Supplemental Digital Content is available in the text
Keywords: 18F-FDG PET/CT, Coxiella burnetii, diagnosis, focalized persistent infection, Q fever
Abstract
Because Q fever is mostly diagnosed serologically, localizing a persistent focus of Coxiella burnetii infection can be challenging. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) could be an interesting tool in this context.
We performed a retrospective study on patients diagnosed with C burnetii infection, who had undergone 18F-FDG PET/CT between 2009 and 2015. When positive 18F-FDG PET/CT results were obtained, we tried to determine if it changed the previous diagnosis by discovering or confirming a suspected focus of C burnetii infection.
One hundred sixty-seven patients benefited from 18F-FDG PET/CT. The most frequent clinical subgroup before 18F-FDG PET/CT was patients with no identified focus of infection, despite high IgG1 serological titers (34%). For 59% (n = 99) of patients, a hypermetabolic focus was identified. For 62 patients (62.6%), the positive 18F-FDG PET/CT allowed the diagnosis to be changed. For 24 of them, (38.7%), a previously unsuspected focus of infection was discovered. Forty-two (42%) positive patients had more than 1 hypermetabolic focus. We observed 21 valvular foci, 34 vascular foci, and a high proportion of osteoarticular localizations (n = 21). We also observed lymphadenitis (n = 27), bone marrow hypermetabolism (n = 11), and 9 pulmonary localizations.
We confirmed that18F-FDG PET/CT is a central tool in the diagnosis of C burnetii focalized persistent infection. We proposed new diagnostic scores for 2 main clinical entities identified using 18F-FDG PET/CT: osteoarticular persistent infections and lymphadenitis.
1. Introduction
Q fever is a worldwide zoonosis caused by the bacterium Coxiella burnetii. Since the first studies on Q fever, a dichotomy has been established between “acute Q fever” and “chronic Q fever.”[1] The term chronic Q fever was used due to the inability to determine the infected site in patients with persistent symptoms or a positive serology with an increase in phase I IgG, suggesting an active infection.[2] The term “chronic Q fever” is, however, misleading because it combines many different clinical entities under serological criteria.[3] Serological cut-offs alone are not sufficient to determine the persistence of C burnetii infection. This phenomenon is illustrated by the Q fever epidemic in French Guiana, where patients with primary Q fever presented high levels of phase I IgG with no systematic clinical progression towards a persistent focalized infection.[4] In France, C burnetii infection is endemic, but localized outbreaks and hyperendemic foci are described .[5] The disease is more often diagnosed in the Southeast of France where the French National Referral Center for Q fever is located.[5]
Endocarditis and vascular infections represent the majority of the described focalized persistent infections.[6,7] Several other localizations have been described, but less frequently, such as joint and bone infections,[8,9] lymphadenitis,[10] pericarditis, lung pseudo-tumor, and gall bladder infection.[11] In the case of endocarditis and vascular infections, definition scores have been elaborated, in which the 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) helps detecting infection focus (Table 1).[3] Thanks to an early diagnosis strategy, prophylaxis, and treatment, the prognosis of C burnetii endocarditis has drastically changed in our center.[6,12] The mortality rate has fallen from 60% to 5%.[6] However, vascular infections remain a very severe entity, with high mortality rates (up to 25%) and requiring surgical treatment.[7] In C burnetii joint and lymph node infections, very little is known about prognosis and treatment.[8–10] These differences in prognosis and treatment between the types of focalized Q fever infections illustrate the inaccuracy of grouping them under the global term of “chronic Q fever.”
Table 1.
Definition criteria for C burnetii endocarditis, vascular infections, and prosthetic joint arthritis.

Nonetheless, in some circumstances, clinical symptoms and/or high IgGI antibodies persist without evident focus of infection. Physicians are confronted with therapeutic challenge, which is whether to treat a potentially fatal infection without knowing the site of infection or not. Moreover, classical morphological tools often fail to identify C burnetii infection because anatomical changes can be very slight. For example, in C burnetii persistent endocarditis, typical vegetation is observed in only 30% of cases, and echocardiography detected a valvular insufficiency in 75% of cases.[6] Vascular infections can be revealed only by aneurysm or vascular graft rupture.[13]
18F-fluorodeoxyglucose positron emission tomography/computed tomography is an imaging modality that allows measurement of metabolic activity within an organ, obtained from the emission of positrons after disintegration of the injected radioactive product. As the majority of the malignant cells have high glycolytic activity, detection of their hypermetabolism was first used in clinical oncology.[14] Recently, it has been used for the identification of inflammatory and infectious processes because they also result in significant FDG uptake by the inflammatory cells. 18F-FDG PET/CT has been used for the detection and monitoring of fever of unknown origin (FUO) and in a growing number of infections.[15,16] Regarding C burnetii, around 10 references are found in the literature reporting the use of 18F-FDG PET/CT. Among these references, Barten et al reported 15 patients with C burnetii endocarditis and vascular infections.[17] Other reports describe hepatic, bone marrow, lymphadenitis, articular, and prostatic uptake of 18F-FDG PET/CT.[8,18–24]18F-FDG PET/CT has been included as a criterion in the definition scores for C burnetii endocarditis, articular prosthesis, and vascular infections. However, this definition was based on a very limited number of patients, and its utility in detection of other foci of infection has not been assessed.
Herein, our objective was to describe the different foci that could be detected in patients with persistent C burnetii infection. Thanks to this description, our secondary objective was to assess if 18F-FDG PET/CT allowed the detection of a focus of infection in patients with unlocalized persistent C burnetii infection.
