Abstract
Introduction:
Acute pancreatitis (AP) is an uncommon disease with a wide clinical course varying from mild and self-limiting to severe with eventual death. However, death caused by AP is rare. Most cases of AP reported in the English-language literature are based on clinical data; few are medico-legal studies.
Case presentation:
The author recently experienced a case of sudden unexpected death in a young man caused by extensive severe hemorrhagic AP secondary to cholelithiasis, not chronic alcoholism, which is a much more prominent etiology of AP in medico-legal perspectives. The deceased had complained of dizziness, nausea, and fatigue without significant abdominal pain for about 1 week and received some home medications for symptomatic treatment including an antibiotic drug from a clinic just 2 days prior to his death. He had complained of lower extremity weakness, intense thirst, and subsequently collapsed and was brought to a nearby hospital where he was pronounced dead shortly after his admission following unsuccessful advanced cardiopulmonary resuscitation attempts.
Conclusion:
This case is herein reported with an extensive review of the pertinent literature to highlight the findings of the case and raise awareness within the medico-legal profession and also the medical profession.
Keywords: acute pancreatitis, autopsy, gall stone, sudden death
1. Introduction
Acute pancreatitis (AP) is an uncommon disease defined as an inflammatory disorder of the pancreas. It is characterized by a wide clinical course varying from mild and self-limiting to severe with eventual death. However, death caused by AP is rare. Such deaths may occur either early within the first week due to hypovolemic shock or late in subsequent weeks due to marked enzyme activation, bleeding, or infection.[1] Most deaths published in the English–language literature are based on clinical studies; few are medico-legal studies. According to autopsy data, the incidence of fatalities caused by AP ranges from 0.2% to 2.5% of sudden natural deaths.[2–6] The authors recently experienced a case of sudden unexpected death in a young man due to extensive severe hemorrhagic AP and reported herein.
2. Presenting concerns
A 38-year-old man suddenly collapsed and was brought to the emergency department of a nearby hospital. According to the information obtained from his friend who presented at the hospital, the deceased had complained of dizziness, nausea, and fatigue without significant abdominal pain for about 1 week prior to his death. The deceased then went to a nearby clinic where he received some home medications for symptomatic treatment and an antibiotic drug (doxycycline) just 2 days prior to hospital arrival. On the day of his arrival to the hospital, the deceased complained of lower extremity weakness and intense thirst and subsequently collapsed.
3. Clinical findings
Upon arrival to the hospital, the deceased was unconscious and his vital signs were as follows: pulse, 82 beats/min; blood pressure, 112/77 mm Hg; respiratory rate, 22 breaths/min; temperature, 37.5°C; and oxygen saturation (SpO2), 79% to 86%. His bedside blood sugar concentration was high as measured by Dextrostix.
4. Diagnostic and therapeutic focus and assessment
The deceased was pronounced dead shortly after his admission following unsuccessful attempts performing advanced cardiopulmonary resuscitation. Some medical investigations were performed at the hospital prior to his death. His complete blood count showed the following results: hemoglobin concentration, 17.9 g/dL; hematocrit, 54.8%; white blood cell count, 17,800/μL (neutrophils, 87.1%; lymphocytes, 5.5%); and platelet count, 189,000/μL. His kidney function test showed a blood urea nitrogen concentration of 40 mg/dL, creatinine concentration of 4.6 mg/dL, and ketone concentration of 5.9 mmol/L. His electrolyte analysis showed a sodium concentration of 154.5 mmol/L, potassium concentration of 5.91 mmol/L, chloride concentration of 109 mmol/L, and bicarbonate level of +8 mmol/L. His coagulogram was in normal range. Brain computed tomography and chest x-rays showed no abnormalities.
5. Follow-up and outcomes
At autopsy, external examination of the deceased revealed a very obese man with a body mass index of 40.3 kg/m2 (193 cm in length and 150 kg in weight). Minimal peripheral cyanosis was observed. There was no evidence of injury in any external regions of the body. Internal examination disclosed a normal scalp and skull. The brain showed marked general congestion and minimal focal subarachnoid hemorrhage. Examination of the chest cavity showed no evidence of trauma. The heart showed mild cardiomegaly with mild dilatation of the left ventricle. The aorta and coronary arteries revealed mild atherosclerotic changes. Gross sections of the heart demonstrated no evidence of old or recent myocardial infarction. The lungs exhibited marked congestion and edema. No pleural effusion was detected in either thoracic cavity.
