Abstract
The formation of beta-amyloid in the brains of individuals with Alzheimer disease requires the proteolytic cleavage of a membrane-associated precursor protein. The proteases that may be involved in this process have not yet been identified. Cathepsins are normally intracellular proteolytic enzymes associated with lysosomes; however, when sections from Alzheimer brains were stained by antisera to cathepsin D and cathepsin B, high levels of immunoreactivity were also detected in senile plaques. Extracellular sites of cathepsin immunoreactivity were not seen in control brains from age-matched individuals without neurologic disease or from patients with Huntington disease or Parkinson disease. In situ enzyme histochemistry of cathepsin D and cathepsin B on sections of neocortex using synthetic peptides and protein substrates showed that senile plaques contained the highest levels of enzymatically active cathepsin. At the ultrastructural level, cathepsin immunoreactivity in senile plaques was localized principally to lysosomal dense bodies and lipofuscin granules, which were extracellular. Similar structures were abundant in degenerating neurons of Alzheimer neocortex, and cathepsin-laden neuronal perikarya in various stages of disintegration could be seen within some senile plaques. The high levels of enzymatically competent lysosomal proteases abnormally localized in senile plaques represent evidence for candidate enzymes that may mediate the proteolytic formation of amyloid. We propose that amyloid precursor protein within senile plaques is processed by lysosomal proteases principally derived from degenerating neurons. Escape of cathepsins from the stringently regulated intracellular milieu provides a basis for an abnormal sequence of proteolytic cleavages of accumulating amyloid precursor protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azaryan A., Barkhudaryan N., Galoyan A. Some properties of human and bovine brain cathepsin B. Neurochem Res. 1985 Nov;10(11):1511–1524. [PubMed] [Google Scholar]
- Bahmanyar S., Higgins G. A., Goldgaber D., Lewis D. A., Morrison J. H., Wilson M. C., Shankar S. K., Gajdusek D. C. Localization of amyloid beta protein messenger RNA in brains from patients with Alzheimer's disease. Science. 1987 Jul 3;237(4810):77–80. doi: 10.1126/science.3299701. [DOI] [PubMed] [Google Scholar]
- Banay-Schwartz M., Dahl D., Hui K. S., Lajtha A. The breakdown of the individual neurofilament proteins by cathepsin D. Neurochem Res. 1987 Apr;12(4):361–367. doi: 10.1007/BF00993246. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz L. I., Rodriguez W., Paskevich P., Mufson E. J., Schenk D., Neve R. L. The amyloid precursor protein is concentrated in neuronal lysosomes in normal and Alzheimer disease subjects. Exp Neurol. 1989 Dec;106(3):237–250. doi: 10.1016/0014-4886(89)90156-8. [DOI] [PubMed] [Google Scholar]
- Bergeron C., Ranalli P. J., Miceli P. N. Amyloid angiopathy in Alzheimer's disease. Can J Neurol Sci. 1987 Nov;14(4):564–569. [PubMed] [Google Scholar]