2. Patients and methods
2.1. Case definition
The French National Reference Center for Q fever receives samples for C burnetii testing[4] from the entire country. Between January 2009 and June 2015, 1555 patients were tested positive for Q fever in our center. Clinical and laboratory data were collected prospectively for all patients—thanks to a standardized questionnaire. For patients who did not benefit from a medical monitoring by our center in our center, data were collected over the phone to complete the standardized questionnaire.
All patients with an active C burnetii infection who benefited from a 18F-FDG PET/CT were included in our study (Fig. 1 and eFig. 1). Among these patients, several subgroups were identified and differentiated according to the diagnosis before 18F-FDG PET/CT: primary C burnetii infection was defined by the association of clinical symptoms (fever and/or hepatitis and/or pneumonia) with serologic criteria for phase II IgG levels ≥200 and phase II IgM levels ≥50, or by a Polymerase Chain Reaction (PCR) and no endocarditis. Possible or definite C burnetii endocarditis, vascular infection, and joint prosthesis infection were defined according to the recent criteria (Table 1).[3,8] The rest of the cases were patients with persistent elevated phase I IgG (≥800) for more than 3 months without any focus of infection at clinical examination and transthoracic echocardiography.
Figure 1.
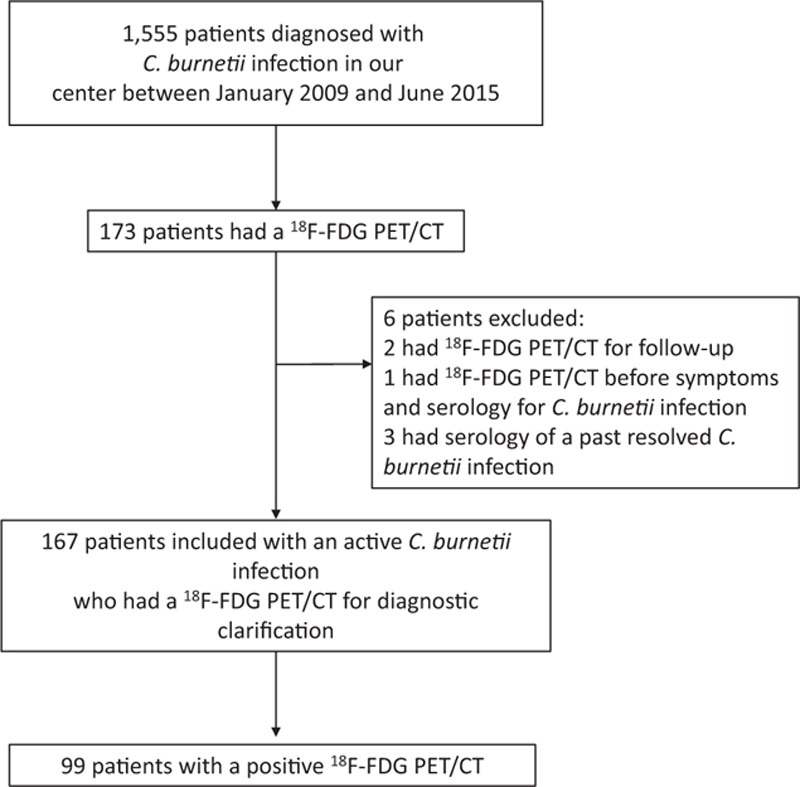
Flow chart.
We excluded patients for whom 18F-FDG PET/CT was performed before the onset of symptoms that motivated the serology. Patients with serology indicative of a past resolved C burnetii infection were also excluded, and 18F-FDG PET/CT examinations that were performed for follow-up were excluded (Fig. 1).
The study was approved by the local ethics committee (Comité de Protection des Personnes Sud Mediterranée 1). All patients gave informed consent.
2.2. Diagnosis of Coxiella burnetii infection
We used an indirect immunofluorescence assay to quantify IgG, IgM, and IgA titers against phase I and phase II, as previously described .[25] DNA was extracted using the QIAamp Tissue Kit (QIAGEN GmbH, Hilden, Germany), and these extracts were used as templates for PCR amplification as previously described.[26] Culture, immunohistochemistry, and fluorescent in situ hybridization (FISH) targeting C burnetii 16S rRNA were performed.[10,26]
2.3. 18F-FDG PET/CT
18F-fluorodeoxyglucose positron emission tomography/computed tomography was performed in the fasting state for at least 6 hours and the glucose level was lower than 150 mg/dL. An FDG dose of 4 to 5 MBq/kg was administered intravenously and imaging was performed 60 minutes after injection in accordance with each center's protocol. The images were analyzed visually and semiquantitatively by measuring the maximum standardized uptake value (SUV-max). Hypermetabolic 18F-FDG activity was considered as a potential site of infection when it did not correspond to physiological uptake (myocardial, liver, bladder, ureter, kidney, and gastrointestinal foci). 18F-FDG PET/CT was performed in several centers without a common interpretation.
When the 18F-FDG PET/CT was performed in another center, the protocol for 18F-FDG PET/CT, images, and interpretation were collected retrospectively. When images were not available, reports alone were collected.
2.4. Main outcome: change of diagnosis after 18-FDG PET/CT
We considered that the 18F-FDG PET/CT results allowed the diagnosis to be changed when a previously unknown localization of the infection was discovered, or when a possible endocarditis or vascular infection was confirmed.
2.5. Statistical analysis
Descriptive statistics for continuous variables are represented as median. Categorical variables are reported in terms of the number and percentages of patients affected. Variables were calculated using SPSS 22 Statistics Software.
3. Results
One hundred sixty-seven patients with C burnetii active infection had a 18F-FDG PET/CT performed, including 37 women (22%) and 130 men (78%). The mean age of patients was 58.4 ± 16 years. The type of C burnetii active infection before 18F-FDG PET/CT were: persistent elevated phase I IgG for more than 3 months for 57 patients (34%), possible endocarditis for 39 patients (23%), definite endocarditis or vascular infection for 31 patients (19%), primary Q fever for 25 patients (11.3%), possible vascular infection for 14 patients (8%), and possible osteo-articular infection for 1 patient (0.5%).
