Abstract
To evaluate the clinical usefulness of surgical resection of persistent malignant-looking ground-glass-opacity nodules (GGN) without a preoperative tissue diagnosis.
From September 1998 to November 2011, we retrospectively enrolled 288 patients (126 men, 162 women; mean age, 59.3 years) with lung adenocarcinoma proven by surgery and which appeared as GGN on chest computed tomography (CT, ground-glass-opacity [GGO] proportion >20%). We divided the study subjects into 2 groups: patients with a preoperative tissue diagnosis (PTD group, n = 207) and patients without a preoperative tissue diagnosis (No-PTD group, n = 81). In patients with GGN having GGO ≥ 90% (n = 140), we divided them into 2 groups: PTD group (n = 83) and No-PTD group (n = 57). The clinical and surgical outcomes were compared between the 2 groups.
In 204 patients who underwent lobectomy for stage Ia lung cancer, there was no significantly different recurrence-free survival between the 2 groups (P = 0.721). A significantly lower percentage of No-PTD group waited >14 days for surgery (77.8% vs 87.9%, P = 0.030) and were hospitalized for >7 days (56.8% vs 89.9%, P < 0.001). They showed a shorter mean surgery time (136.9 vs 155.0 minutes, P = 0.019). In patients with GGN having GGO ≥ 90%, the results were the same as those of all of the study subjects.
No-PTD group can gain benefits perioperatively, showing no different recurrence-free survival with PTD group in stage Ia lung cancer.
Keywords: computed tomography, ground-glass-opacity, lung cancer, surgery
1. Introduction
Lung cancer was the leading cause of cancer mortality worldwide. Due to recent advances in diagnostic imaging modalities such as computed tomography (CT), the detection of small, indeterminate lung nodules has increased.[1] In particular, the ability to detect ground-glass-opacity nodules (GGN) has increased.[2] As many previous studies have revealed, persistent GGN, especially those with a solid component, are likely a malignancy such as adenocarcinoma.[3,4]
The detection of indeterminate nodules on CT scans indicates the needs for further clinical evaluations. Percutaneous needle biopsy of lung nodules has been established as a high diagnostic performance modality.[5,6] However, nodules with a higher ground-glass-opacity (GGO) component tend to show relatively a lower diagnostic accuracy (51.2%–82.0%).[7–9] Also, several previous studies had reported serious complications associated with percutaneous needle biopsy, especially in patients with high comorbidities and poor lung function.[10,11] Therefore, we need to assess the balance of the risks and benefits when we encounter each case of percutaneous needle biopsy. Moreover, many researchers suggest that sublobar resection would be sufficient for pure GGN as they rarely accompany nodal metastasis and they often appear as multiple lesions which may not be treated using lobectomy.[12,13] However, the clinical usefulness of surgical resection of persistent malignant-looking GGN without preoperative tissue diagnosis is not still thoroughly investigated.
The purpose of this study is to evaluate the clinical usefulness of surgical resection of persistent malignant-looking GGN without preoperative tissue diagnosis, by comparing the clinical and surgical outcomes in patients with (PTD group) and without a preoperative tissue diagnosis (No-PTD group).
2. Materials and methods
This study was approved by the institutional review board of our medical institution. The acquisition of informed consent was waived due to the retrospective study design.
2.1. Patients
From September 1998 to November 2011, lung adenocarcinoma in 1813 patients was diagnosed by surgical pathology at our institution. Of these patients, 307 patients showed 320 GGNs having a percentage of the GGO component within the nodule >20% on preoperative CT scans.[14] GGN was defined as a nodule showing a hazy, increased attenuation that did not obliterate the underlying bronchial or vascular structures, as seen on thin-section CT.[15] By semiquantitative measurements using 10% scale units, the proportion of GGO of a GGN was measured, and patients with GGN having GGO ≤ 20% were excluded as it was considered to be obviously subsolid nodules.[14] Of these 307 patients with 320 GGNs, 19 patients with 20 GGNs were excluded due to no follow-up CT scanning after surgery. Finally, we enrolled 288 patients with 300 GGNs, that is, 126 men (mean age, 61.6 years; range, 36–86 years) and 162 women (mean age, 57.4 years; range, 30–77 years) with a mean age of 59.3 years (range, 30–86 years) (Fig. 1). Of these 288 patients, 9 (3.1%) patients had more than 1 surgically proven GGN (range, 2–5). GGNs seen on CT, but had not been confirmed pathologically, were not reviewed in this study.
Figure 1.
