Abstract
Benefits and harms of long-term anticoagulant therapy (AT) after acute pulmonary embolism (PE) are poorly known. The aim of this study was to investigate the outcome of patients with PE treated with AT for 5 years according to American College of Chest Physicians (ACCP) guidelines.
Patients with both unprovoked and secondary PE were consecutively enrolled in a “real life” study. After a 12-month AT, they continued or stopped the treatment according to ACCP guidelines, and were followed-up for 5 years. Outcomes were all-cause mortality, recurrence, and fatal recurrence under AT.
Of the original consecutive 585 patients, 471 were included (83 dead, 31 lost during the 1st year). Of these, 361 (76.6%) continued AT. During 5 years, death occurred in 109 (30.2%) patients, with a mortality rate of 8.00 events/100 person-years of follow-up; recurrence in 34 (9.4%), with an incidence rate of 2.58 events/person-years; fatal recurrence in 13 (3.6%), with an incidence rate of 0.95 events/person-years. The case fatality rate for recurrence was 38.2%. In the subgroup of patients with unprovoked PE, the chance of dying was significantly lower (RR 0.35; 95% confidence interval 0.24–0.53) and the tendency to fatal recurrence (not significantly) greater (0.11 events/100 person-years vs 0.07 events/100 person-years) than in the remaining patients. Major bleeding occurred in 5 (1.3%) patients. The case fatality rate for bleeding was 14.3%.
During 5-year AT, 30% of patients dies, 10% experiences recurrences, and 5% has fatal recurrences. According to guidelines, most patients need to continue AT; the case fatality rate for bleeding is lower than that for recurrence.
Keywords: anticoagulants, follow-up studies, guidelines, pulmonary embolism, recurrence
1. Introduction
Pulmonary embolism (PE) is potentially lethal in the acute phase and prone to recur during the short- or long-term follow-up.[1–5] After anticoagulant therapy (AT) discontinuation, the rate of recurrences increases with time; therefore, the extension of therapy could be ideally useful in several patients but is limited by the risk of bleeding. Indeed, the case fatality rate for major bleeding in patients treated with warfarin for more than 3 months has been reported to be 9.1%,[6] higher than that due to recurrent venous thromboembolism (VTE) (3.6%).[7]
Unprovoked nature of PE,[8–11] recurrence,[12,13] presence of cancer and chemoradiotherapy,[10,14,15] persistence of residual venous thrombotic material,[16,17] and presence of chronic medical illnesses[10,18] have been considered as potential causes for increased recurrences and mortality after discontinuation of treatment and warrant, therefore, prolonged AT. Unfortunately, data on recurrence/mortality and bleeding in patients with PE under prolonged anticoagulation are not consistently reported in literature. Indeed, most studies investigating the late outcome of PE include patients in whom AT has been administered for 3 to 6 months and stopped months or years before the investigation was made[8,19,20]; other studies include patients enrolled retrospectively, in whom AT has been made for variable periods of time.[9,21]
The main purpose of the present study was to prospectively investigate mortality, recurrence, and bleeding in patients with PE who continue AT for 5 years of follow-up, according to American College of Chest Physicians (ACCP) guidelines.[22] We also evaluated risk factors for mortality, recurrence, and bleeding.
2. Methods
This is a single-center, prospective, observational study (named PISA-PEET, Pulmonary Embolism Extension Therapy Study) performed in an academic teaching hospital. Here we report the results of the first 5-year follow-up. When the study was designed, the approval of the Ethical Committee was not required for observational studies. All patients gave informed consent for the anonymous use of their clinical data for research purposes.
All consecutive patients with a diagnosis of PE made by chest computed tomography between January 2001 and December 2005 were enrolled. Patients were classified as having secondary PE if they reported any of the following risk factors: recent (<3 months) major surgical procedure; leg trauma or fracture; bedridden (>1 week); pregnancy; childbirth (within the previous 3 months); estrogens therapy; and active (recent diagnosis or under treatment) cancer. All patients without the above-mentioned risk factors were regarded as having unprovoked PE.
All patients underwent baseline perfusion lung scintigraphy and lower limb ultrasound to detect deep venous thrombosis (DVT).
