating both types of measures (Campbell & Ehlert, 2012).
Pet dog presence significantly buffered children’s rise in perceived stress compared to children in the alone or parent present conditions. The non-evaluative nature of dog companionship may explain its buffering effect on children’s perceived stress (Allen et al., 1991). Social evaluation is one of the major triggers for socially-related anxiety problems (Rapee & Heimberg, 1997). During middle childhood, children increasingly engage in social comparison and incorporate others’ feedback into their self-concept (Cole et al., 2001; Harter, 1998); these emerging abilities make children more sensitive to social evaluation related stressors compared to younger children. Because dog companions are by nature not evaluative in the same way as human companions, they may buffer perceived stress more effectively, at least under some conditions. This hypothesis is consistent with a study conducted with female adults performing a mental arithmetic task which showed the highest autonomic responses in the presence of a female friend, the lowest response with a pet dog present, with intermediate levels in an alone condition (Allen et al., 1991). Although perceived stress was not assessed in that study, the results are strikingly similar to the perceived stress results observed with children in the present study.
The main effect of pet presence on buffering children’s perceived stress did not extend to children’s cortisol response. Cortisol change over time did not significantly differ by experimental condition; in fact, mean cortisol (averaged across all time points) demonstrated modestly higher average cortisol levels in the pet present condition. Examining dyadic interaction between child and pet in the pet present condition was revealing. For children in the pet present condition, the more children engaged in child-solicited petting, and the less dogs engaged in spontaneous proximity-seeking behavior, the lower the cortisol response. Petting may have directly induced physiologic changes that were independent of children’s conscious perceptions of stress. The observed association of petting with cortisol is consistent with experimental studies in adults that manipulate petting and other aspects of positive social interaction. Those studies have shown positive physiological benefits for petting and social interaction, including increased β-endorphins, prolactin, β-phenylethylamine, oxytocin, and dopamine, and a reduction in cortisol (Miller et al., 2009; Nagasawa et al., 2015; Odendaal & Meintjes, 2003).
With respect to the dogs’ proximity-seeking behavior, the direction of effects was counter to our expectations. There are at least two potential explanations for the observed association. One stems from dogs’ known ability to discriminate human behaviors (Udell, Dorey, & Wynne, 2010) and potentially biochemical signals (Guest, 2013; McCulloch et al., 2006). It is possible that dogs may have sensed children’s elevated stress responses and dog proximity-seeking behaviors might have been a response to those signals. Another possibility is that dogs’ proximity-seeking behavior may have contributed to the child’s heightened cortisol responses by adding to the cognitive and behavioral burden on the child during the TSST-C.
The dyadic interaction findings may shed light on why prior studies examining a potential buffering effect of dog presence on cortisol have yielded inconsistent results (Lass-Hennemann et al., 2014; Polheber et al., 2014). The significant associations of cortisol response with multiple aspects of dyadic interaction observed in the present study suggest discrepancies in the literature may be due to unmeasured variation in human-animal interaction.
The present results should be interpreted in light of some limitations. First, the self-report measure of perceived stress was completed at the conclusion of the TSST-C protocol rather than during the TSST-C. This design was implemented for two reasons. First, it preserved the TSST-C paradigm without interruption or modification. Second, it enabled all children, regardless of testing condition, to freely report on their perceived stress levels while not in the presence of their social support figure. This prevented a potential confound if some children were asked about perceived stress while the support figure was still present. It is possible that completing the perceived stress measure at the conclusion of the protocol may have inadvertently led to recall bias in perceived stress. However, children did not differ by condition on baseline perceived stress. Also, all families brought their pet dog to the lab and were not aware that there were multiple conditions to which participants could have been assigned. Whereas it is possible that recall bias may have occurred globally for all participants, there was no a priori reason or evidence that this would have differed by experimental condition.
Second, the sample of participants was largely comprised of White, middle-class families and the generalizability of the findings warrants further study. It is unknown whether the stress-buffering effect would differ among other ethnic or socioeconomic groups who may have different experiences with respect to stress exposures (Evans & English, 2002). Third, the participants in this study were typically developing children prescreened for any known medical conditions. Therefore, whether these findings generalize to clinical populations is unknown. Animals, and especially dogs, are already used for therapeutic purposes for children requiring hospitalization or with developmental disabilities (Anderson & Olson, 2006; Limond, Bradshaw, & Cormack, 1997; Martin & Farnum, 2002; Wu, Niedra, Pendergast, & McCrindle, 2002). Demonstrating the impact of pet dog presence on stress responses among typically developing children provides the basic science needed to provide a foundation for research in special populations. Finally, this study did not select for particular dog breeds. Thus, children in the study were diverse with respect to breed of dogs owned. Although the results did not show any effect of breed class on any of the key measures, future studies should include more rigorous testing among pre-selected breeds differing in morphology or general function (e.g., companionship vs. working) for direct comparison to determine whether there are breed differences in dogs’ ability to buffer children’s stress responses.