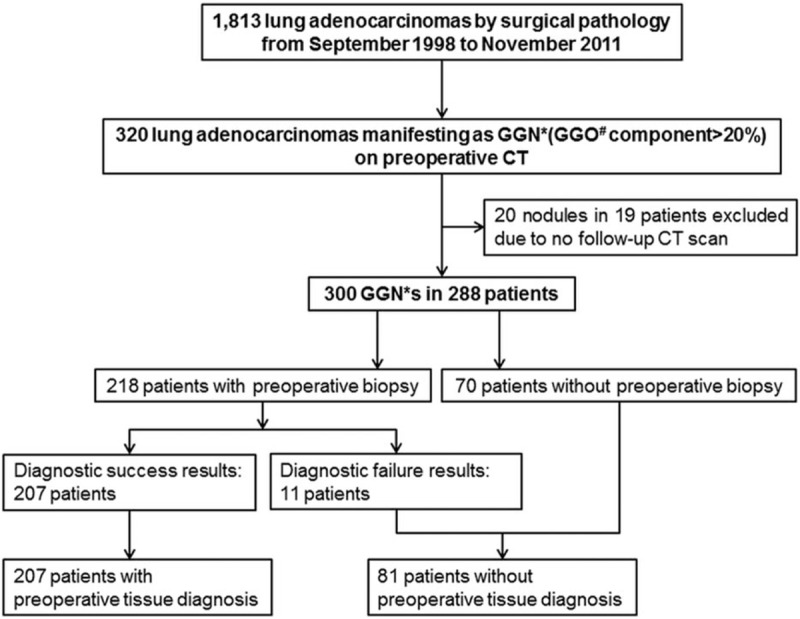
Patients’ enrollment.
We reviewed clinical parameters such as patient age, sex, smoking history, pulmonary function tests, clinical stage, pathologic stage, and mortality by reviewing electronic medical records.
Among the 288 study subjects, 218 underwent percutaneous needle biopsy. To evaluate the clinical advantage of surgical resection of persistent malignant-looking GGN without a preoperative tissue diagnosis, we divided the study subjects into the 2 groups, that is, PTD group (n = 207) in which tissue diagnosis was done by percutaneous lung biopsy; No-PTD group (n = 81) in which percutaneous lung biopsy was not performed (n = 70) or it turned out failed (n = 11).
2.2. CT protocol and image interpretation
From September 1998 to November 2011, chest CT scans were performed using 1 of 4 CT systems (Sensation 16 or 64; Siemens, Erlangen, Germany), (LightSpeed 4 or 64; GE Healthcare, Milwaukee, WI). The scan parameters were 120 kV and 100 effective mA with dose modulation, and the reconstruction intervals were 5-mm thickness and a 5-mm interval without a gap using the standard algorithm and 1-mm reconstruction with a 5-mm gap using the high-frequency algorithm until 2009. Then the reconstruction intervals were 3-mm thickness and a 3-mm interval without a gap using the standard algorithm and a 1-mm reconstruction with a 5-mm gap using the high-frequency algorithm. After intravenous administration of 100 mL of contrast material at a rate of 2.5 mL/second using a power injector, CT scanning was performed with a 50 seconds delay. All images were viewed at the lung window (width, 1500 HU; level 700 HU) and mediastinal window (width, 450 HU; level 50 HU) setting.
Two radiologists (14 and 6 years of clinical experience, respectively, performing the chest imaging) retrospectively reviewed the preoperative CT images obtained on the nearest date to the surgery date in terms of the following GGN parameters: size (diameter across the longest axis), lobar location referring to the anatomical lobes (upper, middle, or lower lobe), proportion of GGO within a GGN, and the presence of pleural/fissural retraction or invasion. The proportion of GGO of a GGN was semiquantitatively measured manually using 10% scale units by 2 radiologists. Because several previous studies had reported the different diagnostic performances of percutaneous needle biopsy between GGN having GGO ≥ 90% and GGN having GGO < 90%,[8,9] the subgroup analysis for GGN having GGO ≥ 90% (n = 140) was done. The clinical stage of each malignant GGN was assessed on CT imaging according to the 7th TNM stage.[16]
2.3. Assessment of recurrence-free survival
The mean postoperative follow-up period was 43.5 ± 20.8 months (range, 1.0–129.0). The mean postoperative follow-up periods in the PTD group and in the No-PTD group were 42.5 ± 20.6 and 46.1 ± 21.0 months, respectively.
In order to evaluate the clinical outcomes of patients who had persistent malignant-looking GGNs, recurrence-free survival was compared between the PTD group and the No-PTD group. Comparison of recurrence-free survival was limited in patients who underwent lobectomy and had the same pathologic stage.
2.4. Assessment of perioperative clinical and surgical outcomes
We reviewed the parameters related to surgery, including the types of surgery, that is, lobectomy, segmentectomy or wedge resection, the histologic diagnosis, pathologic TNM stage, surgery date, length of the surgery, and postoperative complications. The waiting time interval for surgery and the hospital stay duration were calculated from the patient's admission date, surgery date, and discharge date. The waiting time interval for surgery was defined as the time interval between the 1st day the patient presented to the clinician and the day of surgery for the resection of the lung nodule. The hospital stay duration was defined as the total length of the patient's in-patient hospital stay for the preoperative work-up and surgery. In the PTD group (n = 207), the diagnostic results of percutaneous needle biopsy and biopsy-related complications, that is, pneumothorax, hemorrhage, or major bleeding, were also evaluated. In addition, we assessed the 2nd surgery rate between the PTD group and the No-PTD group. Second surgery was defined for when completion lobectomy was performed after resection of malignant GGN. Second surgery did not include operation conversion such as from video-assisted thoracoscopic surgery to open thoracotomy.
In patients with GGN having GGO ≥ 90% (n = 140), we also calculated these surgical and clinical parameters in both groups.