Following the initial therapy with unfractionated heparin or low-molecular weight heparin (LMWH) or fondaparinux for 7 to 10 days, vitamin K antagonists (VKA) or LMWH as indicated were continued for 12 months. After 12 months, patients were recommended to continue or stop anticoagulation solely according to the 2001 ACCP guidelines[22] and then followed for 5 years (follow-up period). Patients with unprovoked PE, previous episodes of PE, active cancer, atrial fibrillation, or chronic diseases leading to immobilization were recommended extended therapy. Therapeutic range for patients on VKA was estimated by International normalized ratio (INR) between 2-3.
2.1. Follow-up
Recurrence of PE and/or DVT was suspected on a clinical basis. The diagnosis of PE recurrence was made by perfusion lung scintigraphy according to previously published criteria[23]; briefly, the diagnosis of recurrence was made when 1 or more new segmental defects were detected on lung scan. The diagnosis of DVT recurrence was made according to standard criteria[24]; briefly, the criteria were represented by abnormal results on compression ultrasonography (proximal veins) in the controlateral leg or, in the ipsilateral leg, a newly noncompressible venous segment or an increase of 4 mm or more in the diameter of the thrombus (proximal veins) on ultrasonography.
The mortality registry of the Tuscany Region was consulted for mortality; both all causes and PE death were searched according to the ICD-9-CM classification. Recorded causes of death were active cancer (140–239), cardiovascular diseases (4100, 4280, 4029), PE (4151), liver insufficiency (5715), chronic respiratory diseases (4912, 4928), neurological diseases (3212, 3310, 325), bleeding (4310, 5789), and renal insufficiency (5856).
Bleeding was investigated in all patients, either at the time of follow-up visits or under the patient's request and categorized as major or minor according to standard criteria.[25]
2.2. Data analysis
Continuous variables were expressed as mean ± standard deviation and categorical variables as percentages.
Annual rate for recurrent PE, death for all causes and for fatal PE were computed. The Kaplan–Meier method was used to determine the time course of events.
Predictors of adverse events were investigated by univariate and multivariate analyses performed using the Cox proportional hazards regression model. Categorical variables were included in the model as dummy variables. Variables that were significantly associated with the risk of death for all causes and for fatal recurrence and with the risk of PE recurrence on univariate analysis were selected in a multivariate Cox proportional hazard model to identify features independently associated with events. In detail, we estimated the power of any association between mortality for all causes, mortality for fatal recurrence and PE recurrence, and the following parameters: age, gender, presence of associated DVT, previous VTE, presence of cardiovascular comorbidities and active cancer, and unprovoked PE. All statistical tests were 2-tailed; a P < 0.05 was considered as significant. Statistical analysis was performed with SPSS 16.0 (SPSS Inc., Chicago, IL).
3. Results
Among the original 585 consecutive patients with the diagnosis of PE, 114 (19.5%) were lost during the 1st year of treatment (83, 72.8% died and 31, 27.2% did not return to control) (Fig. 1).
Figure 1.
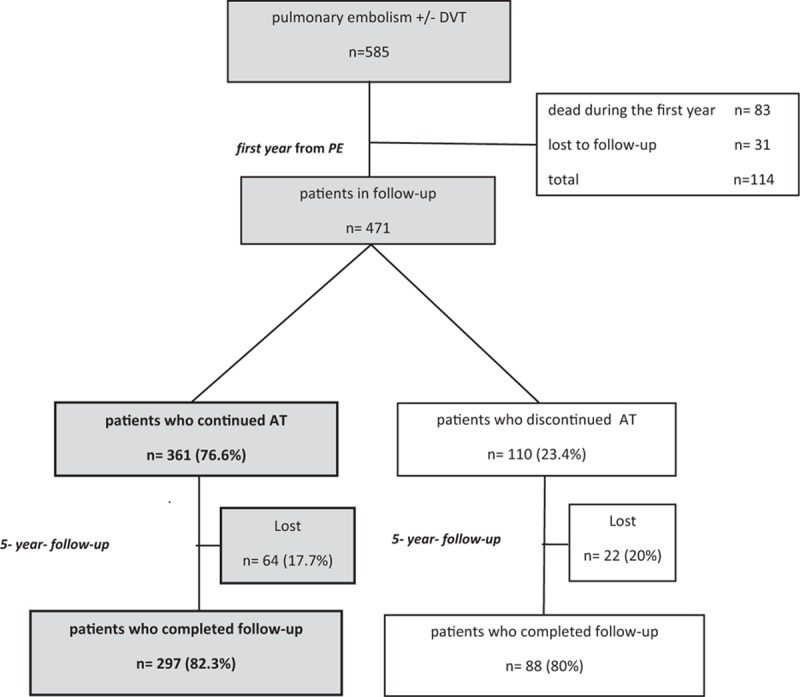
Patient flow diagram of the study. AT = anticoagulant therapy, DVT = deep vein thrombosis, PE = pulmonary embolism.
