Supplemental Digital Content is available in the text
Keywords: bevacizumab, hemorrhage, meta-analysis, metastatic colorectal cancer
Abstract
Background:
As an important antivascular endothelial growth factor monoclonal antibody, bevacizumab has been administrated for the treatment of cancer patients. Hemorrhage, one of the common adverse events of angiogenesis inhibitors, sometimes is also fatal and life-threatening. We aimed at determining the incidence and risk of hemorrhage associated with bevacizumab in patients with metastatic colorectal cancer (mCRC).
Methods:
We searched PubMed, EMBASE, and the Web of Science databases for relevant randomized controlled trials (RCTs). The overall incidence, overall relative risk (RR), and 95% confidence interval (CI) were calculated by using a random-effects or fixed-effects model based on the heterogeneity of selected trials.
Results:
A total of 10,555 mCRC patients from 12 RCTs were included in our study. The overall incidence of hemorrhage was 5.8% (95% CI 3.9%–7.8%). Bevacizumab significantly increased the overall risk of hemorrhage with an RR of 1.96 (95% CI 1.27–3.02). The RR of all-grade hemorrhage was 2.39 (95% CI 1.09–5.24) and 1.41 (95% CI 1.01–1.97) for high-grade hemorrhage. The risk of hemorrhage associated with bevacizumab was dose-dependent with an RR of 1.73 (95% CI 1.15–2.61) for 2.5 mg/kg/wk and 4.67 (95% CI 2.36–9.23) for 5 mg/kg/wk. More importantly, the RR of hemorrhage for treatment duration (<= 6 months and > 6 months) based on subgroup analysis was 4.13 (95% CI 2.58–6.61) and 1.43 (95% CI 0.96–2.14), respectively.
Conclusion:
The addition of bevacizumab to concurrent antineoplastic in patients with mCRC significantly increased the risk of hemorrhage. The dose of bevacizumab may contribute to the risk of hemorrhage. And the 1st 6 months of treatment may be a crucial period when hemorrhagic events occur.
1. Introduction
Angiogenesis, the formation of new vessels, has been proved as an indispensable intermediate process for tumor growth, metastasis, and progression.[1,2] For this reason, multiple investigations have been focused on antiangiogenesis to excogitate its inhibitors for tumor therapy. Vascular endothelial growth factor (VEGF) family consists of VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, placenta growth factor(PIGE)-1, and PIGF-2.[3,4] VEGF has been one of the most promising targets for tumor therapy. Bevacizumab (Avastin; Genentech, South San Francisco, CA) was approved for the treatment of various malignancies by the United States Food and Drug Administration for being a recombinant humanized monoclonal antibody that neutralizes VEGF-A. Bevacizumab has been effective in prolonging survival time for the treatment of metastatic colorectal cancer (mCRC) when used in combination with chemotherapy.[5–12] Also it has shown prominent effects in other neoplasms such as metastatic renal cancer,[13,14] nonsmall-cell-lung cancer,[15–18] metastatic breast cancer,[19–23] ovarian cancer,[24,25] cervical cancer,[26] advanced pancreatic cancer,[27] hepatocellular carcinoma,[28] and follicular lymphoma.[29]
However, just as with many other therapeutic agents, the significant adverse effects of bevacizumab should also be taken into consideration such as hypertension, gastrointestinal perforations, wound healing complications, bleeding, and proteinuria (http://www.gene.com/patients/medicines/avastin). Among all the side effects, hemorrhagic events are frequently reported in clinical trials associated with bevacizumab.[5–12,30–33] Unfortunately, the development of risk of hemorrhagic events during the treatment with bevacizumab in cancer patients has not been elucidated clearly. Two studies tried to demonstrate that bevacizumab increased the risk of high-grade hemorrhage in mCRC patients but did not obtain positive results. The earlier one[34] in 2010 included 6 trials associated with mCRC and the subgroup analysis showed that the relative risk (RR) of high-grade hemorrhage was 1.45 (95% confidence interval [CI] 0.88–2.38, see in Table 3) which meant bevacizumab did not increase the risk of high-grade hemorrhage in mCRC patients. Similarly, the other one[35] in 2011 included 8 trials associated with mCRC. The RR of high-grade hemorrhage was 1.23 (95% CI 0.80–1.89, see in Fig. 2). As for mCRC patients, high-grade hemorrhage such as gastrointestinal perforation was commonly fatal and life-threatening. Therefore, there was a great necessity to confirm the risk of hemorrhage in mCRC patients treated with bevacizumab.
Table 3.
Incidence and relative risk of hemorrhage in mCRC patients treated with bevacizumab based on subgroup analysis.
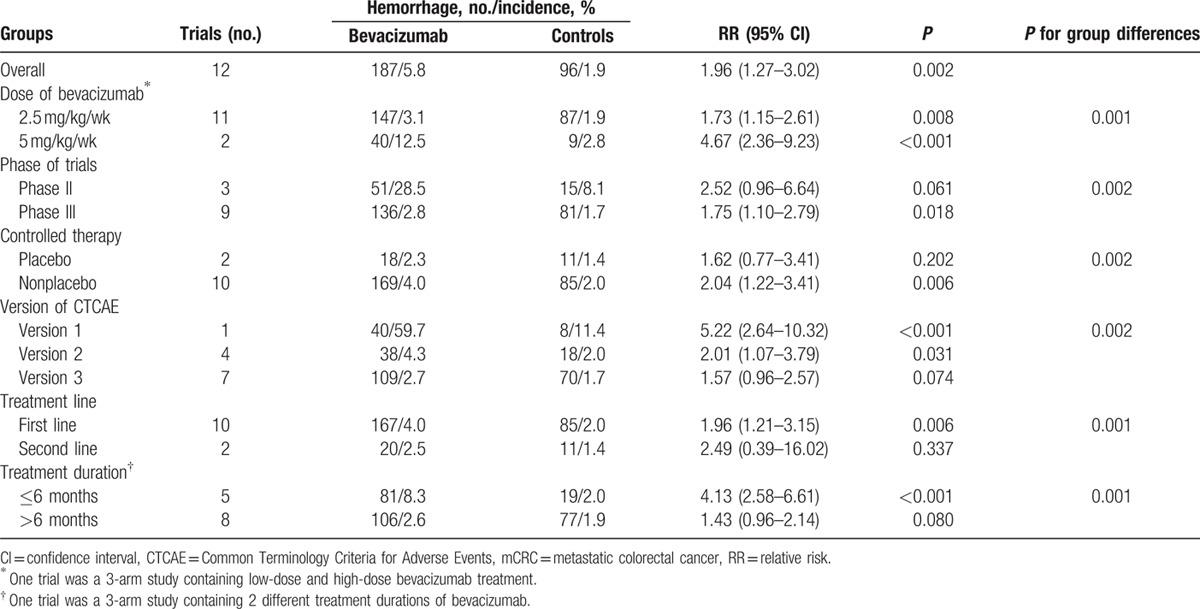
Figure 2.