2.5. Statistical analysis
Statistical analysis was performed using statistical software (SPSS version 21.0; SPSS, Chicago, IL). A P value <0.05 was considered statistically significant.
To analyze the clinical effect of surgical resection of persistent malignant-looking GGN without a preoperative tissue diagnosis, the recurrence-free survivals were calculated and compared between the PTD group and the No-PTD group using the Kaplan–Meier analysis and the log-rank testing. We used a Cox proportional hazards model with age, sex, smoking history, nodule size, GGO proportion, the presence of pleural/fissural retraction, and the methods to determine final diagnosis (PTD group vs No-PTD group) as covariates to test for difference in recurrence-free survival. In addition, surgical and clinical outcomes including the waiting time interval for surgery, hospital stay duration, surgery time, postoperative complications, and the 2nd surgery rate were compared by the univariate analysis using the Student t tests and Fisher exact tests.
3. Results
Of these 288 patients, 207 patients had a preoperative tissue diagnosis and 81 patients had no preoperative tissue diagnosis. The characteristics of all of the 288 patients are summarized in Table 1. The mean size of the nodules in the PTD group (n = 207) was 22.3 mm (range, 7–66 mm), and the mean size of the nodules in the No-PTD group (n = 81) was 14.6 mm (range, 6–39 mm), with a mean overall size of 20.2 mm. In 9 patients with multiple GGNs, 6 GGNs in the same lobe and 6 GGNs in the different lobe were surgically resected.
Table 1.