This study intentionally recruited dog-owning families. This was done to avoid the challenges in interpretation of previous studies combining dog owners vs. non-owners. A primary focus of this study was to test whether having a pet dog present would buffer child owners’ stress responses to a novel stressor. Among dog-owning children, future research is warranted examining whether child characteristics, such as temperament or their relationship quality with support figures, impacts children’s stress responses. Finally, our focus on the stress-buffering effects of pets among dog-owning families differs from the equally interesting question of whether there are potential socioemotional benefits of dog ownership for children in general.
The present study adds to the small but growing literature on HAI in several ways. It is the first study to rigorously test and demonstrate a stress buffering effect of pet dog presence on children’s perceived stress during a stressful task. Second, it represents the first study to empirically test the impact of pet dogs on cortisol responses in an unselected sample of typically developing children. The focus on middle childhood highlights a developmental phase during which children’s social support figures are expanding beyond caregivers but their emotional and biological responses to stress are still maturing, as are their emotion regulation capacities. The findings of this study support the notion that pets function as social support figures in middle childhood (Bryant, 1985). Given that stress responses during childhood predict emotional and health problems across the spectrum of internalizing and externalizing during childhood and adulthood, understanding the potential protective effects of social buffering is critical to understanding long-term risk for stress-related disorders. Third, given the HAI literature on stress buffering has been fraught with methodological limitations, including combining pet and non-pet owners, the use of unfamiliar dogs without a history of social support with study participants, a lack of systematic inquiry in perceived stress and cortisol stress responses, and no assessments of dog behaviors, the present study advances our understanding of the impact of pet dogs on stress responses more generally. In sum, this study demonstrated that among typically developing children, perceived stress during a novel stressor is buffered in the presence of a pet dog. It also demonstrated that when children are under stress with their pet dog present, the cortisol response to stress is associated with both the degree to which children solicit and pet their dogs and the degree to which dogs seek proximity to their child owners. These findings provide clear and compelling evidence that pet dogs have direct socioemotional and physiological effects on children in the face of a novel stressor.
Acknowledgments
This research was supported by the National Institute of Health grant HD071288.
References
- Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: Implications of cross-informant correlations for situational specificity. Psychological Bulletin. 1987;101:213–232. doi: 10.1037/0033-2909.101.2.213. [DOI] [PubMed] [Google Scholar]
- Allen K, Blascovich J, Mendes WB. Cardiovascular reactivity and the presence of pets, friends, and spouses: The truth about cats and dogs. Psychosomatic Medicine. 2002;64:727–739. doi: 10.1097/01.psy.0000024236.11538.41. [DOI] [PubMed] [Google Scholar]
- Allen KM, Blascovich J, Tomaka J, Kelsey RM. Presence of human friends and pet dogs as moderators of autonomic responses to stress in women. Journal of Personality and Social Psychology. 1991;61:582–589. doi: 10.1037/0022-3514.61.4.582. [DOI] [PubMed] [Google Scholar]
- American Pet Products Association. Household penetration rates for pet ownership in the United States from 1988 to 2013. Statista-The Statistics Portal. 2014 Retrieved October 24, 2014, from http://www.statista.com/statistics/198086/us-household-penetration-ratesfor-pet-owning-since-2007/
- Anderson KL, Olson MR. The value of a dog in a classroom of children with severe emotional disorders. Anthrozoös: A Multidisciplinary Journal of the Interactions of People & Animals. 2006;19:35–49. [Google Scholar]
- Barker SB, Wolen AR. The benefits of human-companion animal interaction: A review. Journal of Veterinary Medical Education. 2008;35:487–495. doi: 10.3138/jvme.35.4.487. [DOI] [PubMed] [Google Scholar]