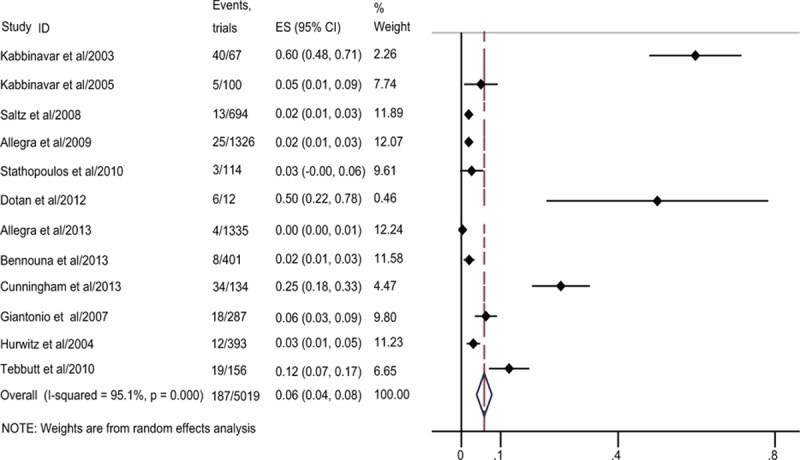
Forest plot of overall incidence of hemorrhage combined all-grade and high-grade hemorrhage in metastatic colorectal cancer (mCRC) treated with bevacizumab.
Considering that the 2 studies were both limited by a small number of randomized controlled trials (RCTs) for mCRC, and increasing RCTs have been focused on the efficacy and safety of bevacizumab in patients with mCRC since the 2 studies were published, we proposed to combine all the related RCTs to perform an updated meta-analysis to verify the overall incidence and risk of hemorrhage associated with bevacizumab in patients with mCRC.
2. Materials and methods
2.1. Data sources
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (Supplementary Table S1) statement,[36] we performed an independent review of citations from PubMed between January 1, 2000 and August 1, 2015 using the keywords “bevacizumab,” “avastin,” “clinical trials,” “neoplasm.” The trial type was limited to randomized, controlled clinical trials published in English. Our search strategies also contained the following text words such as “hemorrhagic events,” “bleeding,” “angiogenesis,” “vascular endothelial growth factor”(VEGF), “colorectal,” “colon,” “rectal,” “large bowel,” “side effect,” “adverse event.” We also independently searched through Embase and the Web of Science between January 1, 2000 and August 1, 2015 to make sure that all eligible trials were included. All reference lists of the related trials and reviews were searched for additional eligible studies. When there were duplicated publications, we compared them and selected the most adequate one for further analysis. We contacted the authors to make sure there were no any omissions about the information when no bleeding was reported in the published studies. All analyses in our study were based on previous published studies, thus no ethical approval and patient consent are required.
2.2. Study selection
The purpose of the study was to determine the risk of hemorrhage associated with bevacizumab in patients with mCRC. Therefore, only RCTs that directly compared mCRC patients with or without bevacizumab were eligible. Phase I and single-arm phase II were excluded for lacking of control groups. Specifically, clinical trials that met the following criteria were included for further analysis: prospective, randomized, controlled phase II or III trials in patients with mCRC; random assignment of participants to bevacizumab or control groups using placebo or concurrent therapy in addition to chemotherapeutic or/and biological agent; and adequate data including event or incidence of hemorrhage and sample size for analysis.