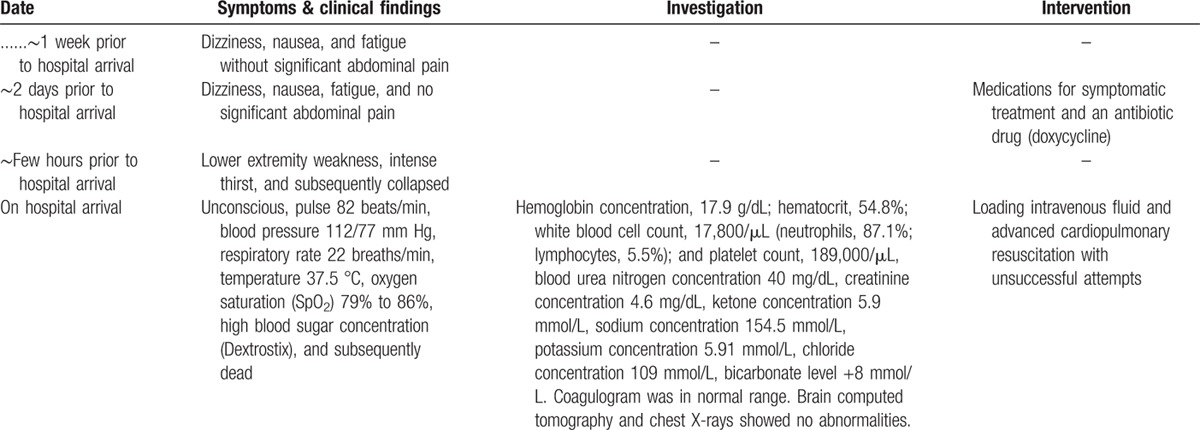
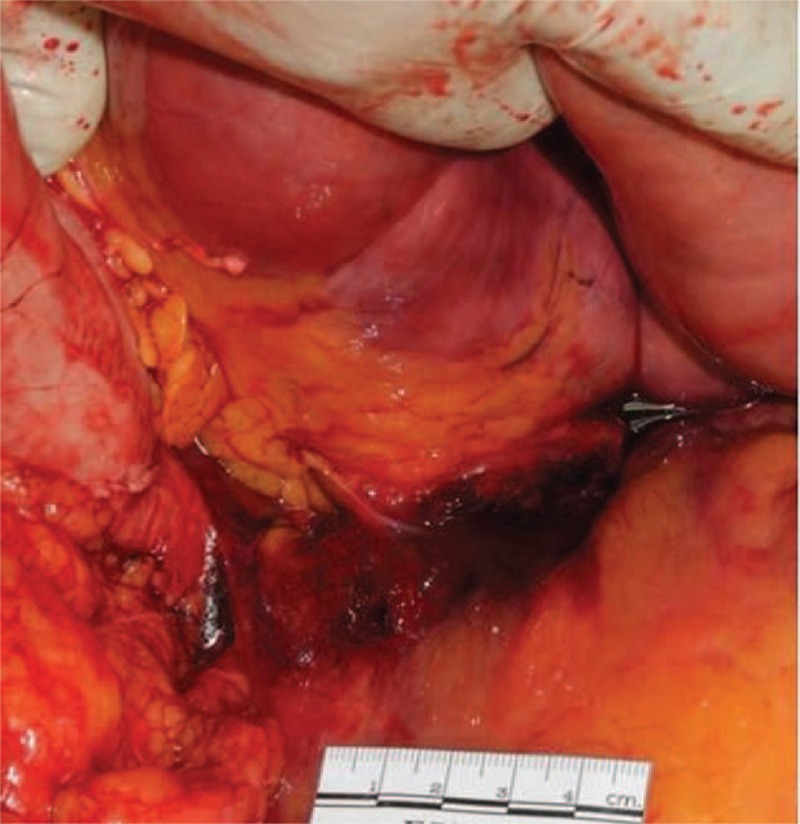
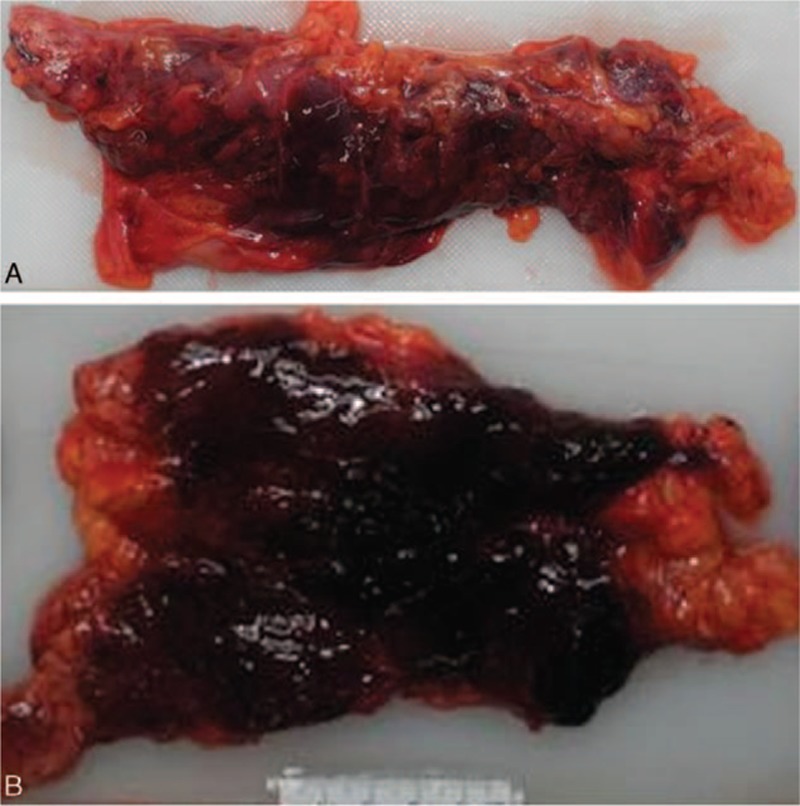
Examination of the abdominal cavity disclosed no signs of injury. Approximately 20 ml of brownish fluid was present in the peritoneal cavity just below the pancreatic sac (Fig. 1). The pancreas was 120 g in weight, soft, pulpy, and edematous with extensive fat necrosis of the peripancreatic tissue and large areas of frank hemorrhage on the external surface. The pancreatic cut surface revealed massive hemorrhagic necrosis of pancreatic tissue along its head, body, and tail (Fig. 2). The duodenal loop appeared normal. The liver showed scattered pale yellowish patches and a smooth surface, and weighed 2650 g. The gall bladder was markedly enlarged with a thickened wall and contained multiple blackish calculi weighing 10 g in total (Fig. 3). Patency of the bile ducts was tested by compressing the distended gall bladder. The common bile duct and pancreatic ducts appeared normal without any obstructed material. The remaining internal organs were otherwise unremarkable.
Figure 1.
Examination of the abdominal cavity disclosed ∼20 mL of brownish fluid in the peritoneal cavity just below the pancreatic sac.
Figure 2.
Gross view of the pancreas (A) showing a soft, pulpy, edematous appearance with extensive fat necrosis of the peripancreatic tissue and large areas of frank hemorrhage on its external surface, and (B) showing massive hemorrhagic necrosis of pancreatic tissue along its head, body, and tail.
Figure 3.
Gross view of the gall bladder showing a markedly enlarged bladder with a thickened wall. Multiple blackish calculi, weighing 10 g in total, were present.
Microscopic examination of the heart showed some myocyte hypertrophy, and most of the myocytes contained gold–yellowish pigments. Microscopic examination of both lungs showed diffuse alveolar damage with intense capillary congestion, interstitial edema, and hemorrhage. Microscopic sections of the pancreas showed extensive areas of coagulation necrosis of the pancreatic tissue, including the acini and islets. The tissue was mostly overwhelmed with extravasated blood that extended into the peripancreatic adipose tissue, and numerous adjacent polynucleated white blood cells were present, suggestive of severe hemorrhagic AP (Fig. 4). There was no evidence of pre-existing chronic pancreatitis or healed previous episodes of the disease in any area of the pancreas. Microscopic examination of liver sections showed degenerative patches of hepatocytes containing small, moderate, or large vacuoles within their cytoplasm and scattered throughout the tissue. Microscopic examination of gall bladder sections showed multiple patches of chronic inflammatory cells and Rokitansky–Aschoff sinuses on the wall. Microscopic examination of both kidneys displayed evidence of bilateral hypertensive degeneration of a few glomeruli.