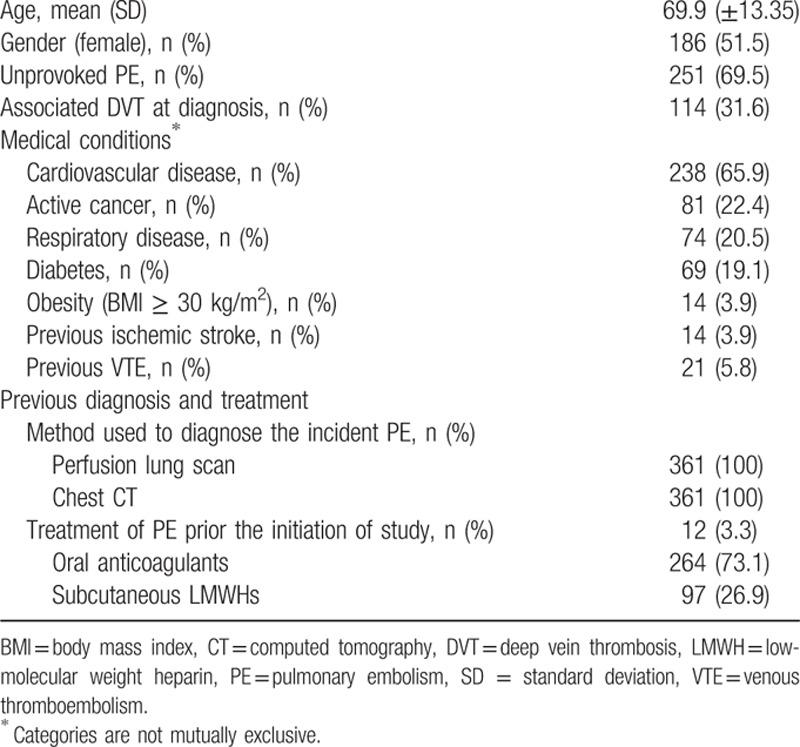
Among the remaining 471 patients, a group of 361 (76.6%) continued AT because they had unprovoked PE (251, 69.5%), recurrence during the 1st year before inclusion (4, 1.1%), presence of active cancer (81, 22.4%), atrial fibrillation (11, 3%), or disabling chronic diseases (14, 3.9%) (Fig. 1). Of these, 64 were lost during the 5-year follow-up (Fig. 1). Patients were treated with VKA in 268 (74.2%) cases and with LMWH in 93 (25.8%) cases. In 153 (42.4%) cases, AT was associated with an antiplatelet drug. No patients had vena cava filter placement. Baseline data of patients are reported in Table 1.
Table 1.
Baseline patients’ characteristics.
3.1. Five-year outcome
3.1.1. All-cause mortality
During the follow-up period, 109 (30.2%) deaths were recorded; of them, 34 occurred in the 1st year (cumulative mortality 9.4%, 95% confidence interval [CI] 6.8–12.9), 19 in the 2nd year (cumulative mortality 15.0%, 95% CI 11.7–19.2), 21 in the 3rd year (cumulative mortality 21.7%, 95% CI 17.6–26.5), 24 in the 4th year (cumulative mortality 29.7%, 95% CI 25.1–35.1), and 11 in the 5th year (cumulative mortality 33.6%, 95% CI 28.7–39.1).
Patients had an all-cause mortality rate of 8.00 events/100 person-years of follow-up. The cause of death was active cancer in 46 (42.2%) cases, cardiovascular disease in 31 (28.4%), recurrence of PE in 13 (11.9%), liver insufficiency in 5 (4.6%), chronic respiratory diseases in 4 (3.7%), neurological disease in 4 (3.7%), major bleeding in 3 (2.8%), and renal insufficiency in 3 (2.8%).
The expected cumulative all-cause mortality probability is reported in Fig. 2. This was 0.35 at the 5th year.
Figure 2.