2.3. Qualitative assessment
Assessment and calculation of the quality of RCTs included in the meta-analysis was based on the Jadad scale.[37] One point would be awarded for the mention of randomization, blinding, and relevant data on study withdrawals, respectively. And 1 point would be awarded when the randomization or blinding was applied correctly or else 1 point would be deducted. At last, no points were awarded if no data were provided on the methodology of the above-mentioned procedures. The full score of an RCT was 5. An RCT with a score >2 was regarded as an RCT of good quality.[38]
2.4. Data extraction and clinical endpoints
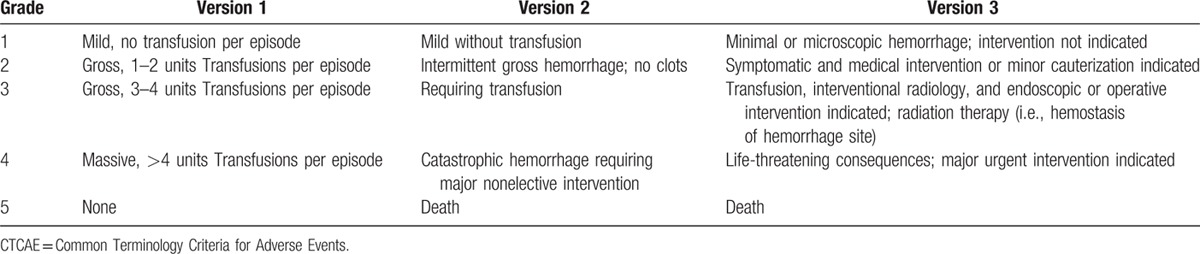
Data abstraction was performed independently by 2 reviewers (XQZ and XLT) based on Preferred Reporting Items for Systematic Reviews and Meta-Analysis (Supplementary Table S1) statement,[36] and any contradiction between the 2 reviewers was resolved by consensus. The following information of each study was extracted: author's name, publication year, phase of trial, number of enrolled patients, treatment arms, median age, median treatment duration, median progression-free survival, median overall survival, bevacizumab dose, median duration of follow-up, and number of hemorrhagic events. Hemorrhage in these trials was reported according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE, version 1, 2, or 3; http://ctep.cancer.gov). All of the 3 versions were almost similar in grading the toxicity of a medication leading to hemorrhage (Table 1). And the following adverse events were considered as bleeding: ecchymosis or petechiae, purpura, eye hemorrhage, epistaxis, gum hemorrhage, gastrointestinal hemorrhage, injection-site hemorrhage, hemothorax, melena, menorrhagia, metrorrhagia, colorectal hemorrhage, retroperitoneal hemorrhage, central nervous system hemorrhage, and vaginal hemorrhage. Consistent with the report of hemorrhagic events in these studies, we divided hemorrhage into all-grade hemorrhage and high-grade hemorrhage (grade 3–5).
Table 1.
National Cancer Institute CTCAE version 1, 2, and 3 for hemorrhage.
2.5. Statistical analysis
All statistical analyses were conducted using Stata version 12.0 software (Stata Corporation, College Station, TX). The number of patients with hemorrhagic events and the number of patients receiving bevacizumab were extracted from all the selected clinical trials. The proportion of patients with bleeding and 95% CI were derived for each trial. For the RR of hemorrhage, patients assigned to bevacizumab were compared only with those who assigned to a control group in the same trial. A dose–effect relationship for hemorrhage was evaluated by dividing the bevacizumab treatment into 2 kinds of types including 2.5 mg/kg/wk which was balanced by 5 or 7.5 mg/kg per dose per schedule and 5 mg/kg/wk which was balanced by 10 or 15 mg/kg per dose per schedule. We estimated the statistical heterogeneity among selected studies by using the Q statistic,[39] and inconsistency was quantified with the I2 statistic. Heterogeneity would be considered statistically significant if Pheterogeneity < 0.05. Data were analyzed using a random-effects model when heterogeneity existed and a fixed-effects model was applied for lacking of heterogeneity. Any statistical test would be considered significant when a P value < 0.05. To explore the possible reasons for heterogeneity, we performed subgroup analysis based on phase of trial, controlled therapy, treatment line, treatment duration, and version of CTCAE. Additionally, we conducted sensitivity analysis by excluding 1 trial sequentially to compare the effect of each trial on the overall effect estimate. At last, publication bias was estimated by using the Begg and Egger tests[40,41] and funnel plots.
3. Results
3.1. Search results
Based on our searching strategies, a total of 350 potentially relevant studies were acquired. The selection process is presented in Fig. 1. Seventeen articles were eligible for further evaluation after 249 articles were excluded including reviews, meta-analyses, commentaries, letters, case reports, and observational studies. Five of the 17 articles were excluded because of the following reasons, 2 single-arm phase II trials, 2 both control and treatment groups received bevacizumab, and 1 had no adequate data for the assessment of hemorrhage. Finally, 12 articles were included for the meta-analysis consisting of 3 phase II trials and 9 phase III trials.
Figure 1.
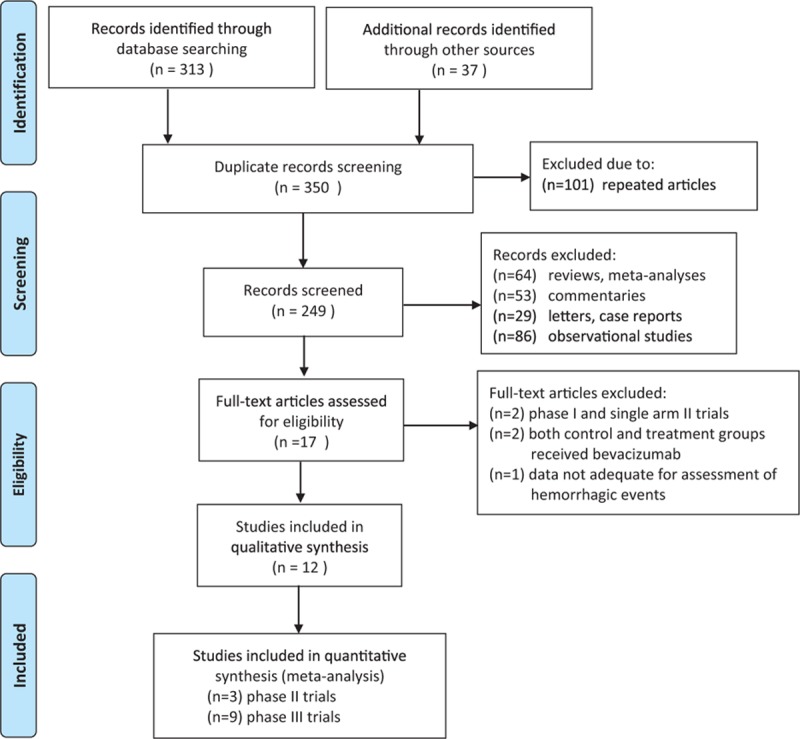
Selection process of randomized controlled trials (RCTs) included in the meta-analysis.
