Abstract
Personal mastery is an important determinant in shaping physical health across middle and late life. The modified effect of mastery on relation between frailty and adverse health outcome remains unclear. The main purpose of this study was to evaluate the prognostic role of mastery on frailty among older people by using a nationwide representative population-based cohort. In total, 715 community-dwelling participants aged 54 years and over recruited in 2000 and received second visit 6 years later. Personal mastery was represented by the Pearlin mastery score, and frailty was defined by modified Fried criteria. Multivariate generalized linear mixed analysis was used to examine the association interaction between frailty and Pearlin mastery scores for activities of daily living decline. Overall, prevalence of frailty and prefrail were 9.7% and 48.8%. In a 6-year period, 94 participants (13.1%) experienced functional decline. Compared with function nondecliners, function decliners had greater proportion of frailty (26.6% vs 7.1%; P < 0.001) and lesser mastery score (17.2 vs 18.7; P < 0.001). After adjusting with basic demography, healthy behavior, cognitive function, and multimorbidity, frailty status and mastery were significantly interacted (coefficient estimate: −0.80, standard error: 0.23, P = 0.001). The negative coefficient estimate indicated that self-control, that is, self-mastery, may attenuate the adverse effects of frailty on functional outcomes. Similar results were shown when subjects with baseline functional deficits were excluded for analysis. In conclusion, high self-mastery attenuates adverse effects of frailty on functional decline.
Keywords: frailty, mastery, physical function, self-control belief
1. Introduction
It is widely accepted that functional status is at central position of health for older people.[1] Maximizing or maintaining functional independence of old people is a priority of public health for seniors in the era of rapid population aging. Prevalence of disability increases with aging, which is closely related to higher risk of mortality and institutionalization, and may be modifiable through preventive interventions.[2] It has been reported that frail older people would benefit most from preventive interventions than the disabled ones.[3] Therefore, early identification and early intervention at the predisability stage, that is, frailty, may be the most important strategy to prevent progressively functional decline or to maintain their physical independence.[4] Frailty has been described as a vulnerable state that was featured by accumulated deficits in multiple inter-related systems and decreased physiological reserve.[5,6] Piles of studies suggested that frailty was closely associated with sarcopenia, immunosenescence, functional decline, and mortality of older people.[7–12] Moreover, frailty may be a reversible state by intensive physical interventions, which make frailty an important issue in public health.[9,13]
Apart from the negative impact of functional decline on overall health status, increasing studies were designed to examine the protective roles of maintaining physical health, especially in the field of positive psychological factors.[14–18] Personal control belief, also known as mastery, has been considered as a positive factor for psychological well-being on senior health.[19] It was defined as people's beliefs regarding the extent to which they would control their life-chances instead of being ruled fatalistically.[20,21] Evidences suggested that self-mastery would shape physical health across middle and late life,[15,16,22] and also was a protective factor against mortality.[23] Penninx et al[24] reported that disabled older women with higher emotional vitality and higher sense of mastery would have better chance to maintain physical function and better survival. In a study of 626 community-dwelling older adults, people with poorer sense of personal mastery were at higher risk of subsequent functional decline of lower limbs.[25] This association was also observed in old frail people during hospitalization. A study of 172 older people admitted to the geriatric evaluation and management unit showed that frail older people with lower mastery would increase their hospital length of stay, risk of mortality, and prehospitalization.[14] However, findings from the Longitudinal Aging Study of Amsterdam suggested that psychological resources may not modify the effect of frailty on functional decline in a 3-year period.[26] Results for personal mastery, modifying the effects of frailty on functional decline, were inconsistent.[14,26] Therefore, the present study was intended to test the hypothesis that higher mastery would attenuate the adverse effect of frailty on function decline through a nationwide population-based cohort study.
2. Materials and methods
2.1. Study design and participants
The present study used the data of the Social Environment and Biomarkers of Aging Study (SEBAS)—a national representative population-based cohort sample with Taiwanese participants aged over 54 years, which started in 2000. SEBAS was randomized subsampling from the 1999 wave of the Taiwan Longitudinal Study of Aging (TLSA). TLSA is a national longitudinal survey designed to understand physical, mental, and social health of middle and older adults. TLSA started since 1989, and re-interviewed subjects every 3 to 4 years. SEBAS randomly sampled the middle-aged and older people from the 1999 wave of TLSA. The details of sampling and data collection procedures were described elsewhere.[27] All participants signed written informed consent and received face-to-face interview by well-trained research nurses to collect basic demographic data and clinical assessments. The whole study was approved by the Joint Institutional Review Boards of Taiwan, and also at Princeton University and Georgetown University.