Figure 4.
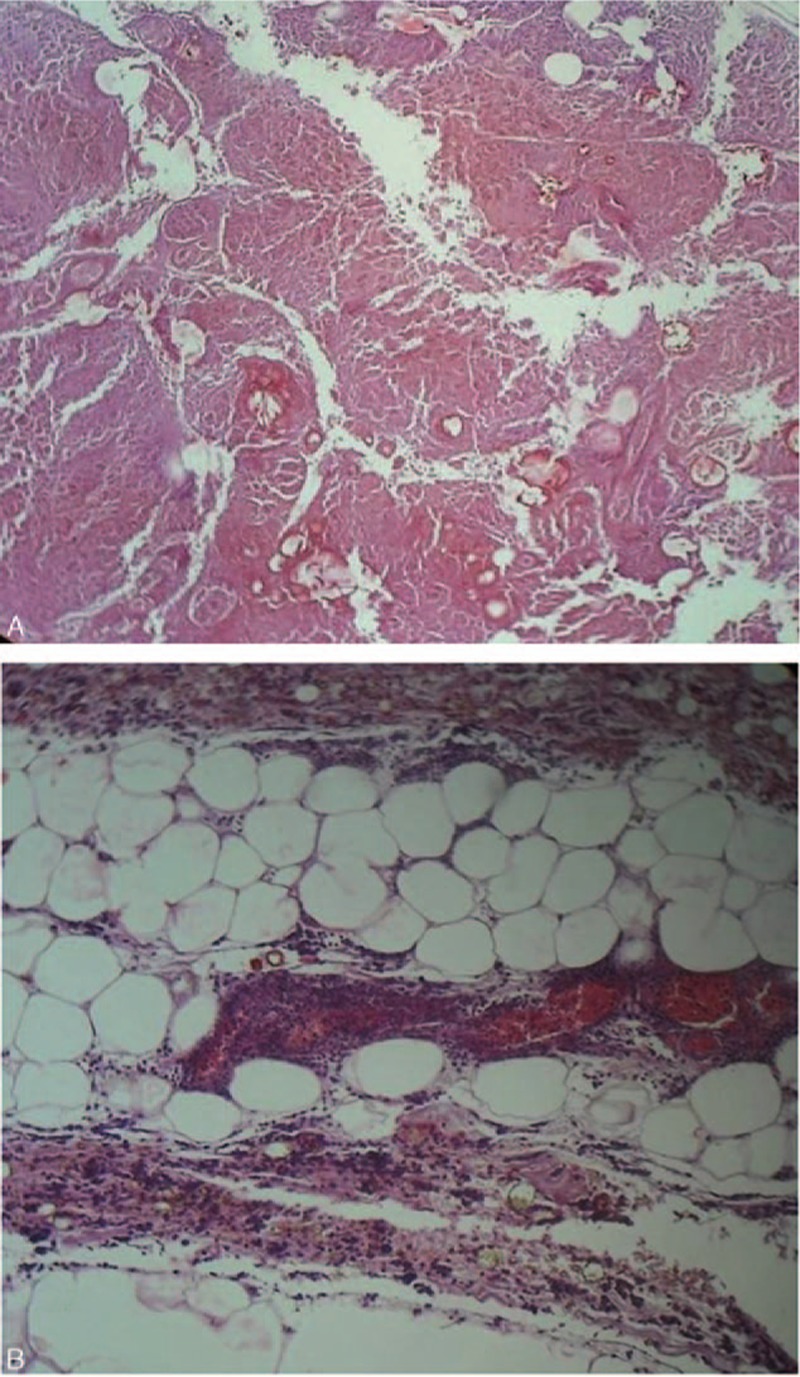
Microscopic view of the pancreas showing (A) the pancreatic tissue totally necrosis and mostly overwhelmed with extravasated blood (hematoxylin–eosin staining, original magnification, ×40), and (B) the extension into the peripancreatic adipose tissue with numerous polynucleated white blood cells (hematoxylin–eosin staining, original magnification, ×100), suggestive of severe hemorrhagic acute pancreatitis.