3.2. Study quality
Randomized treatment allocation sequences were generated in all trials. Patients were enrolled on the basis of specific eligibility criteria for each study. None was double-blinded and placebo controlled. Two trials had placebo as controls,[7,9] and the rest of the trials had active controls.[5,6,8,10–12,30–33] Hemorrhagic events were assessed and recorded according to the National Cancer Institute's Common Toxicity Criteria version 1, 2, or 3. Version 1 was used in only 1 trial.[5] Version 2 was used in 4 trials.[6–8,10] Version 3 was used in 7 trials.[9,11,12,30–33] Follow-up time was not specified in 3 trials.[5,6,30] All the scores of the 12 selected trials were >2 and acceptable.
3.3. Publication bias
No evident publication bias was detected for the RR of hemorrhage among the 12 articles in this study by either Begg tests or Egger tests (P = 0.19 for Begg tests; P = 0.14 for Egger tests). Also a funnel plot with relatively symmetric inverse funnel distribution was obtained.
3.4. Patients
The main characteristics of the 12 selected trials are presented in Table 2. A total of 10,555 patients were included for meta-analysis. The patients were all histologically confirmed with mCRC. Other inclusion criteria included an age of at least 18 years and most of the Eastern Cooperative Oncology Group performance status[42] of 0 and 1. Patients were also required to have adequate hematologic, hepatic, and renal functions. The exclusion criteria included clinically significant bleeding diathesis and cardiovascular disease, clinical detectable ascites, a history of major surgery within 28 days, serious nonhealing wounds, ulcer, or bone fracture, use of full-dose anticogulants or thrombolytics, central nervous system metastases, and pregnancy. All the patients in the selected trials were randomly assigned to either control or bevacizumab group and one of the 3-arm studies had 2 arms of bevacizumab dividing into 2 different dose levels.[5]
Table 2.
Characteristics of the 12 RCTs included in the updated meta-analysis.
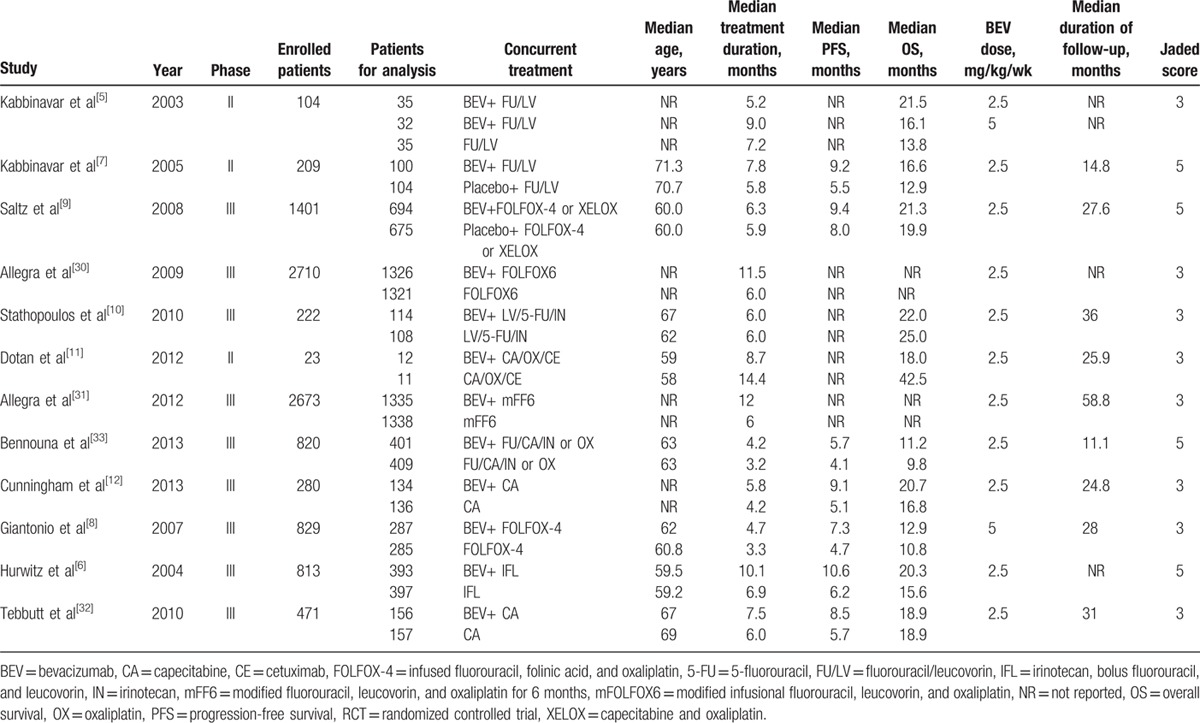
3.5. The overall incidence of hemorrhagic events with bevacizuamb
Together 5019 patients receiving bavacizumab in the 12 RCTs was used to calculate the overall incidence. And 187 hemorrhagic events occurred among these patients treated with bevacizumab. The highest incidence (6.3%; 95% CI 3.5%–9.1%) was obtained in the trial conducted by Giantonio et al,[8] and the lowest incidence (0.3%; 95% CI 0.0%–0.6%) was obtained in the trial conducted by Allegra et al.[31] Using a random-effects model, the overall incidence of hemorrhagic events in patients receiving bevacizumab was 5.8% (95% CI 3.9%–7.8%; Fig. 2).
3.6. RR of hemorrhagic events with bevacizumab
To figure out the specific contribution of bevacizumab to the risk of hemorrhage, we calculated the overall RR of hemorrhage events associated with bevacizumab.
Together 10,030 patients (bevacizumab 5019, controls 5011) were included in the 12 RCTs for calculating the overall RR of hemorrhagic events that combined all-grade and high-grade hemorrhage. All the trials had hemorrhagic events either for all-grade or high-grade or even both, and only one had no hemorrhagic events in the control group.[10] Using a random-effects model, the overall RR of hemorrhagic events was 1.96 (95% CI 1.27–3.02, Fig. 3A).
Figure 3.