Among 1713 respondents sampled from TLSA for SEBAS in 2000, 1497 (92% response rate) of them were interviewed and 1023 (69% of those interviewed) received complete face-to-face baseline assessments, which consisted the 2000 wave of SEBAS. Among them, 20 participants (1.9%) with incomplete data in components of frailty, 35 (3.4%) with missing Perlin mastery scores, and 3 participants with incomplete data were excluded. During the 6-year follow-up period, there were 250 participants lost to follow-up. Data of 715 subjects with complete clinical information, components of frailty, and mastery scores were used for this study (Fig. 1).
Figure 1.
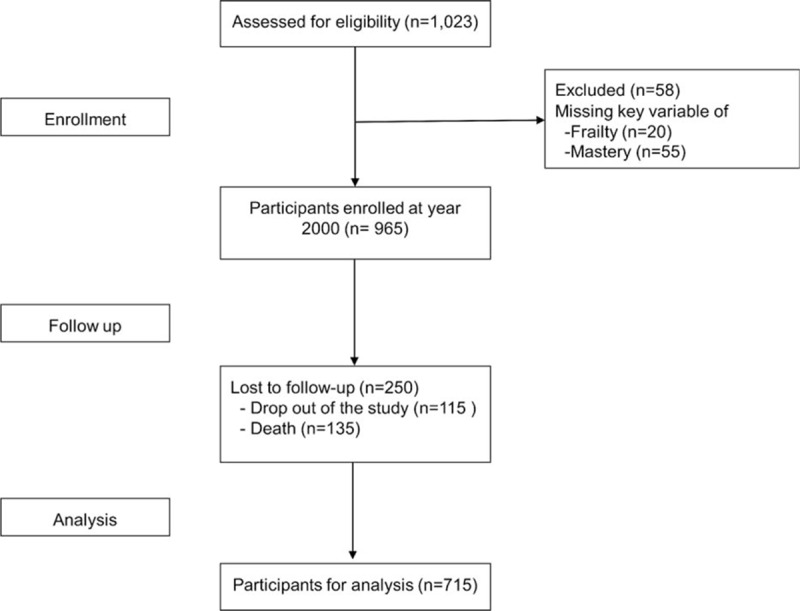
Flow chart of the study.
2.2. Definition of Frailty
At the first wave of SEBAS conducted in 2000, no widely accepted operational definition of frailty was available. To fit the longitudinal cohort data, 5 phenotypic components of frailty were selected based on the concept of frailty originated from the Cardiovascular Health Study.[6] Similar modifications had been done in previous studies for definition of frailty.[28–30] Among the 5 components, weight loss was substituted by poor appetite. The question from modified 10-item Center for Epidemiological Studies—Depression scale,[31] stated with modified sentences as “In the past week, have you experienced the following situations or feelings?— not interested in eating, have a poor appetite” was used. If the answer was yes, the interviewer would ask “How often in the past week did you feel this way?” Sometimes (2–3 d/wk) or often (>4 d/wk) would be defined as frailty in terms of weight loss phenotype. In contrast, none or rare (1 d/wk) would be robust. Similar definitions were also used adopted in the large-scale Survey of Health, Ageing, and Retirement in Europe (SHARE) study.[29] Exhaustion was evaluated by the 2 questions from 2 modified Center for Epidemiological Studies—Depression scale questions, that is, “In the past week, have you experienced that feel that doing anything was exhausting?” and “In the past week, have you experienced that unable to gather your energy to do things?” If the answer was yes, then the interviewer would ask how often it happened. Participants answering sometimes (2–3 d/wk) or often (>4 d/wk) for the abovementioned questions were categorized as having exhaustion. Slowness of walking was measured by the Nagi questionnaire.[32,33] The participants were asked. “Did you have any difficult in walking 200 to 300 meters?” They would be designated as slow, if the answer was yes. Weakness of hand grip strength was also surrogated by the Nagi questionnaire—“Did you have any difficulty in picking up or twisting using your fingers?” If the answer was yes to this question, the participants would be assigned as weak. Participants without any physical activity or physical activity less than once a week were considered as physically inactive in this study. Those who had none, 1, or 2 and 3, or more phenotypes would be classified under robust, prefrail, and frail groups, respectively.
2.3. Mastery (personal belief)
Perceived self-control was assessed by Pearlin mastery scale,[21] which is a 4-point Likert scale (1 = strongly agree to 4 = strongly disagree) and has good validity and reliability for mastery measurement.[34,35] Items asked participants to rate the extent to which they believe their life-chances are under their own control instead of destination ruling. A total score was summarized by all 7 items, ranging from 7 to 28. The higher scores indicated the higher level of self-mastery. Cobranch α at SEBAS 2000 and 2006 were 0.60 and 0.95, respectively.