18F-fluorodeoxyglucose positron emission tomography/computed tomography revealed positive hypermetabolism for 99 patients (59%). Fifty-seven of these patients (34.7% of all patients) had 1 hypermetabolism, 42 patients (15%) had 2 hypermetabolic foci, 10 (6%), and 3 patients (1.8%), respectively, had 3 and 4 hypermetabolic foci. The highest number of infectious foci located in 1 person was 5, which were found in 3 patients.
3.1. Osteoarticular localizations
Osteoarticular hypermetabolism was identified in 21 patients (Fig. 2 and Table 2). Osteoarticular localizations as the main focus of infection were observed in 8 cases (Fig. 2, Tables 2 and 3). Three infections involved a joint prosthesis. For 2 patients, we observed an acromioclavicular hypermetabolism, and 1 other patient had shoulder involvement (Figs. 2 and 3). One patient had tenosynovitis and another had an isolated spondylodiscitis.
Figure 2.
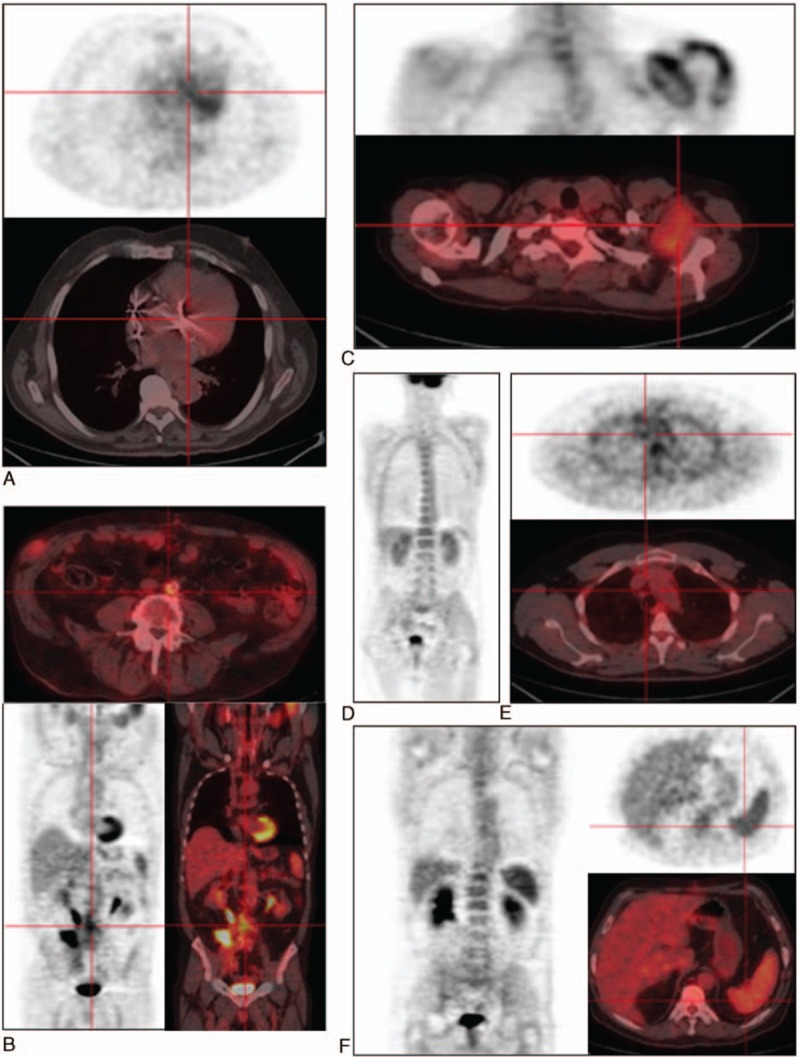
Hypermetabolic foci of Coxiella burnetii infection identified by 18F-FDG PET/CT. A, Aortic valve hypermetabolism during definite Q fever endocarditis; B, abdominal aortic hypermetabolism during definite Q fever vascular infection; C, bursitis, arthritis foci during Q fever osteoarticualr infection; D, bone marrow hypermetabolism during Q fever; E, Q fever lymphadenitis identified with PET scan; F, spleen hypermetabolism during Q fever. 18F-FDG PET/CT = 18F-fluorodeoxyglucose positron emission tomography/computed tomography.
Table 2.
Description of 18F-FDG foci.
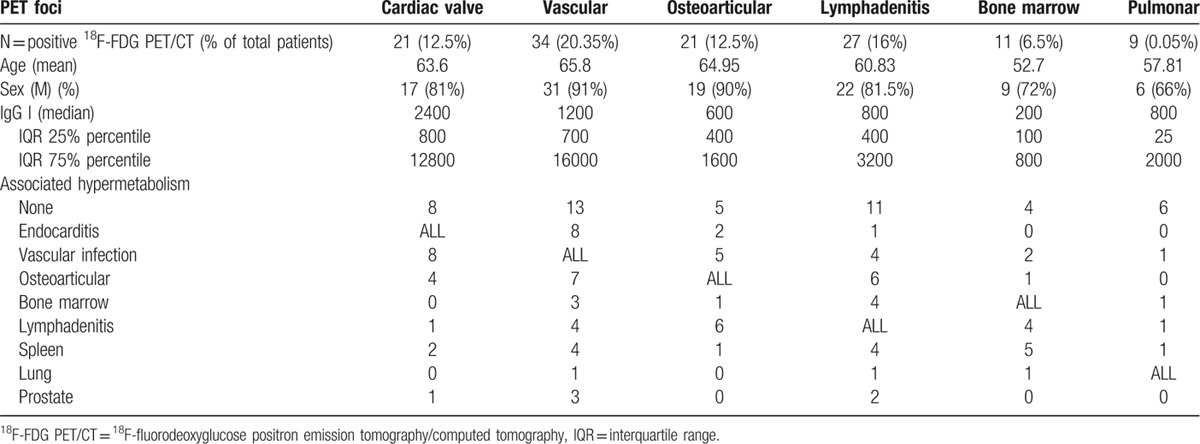
Table 3.