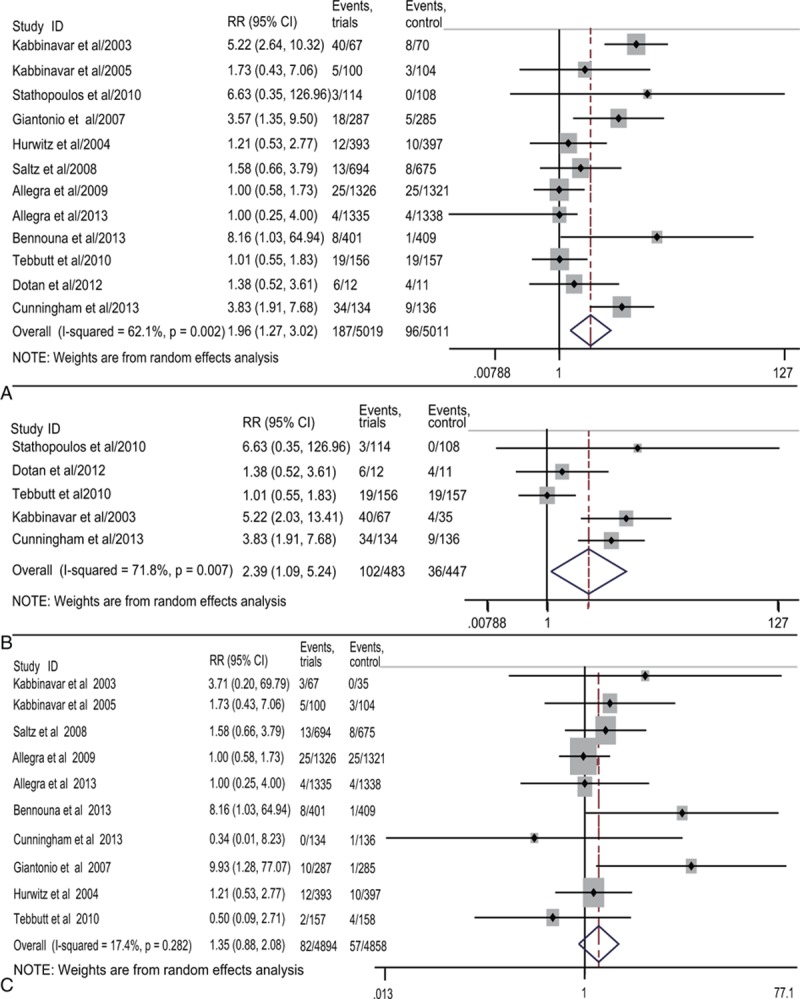
Forest plots of relative risk (RR) of hemorrhage associated with bevacizumab versus the controls. The overall RR of hemorrhage (A) was calculated using a random-effects model. The RR of all-grade hemorrhage (B) and high-grade hemorrhage (C) was calculated using random- and fixed-effects models, respectively.
The RR of hemorrhagic events for all-grade and high-grade were also calculated separately. Five of the 12 trials were included to calculate the overall RR of all-grade hemorrhagic events, and the RR was 2.39(95% CI 1.09–5.24, Fig. 3B), suggesting the treatment with bevacizumab had a higher risk compared with the control. For the high-grade hemorrhagic events, 10 trials had available data for calculation and the RR was 1.41 (95% CI 1.01–1.97, Fig. 3C).
Thus, the conclusions from RR of all-grade and high-grade hemorrhage were consistent with the overall RR that combined all-grade and high-grade hemorrhage, demonstrating that bevacizumab increased the risk of hemorrhagic events.
3.7. Bevacizumab dosage and the risk of hemorrhagic events
We determined the dose of bevacizumab contributed to the RR of hemorrhagic events. The dose of bevacizumab was divided into low dosage (2.5 mg/kg/wk) and high dosage (5 mg/kg/wk). The RR of 2.5 mg/kg/wk was 1.73 (95% CI 1.15–2.61, Fig. 4A) and the RR of 5 mg/kg/wk was 4.67 (95% CI 2.36–9.23, Fig. 4A). The differences between the 2 groups were significant (P = 0.001; Table 3). Besides, the higher risk of high dosage than low dosage suggested that the risk of hemorrhage associated with bevacizumab was dose-dependent.
Figure 4.
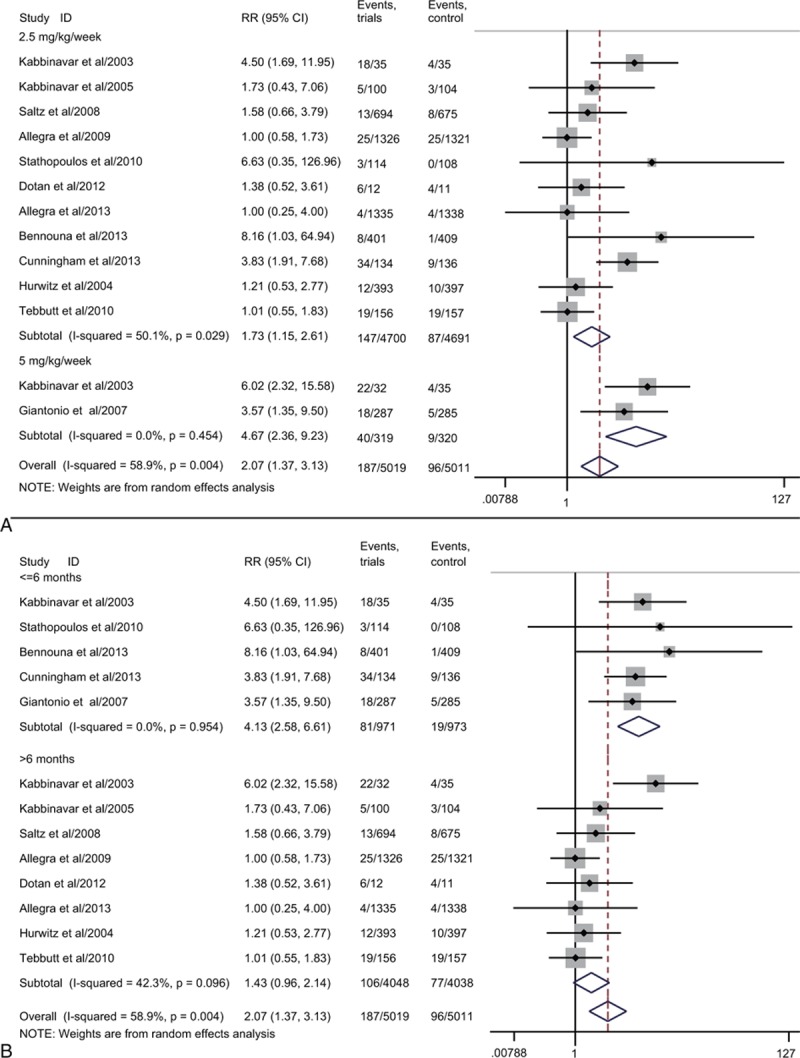
Subgroup analysis of relative risk (RR) of hemorrhage based on the dose of bevacizuamb (2.5 and 5 mg/kg/wk, A) and treatment duration (≤6 and >6 months, B). Random-effects models were used.