Summary of clinical and radiological characteristics of 288 patients.
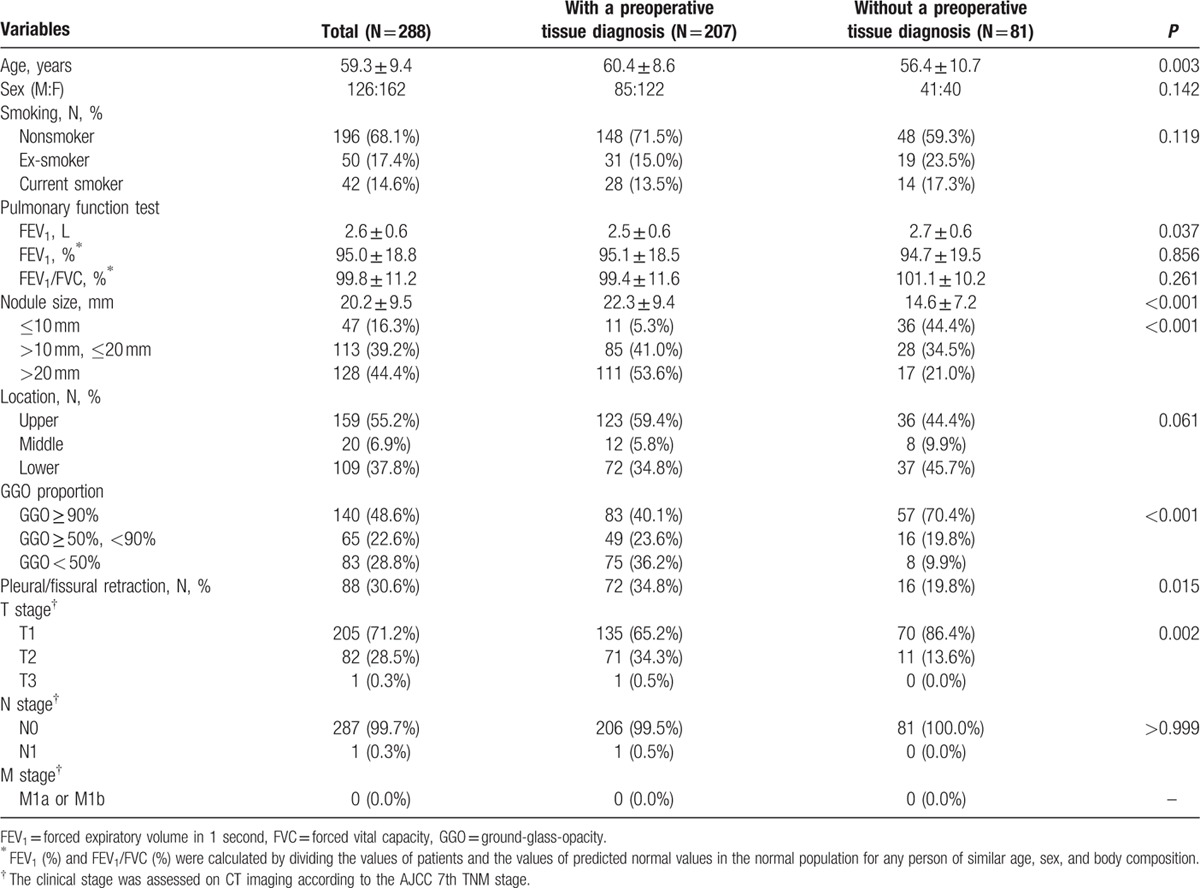
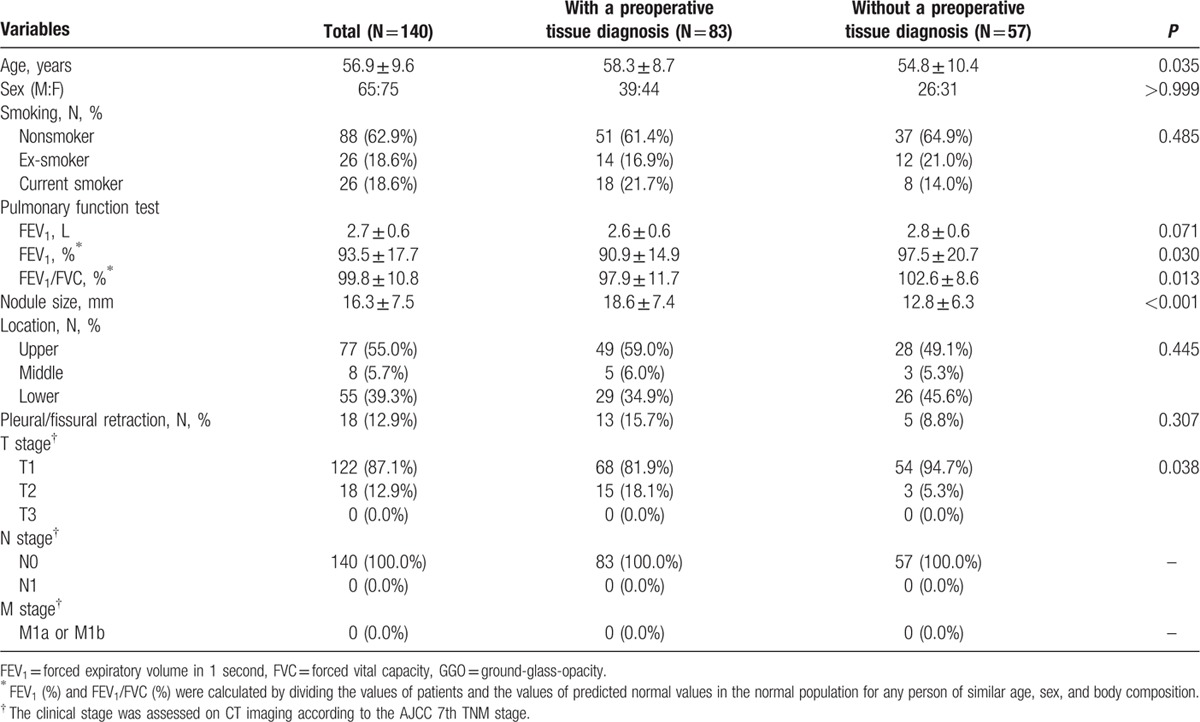
Regarding the proportion of GGO within a GGN, the percentage of patients with GGO ≥ 90% was significantly higher in the No-PTD group than in the PTD group (70.2% [59/84] vs 42.1% [91/216], P < 0.001). The characteristics of the 140 patients with a GGN having GGO ≥ 90% are summarized in Table 2.
Table 2.
Summary of clinical and radiological characteristics of the 140 patients with GGN having GGO ≥ 90%.
3.1. Recurrence-free survival
In patients who underwent lobectomy for stage Ia lung cancer (n = 204), there was no significant difference in the recurrence-free survival time between the PTD group and the No-PTD group (P = 0.721, Fig. 2). The estimated 5 year, recurrence-free survival rate was 91.0% in the No-PTD group and 88.8% in the PTD group. The proportional hazards model showed that there was no significant covariate among these 7 covariates (P ≥ 0.060, Fig. 2). The methods to determine final diagnosis (PTD group vs No-PTD group) was not a significant factor associated with recurrence-free survival (hazard ratio = 1.00 [95% confidence interval, 0.26–3.91], P = 0.998).
Figure 2.
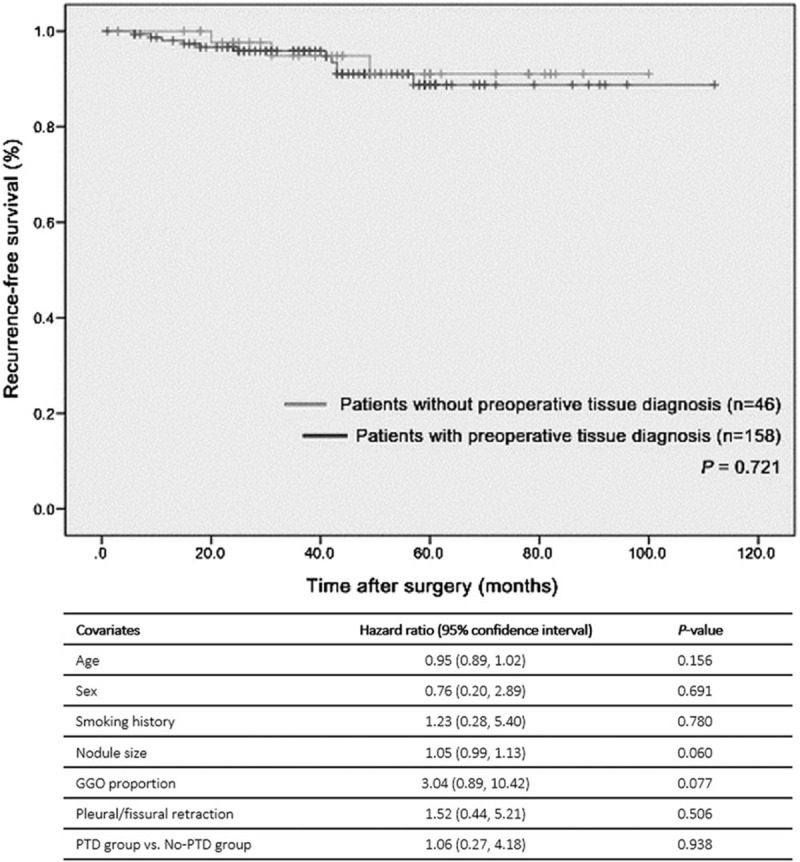
Recurrence-free survival time after surgery: 204 patients who underwent lobectomy for stage Ia lung cancer. There was no significant covariate among these seven covariates in the proportional hazards model. GGN = ground-glass-opacity nodule, GGO = ground-glass-opacity, PTD = preoperative tissue diagnosis.