Toxicological analyses were negative. Postmortem laboratory investigation performed 14 h after death showed a blood ketone concentration of 1.7 mmol/L, creatinine concentration of 4.63 mg/dL, blood urea nitrogen concentration of 42.8 mg/dL, glucose concentration of 765 mg/dL, amylase concentration of 1875 U/L, and lipase concentration of 3709 U/L.
The cause of death in this case was attributed to extensive severe hemorrhagic AP secondary to cholelithiasis.
6. Discussion
According to a large clinical-based study,[1] AP is most frequently found in middle-aged and older individuals, not as in the present case whose age was still <40 years. Biliary tract disease is a common etiological factor of AP in women, not in men like a present case, whereas chronic alcoholism is a common etiology in men. Also, the main etiological factor of fatal AP among people who died outside the hospital was alcohol abuse.[3] Other less frequent etiological factors of AP include idiopathic and miscellaneous conditions such as roundworm infestation,[7] scrub typhus,[8] and amyloidosis.[9] Trauma-induced AP can also occur, particularly after a surgical procedure or abdominal blunt force injury, resulting in injury to the pancreas. As may be expected, alcohol abuse is the most common cause of AP found in autopsy-based studies (approximately 70% of cases);[2,5] gallstones are found much less frequently (7%).[2–6] AP is, therefore, a severe complication of gallstone disease, even that involving small gallstones (≤5 mm), with a considerable mortality.[10]
Classical symptoms of AP include nausea, vomiting, and upper abdominal pain that can radiate to the back. The abdominal pain, which is the most frequent symptom, may vary widely in intensity from the typical sharp, incapacitating pain to mild bearable pain with a more or less asymptomatic onset.[11] AP is diagnosed based on clinical features, imaging studies, and laboratory investigation, the latter of which reveals elevated serum amylase and lipase concentrations at least 3 times higher than the normal levels. Additionally, an elevated serum creatinine concentration (>2.0 mg/dL) and blood glucose level (>250 mg/dL) are significant predictors of a fatal outcome,[12] as in the present case. The deceased presented with signs of hypovolemic shock and multiple organ dysfunction syndromes (MODS) shortly after admission to the hospital and subsequently died in the emergency room of the hospital. According to the Atlanta Classification System, MODS are clinically associated with severe AP (SAP).[13] A lipase level of >1000 U/L upon admission also indicates SAP.[12] Because of its abrupt onset, varied and nonspecific symptoms, and rapid death, the diagnosis of SAP-related death may not be made until autopsy,[2] as in the present case. Deaths due to SAP are likely to be more frequent than reported; however, they are seldom recognized or missed diagnosed, either because full autopsy is not performed or because they are overlooked or confused with postmortem changes of the pancreas even when a complete autopsy is conducted.
Although the severity of AP is variable and does not always correlate with structural damage to the pancreas,[14] pancreatic necrosis of >30% is associated with mortality rates of 11% to 25%.[15] A higher mortality rate is presumed if the lesion occurs in the head of the pancreas.[16] Nonetheless, the mortality rate of SAP varies from 6% to 55% depending on the treatment settings.[17–19] Most deaths from SAP in its early coarse are caused by MODS, as in the present case. This phenomenon is most likely due to the overwhelming systemic insult caused by cytokines and other inflammatory mediators[20–22] via various mechanisms including stasis, intravascular coagulation, or endothelium disruption.[23] Furthermore, the inflamed pancreas can be infected by translocation of bacteria either internally or externally.[5] Therefore, the presence of either MODS or infected pancreatic necrosis indicates SAP, and the presence of both indicates an extremely severe condition with a double relative risk of mortality.[24] Obesity, as in the present case, is another important prognostic factor for AP due to its potential cause of severe hypertriglyceridemia which can induce AP,[25] and an independent risk factor for mortality in SAP[26,27] because of its higher incidence of local and systemic complications.