3.8. RR of hemorrhagic events based on the subgroup analysis
To explore the potential factors for the heterogeneity, subgroup analysis was performed according to phase of trial (random phase II and phase III), controlled therapy (placebo and nonplacebo), treatment line (1st line and 2nd line), treatment duration (<=6 and >6 months), and versions of hemorrhagic events grading according to the National Cancer Institute CTCAE (version 1, 2, and 3).
First, we performed a subgroup analysis stratified according to phase of trial (Supplementary Figure S1A). The RR of hemorrhage for phase III was 1.75 (95% CI 1.10–2.79, P = 0.02) while the phase II was 2.52 (95% CI 0.96–6.64, P = 0.06). As for controlled therapy (Supplementary Figure S1B), the RR of hemorrhage for nonplacebo group was 2.04 (95% CI 1.22–3.41, P = 0.01) while placebo group was 1.62 (95% CI 0.77–3.41, P = 0.20). In addition, we did subgroup analysis based on the treatment line (Supplementary Figure S2A). The RR of hemorrhage treated with bevacizumab as 1st-line therapy was 1.95 (95% CI 1.21–3.15, P = 0.01) versus 2.49 (95% CI 0.39–16.02, P = 0.34) for the 2nd-line therapy. And then we carried out a subgroup analysis stratified according to the treatment duration (>6 months as long period and <=6 months as short period; Fig. 4B). The RR of hemorrhage for long period was 1.43 (95% CI 0.96–2.14, P = 0.08) and the short period was 4.13 (95% CI 2.58–6.61, P < 0.001). There were significant differences between the 2 groups (P = 0.001; Table 3). Finally, a subgroup analysis stratified according to the version of CTCAE (Supplementary Figure S2B) was conducted. The results showed a significantly increased risk of hemorrhage as the RR of CTCAE version 2 was 2.01 (95% CI 1.07–3.79, P = 0.03) and CTCAE version 3 was 1.57 (95% CI 0.96–2.57, P = 0.07). The RR of CTCAE version 1 was 5.22 (95% CI 2.64–10.32, P < 0.001).
3.9. RR of hemorrhagic events based on the sensitivity analysis
We also performed a sensitivity analysis to identify the influence of each study on the overall RR of hemorrhage by omitting 1 trial sequentially. Our results demonstrated that the heterogeneity could not decrease significantly by eliminating any one of the trials. Then we tried to excluded 2 trials, Kabbinavar et al[5] and Cunningham et al,[12] that influenced the heterogeneity significantly (Supplementary Figure S3A). Then the overall RR was 1.39 (95% CI 1.00–1.92; Supplementary Figure S3B), and there were no significant differences among these trials (I2 = 15.2%, P = 0.30). We proposed this might be the source of heterogeneity.
4. Discussion
Two previous meta-analyses regarding the risk of hemorrhage among mCRC patients treated with bevacizumab have been published in 2010 and 2011, respectively. Hapani et al[34] firstly estimated the risk of serious hemorrhage treated with bevacizumab in mCRC patients but failed to demonstrate that bevacizumab increased the risk of high-grade hemorrhage. Also the results from Hang et al[35] suggested that bevacizumab did not increase the risk of high-grade hemorrhage. However, the results from these 2 studies might not be credible because of several limitations as follows. First, the number of RCTs involved in mCRC in both previous meta-analyses were small (6 in Hapani study and 8 in Hang study). Second, both of the studies did not perform further analysis for certain tumor type and were lack of further investigations about confounding factors of hemorrhage. Third, most of the RCTs used CTCAE version 1 and 2 to grade hemorrhagic events. Although version 1, 2, and 3 of CTCAE was similar, version 3 was more complete than previous versions and applied by 7 RCTs in our updated studies.
VEGF, being a multifunctional cytokine that plays an important role on regulating the angiogenesis process, is a pivotal target for cancer therapy. Although the mechanisms of hemorrhage regarding the anti-VEGF monoclonal antibody have not been clearly clarified, the interaction of bevacizumab and VEGF could be one possible interpretation. When bevacizuamb combined with VEGF, the renewal of endothelial cells decreased, namely, the formation of new vessels was inhibited. This might increase the risk of hemorrhage when the vessels are damaged such as trauma. Also when the maintaining of vascular integrity was disrupted by bevacizumab, the underlying matrix, and collagen exposed and contributed to hemorrhage or thrombosis or both.[43] A meta-analysis has demonstrated that the addition of bevacizuamb to standard antineoplastic therapy increased the risk of venous thromboembolism with an RR of 1.33 (95% CI 1.13–1.56).[44] In addition, bevacizumab could promote the apoptosis of endothelial cells and made substantial subendothelial matrix deposit. This might facilitate the access to hemorrhage.[45] Indeed, infinite proliferation and invasion were the main characteristics of specific tumors that contributed to tumor necrosis. This might lead to the formation of cavity that increased the risk of hemorrhage.