Details of the 8 patients with osteoarticular hypermetabolism as the main focus of infection.
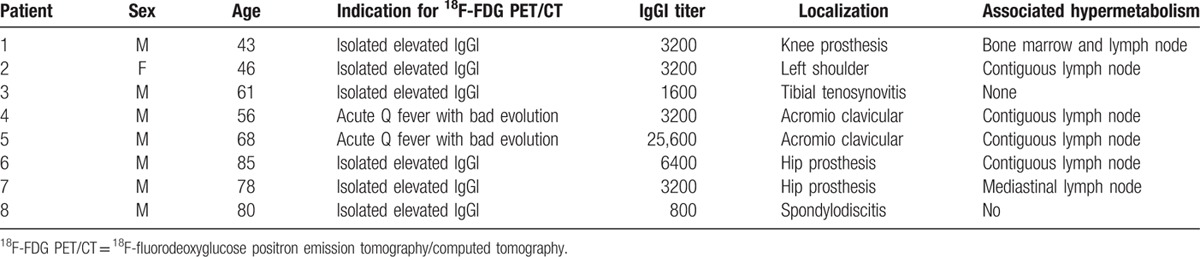
Figure 3.
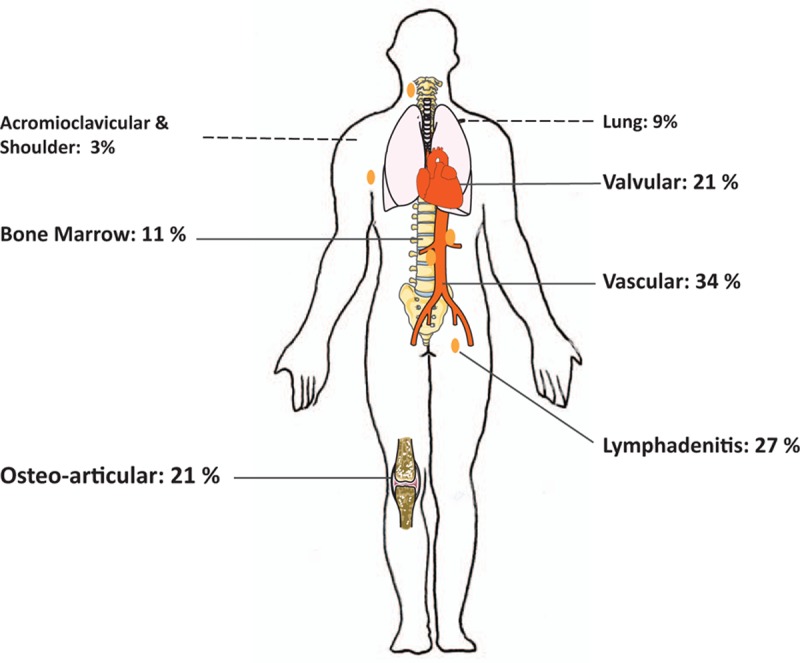
Distribution of Q fever foci identified by 18F-FDG PET/CT. 18F-FDG PET/CT = 18F-fluorodeoxyglucose positron emission tomography/computed tomography.
Thirteen osteoarticular hypermetabolisms were associated with other hypermetabolic foci. In this context, we found a majority of spondylodiscitis (n = 9) complicating endocarditis or vascular infection (Table 3).
3.2. Lymphadenitis
Lymphadenitis hypermetabolism was identified in 27 patients (Figs. 2 and 3). Lymphadenitis was the sole focus for 11 patients, among which 7 (25%) also presented a primary C burnetii infection, and the remaining 4 were cases of isolated persistent lymphadenitis (14.8%). Lymphoma was diagnosed in 2 patients with lymphadenitis hypermetabolism.
Lymphadenitis hypermetabolism was associated with another persistent focalized infection in 16 cases (59%), with 5 patients presenting 3 or more concomitant foci. Cardiovascular foci were present in 5 cases (1 endocarditis, 4 vascular infections), osteoarticular foci in 6 cases (22%), and other foci are detailed in Table 2.
3.3. Endocarditis
A total of 21 patients (21%) showed a hypermetabolism suggesting endocarditis, including 6 hypermetabolisms on a native valve, 14 hypermetabolisms on a prosthetic valve, and 1 hypermetabolism on a pacemaker (Figs. 2 and 3). Before the 18F-FDG PET/CT, these patients had possible endocarditis (n = 13), definite endocarditis (n = 3), persistent IgG1 (n = 3), suspicion of osteoarticular infection (n = 1), and suspicion of vascular infection (n = 1).
3.3.1. Endocarditis with aortic hypermetabolism and other embolic localizations
Eight patients with endocarditis had a simultaneous aortic hypermetabolism (6 Bentalls and 2 mycotic aneurysms) (Table 2). One of these patients had an associated spondylodiscitis and psoas abscess. One patient had a simultaneous spondylodiscitis and 3 had other articular foci.