In the subgroup of patients with GGN having GGO ≥ 90% who underwent lobectomy for stage Ia lung cancer (n = 98, Fig. 3), the No-PTD group tended to have a longer, recurrence-free survival time than the PTD group.
Figure 3.
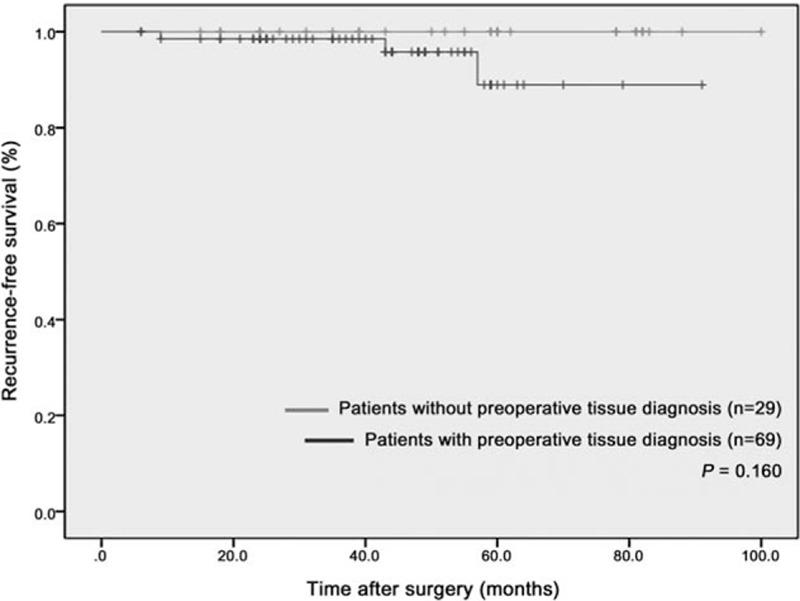
Recurrence-free survival time after surgery: 98 patients with GGN having GGO ≥ 90% who underwent lobectomy for stage Ia lung cancer. GGN = ground-glass-opacity nodule, GGO = ground-glass-opacity.
3.2. Perioperative clinical and surgical outcomes
In total 288 patients, the type of surgery was classified into lobectomy (83.3%, 240/288), segmentectomy (3.8%, 11/288), and wedge resection (12.8%, 37/288). Most patients underwent lobectomy in the PTD group (92.8%, 192/207) and the No-PTD group (59.3%, 48/81), but the proportion of wedge resection for the PTD group was significantly lower than that for the No-PTD group (3.9% [8/207] vs 35.8% [29/81], P < 0.001).
The clinical and surgical outcomes in all of the 288 patients are shown in Table 3. The waiting time interval for surgery was reduced in the No-PTD group. The percentage of patients who waited more than 14 days were significantly lower in the No-PTD group than in the PTD group (77.8% [63/81] vs 87.9% [182/207], P = 0.030). Regarding the hospital stay duration, the percentage of patients who stayed more than 5 days were significantly lower in the No-PTD group than in the PTD group (56.8% [46/81] vs 89.9% [186/207], P < 0.001). The mean surgery time for the No-PTD group was significantly shorter than for the PTD group (136.90 vs 155.0 minutes, P = 0.019). There was no major postoperative complication in either group. The postoperative complications rate was lower in the No-PTD group, although there was no statistically significant difference (7.4% [6/81] vs 7.2% [15/207], P = 0.962). In the 240 patients undergoing lobectomy, there was no significant difference in the waiting time interval for surgery, hospital stay duration, or mean surgery time between the PTD group (n = 192) and the No-PTD group (n = 48) (P > 0.05). The 2nd surgery was only performed in the No-PTD group (26.3%, 15/81).
Table 3.
Comparisons of clinical and surgical outcomes in total 288 patients and in the 140 patients with GGN having GGO ≥ 90%.