2.4. Physical function (activity of daily living)
At both waves of SEABS, participants were asked if they have any perceived difficult in activities of daily living (ADLs), including bathing, dressing, eating, transferring (transferring from bed to a chair), mobilization (moving around in the house), and toileting.[34,36] A simple sum of these 6 measures ranged from 0 to 6, and higher scores indicated greater limitation of physical function. No physical function limitation was defined as limitation of ADLs equaled to 0.
2.5. Covariates
Factors associated with physical function were selected as covariates. Covariates included age (<65 years and ≥65 years), sex, education (no schooling, elementary school, or middle school and above), smoke (smoker and nonsmoker), drink (drinker and nondrinker), cognitive function, and multimorbidities. Cognitive function was evaluated with Short Portable Mental Status Questionnaire,[37] which ranged from 1 to 10, and higher score indicated greater deficits of cognitive function. Multimorbidity was taken regards as a surrogate of individual's general health.[38] There were 14 chronic conditions, self-report physician diagnosed, in SEBAS, including hypertension, diabetes, heart disease, stroke, cancer, pulmonary disease, gastric disease, liver disease, arthritis, kidney disease, gout, cataract, degenerative joint disease, and hip fracture. Those who had 2 or more conditions were referred as having multimorbidity.
2.6. Statistical analysis
In this study, the numerical variables were expressed by mean ± standard deviation, and categorical variables were expressed by frequency and rate. Statistical analysis was performed with SAS 9.4 (The SAS Institute, Cary, NC). Those whose ADL deficits increased in the 6-year period were assigned to functional decline group, and others were included in the nondecline group. For comparisons between the 2 groups—numerical variables and categorical variables—Student t test, chi-square, and Fisher exact test were used when appropriate. Because of correlated data nature, generalized linear mixed model analysis (GLMM) was used.[39] Univariate GLMM was used to explore the impact of each variable on ADL change. Multivariate GLMM was used for investigation the relationship between frailty and physical function changes. The interaction between frail conditions and mastery score (robust/prefrail/frail × mastery) were tested by multivariate GLMM. We stratified participants into higher and lower mastery groups by mean mastery score at baseline, and then examined the impact of frailty on physical function changes. A secondary analysis was performed when participants with any disability at baseline were all excluded. A P value (2-tailed) less than 0.05 was considered statistically significant.
3. Results
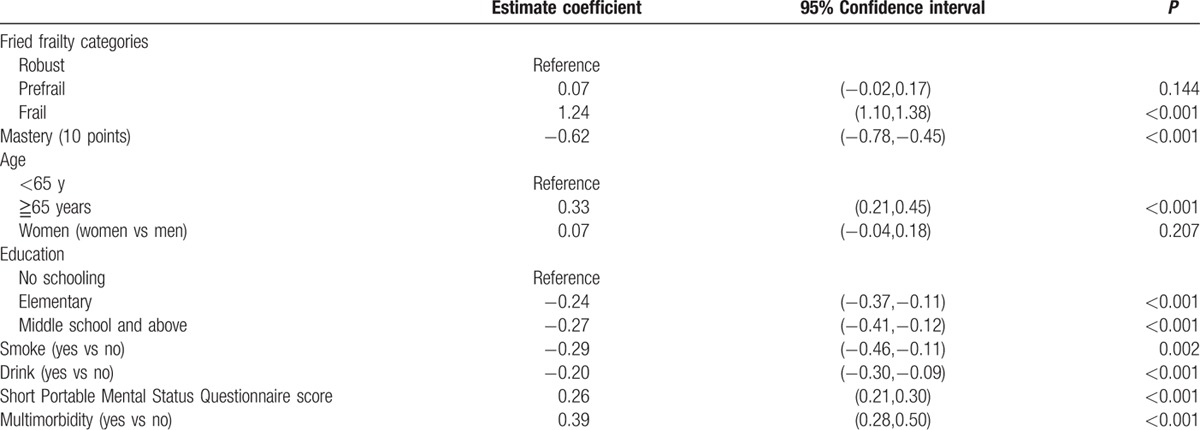
Table 1 showed characteristics of participants at baseline and follow-up after 6 years. In general, participants (age 66.5 years, standard deviation 7.3 years, ranged from 54 to 80 years) became frailer, had worsen cognitive function, poor physical function, and higher proportion of multimorbidity. Compared with subjects without functional decline, those having functional decline were more likely to be older, women, having poorer cognitive function, less drinking, higher chance of frailty, and no schooling (Table 2). Univariate GLMM analysis was used to explore possible predictors for functional decline, and the results are summarized in Table 3. In a 6-year follow-up period, frail people would lose 1.2 items of ADLs than robust ones. On the other hand, the increasing of every 10 points of mastery score would attenuate 0.6 item of ADL loss. Higher education, smoking, and drinking were negatively associated with functional decline. The association between smoking, drinking, and functional changes disappeared after adjusting age and sex. Short Portable Mental Status Questionnaire score and multimorbidity were positively associated with functional decline.