3.4. Vascular infections
Twenty-six patients (26%) had a vascular hypermetabolism without endocarditis. Four of these patients had associated hyperfixating spondylodiscitis and psoas abscesses (Figs. 2 and 3). Diagnosis subgroups before18F-FDG PET/CT were: possible vascular infections in 11 cases, definite vascular infections in 4 cases, possible endocarditis in 3 cases, definite endocarditis in 3 cases, persistent IgGI in 3 cases, and primary Q fever in 2 cases.
3.5. Bone marrow
Eleven patients presented an increased bone marrow uptake (Figs. 2 and 3). Among them, 4 presented a primary Q fever infection, 6 had a persistent cardiovascular focalized infection, 1 had an osteoarticular infection, 5 had a concomitant spleen hypermetabolism, and 4 had a concomitant lymphadenitis uptake. Four patients presented bone marrow uptake as the unique hypermetabolic focus (2 in a context of primary infection and 2 associated with nonhypermetabolic possible and definite endocarditis).
3.6. Pulmonary localization
For 9 patients, we observed a pulmonary hypermetabolism (Fig. 3). Five patients displayed conventional lobar pneumonia, and 2 had a hypermetabolic nodule. Four of them had a 18F-FDG PET/CT in a context of primary Q fever (Table 2).
3.7. Other hypermetabolic foci
We observed the following other hypermetabolic foci: prostatic (5 patients), thyroid (4 patients), and laryngeal (4 patients). These foci were always associated with another main focus of infection.
3.8. Clinical relevance of 18F-FDG PET/CT in the localization of Coxiella burnetii persistent focalized infection
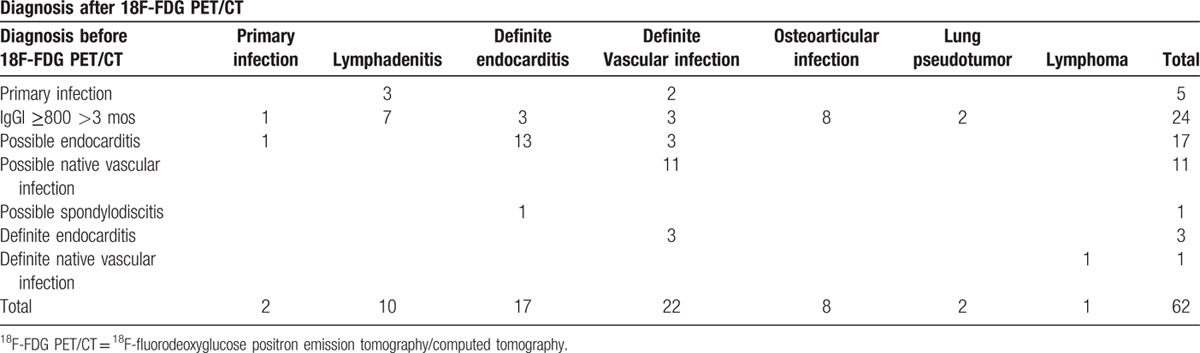
Positive 18F-FDG PET/CT allowed the diagnosis to be changed for 62 patients (62.6%). When the following 2 groups of patients were pooled, the first group being patients with isolated persistent elevated IgGI for more than 3 months and the second group being patients with possible endocarditis (n = 96), the diagnosis was changed in 55% of the patients—thanks to 18F-FDG PET/CT. For patients with persistent isolated IgG1 (n = 57), the most frequent entities were an osteo-articular infection focus (n = 8, 30.7%) (Table 4) and lymphadenitis (n = 7, 26.9%) followed by endocarditis (n = 3), vascular infections (n = 3), lung pseudotumor (n = 2), and pulmonary hypermetabolism evocative of primary infection (n = 1; Figs. 2 and 3, Table 4). Six definite vascular infections were discovered in a context of suspicion of endocarditis (Table 4).
Table 4.
Cases of change in diagnosis after 18F-FDG PET/CT.
4. Discussion
We here report the largest case series of Q fever patients benefiting from a 18F-FDG PET/CT. The mean age of patients (58 years) and the male predominance is concordant with the classic epidemiology of symptomatic Q fever.[27] More than half of these patients showed a positive 18F-FDG PET/CT, and this examination allowed the diagnosis to be changed in 62.6% of cases. Regarding hypermetabolism, it is that a high proportion of patients (42%) present 2 or more foci of fixation, reflecting the systemic nature of the C burnetii infection.
Because no gold standard imaging technique exists in the detection of C burnetii foci of infection, no statistical comparison could be made to assess the sensitivity and specificity of 18F-FDG PET/CT, and this represents 1 limitation of our study. Patients diagnosed in our center may be followed in other cities, so that no common interpretation of 18F-FDG PET/CT results was performed. This is another limitation of our study.
For patients with persistent elevated IgGI levels, we observed a focus of infection in 38.7% of cases. One striking finding is that the majority of these patients had an osteoarticular focus of infection (33%). This is an important result since osteoarticular Q fever infections have been considered to be rare occurrences, representing about 2% of Q fever cases.[28] The most widely reported localizations in the literature were osteomyelitis[21,29] and isolated spondylodiscitis.[30] Two cases of tenosynovitis of the wrist[30] and Q fever infections of a joint prosthesis have been reported.[8,31] We found only 1 case of isolated spondylodiscitis. All other cases of spondylodiscitis were associated with vascular infections or endocarditis. This result confirms that isolated C burnetii spondylodiscitis is quite rare. We observed 2 cases of acromioclavicular hypermetabolism with contiguous lymphadenopathy. Only 1 similar case of Q fever subacromial bursitis has been reported.[9] These 2 additional cases suggest a new Q fever clinical entity. We also reported here the fourth case of C burnetii tenosynovitis.[9,30] Thus, we suggest a new definition score for C burnetii osteoarticular infections (Table 5, part I). Definite criteria for diagnosis are microbiological proof (by PCR, culture, FISH, or immunohistochemistry) of infection in a bone or joint biopsy or joint fluid aspirate. Major and minor criteria are detailed in Table 5 (part I). Definite diagnosis of Q fever osteoarticular infection is defined by the presence of either 1 definite criterion, 2 major criteria, or 1 major and 3 minor criteria (Table 5, part I).