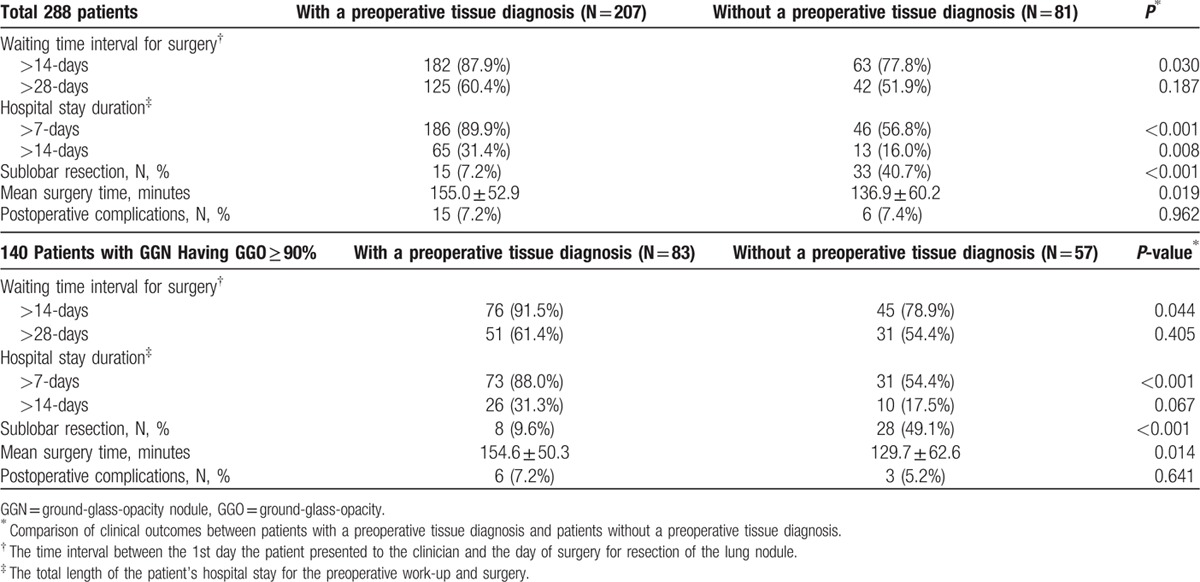
In the 140 patients with GGN having GGO ≥ 90% (Table 3), the percentage of patients who waited for surgery for more than 14 days and stayed in the hospital for more than 7 days were significantly lower in the No-PTD group than in the PTD group (78.9% [45/57] vs 91.5% [76/83], P = 0.044; 54.4% [31/57] vs 88.0% [73/83], P < 0.001). The 2nd surgery was only performed in the No-PTD group (15.8%, 9/57).
4. Discussion
We have assessed the clinical and surgical advantages of surgical resection of persistent malignant-looking GGN without a preoperative tissue diagnosis. Our study demonstrated that surgical resection without a preoperative tissue diagnosis did not have any disadvantages compared with that seen in the PTD group in terms of recurrence-free survival. These results are similar to those of a previous study of Sihoe et al,[17] showing no significantly different recurrence-free survival between the 2 groups in patients who underwent lobectomy for nonsmall-cell lung cancer (stage Ia). In addition, Nakajima et al[18] reported that intraoperative diagnosis followed by the consecutive resection of lung cancer may be beneficial for improving the surgical outcomes compared with PTD group. Because persistent malignant-looking GGN had the low likelihood of N2 nodal or distant metastasis,[19,20] the benefits for surgical resection of persistent malignant-looking GGN without a preoperative tissue diagnosis could be lower than those for solid lung nodules. Recently, considering these natures of malignant GGN, several recent studies have focused on the limited surgical resections for patients with malignant GGN.[12,21–23] These could be mainly explained by the high possibility of malignancy in the persistent GGN[4,24] and the close correlations between the CT findings and pathologic findings of malignant GGN.[25,26] Regarding the diagnostic outcome of biopsy, the nodules with a higher proportion of a GGO component tended to show relatively lower diagnostic accuracy, showing 51.2% to 57.1% accuracy for fine-needle aspiration,[7,8] and 73% concordance rate in malignant and premalignant lesions between core-need and surgical biopsy.[9] Percutaneous needle biopsy of GGNs has a major potential limitation as it may not be feasible due to limited cytologic or even histologic sampling used to differentiate between a purely lepidic pattern and stromal invasion.[12,13] Therefore, surgical resection of persistent malignant-looking GGN without a preoperative tissue diagnosis might be possible by the preoperative CT imaging diagnosis.
Surgical resection without a preoperative tissue diagnosis has many clinical and surgical advantages, including reducing the waiting time interval for surgery, hospital stay duration, and mean surgery time. Although it was difficult to directly compare the results of previous studies due to different definitions, our study had a similar tendency to reduce the waiting time interval for surgery, hospital stay duration, and mean surgery time.[17,27] Considering the higher percentage of sublobar resection in the No-PTD group, this could affect the clinical outcomes because none of the clinical outcomes were significantly different between the No-PTD group and the PTD group in patients who underwent lobectomy. Although the time for an intraoperative biopsy and analysis of frozen specimen would be additionally needed in the No-PTD group, it did not significantly add to the mean surgery time. In other words, surgical resection without a preopreative tissue diagnosis could be useful without prolonging the mean surgery time.