We performed this updated meta-analysis aiming at exploring the overall incidence and RR of hemorrhagic events associated with bevacizuamb in patients with mCRC so that physicians and patients can get a better understanding about the risk of hemorrhage. Our updated meta-analysis provided a greater statistical power for evaluating the risk of hemorrhage treated with bevacizumab in mCRC patients because a total of 10,555 patients from 12 RCTs were included. Our results showed that the overall incidence of 5.8% (95% CI 3.9%–7.8%) was much higher compared with the 2 previous studies, showing the incidence of 2.8% (95% CI 2.1%–3.7%)[34] and 2.7% (95% CI 2.1%–3.6%).[35] More importantly, as for all-grade and high-grade hemorrhage, the RR was 2.39 (95% CI 1.09–5.24) and 1.41 (95% CI 1.01–1.97), respectively. And when combined all-grade and high-grade hemorrhage, the RR was 1.96 (95% CI 1.27–3.02). Thus, we could draw a powerful conclusion that bevacizumab significantly increased the risk of hemorrhage in patients with mCRC.
In consideration of the potential risk factors of hemorrhage associated with bevacizumab, we 1st determined that bevacizumab dosage contributed to the risk of hemorrhage. Our subgroup analysis demonstrated that high dose of bevacizumab (5 mg/kg/wk) much more significantly increased the risk of hemorrhage than low dose of bevacizumab (2.5 mg/kg/wk) with the RR of 4.67 (95% CI 2.36–9.23) versus 1.73 (95% CI 1.15–2.61). This result suggested that the risk of hemorrhage associated with bevacizumab was dose-dependent, and bevacizumab dosage could be one of the risk factors. A close supervision of the development of hemorrhage was strongly recommended during the treatment especially in a high dosage.
Given that the risk factors of bevacizumab were not fully elucidated, we also did additional subgroup analyses to figure out the potentially relevant risk factors. Interestingly, we found that the treatment duration for short period (<=6 months) increased much higher risk of hemorrhage with an RR of 4.13 (95% CI 2.58–6.61) despite that the RR of long period (>6 months) was 1.43 (95% CI 0.96–2.14). Therefore, we suggested that clinicians and patients should be aware of the high risk of hemorrhage during the 1st 6 months treatment with bevacizumab. Patients might have developed good tolerance of hemorrhage associated with bevacizumab after 6 months. Unfortunately, in terms of the results of subgroup stratified according to phase of trial, controlled therapy, treatment line, and version of CTCAE, no exact evidence demonstrated these were risk factors of hemorrhage. Our findings might be limited by the sample size and more trials were needed to identify these results.
We did subgroup analyses to explore the source of heterogeneity but our results showed that subgroups such as phase of trial, controlled therapy, treatment line, and CTCAE version could not give credible interpretations. Then we did sensitivity analysis and demonstrated that the 2 trials[5,12] could be the source of heterogeneity because statistics ranged from I2 = 62.1%, P = 0.002 to I2 = 15.2%, P = 0.30 when the 2 trials were excluded. Then we further investigated the 2 trials and found that there were no high-grade hemorrhagic events reported in the 2 trials. The possible explanation might be that the criteria of grading hemorrhage were different among these trials. Since the version of the National Cancer Institute's Common Terminology Criteria renewed every several years, we could not determine which version to be used for individual trials.
However, limitations are inevitable in any meta-analysis because a meta-analysis is at the study level and our study is not exceptional. First, confounding factors belonging to the baseline characteristics of patients such as sex, age, race, or geographical distribution could not be assessed because data on these aspects were not commonly published. As for age, Aprile et al[46] suggested that older patients should not represent a stringent limit for the employ of antiangiogenic agents. Also, patients who were regularly received anticoagulants (e.g., warfarin), antiplatelet therapy (e.g., aspirin), or hematological therapies were often excluded by investigators. Although a study published by Leighl et al[47] suggested that bevacizumab did not increase the risk of severe bleeding in cancer patients who received therapeutic anticoagulation, we could not identify this conclusion in our study. Second, the time that hemorrhage occurred among patients in individual trials was commonly different because bevacizumab could generally prolong the overall survival or progression-free survival, and this might lead to an observation time bias. Third, in terms of patients with metastatic malignancies, metastasis sites might also increase the risk of hemorrhage because the metastasis sites could affect the functions of corresponding organs. Indeed, patients with adequate hematologic, hepatic, and renal functions were included in most of the trials which meant those who had corresponding organ dysfunctions were generally excluded. So, the risk of hemorrhage associated with bevacizumab among these patients was still not estimated and more studies should be focused on this issue. Fourth, Huang et al mentioned that the most common site of high-grade hemorrhage associated with bevacizumab in colorectal cancer was gastrointestinal/rectal and the primary tumor site was associated with the risk of high-grade hemorrhage because the rectum site had a higher rate than colon site.[35] And this conclusion could not be identified in our study either. Finally, although we did subgroups based on treatment duration, the conclusion that the 1st 6 months was a critical period with a high risk of hemorrhage might not be strongly supported by our results because the sample size was small. As for controlled therapy subgroup, there were only 2 trials that used placebo as for controls. More trials should be conducted to confirm these issues.