- Braak H., Braak E., Ohm T., Bohl J. Silver impregnation of Alzheimer's neurofibrillary changes counterstained for basophilic material and lipofuscin pigment. Stain Technol. 1988 Jul;63(4):197–200. doi: 10.3109/10520298809107184. [DOI] [PubMed] [Google Scholar]
- Bradley J. D., Whitaker J. N. Isolation and characterization of cathepsin B from bovine brain. Neurochem Res. 1986 Jun;11(6):851–867. doi: 10.1007/BF00965209. [DOI] [PubMed] [Google Scholar]
- Castaño E. M., Frangione B. Human amyloidosis, Alzheimer disease and related disorders. Lab Invest. 1988 Feb;58(2):122–132. [PubMed] [Google Scholar]
- Cohen M. L., Golde T. E., Usiak M. F., Younkin L. H., Younkin S. G. In situ hybridization of nucleus basalis neurons shows increased beta-amyloid mRNA in Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1227–1231. doi: 10.1073/pnas.85.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. M., Huynh T. V., Saitoh T. Evidence for lysosomal processing of amyloid beta-protein precursor in cultured cells. Neurochem Res. 1989 Oct;14(10):933–939. doi: 10.1007/BF00965926. [DOI] [PubMed] [Google Scholar]
- Cole G., Masliah E., Huynh T. V., DeTeresa R., Terry R. D., Okuda C., Saitoh T. An antiserum against amyloid beta-protein precursor detects a unique peptide in Alzheimer brain. Neurosci Lett. 1989 May 22;100(1-3):340–346. doi: 10.1016/0304-3940(89)90710-6. [DOI] [PubMed] [Google Scholar]
- Coria F., Castaño E. M., Frangione B. Brain amyloid in normal aging and cerebral amyloid angiopathy is antigenically related to Alzheimer's disease beta-protein. Am J Pathol. 1987 Dec;129(3):422–428. [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Dolbeare F. A., Smith R. E. Flow cytometric measurement of peptidases with use of 5-nitrosalicylaldehyde and 4-methoxy-beta-naphthylamine derivatives. Clin Chem. 1977 Aug;23(8):1485–1491. [PubMed] [Google Scholar]
- Dolbeare F., Vanderlaan M. A fluorescent assay of proteinases in cultured mammalian cells. J Histochem Cytochem. 1979 Nov;27(11):1493–1495. doi: 10.1177/27.11.512330. [DOI] [PubMed] [Google Scholar]
- Dyrks T., Weidemann A., Multhaup G., Salbaum J. M., Lemaire H. G., Kang J., Müller-Hill B., Masters C. L., Beyreuther K. Identification, transmembrane orientation and biogenesis of the amyloid A4 precursor of Alzheimer's disease. EMBO J. 1988 Apr;7(4):949–957. doi: 10.1002/j.1460-2075.1988.tb02900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisow M. J., D'Arcy Hart P., Young M. R. Temporal changes of lysosome and phagosome pH during phagolysosome formation in macrophages: studies by fluorescence spectroscopy. J Cell Biol. 1981 Jun;89(3):645–652. doi: 10.1083/jcb.89.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf M., Baici A., Sträuli P. Histochemical localization of cathepsin B at the invasion front of the rabbit V2 carcinoma. Lab Invest. 1981 Dec;45(6):587–596. [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., George L., Tung Y. C., Kim K. S., Wisniewski H. M. Amyloid protein and neurofibrillary tangles coexist in the same neuron in Alzheimer disease. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2853–2857. doi: 10.1073/pnas.86.8.