For solid lung nodules, previous studies reported that the percentage of benign disease in No-PTD group ranged from 7.8% to 15.0%.[17,27] In contrast to solid lung nodules, which are concerned about the unnecessary operation for benign lung nodules, persistent malignant-looking GGNs are more concerned about the false-negative result for percutaneous needle biopsy, as persistent malignant-looking GGNs are classified into malignant-looking GGNs after surveillance CT for a minimum of 3 years according to the management guideline for GGNs,[28,29] and the low diagnostic accuracy in GGNs having GGO ≥ 90% is frequently reported due to the low cellularity of GGNs.[8] Our study showed a 5.0% false-negative result rate. This result is similar to the result from Kim et al,[9] reporting the diagnostic accuracy of CT-guided core-needle biopsy for GGNs (90.5%).
The 2nd surgery was performed in 26.3% of the No-PTD group. In light of a recent data documenting the markedly improved 5-year survival of patients with GGN, especially pure GGN, the potential role of limited surgical resections, including partial wedge resections and segmentectomy, has become under renewed scrutiny.[12,21–23,30–33] In our study, we noted that only 1 2nd surgery was performed after 2010. Therefore, the change of surgical management for persistent malignant-looking GGN could affect the result, referring to the high 2nd surgery rate.
There are several limitations to our study. First, its retrospective design could be a limitation such as a selection bias. Considering the fact that the power of our analyses ranged from 63% to 99%, some of our results have a limitation to compare the clinical and surgical outcomes between the 2 groups (PTD group vs No-PTD group). In addition, there were 5 significantly different clinical factors, including age, nodule size, GGO proportion, pleural/fissural retraction, and T stage, between the 2 groups. In order to minimize and adjust these limitations, we consecutively included all of the patients with persistent malignant-looking GGNs within the specific period of 13 years, and performed a Cox proportional hazards model with these clinical factors as a covariate to test for difference in recurrence-free survival. A further large and prospective cohort study would be needed to validate these results. Second, in order to determine the necessity of preoperative tissue diagnosis of persistent malignant-looking GGN, it is ultimately necessary to evaluate the futile surgery rate in all patients with pathologically proven benign and malignant diseases. However, in our study, we focused the perioperative clinical and surgical advantages of surgical resection of persistent malignant-looking GGN without a preoperative tissue diagnosis and included patients with pathologically proven lung adenocarcinomas. Third, the CT imaging techniques were heterogeneous, as our study cohort included the study subjects over a period of 13 years. Although current guideline emphasized that it is necessary to establish the characteristics of GGNs using contiguous thin CT sections (1 mm),[28,34] all CT examinations could not follow this current guideline due to the retrospective study design. In our study, there was no critical limitation to characterize GGNs considering that the mean size of GGNs was 20.2 mm. Also, we included all CT examinations as our study subjects without any selection loss.
In summary, we found that the No-PTD group did not have any disadvantages in clinical outcomes, showing no different recurrence-free survival from that of the PTD group. Also, No-PTD group had perioperative clinical and surgical benefits, which include reduction of waiting time interval for surgery, hospital stay duration, and surgery time. Therefore, surgical resection of persistent malignant-looking GGN without a preoperative tissue diagnosis could provide many clinical and surgical advantages perioperatively without the recurrence-free survival time shortening.
Footnotes
Abbreviations: CT = computed tomography, GGN = ground-glass-opacity nodules, GGO = ground-glass-opacity, No-PTD group = patients without a preoperative tissue diagnosis, PTD group = patients with a preoperative tissue diagnosis.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Sato S, Koike T, Yamato Y, et al. Diagnostic yield of preoperative computed tomography imaging and the importance of a clinical decision for lung cancer surgery. Gen Thorac Cardiovasc Surg 2010; 58:461–466. [DOI] [PubMed] [Google Scholar]
- 2.Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002; 178:1053–1057. [DOI] [PubMed] [Google Scholar]
- 3.Nakata M, Saeki H, Takata I, et al. Focal ground-glass opacity detected by low-dose helical CT. Chest 2002; 121:1464–1467. [DOI] [PubMed] [Google Scholar]
- 4.Kim HY, Shim YM, Lee KS, et al. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology 2007; 245:267–275. [DOI] [PubMed] [Google Scholar]
- 5.Westcott JL. Percutaneous transthoracic needle biopsy. Radiology 1988; 169:593–601. [DOI] [PubMed] [Google Scholar]
- 6.Tsukada H, Satou T, Iwashima A, et al. Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. AJR Am J Roentgenol 2000; 175:239–243. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu K, Ikeda N, Tsuboi M, et al. Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Lung Cancer 2006; 51:173–179. [DOI] [PubMed] [Google Scholar]