In summary, our updated meta-analysis of 12 RCTs demonstrated that the addition of bevacizumab to treatment in patients with mCRC significantly increased the risk of hemorrhage. The probable risk factors may be the dose of bevacizumab. Our findings would be helpful for physicians and patients to be aware of that hemorrhagic events may occur at any time during the treatment with bevacizumab in mCRC patients especially in the 1st 6 months. Further studies should be performed to investigate the prevention and management of hemorrhage in mCRC patients treated with bevacizumab.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CTCAE = Common Terminology Criteria for Adverse Events, mCRC = metastatic colorectal cancer, RCT = randomized controlled trial, RR = relative risk, VEGF = vascular endothelial growth factor.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002; 29 (6 Suppl 16):15–18. [DOI] [PubMed] [Google Scholar]
- 2.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005; 23:1011–1027. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 1997; 18:4–25. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003; 9:669–676.06//print. [DOI] [PubMed] [Google Scholar]
- 5.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003; 21:60–65. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 7.Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol 2005; 23:3697–3705. [DOI] [PubMed] [Google Scholar]
- 8.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007; 25:1539–1544. [DOI] [PubMed] [Google Scholar]
- 9.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008; 26:2013–2019. [DOI] [PubMed] [Google Scholar]
- 10.Stathopoulos G, Batziou C, Trafalis D, et al. Treatment of colorectal cancer with and without bevacizumab: a phase III study. Oncology 2010; 78:376–381. [DOI] [PubMed] [Google Scholar]
- 11.Dotan E, Meropol NJ, Burtness B, et al. A phase II study of capecitabine, oxaliplatin, and cetuximab with or without bevacizumab as frontline therapy for metastatic colorectal cancer. A Fox Chase extramural research study. J Gastrointest Cancer 2012; 43:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol 2013; 14:1077–1085. [DOI] [PubMed] [Google Scholar]
- 13.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti–vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003; 349:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 2008; 370:2103–2111. [DOI] [PubMed] [Google Scholar]
- 15.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004; 22:2184–2191. [DOI] [PubMed] [Google Scholar]
- 16.Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med 2006; 355:2542–2550. [DOI] [PubMed] [Google Scholar]
- 17.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non0-small-cell lung cancer: AVAil. J Clin Oncol 2009; 27:1227–1234. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Chavez A, Young T, Fages S, et al. Bevacizumab maintenance in patients with advanced non–small-cell lung cancer, clinical patterns, and outcomes in the Eastern Cooperative Oncology Group 4599 Study: results of an exploratory analysis. J Thorac Oncol 2012; 7:1707–1712. [DOI] [PubMed] [Google Scholar]
- 19.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 2005; 23:792–799. [DOI] [PubMed] [Google Scholar]
- 20.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007; 357:2666–2676. [DOI] [PubMed] [Google Scholar]
- 21.Gray R, Bhattacharya S, Bowden C, et al. Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol 2009; 27:4966–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brufsky AM, Hurvitz S, Perez E, et al. RIBBON-2: A randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2–negative metastatic breast cancer. J Clin Oncol 2011; 29:4286–4293. [DOI] [PubMed] [Google Scholar]
- 23.Martín M, Loibl S, von Minckwitz G, et al. Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer: the letrozole/fulvestrant and avastin (LEA) study. J Clin Oncol 2015; 33:1045–1052. [DOI] [PubMed] [Google Scholar]
- 24.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011; 365:2484–2496. [DOI] [PubMed] [Google Scholar]
- 25.Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol 2015; 16:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tewari KS, Sill MW, Long HJ, III, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 2014; 370:734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010; 28:3617–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015; 16:859–870. [DOI] [PubMed] [Google Scholar]
- 29.Hainsworth JD, Greco FA, Raefsky EL, et al. Rituximab with or without bevacizumab for the treatment of patients with relapsed follicular lymphoma. Clinl Lymphoma Myeloma Leuk 2014; 14:277–283. [DOI] [PubMed] [Google Scholar]
- 30.Allegra CJ, Yothers G, O’Connell MJ, et al. Initial safety report of NSABP C-08: a randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol 2009; 27:3385–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allegra CJ, Yothers G, O’Connell MJ, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol 2013; 31:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tebbutt NC, Wilson K, Gebski VJ, et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol 2010; 28:3191–3198. [DOI] [PubMed] [Google Scholar]
- 33.Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013; 14:29–37. [DOI] [PubMed] [Google Scholar]
- 34.Hapani S, Sher A, Chu D, et al. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology 2010; 79:27–38. [DOI] [PubMed] [Google Scholar]
- 35.Hang XF, Xu WS, Wang JX, et al. Risk of high-grade bleeding in patients with cancer treated with bevacizumab: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2011; 67:613–623. [DOI] [PubMed] [Google Scholar]
- 36.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151:264–269. [DOI] [PubMed] [Google Scholar]
- 37.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998; 352:609–613. [DOI] [PubMed] [Google Scholar]
- 38.Moher D, Jadad AR, Tugwell P. Assessing the Quality of Randomized Controlled Trials: Current Issues and Future Directions. Int J Technol Assess Health Care 1996; 12:195–208. [DOI] [PubMed] [Google Scholar]
- 39.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol 2005; 28:123–137. [DOI] [PubMed] [Google Scholar]
- 40.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 1088–1101. [PubMed] [Google Scholar]
- 41.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–656. [PubMed] [Google Scholar]
- 43.Kamba T, McDonald D. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 2007; 96:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rani Nalluri S, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 2008; 300:2277–2285. [DOI] [PubMed] [Google Scholar]
- 45.Jeanine MR, Marlies HL, Els W, et al. The molecular basis of class side effects due to treatment with inhibitors of the VEGF/VEGFR pathway. Curr Clin Pharmacol 2008; 3:132–143. [DOI] [PubMed] [Google Scholar]
- 46.Aprile G, Fontanella C, Lutrino ES, et al. Angiogenic inhibitors for older patients with advanced colorectal cancer: does the age hold the stage? World J Gastroenterol 2013; 19:2131–2140.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leighl NB, Bennouna J, Yi J, et al. Bleeding events in bevacizumab-treated cancer patients who received full-dose anticoagulation and remained on study. Br J Cancer 2011; 104:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