Table 1.
Characteristics of participants at baseline and 6-year follow-up.
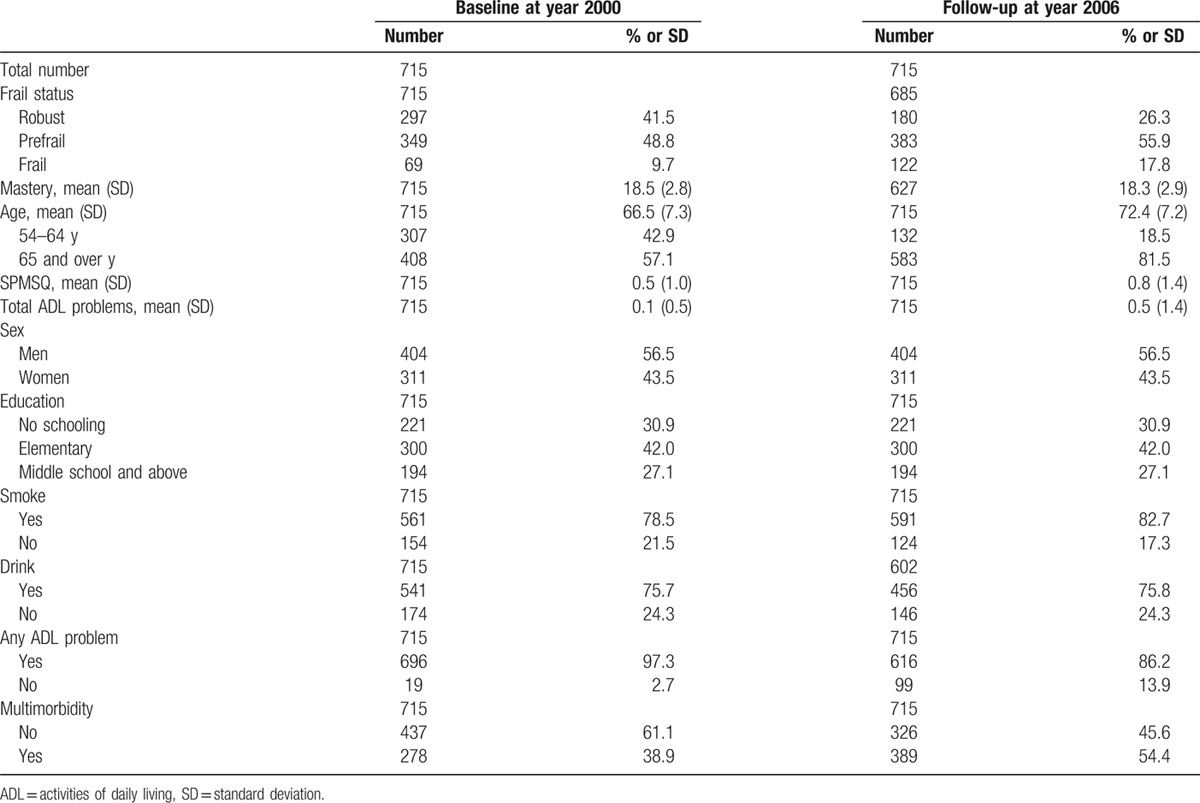
Table 2.
Comparison of functional decliner and nondecliner on baseline clinical characteristics.