Table 5.
Definition criteria of C burnetii focalized infection.
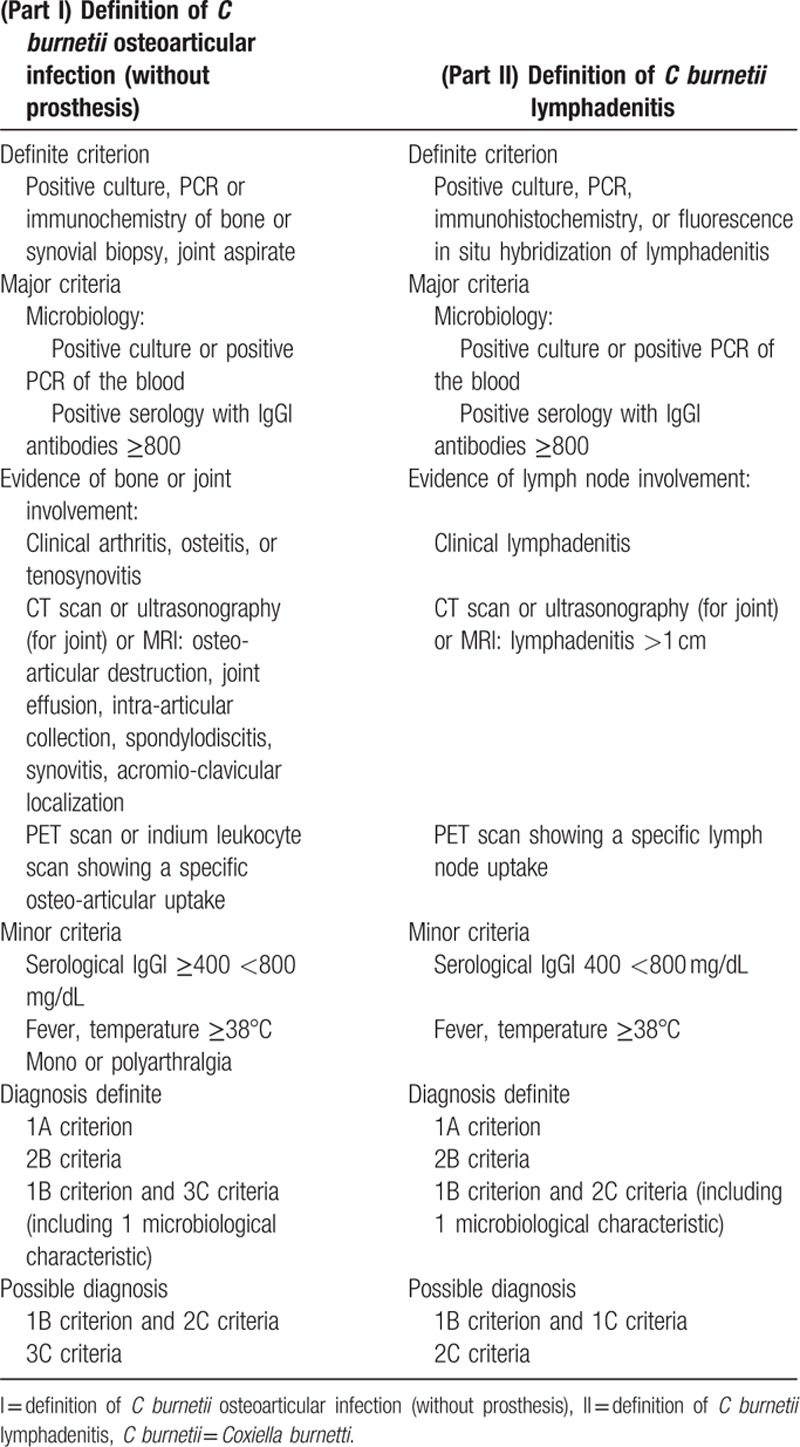
Q fever lymphadenitis was described in the literature as a proven microbiological focus of Q fever. C burnetii was identified within lymph nodes by PCR, immunohistochemistry, and FISH (eFig. 2).[32] The use of 18F-FDG PET/CT, however, has been anecdotally described in this setting. In a recent study, we described 59 cases of lymphadenitis associated with C burnetii infection, among which 42% were associated with persistent focalized infection.[33] Moreover, we recently demonstrated that C burnetii may predispose to lymphomagenesis.[32]18F-FDG PET/CT is therefore a tool of choice for monitoring C burnetii lymphadenitis. Thus, we suggest a diagnostic score for C burnetii persistent lymphadenitis (Table 5, part II). C burnetii lymphadenitis is definite when the bacteria have been identified within lymph nodes by culture, PCR, immunohistochemistry, or FISH, or when 2 major criteria are fulfilled.