- Beetz A, Julius H, Turner D, Kotrschal K. Effects of social support by a dog on stress modulation in male children with insecure attachment. Frontiers in Psychology. 2012;3:352. doi: 10.3389/fpsyg.2012.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beetz A, Uvnäs-Moberg K, Julius H, Kotrschal K. Psychosocial and psychophysiological effects of human-animal interactions: The possible role of oxytocin. Frontiers in Psychology. 2012;3:234. doi: 10.3389/fpsyg.2012.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bryant BK. The neighborhood walk: Sources of support in middle childhood. Monographs of the Society for Research in Child Development. 1985;50:1–122. [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Campbell J, Ehlert U. Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37:1111–1134. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Caserta TA, Punamäki RL, Pirttilä-Backman AM. The buffering role of social support on the psychosocial wellbeing of orphans in Rwanda. Social Development. doi: 10.1111/sode.12188. (in press) Advance online publication. [DOI] [Google Scholar]
- Cillessen AHN, Bellmore AD. Accuracy of social self-perceptions and peer competence in middle childhood. Merrill-Palmer Quarterly. 1999;45:650–676. Retrieved from http://www.jstor.org/stable/23093376. [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98:310–357. doi: 10.1037/0033-2909.98. [DOI] [PubMed] [Google Scholar]
- Cole DA, Maxwell SE, Martin JM, Peeke LG, Seroczynski AD, Tram JM, et al. The development of multiple domains of child and adolescent self-concept: A cohort sequential longitudinal design. Child Development. 2001;72:1723–1746. doi: 10.1111/1467-8624.00375. [DOI] [PubMed] [Google Scholar]
- Cole PM, Michel MK, Teti LO. The development of emotion regulation and dysregulation: A clinical perspective. Monographs of the Society for Research in Child Development. 1994;59:73–100. doi: 10.2307/1166139. [DOI] [PubMed] [Google Scholar]
- Daly B, Morton LL. An investigation of human–animal interactions and empathy as related to pet preference, ownership, attachment, and attitudes in children. Anthrozoös: A Multidisciplinary Journal of the Interactions of People & Animals. 2006;19:113–127. [Google Scholar]
- Demello LR. The effect of the presence of a companion-animal on physiological changes following the termination of cognitive stressors. Psychology & Health. 1999;14:859–868. doi: 10.1080/08870449908407352. [DOI] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dieleman GC, van der Ende J, Verhulst FC, Huizink AC. Perceived and physiological arousal during a stress task: Can they differentiate between anxiety and depression? Psychoneuroendocrinology. 2010;35:1223–1234. doi: 10.1016/j.psyneuen.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL, Eggum ND. Emotion-related self-regulation and its relation to children’s maladjustment. Annual Review of Clinical Psychology. 2010;6:495–525. doi: 10.1146/annurev.clinpsy.121208.131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Folkman S, Lazarus RS, Dunkel-Schetter C, DeLongis A, Gruen RJ. Dynamics of a stressful encounter: Cognitive appraisal, coping, and encounter outcomes. Journal of Personality and Social Psychology. 1986;50:992–1003. doi: 10.1037//0022-3514.50.5.992. doi:1037/0022-3514.50.5.992. [DOI] [PubMed] [Google Scholar]
- Friedmann E, Katcher AH, Thomas SA, Lynch JJ, Messent PR. Social interaction and blood pressure: Influence of animal companions. The Journal of Nervous and Mental Disease. 1983;171:461–465. doi: 10.1097/00005053-198308000-00002. [DOI] [PubMed] [Google Scholar]
- Grazzani I, Ornaghi V. How do use and comprehension of mental-state language relate to theory of mind in middle childhood? Cognitive Development. 2012;27:99–111. doi: 10.1016/j.cogdev.2012.03.002. [DOI] [Google Scholar]
- Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/S0048577201393198. [DOI] [PubMed] [Google Scholar]
- Guest C. Canine olfactory detection of human disease. In: Grassberger M, Sherman RA, Gileva OS, Kim CMH, Mumcuoglu KY, editors. Biotherapy-History, principles and practice. London: Springer; 2013. pp. 285–302. Retrieved from http://link.springer.com/chapter/10.1007/978-94-007-6585-6_11. [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/S03064530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall N, Liu J, Kertes DA, Wynne CDL. Behavioral and self-report measures influencing children’s reported attachment to their dog. Anthrozoös: A Multidisciplinary Journal of the Interactions of People & Animals. 2016;29:137–150. doi: 10.1080/08927936.2015.1088683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel P, Petermann F. Perceived stress, coping, and adjustment in adolescents. Journal of Adolescent Health. 2006;38:409–415. doi: 10.1016/j.jadohealth.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Hansen KM, Messinger CJ, Baun MM, Megel M. Companion animals alleviating distress in children. Anthrozoös: A Multidisciplinary Journal of the Interactions of People & Animals. 1999;12:142–148. [Google Scholar]