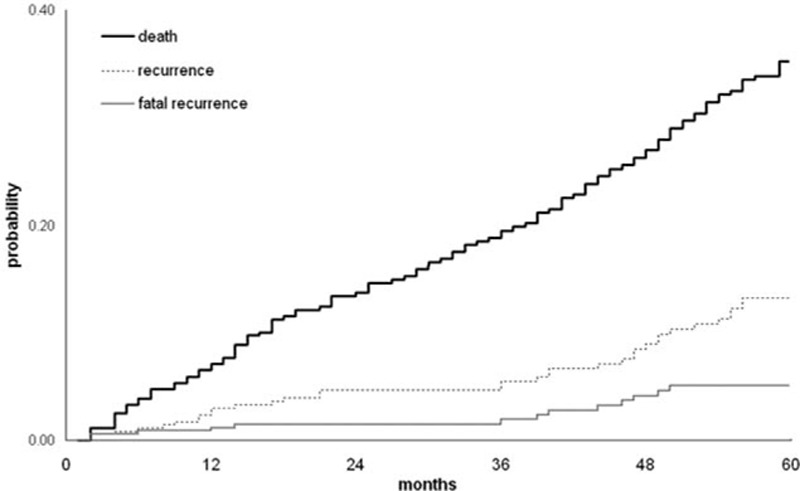
Probability of all-cause death, recurrence, and fatal recurrence.
3.1.2. Recurrence
Thirty-four (9.4%) recurrences were observed during the follow-up period. Recurrences occurred as PE alone in 29 (85.3%) cases, PE associated with DVT in 3 (8.8%), and isolated DVT in 2 (5.9%). Of the total, 15 episodes occurred in the 1st year (cumulative incidence 4.3%, 95% CI 2.6–6.9), 1 in the 2nd year (cumulative incidence 4.6%, 95% CI 2.8–7.3), 6 in the 3rd year (cumulative incidence 6.8%, 95% CI 4.5–10.1), 6 in 4th year (cumulative incidence 9.3%, 95% CI 6.5–13.3), and 6 in 5th year (cumulative incidence 12.2%, 95% CI 8.8–16.8). The incidence of recurrence rate was of 2.58 events/100 person-years of follow-up. The expected cumulative probability of recurrence is reported in Fig. 2. This was 0.10 at the 5th year. Patients who experienced recurrence were on VKA in 19 (55.9%) cases and on LMWH in 15 (44.1%) cases.
3.1.3. Fatal recurrence
Patients experienced 13 (3.6%) fatal recurrences during the follow-up period; of them, 5 occurred in the 1st year (cumulative incidence 1.4%, 95% CI 0.6–3.3), 3 in the 3rd year (cumulative incidence 2.5%, 95% CI 1.3–4.9), and 5 in the 4th year (cumulative incidence 4.6%, 95% CI 2.7–7.9). The incidence of fatal recurrence rate was of 0.95 events/100 person-years of follow-up. Case fatality rate for recurrence was 38.2%. There was no statistically significant difference in the occurrence of fatal recurrence (6 patients, 6.5% vs 7 patients, 2.6%, P = 0.087, chi-squared test) between patients treated with LMWH and VKA. The expected cumulative probability of fatal recurrence is reported in Fig. 2. This was 0.5 at the 5th year.
3.1.4. Role of unprovoked PE on patients’ outcome
In order to evaluate whether the risk of mortality and recurrence was different when therapy was extended due to unprovoked PE (251, 69.5%, patients) or to active cancer or chronic medical illness (95, 26.3%, patients), the 2 groups were analyzed separately. Results are reported in Table 2. Patients with unprovoked PE had a significantly lower mortality (incidence rate ratio 0.35; 95% CI 0.24–0.53). However, patients with unprovoked PE showed greater tendency, albeit not statistically significant, to fatal recurrence (0.11 events/100 person-years vs 0.07 events/100 person-years; incidence rate ratio 1.57; 95% CI 0.34–14.72) (Table 2).
Table 2.
Adverse events in patients with unprovoked PE vs those with active cancer or chronic medical illness.

3.1.5. Bleeding
During the follow-up period, 21 (5.8%) episodes of bleeding occurred; of them, 9 in the 1st year (cumulative incidence 2.7%, 95% CI 1.4–5.1), 4 in the 2nd year (cumulative incidence 3.9%, 95% CI 2.3–6.7), 1 in the 3rd year (cumulative incidence 4.2%, 95% CI 2.5–7.1), 4 in 4th year (cumulative incidence 5.7%, 95% CI 3.6–8.9), and 3 in 5th year (cumulative incidence 8%, 95% CI 5.1–12.5) (Fig. 3).