Bone marrow uptake was observed in both primary and persistent focalized infection and was associated in almost 50% of cases with spleen hypermetabolism, reflecting the lymphoid tropism of C burnetii. Bone marrow involvement during Q fever has been reported in cases of pancytopenia, hemophagocytic syndrome with aspects of doughnut granuloma,[19,33] and has also recently been described as a diffuse bone marrow 18F-FDG PET/CT hypermetabolism.[20,34]
As mentioned in the literature, we found that 18F-FDG PET/CT is particularly useful in the diagnosis of prosthetic valve endocarditis.[35] Of 21 patients with positive valvular 18F-FDG PET/CT hypermetabolism, over two-thirds had a prosthetic cardiac valve. We described 1 case with pacemaker hypermetabolism. In over two-thirds of cases (71%), valvular hypermetabolism required us to change the diagnosis by confirming or revealing an endocarditis. This is particularly interesting in C burnetii endocarditis, where typical echocardiography findings such as vegetations are frequently lacking.[2] One-third of patients presented associated vascular or osteoarticular foci, supporting the usefulness of 18F-FDG PET/CT in the detection of extracardiac complications of infective endocarditis.[36]
Thanks to 18F-FDG PET/CT, we detected 34 vascular foci, 15 of them involving a vascular prosthesis (44%). In 6 cases, these vascular foci involved a Bentall graft, so that these infections were systematically considered to be associated with prosthetic endocarditis, and 2 cases showed an associated hypermetabolism on a native valve. This shows that vascular C burnetii infections cover 2 different entities: primary infection of a pre-existing aneurysm or vascular graft (which seems to be the more frequent) and real “mycotic aneurysm” as a consequence of Q fever endocarditis. Historically, the definition of “mycotic aneurysm” was provided by Osler in 1885, with the description of a “mushroom-shaped” aneurysm secondary to infectious endocarditis embolism in the arterial wall.[37] These aneurysms are more frequently saccular. Thus, we think that the term “mycotic aneurysm” that has been used generically in several studies dealing with Q fever vascular infections[17] should be used only in the case of associated endocarditis, that is, in cases of valvulopathy associated with a vascular aneurysm in a context of Q fever infection. 18F-FDG PET/CT, which provides a systemic view of infected foci, is a key tool in the distinction of these 2 clinical entities. Some hypermetabolic foci (prostatic, thyroid, and laryngeal) remain of unknown significance, so further studies are required to monitor these foci carefully to understand their meaning and specificity. Our study is 1 more argument for the use of 18-F-FDG PET/CT in the diagnosis of infectious diseases, as recommended by the European regulatory agency,[38] because it allows to change the diagnosis in C burnetii infection for 62% of cases upon discovering or confirming a focus of infection.
Because Q fever is a systemic infectious disease that can affect several organs at once, 18F-FDG PET/CT imaging emerges as a revolutionary tool for localizing all foci of C burnetii infection. Moreover, our work is a new step in demonstrating that the notion of “chronic Q fever” is inadequate because it artificially combines significantly different persistent foci of infection. 18F-FDG PET/CT helps achieve a more accurate identification of infected foci. For each of the foci described, we propose a sampling strategy to confirm the diagnosis of C burnetii infection, which can be made—thanks to several methods such as PCR, culture, immunohistochemistry, and FISH. All these new tools will encourage the development of specific prevention and treatment strategies for each type of C burnetii persistent focalized infection.
Supplementary Material
Acknowledgment
The authors thank Magdalen Lardière for her help in the last corrections of this manuscript.
Footnotes
Abbreviations: 18F-FDG PET/CT = 18F-fluorodeoxyglucose positron emission tomography/computed tomography, C burnetii = Coxiella burnetii.
CE and CM equally contributed to the work and should be considered as co-first authors.
Funding: French National Referral Center for Q fever. Funding sources had no role in the design and conduct of the study.
The authors have no conflicts of interest to declare.
Supplemental Digital Content is available for this article.
References
- 1.Peacock MG, Philip RN, Williams JC, et al. Serological evaluation of O fever in humans: enhanced phase I titers of immunoglobulins G and A are diagnostic for Q fever endocarditis. Infect Immun 1983; 41:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Million M, Raoult D. Recent advances in the study of Q fever epidemiology, diagnosis and management. J Infect 2015; 71 suppl 1:S2–9. [DOI] [PubMed] [Google Scholar]
- 3.Raoult D. Chronic Q fever: expert opinion versus literature analysis and consensus. J Infect 2012; 65:102–108. [DOI] [PubMed] [Google Scholar]
- 4.Eldin C, Mahamat A, Demar M, et al. Q fever in French Guiana. Am J Trop Med Hyg 2014; 91:771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frankel D, Richet H, Renvoisé A, et al. Q fever in France, 1985–2009. Emerg Infect Dis 2011; 17:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Million M, Thuny F, Richet H, et al. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect Dis 2010; 10:527–535. [DOI] [PubMed] [Google Scholar]
- 7.Botelho-Nevers E, Fournier P-E, Richet H, et al. Coxiella burnetii infection of aortic aneurysms or vascular grafts: report of 30 new cases and evaluation of outcome. Eur J Clin Microbiol Infect Dis 2007; 26:635–640. [DOI] [PubMed] [Google Scholar]
- 8.Million M, Bellevegue L, Labussiere A-S, et al. Culture-negative prosthetic joint arthritis related to Coxiella burnetii. Am J Med 2014; 127:786e7–786e10. [DOI] [PubMed] [Google Scholar]