- Harter S. The development of self-representations. In: Eisenberg N, Damon W, editors. Handbook of child psychology: Vol. 3. Social, emotional, and personality development. 5. New York: Wiley; 1998. pp. 102–132. [Google Scholar]
- Havener L, Gentes L, Thaler B, Megel ME, Baun MM, Driscoll FA, et al. The effects of a companion animal on distress in children undergoing dental procedures. Issues in Comprehensive Pediatric Nursing. 2001;24:137–152. doi: 10.1080/01460860118472. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: Diversity, mechanisms, and functions. Frontiers in Neuroendocrinology. 2009;30:470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Cicchetti D, Rogosch F. Salivary biomarker levels and diurnal variation: Associations with medications prescribed to control children’s problem behavior. Child Development. 2007;78:927–937. doi: 10.1111/j.1467-8624.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann AO, Lee AH, Wertenauer F, Ricken R, Jansen JJ, Gallinat J, et al. Dog-assisted intervention significantly reduces anxiety in hospitalized patients with major depression. European Journal of Integrative Medicine. 2009;1:145–148. [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin. 2014;140:256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RE, Price JM. Vulnerability to psychopathology: Risk across the lifespan. 2. New York, NY: Guilford Press; 2010. [Google Scholar]
- Kerns KA, Tomich PL, Kim P. Normative trends in children’s perceptions of availability and utilization of attachment figures in middle childhood. Social Development. 2006;15:1–22. doi: 10.1111/j.1467-9507.2006.00327.x. [DOI] [Google Scholar]
- Kertes DA, Gunnar MR. Evening activities as a potential confound in research on the adrenocortical system in children. Child Development. 2004;75:193–204. doi: 10.1111/j.1467-8624.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- Kingwell B, Lomdahl A, Anderson W. Presence of a pet dog and human cardiovascular responses to mild mental stress. Clinical Autonomic Research. 2001;11:313–317. doi: 10.1007/BF02332977. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: A overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kotrschal K, Ortbauer B. Behavioral effects of the presence of a dog in a classroom. Anthrozoös: A Multidisciplinary Journal of the Interactions of People & Animals. 2003;16:147–159. [Google Scholar]
- Kruger KA, Serpell JA. Animal-assisted interventions in mental health: Definitions and theoretical foundations. Handbook on Animal-Assisted Therapy: Theoretical Foundations and Guidelines for Practice. 2006;2:21–38. [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C. Ten years of research with the Trier Social Stress Test–Revisited. In: Eddie HJ, Piotr W, editors. Social neuroscience: Integrating biological and psychological explanations of social behavior. New York, NY: Guilford Press; 2007. pp. 56–83. [Google Scholar]
- Kurdek LA. Pet dogs as attachment figures. Journal of Social and Personal Relationships. 2008;25:247–266. [Google Scholar]
- Lakey B, Cohen S. Social support theory and measurement. In: Cohen S, Underwood LG, Gottlieb BH, editors. Social support measurement and intervention: A guide for health and social scientists. New York, NY: Oxford University Press; 2000. pp. 29–52. [Google Scholar]
- Lam CB, McHale SM, Crouter AC. Parent–child shared time from middle childhood to late adolescence: Developmental course and adjustment correlates. Child Development. 2012;83:2089–2103. doi: 10.1111/j.1467-8624.2012.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass-Hennemann J, Peyk P, Streb M, Holz E, Michael T. Presence of a dog reduces subjective but not physiological stress responses to an analogue trauma. Frontiers in Psychology. 2014;5:1010. doi: 10.3389/fpsyg.2014.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen-Feldner EW, Zvolensky MJ, Feldner MT. Behavioral inhibition sensitivity and emotional response suppression: A laboratory test among adolescents in a fear-relevant paradigm. Journal of Clinical Child and Adolescent Psychology. 2004;33:783–791. doi: 10.1207/s15374424jccp3304_13. [DOI] [PubMed] [Google Scholar]