Figure 3.
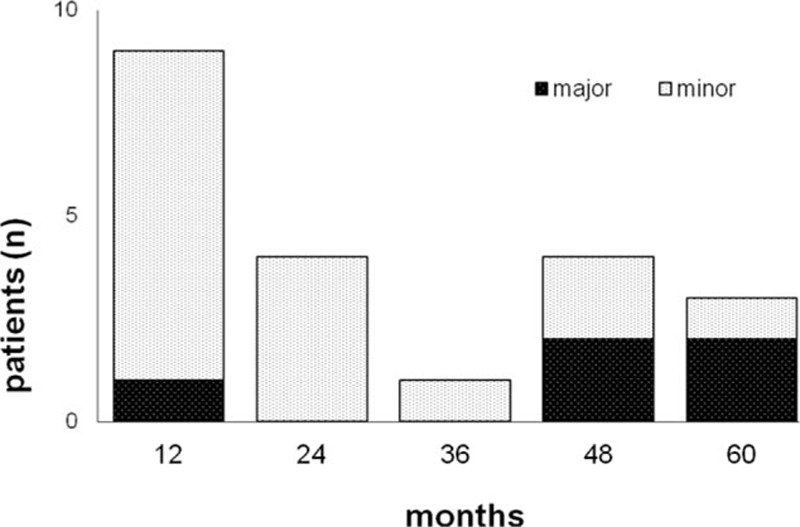
Incidence of bleeding.
Bleedings were classified as major in 5 (1.3%) cases. Major bleeding included 3 (14.3%) fatal episodes, 1 (4.8%) intracranial bleeding, and 1 (4.8%) bleeding leading to hemoglobin reduction of 2 g/dL. Major bleeding occurred in the 1st year in 1 (20%) case, in the 4th year in 2 (40%) cases, and in the 5th year in 2 (40%) cases (Fig. 3). Minor bleeding occurred in the 1st year in 8 (50%) cases, in the 2nd year in 4 (25%) cases, in the 3rd year in 1 (6.2%) case, in the 4th year in 2 (12.5%) cases, and in the 5th year in 1 (6.2%) case (Fig. 3). The case fatality rate of bleeding was 14.3%.
Among patients who experienced bleeding, 16 (76.2%) were on VKA, 3 (14.3%) on VKA in association with an antiplatelet drug, and 2 (9.5%) on LMWH. In patients treated with VKA, INR value was in the therapeutic range (2–3) in 17 (89.5%) cases, out of range (>3) in 2 (10.5%) cases.
Major bleeding occurred in 3 (60%) patients anticoagulated with VKA, in 1 (20%) patient treated with VKA associated with an antiplatelet drug, and in 1 (20%) patient treated with LMWH. All patients treated with VKA had INR value in the therapeutic range.
There was no statistically significant difference in the occurrence of bleeding (3 patients, 3.2% vs 18 patients, 6.7%, P = 0.215, chi-squared test) between patients treated with LMWH and VKA.
3.1.6. Risk factors for mortality, recurrence, and bleeding
In a multivariate Cox proportional hazard model, age (P < 0.0001, 95% CI 1.03–1.07, hazard ratio [HR] 1.05) was independently associated with all-cause mortality (Table 3).
Table 3.
Risk factors for all-cause mortality, recurrence, and fatal recurrence.
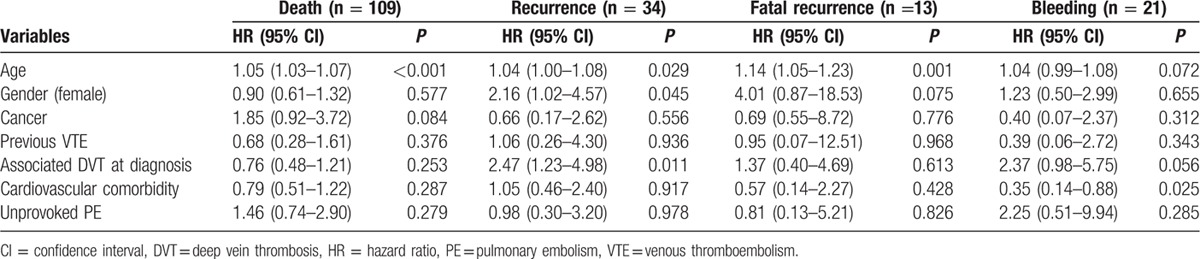
Age (P = 0.029, 95% CI 1.00–1.08, HR 1.04), presence of DVT at diagnosis of PE (P = 0.011, 95% CI 1.23–4.98, HR 2.47) were independently associated with the risk of recurrence (Table 3). Age (P = 0.001, 95% CI 1.05–1.23, HR 1.14) was independently associated with fatal recurrences (Table 3).