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins G. A., Lewis D. A., Bahmanyar S., Goldgaber D., Gajdusek D. C., Young W. G., Morrison J. H., Wilson M. C. Differential regulation of amyloid-beta-protein mRNA expression within hippocampal neuronal subpopulations in Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1297–1301. doi: 10.1073/pnas.85.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Ishii T., Kametani F., Haga S., Sato M. The immunohistochemical demonstration of subsequences of the precursor of the amyloid A4 protein in senile plaques in Alzheimer's disease. Neuropathol Appl Neurobiol. 1989 Mar-Apr;15(2):135–147. doi: 10.1111/j.1365-2990.1989.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Jacques Y. V., Bainton D. F. Changes in pH within the phagocytic vacuoles of human neutrophils and monocytes. Lab Invest. 1978 Sep;39(3):179–185. [PubMed] [Google Scholar]
- Kelényi G. Thioflavin S fluorescent and Congo red anisotropic stainings in the histologic demonstration of amyloid. Acta Neuropathol. 1967 Feb 3;7(4):336–348. doi: 10.1007/BF00688089. [DOI] [PubMed] [Google Scholar]
- Knight C. G. Human cathepsin B. Application of the substrate N-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide to a study of the inhibition by leupeptin. Biochem J. 1980 Sep 1;189(3):447–453. doi: 10.1042/bj1890447. [DOI] [PMC free article] [PubMed] [Google Scholar]
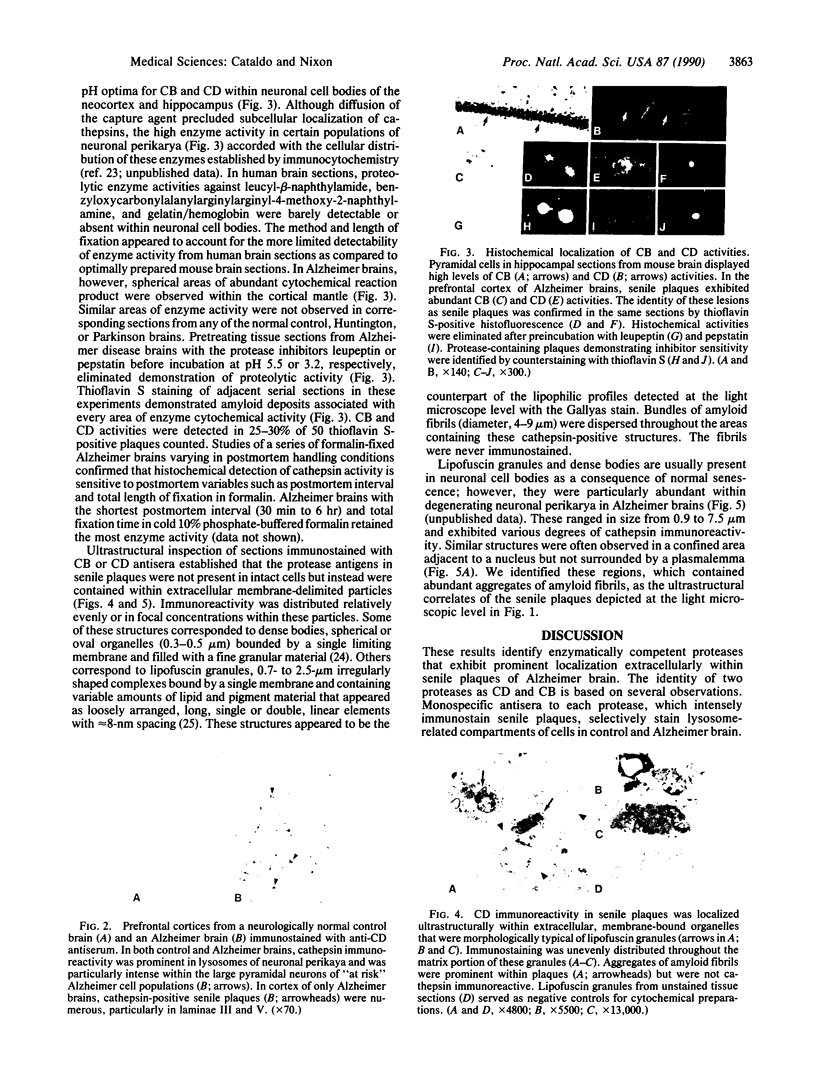
- Majocha R. E., Benes F. M., Reifel J. L., Rodenrys A. M., Marotta C. A. Laminar-specific distribution and infrastructural detail of amyloid in the Alzheimer disease cortex visualized by computer-enhanced imaging of epitopes recognized by monoclonal antibodies. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6182–6186. doi: 10.1073/pnas.85.16.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve R. L., Finch E. A., Dawes L. R. Expression of the Alzheimer amyloid precursor gene transcripts in the human brain. Neuron. 1988 Oct;1(8):669–677. doi: 10.1016/0896-6273(88)90166-3. [DOI] [PubMed] [Google Scholar]
- Nixon R. A., Marotta C. A. Degradation of neurofilament proteins by purified human brain cathepsin D. J Neurochem. 1984 Aug;43(2):507–516. doi: 10.1111/j.1471-4159.1984.tb00928.x. [DOI] [PubMed] [Google Scholar]
- Palmert M. R., Podlisny M. B., Witker D. S., Oltersdorf T., Younkin L. H., Selkoe D. J., Younkin S. G. Antisera to an amino-terminal peptide detect the amyloid protein precursor of Alzheimer's disease and recognize senile plaques. Biochem Biophys Res Commun. 1988 Oct 14;156(1):432–437. doi: 10.1016/s0006-291x(88)80859-3. [DOI] [PubMed] [Google Scholar]
- Perry G., Lipphardt S., Mulvihill P., Kancherla M., Mijares M., Gambetti P., Sharma S., Maggiora L., Cornette J., Lobl T. Amyloid precursor protein in senile plaques of Alzheimer disease. Lancet. 1988 Sep 24;2(8613):746–746. doi: 10.1016/s0140-6736(88)90219-x. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H., Renner I. G. Increased cathepsin B activity in pancreatic juice from a patient with pancreatic cancer. N Engl J Med. 1980 Aug 21;303(8):462–463. doi: 10.1056/nejm198008213030819. [DOI] [PubMed] [Google Scholar]