- Limond JA, Bradshaw JWS, Cormack MKF. Behavior of children with learning disabilities interacting with a therapy dog. Anthrozoös: A Multidisciplinary Journal of the Interactions of People & Animals. 1997;10:84–89. doi: 10.2752/089279397787001139. [DOI] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Martin F, Farnum J. Animal-assisted therapy for children with pervasive developmental disorders. Western Journal of Nursing Research. 2002;24:657–670. doi: 10.1177/019394502320555403. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? coherence among emotion experience, behavior, and physiology. Emotion. 2005;5:175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- McCulloch M, Jezierski T, Broffman M, Hubbard A, Turner K, Janecki T. Diagnostic accuracy of canine scent detection in early-and late-stage lung and breast cancers. Integrative Cancer Therapies. 2006;5:30–39. doi: 10.1177/1534735405285096. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Kennedy C, DeVoe D, Hickey M, Nelson T, Kogan L. An examination of changes in oxytocin levels in men and women before and after interaction with a bonded dog. Anthrozoös: A Multidisciplinary Journal of the Interactions of People and Animals. 2009;22:31–42. doi: 10.2752/175303708x390455. [DOI] [Google Scholar]
- Millot JL, Filiatre JC. The behavioural sequences in the communication system between the child and his pet dog. Applied Animal Behaviour Science. 1986;16:383–390. doi: 10.1016/0168-1591(86)90010-9. [DOI] [Google Scholar]
- Millot JL, Filiatre JC, Gagnon AC, Eckerlin A, Montagner H. Children and their pet dogs: How they communicate. Behavioural Processes. 1988;17:1–15. doi: 10.1016/0376-6357(88)90046-0. [DOI] [PubMed] [Google Scholar]
- Nagasawa M, Mitsui S, En S, Ohtani N, Ohta M, Sakuma Y, et al. Oxytocingaze positive loop and the coevolution of human-dog bonds. Science. 2015;348:333–336. doi: 10.1126/science.1261022. [DOI] [PubMed] [Google Scholar]
- Nagengast SL, Baun MM, Megel M, Leibowitz JM. The effects of the presence of a companion animal on physiological arousal and behavioral distress in children during a physical examination. Journal of Pediatric Nursing. 1997;12:323–330. doi: 10.1016/s0882-5963(97)80058-9. [DOI] [PubMed] [Google Scholar]
- Odendaal JSJ, Meintjes RA. Neurophysiological correlates of affiliative behaviour between humans and dogs. The Veterinary Journal. 2003;165:296–301. doi: 10.1016/S1090-0233(02)00237-X. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Ormel J, Bosch NM, Bouma E, Van Roon AM, Rosmalen JG, et al. Stressed out? associations between perceived and physiological stress responses in adolescents: The TRAILS study. Psychophysiology. 2011;48:441–452. doi: 10.1111/j.1469-8986.2010.01118.x. [DOI] [PubMed] [Google Scholar]
- Polheber J, Matchock R. The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. Journal of Behavioral Medicine. 2014;37:860–867. doi: 10.1007/s10865-013-9546-1. [DOI] [PubMed] [Google Scholar]
- Protopopova A, Gilmour AJ, Weiss RH, Shen JY, Wynne CDL. The effects of social training and other factors on adoption success of shelter dogs. Applied Animal Behaviour Science. 2012;142:61–68. [Google Scholar]
- Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behaviour Research and Therapy. 1997;35:741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- Selman RL. Social-cognitive understanding: A guide to educational and clinical practice. In: Lickona T, editor. Moral development and behavior: Theory, research, and social issues. New York: Holt, Rinehart, & Winston; 1976. pp. 299–316. [Google Scholar]
- Serpell JA. Evidence for an association between pet behavior and owner attachment levels. Applied Animal Behaviour Science. 1996;47:49–60. [Google Scholar]
- Svartberg K, Tapper I, Temrin H, Radesäter T, Thorman S. Consistency of personality traits in dogs. Animal Behaviour. 2005;69:283–291. doi: 10.1016/j.anbehav.2004.04.011. [DOI] [Google Scholar]
- Udell M, Dorey NR, Wynne CDL. What did domestication do to dogs? a new account of dogs’ sensitivity to human actions. Biological Reviews. 2010;85:327–345. doi: 10.1111/j.1469-185X.2009.00104.x. [DOI] [PubMed] [Google Scholar]
- Vaisvaser S, Lin T, Admon R, Podlipsky I, Greenman Y, Stern N, et al. Neural traces of stress: Cortisol related sustained enhancement of amygdalahippocampal functional connectivity. Frontiers in Human Neuroscience. 2013;7:313. doi: 10.3389/fnhum.2013.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgarth C, Boddy LM, Stratton G, German AJ, Gaskell RM, Coyne KP, et al. Pet ownership, dog types and attachment to pets in 9–10 year old children in Liverpool, UK. BMC Veterinary Research. 2013;9:102. doi: 10.1186/1746-6148-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AS, Niedra R, Pendergast L, McCrindle BW. Acceptability and impact of pet visitation on a pediatric cardiology inpatient unit. Journal of Pediatric Nursing. 2002;17:354–362. doi: 10.1053/jpdn.2002.127173. [DOI] [PubMed] [Google Scholar]