No risk factor was identified as associated with bleeding (Table 3). However, cardiovascular morbidity appeared associated with significantly lower risk of bleeding.
4. Discussion
After AT discontinuation in patients with PE, the rate of adverse events increases with time but the AT prolongation may carry important bleeding complications. Therefore, the decision to continue or stop AT is often difficult in such patients and should be based on the careful evaluation of the incidence of adverse events. This “real-life,” prospective, observational study investigated the outcome of patients with PE who continued AT for a long period (5 years) according to ACCP guidelines.[22]
The study shows that, during 5-year anticoagulation, about 1 out of 3 patients died, 1 out of 10 experienced recurrences, and 1 out of 20 had fatal recurrences.
All-cause mortality occurred in 33.6% patients (8.00 events/100 person-years), with a probability of dying of 35%. Several deaths were concentrated in the 1st year (9.4%); however, the mortality remained appreciable during the entire follow-up. Age was independently associated with mortality. Surprisingly, all-cause mortality in our study population under anticoagulation is similar to that reported by other authors in patients who had prophylaxis for only few months.[26,27] This may be explained with the inclusion of patients with cancer and other chronic illnesses (causes apparently unrelated to the anticoagulant prophylaxis) in most published series. A recent multicenter, randomized trial in patients with unprovoked PE treated for a long period (18 months) reports a lower mortality (1.1%).[28] This study, however, excluded patients with short life expectancy such as those affected by cancer or chronic illnesses.
The incidence of PE recurrence in our study was 12.2% (2.58 events/100 person-years of follow-up), with a probability of recurrence of about 10%. Of note, the risk for recurrence increases with age, and in the presence of DVT at the time of the diagnosis of PE; such conditions, therefore, deserve greater caution. Recurrence occurred with greater frequency during the 1st year of follow-up, but it was still appreciable in the late phases. Figures of late recurrence in our series were lower than those reported in patients who stopped prophylaxis early.[3,8,27] This may be explained with the protective effect of AT in our survey.
The incidence of fatal recurrent PE was 4.6% (0.95 events/100 person-years of follow-up), with a probability of dying due to PE of 5%. Mortality due to PE is higher in elderly people. Such mortality occurred at similar rates during the entire follow-up, thus showing that in patients who continue AT beyond 3 months the risk for PE mortality remains almost unchanged despite treatment for at least 5 years. Of note, the case fatality rate for recurrence is 38.2%; therefore, in case of PE recurrence during 5-year follow-up, the chance of death is high, being expected in more than one-third of patients.
It may be of interest that the risk for fatal recurrence of PE is higher in patients in whom the indication for extended therapy was the unprovoked nature of the episode. Indeed, patients with unprovoked PE showed more fatal recurrences than those with active cancer or chronic medical illnesses (4.4% vs 2.1%). The difference between the groups was not significant but this was perhaps due to the small sample size and to the fact that all patients were efficaciously treated. These data confirm that unprovoked PE are at increased risk for fatal recurrence; moreover, they demonstrate for the first time that this is true even under AT up to at least 5 years after acute PE.
Bleedings occurred with a cumulative incidence of 8% and a case fatality rate of 14.3%. Because the case fatality rate for bleeding resulted lower than that for recurrence, the extension of prophylaxis for at least 5 years appears as cost-effective. Bleeding was lower in the above-cited recent study (2.2%).[28] However, in such trial, the bleeding incidence was influenced by the exclusion of patients with predisposing conditions such as cancer, low platelet count, disease resulting in low life expectancy.[28] Major bleeding occurred in 1.4% (5 patients) and fatal in 0.8% (3 patients); other authors reported higher incidences in patients treated and followed for variable periods of time.[19,20,29,30]
The overall, unexpected result of this study is that, according to the guidelines, the large majority of patients with PE continues the AT beyond the period of 3 months commonly recommended. As a matter of fact, the extension of anticoagulation is recommended in about 80% of patients, because they are affected by unprovoked PE, previous VTE, presence of active cancer, atrial fibrillation, or disabling chronic diseases. The high prevalence of patients who continue anticoagulation may be interpreted as a partial failure of the current guidelines, indicating the need for new strategies based on tailored treatments and new anticoagulant molecules.