Sterile pancreatic necrosis requires only conservative medical treatment to maintain the function of organs and correct fluid, electrolyte and acid–base disequilibrium, whereas infected pancreatic necrosis may need more aggressive treatments such as lavage, drainage, and necrosectomy, in combination with conservative management and antibiotics.[1] As such, emphasis placed on the prompt recognition and treatment of fluid, electrolyte and acid–base imbalance in all patients with SAP significantly increases the survival rate.[5] In addition, prophylactic cholecystectomy may be considered to prevent SAP-related deaths secondary to gallstones.[28] However, no major surgery should be performed within 24 hours after admission because of the added stress to organs, which will increase the mortality rate.[29]
Because the aforementioned predictive factors for SAP-related death were present in this case, prompt treatment to maintain organ function and correct the imbalance of physiochemical substances was extremely critical. Although recent advances in diagnosis and treatment strategies have improved the mortality rate in individuals with SAP, the early fulminant type of SAP still exists[30] and results in intractable MODS and early death, as in the present case. These individuals should, therefore, be treated in a highly specialized hospital, not just a local hospital. It is essential to predict the likelihood of early death during the initial admission of such individuals to determine whether hospital transfer or intensive care treatment is needed.
Previous autopsy studies[2,5] have shown that most patients with SAP (60%–89%) die during the very early phase of SAP, as in the present case. In addition to the pathologic lesions of the pancreas in this case, the laboratory investigation findings also indicated that the MODS had occurred prior to the patient's sudden death, which was clearly due to extensive hemorrhagic AP. Interestingly, first episodes of AP, which have higher mortality rates than multiple episodes of AP,[2] was pathologically depicted in this case. Pulmonary edema, which has been considered a major organ failure and an early cause of death in individuals with SAP in both clinical- and autopsy-based studies,[2,5,31–33] was also found in this case. SAP-induced severe systemic inflammatory response,[32] diabetic ketoacidosis,[34] fluid overload, and/or blood transfusion[35] could be responsible for such a phenomenon. In addition, acute hemorrhagic lesions in the pancreas, which are significantly prevalent in these fatalities,[5,31] were also detected in this case.
In conclusion, the authors have reported the present case to arouse the medical and medico-legal profession's awareness of SAP as a cause of death when encountering cases of symptomless or vaguely symptomatic sudden unexpected death, especially involving young men either with or without a history of gallstone treatment. Although many cases of sudden death caused by SAP have classic symptoms, a few have no symptoms at all.[2,4,5,31] Therefore, one should perform a careful and thorough examination of the pancreatic region and consider pancreatitis-related complications when considering the possibility of SAP as a primary cause of sudden death. Prediction of such deaths upon hospital admission may allow treating physicians to be better prepared for difficult situations.
7. Informed consent
According to the policy of our institution, the regulations of our institutional review board, and Thai law regarding the postmortem inquest, there is no need to have the patient or his next of kin provide the informed consent for the publication of this case report.
Footnotes
Abbreviations: AP = acute pancreatitis, MODS = multiple organ dysfunction syndromes, SAP = severe acute pancreatitis.
The article has not been submitted or published elsewhere. However, the article was presented at the Siriraj International Conference in Medicine and Public Health (SICMPH 2016: Innovation in Health) held at Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, during June 13–15, 2016, and will be presented at the 23rd ANZFSS International Symposium on the Forensic Sciences which is going to be held in Auckland, New Zealand, during September 18–23, 2016.