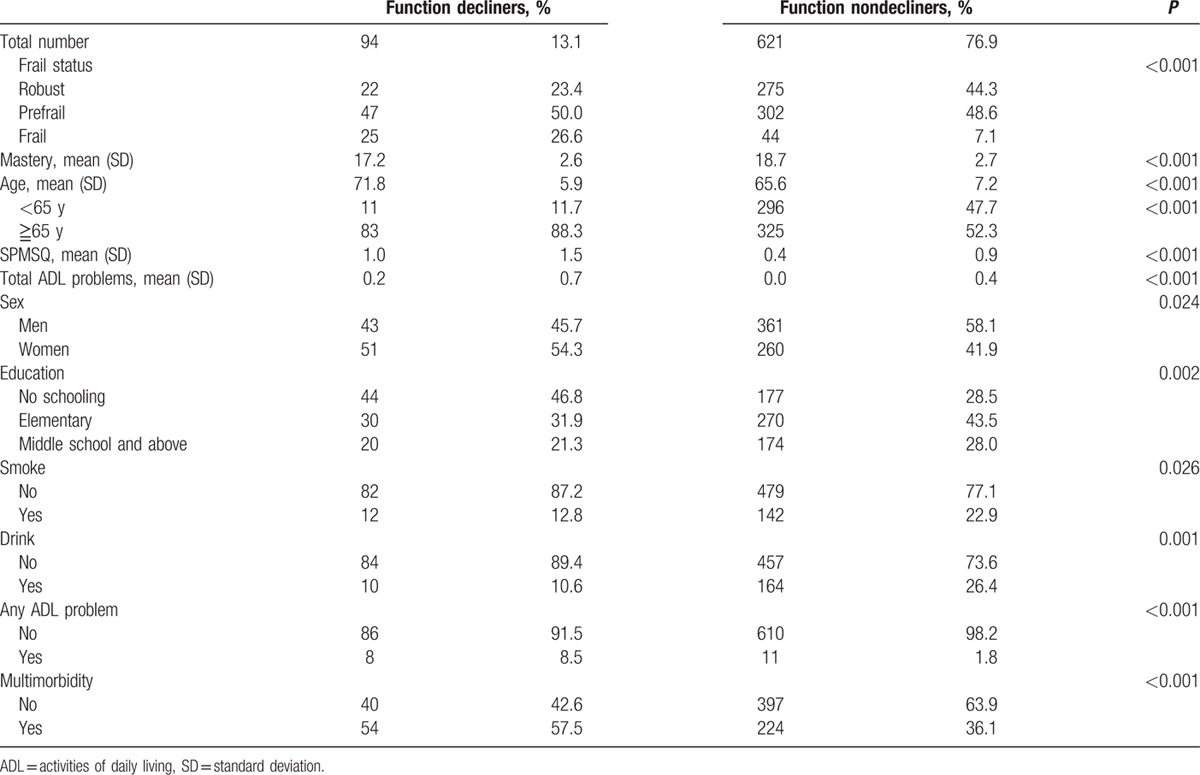
Table 3.
Univariate linear mixed model to explore the association between variables and functional decline.
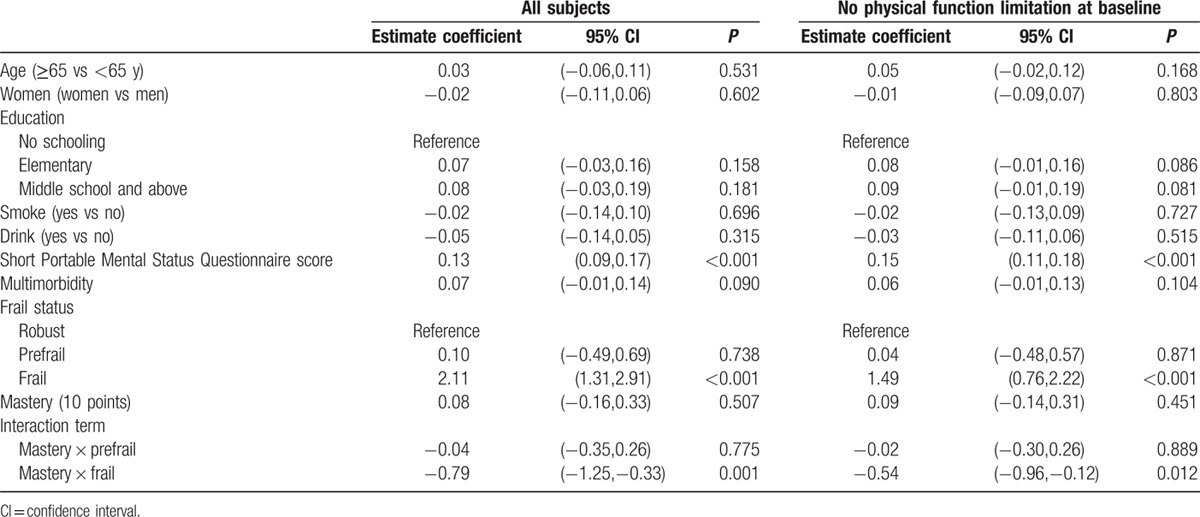
Multivariate GLMM analysis revealed that mastery score was negatively associated with functional decline after adjustment for age, sex, smoking, drinking, education level, cognitive function, and multimorbidity (estimate: −0.22, standard error: 0.08, 95% confidence interval [CI] −0.37, −0.07, P = 0.004). This negative association became statistically insignificant (estimate: −0.04, standard error: 0.07, 95%CI −0.19, 0.10, P = 0.101) when further adjusting frailty. Multivariate GLMM was used to test the interaction between frail conditions and mastery, which showed statistical insignificant for prefrail x mastery (estimate: −0.04, standard error: 0.16, 95% CI −0.35, 0.26, P = 0.775) and significant for frail × mastery (estimate: −0.79, standard error: 0.23, 95% CI −1.25, −0.33, P < 0.001). After excluding participants with any baseline ADL deficit, the interaction between frail × mastery remained significant (estimate: −0.54, standard error: 0.21, 95% CI −0.96, −0.12, P = 0.012) (Table 4).