4.1. Study strengths and limitations
To our knowledge, this is the first study reporting data on the 5-year outcome of patients with PE undergoing to AT throughout the follow-up. This has been possible because a center for the diagnosis and treatment of PE is active at the University Hospital, where patients are followed according to a standard clinical strategy.[31] Other authors reported results on patients followed for years after the discontinuation of 3 to 6 months of prophylaxis[3,8,27,30] or treated for a longer period of time but following a nonstandardized protocol.[30] Recently, a standardized, prospective study reported results on patients with unprovoked PE systematically treated for 18 months. Though well designed and most useful clinically, this study limited the follow-up to a selected population, by excluding patients with a short life expectancy.[28] Our work, instead, can be defined a “real life” study, in that it enrolled all consecutive patients with PE without exclusion criteria, including those with cancer or other relevant comorbidities often excluded in other studies.
A main limitation is that this is a single-center study, with a relatively small sample size. Another limitation would be that all our patients were treated for 1 year before the start of follow-up and this may make less comparable our data with those of studies that treat patients for 3 or 6 months. A third limitation is that our study started before the era of new-generation anticoagulants. Therefore, long-term anticoagulation was made by VKA or LMWH. Recently, new anticoagulants (direct thrombin and direct factor Xa inhibitors) have been made available and their properties investigated in a number of clinical trials.[32–34] The oral route of administration and the remarkable pharmacologic properties, such as the rapid onset and offset of action, the relatively short half-life, the predictable anticoagulant effect, and the lower interaction with food and drugs, make these agents easy to use and, thus, ideal candidates for secondary prevention of VTE, especially in patients requiring long-term treatment. Thus, we cannot exclude that future guidelines might recommend different options, making our present results not applicable to different scenarios.
Footnotes
Abbreviations: ACCP = American College of Chest Physicians, AT = anticoagulant therapy, DVT = deep vein thrombosis, INR = International normalized ratio, LMWH = low-molecular weight heparin, PE = pulmonary embolism, VKA = vitamin K antagonists, VTE = venous thromboembolism.
AP takes the responsibility for the content of the manuscript, including the data and the analysis.
LM, LC, and AP had full access to all of the data in the study; LM, AC, and AP take responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. All authors contributed substantially to the study design; LC and FA are responsible for data analysis and interpretation. LM and AP took the responsibility of writing the manuscript.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Kearon C. Natural history of venous thromboembolism. Circulation 2003; 107:22–30. [DOI] [PubMed] [Google Scholar]
- 2.Nijkeuter M, Söhne M, Tick LW, et al. The natural course of hemodynamically stable pulmonary embolism: clinical outcome and risk factors in a large prospective cohort study. Chest 2007; 131:517–523. [DOI] [PubMed] [Google Scholar]
- 3.Heit JA, Silverstein MD, Mohr DN, et al. The epidemiology of venous thromboembolism in the community. Thromb Haemost 2001; 86:452–463. [PubMed] [Google Scholar]
- 4.Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 2007; 98:756–764. [DOI] [PubMed] [Google Scholar]
- 5.Martinez C, Cohen AT, Bamber L, et al. Epidemiology of first and recurrent venous thromboembolism: a population-based cohort study in patients without active cancer. Thromb Haemost 2014; 112:255–263. [DOI] [PubMed] [Google Scholar]
- 6.Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003; 139:893–900. [DOI] [PubMed] [Google Scholar]
- 7.Carrier M, Le Gal G, Wells PS, et al. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med 2010; 152:578–589. [DOI] [PubMed] [Google Scholar]
- 8.Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1626 patients. Haematologica 2007; 92:199–205. [DOI] [PubMed] [Google Scholar]
- 9.Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heit JA, Mohr DN, Silverstein MD, et al. Predictors of recurrence after deep vein thrombosis and pulmonary embolism. Arch Intern Med 2000; 160:761–768. [DOI] [PubMed] [Google Scholar]