- 8.Hur J, Lee HJ, Nam JE, et al. Diagnostic accuracy of CT fluoroscopy-guided needle aspiration biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2009; 192:629–634. [DOI] [PubMed] [Google Scholar]
- 9.Kim TJ, Lee JH, Lee CT, et al. Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2008; 190:234–239. [DOI] [PubMed] [Google Scholar]
- 10.Laurent F, Michel P, Latrabe V, et al. Pneumothoraces and chest tube placement after CT-guided transthoracic lung biopsy using a coaxial technique: incidence and risk factors. AJR Am J Roentgenol 1999; 172:1049–1053. [DOI] [PubMed] [Google Scholar]
- 11.Saji H, Nakamura H, Tsuchida T, et al. The incidence and the risk of pneumothorax and chest tube placement after percutaneous CT-guided lung biopsy: the angle of the needle trajectory is a novel predictor. Chest 2002; 121:1521–1526. [DOI] [PubMed] [Google Scholar]
- 12.Godoy MC, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology 2009; 253:606–622. [DOI] [PubMed] [Google Scholar]
- 13.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6:244–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuguma H, Mori K, Nakahara R, et al. Characteristics of subsolid pulmonary nodules showing growth during follow-up with CT scanning. Chest 2013; 143:436–443. [DOI] [PubMed] [Google Scholar]
- 15.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246:697–722. [DOI] [PubMed] [Google Scholar]
- 16.Lung Edge SB, Byrd DR, Compton CC. AJCC Cancer Staging Manual. 7th edNew York, NY: Springer; 2010. 253–270. [Google Scholar]
- 17.Sihoe AD, Hiranandani R, Wong H, et al. Operating on a suspicious lung mass without a preoperative tissue diagnosis: pros and cons. Eur J Cardiothorac Surg 2013; 44:231–237.discussion 237. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima J, Sato H, Takamoto S. Does preoperative transbronchial biopsy worsen the postsurgical prognosis of lung cancer? A propensity score-adjusted analysis. Chest 2005; 128:3512–3518. [DOI] [PubMed] [Google Scholar]
- 19.Matsuguma H, Nakahara R, Kondo T, et al. Risk of pleural recurrence after needle biopsy in patients with resected early stage lung cancer. Ann Thorac Surg 2005; 80:2026–2031. [DOI] [PubMed] [Google Scholar]
- 20.Okada M, Nakayama H, Okumura S, et al. Multicenter analysis of high-resolution computed tomography and positron emission tomography/computed tomography findings to choose therapeutic strategies for clinical stage IA lung adenocarcinoma. J Thorac Cardiovasc Surg 2011; 141:1384–1391. [DOI] [PubMed] [Google Scholar]
- 21.Yamato Y, Tsuchida M, Watanabe T, et al. Early results of a prospective study of limited resection for bronchioloalveolar adenocarcinoma of the lung. Ann Thorac Surg 2001; 71:971–974. [DOI] [PubMed] [Google Scholar]
- 22.Nakata M, Sawada S, Saeki H, et al. Prospective study of thoracoscopic limited resection for ground-glass opacity selected by computed tomography. Ann Thorac Surg 2003; 75:1601–1605.discussion 1605–1606. [DOI] [PubMed] [Google Scholar]
- 23.Park JH, Lee KS, Kim JH, et al. Malignant pure pulmonary ground-glass opacity nodules: prognostic implications. Korean J Radiol 2009; 10:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon HE, Fukuhara K, Michiura T, et al. Pulmonary nodules 10 mm or less in diameter with ground-glass opacity component detected by high-resolution computed tomography have a high possibility of malignancy. Jpn J Thorac Cardiovasc Surg 2005; 53:22–28. [DOI] [PubMed] [Google Scholar]
- 25.Aoki T, Nakata H, Watanabe H, et al. Evolution of peripheral lung adenocarcinomas: CT findings correlated with histology and tumor doubling time. AJR Am J Roentgenol 2000; 174:763–768. [DOI] [PubMed] [Google Scholar]
- 26.Yang ZG, Sone S, Takashima S, et al. High-resolution CT analysis of small peripheral lung adenocarcinomas revealed on screening helical CT. AJR Am J Roentgenol 2001; 176:1399–1407. [DOI] [PubMed] [Google Scholar]
- 27.Heo EY, Lee KW, Jheon S, et al. Surgical resection of highly suspicious pulmonary nodules without a tissue diagnosis. Jpn J Clin Oncol 2011; 41:1017–1022. [DOI] [PubMed] [Google Scholar]
- 28.Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013; 266:304–317. [DOI] [PubMed] [Google Scholar]
- 29.Lung CT screening reporting and data system (Lung-RADS). http://www.acr.org/∼/media/ACR/Documents/PDF/QualitySafety/Resources/LungRADS/AssessmentCategories.pdf [Accessed July 1, 2016]. [Google Scholar]
- 30.Asamura H, Suzuki K, Watanabe S, et al. A clinicopathological study of resected subcentimeter lung cancers: a favorable prognosis for ground glass opacity lesions. Ann Thorac Surg 2003; 76:1016–1022. [DOI] [PubMed] [Google Scholar]
- 31.Carretta A, Ciriaco P, Melloni G, et al. Surgical treatment of multiple primary adenocarcinomas of the lung. Thorac Cardiovasc Surg 2009; 57:30–34. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe S, Watanabe T, Arai K, et al. Results of wedge resection for focal bronchioloalveolar carcinoma showing pure ground-glass attenuation on computed tomography. Ann Thorac Surg 2002; 73:1071–1075. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida J, Shirota T, Tanimura A, et al. Clinical pathway for impalpable or small lung lesions treated with coil marking and thoracoscopy. Jpn J Thorac Cardiovasc Surg 2001; 49:108–112. [DOI] [PubMed] [Google Scholar]
- 34.Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics 2007; 27:391–408. [DOI] [PubMed] [Google Scholar]