Table 4.
Multivariate linear mixed model to explore the interaction between frail status and mastery for functional decline among all participants and those without physical function limitations at baseline.
Participants were categorized into higher mastery group (mastery score >18 points) and lower mastery group. The physical function deficits among frail adults was 0.3, which was greater than robust ones (estimate: 0.31, standard error: 0.06, 95% CI −0.19, −0.44, P < 0.001) among higher mastery group and those among frail people was 0.9 greater than robust ones (estimate: 0.91, standard error: 0.11, 95% CI 0.69, 1.13, P < 0.001).
Compared with participants (n = 715) for analysis, those who were excluded (n = 250) from the study were significantly older (70.8 ± 7.6 vs 66.5 ± 7.3 years; P < 0.001), having lower ADL deficits (0.2 ± 0.8 vs 0.1 ± 0.5; P = 0.042), but no statistical difference in mastery score (18.1 ± 3.0 vs 18.5 ± 2.8; P = 0.119), frailty status (12.4% vs 9.7%; χ2 = 1.76, P = 0.415), and multimorbidity (43.6% vs 38.9%, χ2 = 1.72, P = 0.190).
4. Discussion
In this study, we found that frailty and low mastery were associated with functional decline in a 6-year period. Moreover, the present study found that the interaction between higher sense of self-control and frailty was associated with ADL status of community-dwelling older people. To the best of our knowledge, this is the first study that clearly showed mastery would attenuate the adverse effects of frailty on functional decline.
Frailty is a common condition for older people and has become an emerging public challenge in the aging world.[40] In this study, frailty was significantly associated with physical function decline, which was compatible with results of previous studies.[6,10] More importantly, the impact of frailty on physical function changes was lesser in the adults with higher level of mastery. In recent years, study suggested that process of frailty causing adverse health outcomes to older people is potentially reversed by physical intervention.[18,41] Based on our finding, mastery would be an important factor when targeting frailty intervention. Previous studies have shown the significant association of low mastery and functional decline.[14,16,25] In a longitudinal study of 3626 community-dwelling Americans, people with more positive psychological protective factors were associated with better maintenance of functional independence in a 10-year period than those with less positive psychological protective factors.[16] However, this association was not identified among frail older people in the Longitudinal Aging Study of Amsterdam Study (LASA). Hoogendijk et al[26] argued that it takes time to observe the influence of mastery on health, which caused the failure of the LASA study to show the protective effect of mastery in a 3-year follow-up period. In a study of 172 older hospital inpatients, in whom the prevalence of frailty was high (56%), mastery was proposed to be an effective modifier for frailty among them.[14] Nevertheless, results of this study clearly demonstrated that mastery may attenuate the adverse effects of frailty on the health of older people with an extended observation period.
Although the protective effect of mastery against the adverse effect of frailty on physical functional decline was clearly shown, the pathophysiological mechanism between psychological function and physical health remained unclear. Two plausible pathways, that is, behavioral and biological aspects, may help to explain the complex association. Personal mastery would affect the skills of coping and adaptation to stressors, which would determine if an event was stressful or not.[42] More and more evidences suggested that personal perceived control would serve as a facilitator of health status through affecting homeostasis,[43] hormonal, immune process,[17] and cardiometabolic risk factors.[44] Results of this study suggested that higher level of mastery would protect older people from functional decline and modify the adverse effect of frailty on functional decline, which implied the potential role of self-control belief as part of psychological frailty. Moreover, personal mastery is a modifiable factor. Previous studies showed that people receiving training of self-regulation and coping skills would significantly increase their self-control.[19,45] This strengthened the need for screening psychological factors for frailty intervention and the need to arrange related training for self-regulation and coping skills, when we take frailty intervention as an integrated consideration in terms of a biopsychosocial approach, instead of exercise and nutrition only, because of the benefits of physical function and overall health. The current study provided a proposal that personal mastery would be a possible factor of psychological frailty, and further intervention study would be needed to demonstrate.