- 11.Hansson PO, Sorbo J, Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Arch Intern Med 2000; 160:769–774. [DOI] [PubMed] [Google Scholar]
- 12.White RH, Zhou H, Kim J, et al. A population-based study of the effectiveness of inferior vena cava filter use among patients with venous thromboembolism. Arch Intern Med 2000; 160:2033–2041. [DOI] [PubMed] [Google Scholar]
- 13.Schulman S, Wahlander K, Lundstrom T, et al. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N Eng J Med 2003; 349:1713–1721. [DOI] [PubMed] [Google Scholar]
- 14.Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Int Med 2010; 170:1710–1716. [DOI] [PubMed] [Google Scholar]
- 15.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002; 100:3484–3488. [DOI] [PubMed] [Google Scholar]
- 16.Piovella F, Crippa L, Barone M, et al. Normalization rate of compression ultrasonography in patients with a first episode of deep vein thrombosis of the lower limbs: association with DVT recurrence and new thrombosis. Haematologica 2002; 87:515–522. [PubMed] [Google Scholar]
- 17.Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Int Med 2002; 137:955–960. [DOI] [PubMed] [Google Scholar]
- 18.Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the Longitudinal Investigation of Thromboembolism Etiology. Am J Med 2004; 117:19–25. [DOI] [PubMed] [Google Scholar]
- 19.Spencer FA, Emery C, Joffe SW, et al. Incidence rates, clinical profile and outcomes of patients with venous thromboembolism. The Worcester VTE study. J Thromb Thrombolysis 2009; 28:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verso M, Agnelli G, Ageno W, et al. Long-term death and recurrence in patients with acute venous thromboembolism: the MASTER registry. Thromb Res 2012; 130:369–373. [DOI] [PubMed] [Google Scholar]
- 21.Moutzouris JP, Ng AC, Chow V, et al. Acute pulmonary embolism during warfarin therapy and long-term risk of recurrent fatal pulmonary embolism. Thromb Haemost 2013; 110:523–533. [DOI] [PubMed] [Google Scholar]
- 22.Hyers TM, Agnelli G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease. Sixth ACCP Consensus Conference on Antithrombotic Therapy. Chest 2001; 119:176S–193S. [DOI] [PubMed] [Google Scholar]
- 23.Palla A, Ribas C, Rossi G, et al. The clinical course of pulmonary embolism patients anticoagulated for 1 year: results of a prospective, observational, cohort study. J Thromb Haemost 2010; 8:68–74. [DOI] [PubMed] [Google Scholar]
- 24.Prandoni P, Lensing AWA, Bernardi E, et al. The diagnostic value of compression ultrasonography in patients with suspected recurrent deep vein thrombosis. Thromb Haemost 2002; 88:402–406. [PubMed] [Google Scholar]
- 25.Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet 1996; 348:423–428. [DOI] [PubMed] [Google Scholar]
- 26.Ng AC, Chung T, Yong AS, et al. Long-term cardiovascular and noncardiovascular mortality of 1023 patients with confirmed acute pulmonary embolism. Circ Cardiovasc Qual Outcomes 2011; 4:122–128. [DOI] [PubMed] [Google Scholar]
- 27.den Exter PL, van der Hulle T, Lankeit M, et al. Long term clinical course of acute pulmonary embolism. Blood Rev 2013; 27:185–192. [DOI] [PubMed] [Google Scholar]
- 28.Couturaud F, Sanchez O, Pernod G, et al. Six months vs extended oral anticoagulation after a first episode of pulmonary embolism. The PADIS-PE Randomized Clinical Trial. JAMA 2015; 314:31–40. [DOI] [PubMed] [Google Scholar]
- 29.Schulman S, Lindmarker P, Holmström M, et al. Post-thrombotic syndrome, recurrence and death 10 year after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost 2006; 4:734–742. [DOI] [PubMed] [Google Scholar]
- 30.Douketis JD, Gu CS, Schulman S, et al. The risk for fatal pulmonary embolism after discontinuing anticoagulant therapy for venous thromboembolism. Ann Intern Med 2007; 147:766–774. [DOI] [PubMed] [Google Scholar]
- 31.Palla A, Celi A, Marconi L, et al. Venous thromboembolism in cancer: frequently asked questions when guidelines are inconclusive. Cancer Invest 2015; 33:142–151. [DOI] [PubMed] [Google Scholar]
- 32.Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718. [DOI] [PubMed] [Google Scholar]
- 33.The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510. [DOI] [PubMed] [Google Scholar]
- 34.Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368:699–708. [DOI] [PubMed] [Google Scholar]