- Sekhon S. S., Maxwell D. S. Ultrastructural changes in neurons of the spinal anterior horn of ageing mice with particular reference to the accumulation of lipofuscin pigment. J Neurocytol. 1974 Mar;3(1):59–72. doi: 10.1007/BF01111932. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Abraham C. R. Isolation of paired helical filaments and amyloid fibers from human brain. Methods Enzymol. 1986;134:388–404. doi: 10.1016/0076-6879(86)34105-3. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Podlisny M. B., Joachim C. L., Vickers E. A., Lee G., Fritz L. C., Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvén B. Studies on the histochemical "leucine aminopeptidase" reaction. VI. The selective demonstration of cathepsin B activity by means of the naphythylamide reaction. Histochemie. 1968;15(2):150–159. doi: 10.1007/BF00306365. [DOI] [PubMed] [Google Scholar]
- Tate-Ostroff B., Majocha R. E., Marotta C. A. Identification of cellular and extracellular sites of amyloid precursor protein extracytoplasmic domain in normal and Alzheimer disease brains. Proc Natl Acad Sci U S A. 1989 Jan;86(2):745–749. doi: 10.1073/pnas.86.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nostrand W. E., Wagner S. L., Suzuki M., Choi B. H., Farrow J. S., Geddes J. W., Cotman C. W., Cunningham D. D. Protease nexin-II, a potent antichymotrypsin, shows identity to amyloid beta-protein precursor. Nature. 1989 Oct 12;341(6242):546–549. doi: 10.1038/341546a0. [DOI] [PubMed] [Google Scholar]
- Whitaker J. N., Terry L. C., Whetsell W. O., Jr Immunocytochemical localization of cathepsin D in rat neural tissue. Brain Res. 1981 Jul 6;216(1):109–124. doi: 10.1016/0006-8993(81)91281-6. [DOI] [PubMed] [Google Scholar]
- Whitson J. S., Selkoe D. J., Cotman C. W. Amyloid beta protein enhances the survival of hippocampal neurons in vitro. Science. 1989 Mar 17;243(4897):1488–1490. doi: 10.1126/science.2928783. [DOI] [PubMed] [Google Scholar]
- Williams K. R., Williams N. D., Konigsberg W., Yu R. K. Acidic lipids enhance cathepsin D cleavage of the myelin basic protein. J Neurosci Res. 1986;15(2):137–145. doi: 10.1002/jnr.490150203. [DOI] [PubMed] [Google Scholar]
- Wisniewski K. E., Maslinska D. Immunoreactivity of ceroid lipofuscin storage pigment in Batten disease with monoclonal antibodies to the amyloid beta-protein. N Engl J Med. 1989 Jan 26;320(4):256–257. doi: 10.1056/nejm198901263200421. [DOI] [PubMed] [Google Scholar]
- Wong C. W., Quaranta V., Glenner G. G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Hirano A. A comparative study of modified Bielschowsky, Bodian and thioflavin S stains on Alzheimer's neurofibrillary tangles. Neuropathol Appl Neurobiol. 1986 Jan-Feb;12(1):3–9. doi: 10.1111/j.1365-2990.1986.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Dawes L. R., Fisher S., Villa-Komaroff L., Oster-Granite M. L., Neve R. L. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science. 1989 Jul 28;245(4916):417–420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]