Despite all efforts which went into this study, there still are some limitations. An important limitation is the loss of participants to follow-up, which was mostly attributable to unmodifiable cause—death. Those who were lost to follow-up were significantly older having poorer physical function, but no substantial difference in frailty states or level of mastery. These attritions might limit the generalizability of the present study. Nevertheless, the major strength of this study is using a population-based longitudinal cohort which was followed up for a long period, long enough to prove the link between mastery and frailty, which also echoed the LASA that buffering effects of mastery on function might take time.[26]
In conclusion, given the population aging with increasing functional dependence, strategies to prevent function decline are very great important for public health. Findings from this study suggested that a comprehensive frailty intervention program with full consideration of biopsychosocial aspects would include not only exercise and nutrition but also the components of improving coping skills and self-regulation. Further intervention study is needed for further clarification.
Acknowledgments
The authors express their gratitude to the staff from Health Promotion Administration, Ministry of Health and Welfare, Taiwan, for data gathering, and to all the participants for their assistance.
Footnotes
Abbreviations: ADLs = activities of daily living, CI = confidence interval, GLMM = Generalized Linear Mixed Model, LASA = Longitudinal Aging Study of Amsterdam, SEBAS = Social Environment and Biomarkers of Aging Study, SHARE = Survey of Health, Ageing and Retirement in Europe, TLSA = Taiwan Longitudinal Study of Aging.
Funding: This study was supported by the Aging and Health Research Center, National Yang Ming University and Ministry of Science and Technology of Taiwan (MOST 103–2633-B-400–002; and MOST 105–3011-B-010–001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declared no competing interests.
References
- 1.Chatterji S, Byles J, Cutler D, et al. Health, functioning, and disability in older adults: present status and future implications. Lancet 2015; 385:563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen K, Doblhammer G, Rau R, et al. Ageing populations: the challenges ahead. Lancet 2009; 374:1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berrut G, Andrieu S, Araujo de Carvalho I, et al. Promoting access to innovation for frail old persons. IAGG (International Association of Gerontology and Geriatrics), WHO (World Health Organization) and SFGG (Societe Francaise de Geriatrie et de Gerontologie) Workshop: Athens January 20–21, 2012. J Nutr Health Aging 2013; 17:688–693. [DOI] [PubMed] [Google Scholar]
- 4.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013; 381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–156. [DOI] [PubMed] [Google Scholar]
- 7.Eeles EM, White SV, O’Mahony SM, et al. The impact of frailty and delirium on mortality in older inpatients. Age Ageing 2012; 41:412–416. [DOI] [PubMed] [Google Scholar]
- 8.Lee WJ, Chen LK, Tang GJ, et al. The impact of influenza vaccination on hospitalizations and mortality among frail older people. J Am Med Dir Assoc 2014; 15:256–260. [DOI] [PubMed] [Google Scholar]
- 9.Gill TM, Baker DI, Gottschalk M, et al. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med 2002; 347:1068–1074. [DOI] [PubMed] [Google Scholar]
- 10.Vermeulen J, Neyens JC, van Rossum E, et al. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr 2011; 11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WJ, Chen LK, Liang CK, et al. Soluble ICAM-1, independent of IL-6, is associated with prevalent frailty in community-dwelling elderly Taiwanese people. PLoS One 2016; 11:e0157877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen LK, Lee WJ, Peng LN, et al. Recent advances in sarcopenia research in Asia: 2016 update from the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2016; 17:767. [DOI] [PubMed] [Google Scholar]
- 13.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014; 311:2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dent E, Hoogendijk EO. Psychosocial factors modify the association of frailty with adverse outcomes: a prospective study of hospitalised older people. BMC Geriatr 2014; 14:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerstorf D, Heckhausen J, Ram N, et al. Perceived personal control buffers terminal decline in well-being. Psychol Aging 2014; 29:612–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lachman ME, Agrigoroaei S. Promoting functional health in midlife and old age: long-term protective effects of control beliefs, social support, and physical exercise. PLoS One 2010; 5:e13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steptoe A, O’Donnell K, Badrick E, et al. Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: the Whitehall II study. Am J Epidemiol 2008; 167:96–102. [DOI] [PubMed] [Google Scholar]