- 9.Angelakis E, Edouard S, Lafranchi M-A, et al. Emergence of Q fever arthritis in France. J Clin Microbiol 2014; 52:1064–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melenotte C, Million M, Audoly G, et al. B-cell non-Hodgkin lymphoma linked to Coxiella burnetii. Blood 2016; 127:113–121. [DOI] [PubMed] [Google Scholar]
- 11.Maurin M, Raoult D. Q fever. Clin Microbiol Rev 1999; 12:518–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Million M, Walter G, Thuny F, et al. Evolution from acute Q fever to endocarditis is associated with underlying valvulopathy and age and can be prevented by prolonged antibiotic treatment. Clin Infect Dis 2013; 57:836–844. [DOI] [PubMed] [Google Scholar]
- 13.Eldin C, Mailhe M, Lions C, et al. Treatment and prophylactic strategy for Coxiella burnetii infection of aneurysms and vascular grafts: a retrospective cohort study. Medicine (Baltimore) 2016; 95:e2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sweet WH. The uses of nuclear disintegration in the diagnosis and treatment of brain tumor. N Engl J Med 1951; 245:875–878. [DOI] [PubMed] [Google Scholar]
- 15.Spacek M, Belohlavek O, Votrubova J, et al. Diagnostics of “non-acute” vascular prosthesis infection using 18F-FDG PET/CT: our experience with 96 prostheses. Eur J Nucl Med Mol Imaging 2009; 36:850–858. [DOI] [PubMed] [Google Scholar]
- 16.Van Riet J, Hill EE, Gheysens O, et al. (18)F-FDG PET/CT for early detection of embolism and metastatic infection in patients with infective endocarditis. Eur J Nucl Med Mol Imaging 2010; 37:1189–1197. [DOI] [PubMed] [Google Scholar]
- 17.Barten DG, Delsing CE, Keijmel SP, et al. Localizing chronic Q fever: a challenging query. BMC Infect Dis 2013; 13:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chieng D, Janssen J, Benson S, et al. 18-FDG PET/CT scan in the diagnosis and follow-up of chronic Q fever aortic valve endocarditis. Heart Lung Circ 2016; 25:e17–20. [DOI] [PubMed] [Google Scholar]
- 19.Dugdale C, Chow B, Yakirevich E, et al. Prolonged pyrexia and hepatitis: Q fever. Am J Med 2014; 127:928–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vos FJ, Bleeker-Rovers CP, Delsing CE, et al. Bone-marrow uptake of (18)F-FDG during fever. Lancet Infect Dis 2010; 10:509–510.[author reply 510–511]. [DOI] [PubMed] [Google Scholar]
- 21.Merhej V, Cammilleri S, Piquet P, et al. Relevance of the positron emission tomography in the diagnosis of vascular graft infection with Coxiella burnetii. Comp Immunol Microbiol Infect Dis 2012; 35:45–49. [DOI] [PubMed] [Google Scholar]
- 22.Takanami K, Kaneta T, Tamada T, et al. Q fever with lymphadenopathy on F-18 FDG PET. Clin Nucl Med 2008; 33:436–437. [DOI] [PubMed] [Google Scholar]
- 23.Oh M, Baek S, Lee S-O, et al. A case of acute Q fever hepatitis diagnosed by F-18 FDG PET/CT. Nucl Med Mol Imaging 2012; 46:125–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon L, De Martino S, Garnon J, et al. Positron emission tomography to diagnose chronic Q fever. Med Mal Infect 2015; 45:420–422. [DOI] [PubMed] [Google Scholar]
- 25.Healy B, van Woerden H, Raoult D, et al. Chronic Q fever: different serological results in three countries: results of a follow-up study 6 years after a point source outbreak. Clin Infect Dis 2011; 52:1013–1019. [DOI] [PubMed] [Google Scholar]
- 26.Eldin C, Angelakis E, Renvoisé A, et al. Coxiella burnetii DNA, but not viable bacteria, in dairy products in France. Am J Trop Med Hyg 2013; 88:765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tissot-Dupont H, Vaillant V, Rey S, et al. Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis 2007; 44:232–237. [DOI] [PubMed] [Google Scholar]
- 28.Raoult D, Tissot-Dupont H, Foucault C, et al. Q fever 1985–1998 Clinical and epidemiologic features of 1,383 infections. Medicine (Baltimore) 2000; 79:109–123. [DOI] [PubMed] [Google Scholar]
- 29.Nourse C, Allworth A, Jones A, et al. Three cases of Q fever osteomyelitis in children and a review of the literature. Clin Infect Dis 2004; 39:e61–66. [DOI] [PubMed] [Google Scholar]
- 30.Landais C, Fenollar F, Constantin A, et al. Q fever osteoarticular infection: four new cases and a review of the literature. Eur J Clin Microbiol Infect Dis 2007; 26:341–347. [DOI] [PubMed] [Google Scholar]
- 31.Tande AJ, Cunningham SA, Raoult D, et al. A case of Q fever prosthetic joint infection and description of an assay for detection of Coxiella burnetii. J Clin Microbiol 2013; 51:66–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melenotte C, Audoly G, Gorse A, et al. B-cell non-Hodgkin lymphoma linked to Coxiella burnetii. Blood 2016; 127:113–121. [DOI] [PubMed] [Google Scholar]
- 33.Delsol G, Pellegrin M, Familiades J, et al. Bone marrow lesions in Q fever. Blood 1978; 52:637–638. [PubMed] [Google Scholar]
- 34.Alwis L, Balan K, Wright P, et al. Bone marrow involvement in Q fever: detection by fluorine-18-labelled fluorodeoxyglucose PET. Lancet Infect Dis 2009; 9:718. [DOI] [PubMed] [Google Scholar]
- 35.Ricciardi A, Sordillo P, Ceccarelli L, et al. 18-Fluoro-2-deoxyglucose positron emission tomography-computed tomography: an additional tool in the diagnosis of prosthetic valve endocarditis. Int J Infect Dis 2014; 28:219–224. [DOI] [PubMed] [Google Scholar]
- 36.Orvin K, Goldberg E, Bernstine H, et al. The role of FDG-PET/CT imaging in early detection of extra-cardiac complications of infective endocarditis. Clin Microbiol Infect 2015; 21:69–76. [DOI] [PubMed] [Google Scholar]
- 37.Osler W. The Gulstonian lectures on malignant endocarditis. Br Med J 1885; 1:577–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jamar F, Buscombe J, Chiti A, et al. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J Nucl Med 2013; 54:647–658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