- 18.Park-Lee E, Fredman L, Hochberg M, et al. Positive affect and incidence of frailty in elderly women caregivers and noncaregivers: results of Caregiver-Study of Osteoporotic Fractures. J Am Geriatr Soc 2009; 57:627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodin J. Behavioral medicine: beneficial effects of self control training in aging. Appl Psychol 1983; 32:153–181. [Google Scholar]
- 20.Rodin J. Aging and health: effects of the sense of control. Science 1986; 233:1271–1276. [DOI] [PubMed] [Google Scholar]
- 21.Pearlin LI, Lieberman MA, Menaghan EG, et al. The stress process. J Health Soc Behav 1981; 22:337–356. [PubMed] [Google Scholar]
- 22.Turiano NA, Chapman BP, Agrigoroaei S, et al. Perceived control reduces mortality risk at low, not high, education levels. Health Psychol 2014; 33:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Infurna FJ, Ram N, Gerstorf D. Level and change in perceived control predict 19-year mortality: findings from the Americans’ changing lives study. Dev Psychol 2013; 49:1833–1847. [DOI] [PubMed] [Google Scholar]
- 24.Penninx BW, Guralnik JM, Bandeen-Roche K, et al. The protective effect of emotional vitality on adverse health outcomes in disabled older women. J Am Geriatr Soc 2000; 48:1359–1366. [DOI] [PubMed] [Google Scholar]
- 25.Milaneschi Y, Bandinelli S, Corsi AM, et al. Personal mastery and lower body mobility in community-dwelling older persons: the Invecchiare in Chianti study. J Am Geriatr Soc 2010; 58:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoogendijk EO, van Hout HP, van der Horst HE, et al. Do psychosocial resources modify the effects of frailty on functional decline and mortality? J Psychosom Res 2014; 77:547–551. [DOI] [PubMed] [Google Scholar]
- 27.Cornman JC, Glei DA, Goldman N, et al. Cohort profile: The Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan. Int J Epidemiol 2016; 45:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CY, Wu SC, Chen LJ, et al. The prevalence of subjective frailty and factors associated with frailty in Taiwan. Arch Gerontol Geriatr 2010; 50 suppl 1:S43–47. [DOI] [PubMed] [Google Scholar]
- 29.Romero-Ortuno R, Walsh CD, Lawlor BA, et al. A frailty instrument for primary care: findings from the Survey of Health, Ageing and Retirement in Europe (SHARE). BMC Geriatr 2010; 10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos-Eggimann B, Cuenoud P, Spagnoli J, et al. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci 2009; 64:675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boey KW. Cross-validation of a short form of the CES-D in Chinese elderly. Int J Geriatr Psychiatry 1999; 14:608–617. [DOI] [PubMed] [Google Scholar]
- 32.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem. Fund Q Health Soc 1976; 54:439–467. [PubMed] [Google Scholar]
- 33.Nagi SZ, Marsh J. Disability, health status, and utilization of health services. Int J Health Serv 1980; 10:657–676. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein M, Goldman N, Chang M-C, et al. Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan, 2000 and 2006. Inter-university Consortium for Political and Social Research (ICPSR) [distributor] 2014:01–06. [Google Scholar]
- 35.Leung C, Lo SK. Validation of a questionnaire to measure mastery motivation among Chinese preschool children. Res Dev Disabil 2013; 34:234–245. [DOI] [PubMed] [Google Scholar]
- 36.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963; 185:914–919. [DOI] [PubMed] [Google Scholar]
- 37.Erkinjuntti T, Sulkava R, Wikstrom J, et al. Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly. J Am Geriatr Soc 1987; 35:412–416. [DOI] [PubMed] [Google Scholar]
- 38.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380:37–43. [DOI] [PubMed] [Google Scholar]
- 39.Littell R, Milliken G, Stroup W, et al. SAS for Mixed Models. 2nd edCary, NC, USA: SAS Institute Inc; 2006. [Google Scholar]
- 40.Rodriguez-Artalejo F, Rodriguez-Manas L. The frailty syndrome in the public health agenda. J Epidemiol Commun Health 2014; 68:703–704. [DOI] [PubMed] [Google Scholar]
- 41.Cesari M, Vellas B, Hsu FC, et al. A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J Gerontol A Biol Sci Med Sci 2015; 70:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lachman ME, Neupert SD, Agrigoroaei S. The Relevance of Control Beliefs for Health and Aging. 7th edLondon: Elsevier; 2011. [Google Scholar]
- 43.Dockray S, Steptoe A. Positive affect and psychobiological processes. Neurosci Biobehav Rev 2010; 35:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Infurna FJ, Gerstorf D. Perceived control relates to better functional health and lower cardio-metabolic risk: the mediating role of physical activity. Health Psychol 2014; 33:85–94. [DOI] [PubMed] [Google Scholar]
- 45.Jerant A, Moore M, Lorig K, et al. Perceived control moderated the self-efficacy-enhancing effects of a chronic illness self-management intervention. Chronic Illn 2008; 4:173–182. [DOI] [PubMed] [Google Scholar]