General Considerations: The authors are so sure that all patient data have been de-identified. However, this case reported does not need ethical approval from the institutional review board of my institution, Mahidol University, due to the policy of my institution stating that there is no need to have the article like “a case report” approved by the institutional review board. Also, we do not have any competing interests at all.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Beger HG, Rau BM. Severe acute pancreatitis: clinical course and management. World J Gastroenterol 2007; 13:5043–5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsokos M, Braun C. Acute pancreatitis presenting as sudden, unexpected death: an autopsy-based study of 27 cases. Am J Forensic Med Pathol 2007; 28:267–270. [DOI] [PubMed] [Google Scholar]
- 3.Andersson R, Andren-Sandberg A. Fatal acute pancreatitis. Characteristics of patients never reaching hospital. Pancreatology 2003; 3:64–66. [DOI] [PubMed] [Google Scholar]
- 4.Di Maio VJ, Di Maio DJ. Natural death as viewed by the medical examiner: a review of 1000 consecutive autopsies of individuals dying of natural disease. J Forensic Sci 1991; 36:17–24. [PubMed] [Google Scholar]
- 5.Renner IG, Savage WT, 3rd, Pantoja JL, et al. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci 1985; 30:1005–1018. [DOI] [PubMed] [Google Scholar]
- 6.Storck G, Pettersson G, Edlund Y. A study of autopsies upon 116 patients with acute pancreatitis. Surg Gynecol Obstet 1976; 143:241–245. [PubMed] [Google Scholar]
- 7.Rigby HM. Acute hemorrhagic pancreatitis: round worm in pancreatic duct. Bri J Surg 1923; 10:419–420. [Google Scholar]
- 8.Ahmed AS, Kundavaram AP, Sathyendra S, et al. Acute pancreatitis due to scrub typhus. J Glob Infect Dis 2014; 6:31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuroda T, Sato H, Hasegawa H, et al. Fatal acute pancreatitis associated with reactive AA amyloidosis in rheumatoid arthritis with end-stage renal disease: a report of three cases. Intern Med 2011; 50:739–744. [DOI] [PubMed] [Google Scholar]
- 10.Venneman NG, Buskens E, Besselink MG, et al. Small gallstones are associated with increased risk of acute pancreatitis: potential benefits of prophylactic cholecystectomy? Am J Gastroenterol 2005; 100:2540–2550. [DOI] [PubMed] [Google Scholar]
- 11.Kanchan T, Shetty M, Nagesh KR, et al. Acute haemorrhagic pancreatitis—A case of sudden death. J Forensic Leg Med 2009; 16:101–103. [DOI] [PubMed] [Google Scholar]
- 12.Blum T, Maisonneuve P, Lowenfels AB, et al. Fatal outcome in acute pancreatitis: its occurrence and early prediction. Pancreatology 2001; 1:237–241. [DOI] [PubMed] [Google Scholar]
- 13.Bradley EL, 3rd, Maisonneuve P, Lowenfels AB, et al. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993; 128:586–590. [DOI] [PubMed] [Google Scholar]
- 14.Lankisch PG. Natural course of acute pancreatitis: what we know today and what we ought to know for tomorrow. Pancreas 2009; 38:494–498. [DOI] [PubMed] [Google Scholar]
- 15.Balthazar EJ, Robinson DL, Megibow AJ, et al. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990; 174:331–336. [DOI] [PubMed] [Google Scholar]
- 16.Kemppainen E, Sainio V, Haapiainen R, et al. Early localization of necrosis by contrast-enhanced computed tomography can predict outcome in severe acute pancreatitis. Br J Surg 1996; 83:924–929. [DOI] [PubMed] [Google Scholar]
- 17.Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut 2004; 53:1340–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buter A, Imrie CW, Carter CR, et al. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg 2002; 89:298–302. [DOI] [PubMed] [Google Scholar]
- 19.Halonen KI, Pettila V, Leppaniemi AK, et al. Multiple organ dysfunction associated with severe acute pancreatitis. Crit Care Med 2002; 30:1274–1279. [DOI] [PubMed] [Google Scholar]
- 20.Sharma PK, Madan K, Garg PK. Hemorrhage in acute pancreatitis: should gastrointestinal bleeding be considered an organ failure? Pancreas 2008; 36:141–145. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia M, Wong FL, Cao Y, et al. Pathophysiology of acute pancreatitis. Pancreatology 2005; 5:132–144. [DOI] [PubMed] [Google Scholar]
- 22.Garg PK, Madan K, Pande GK, et al. Association of extent and infection of pancreatic necrosis with organ failure and death in acute necrotizing pancreatitis. Clin Gastroenterol Hepatol 2005; 3:159–166. [DOI] [PubMed] [Google Scholar]
- 23.Pastor CM, Matthay MA, Frossard JL. Pancreatitis-associated acute lung injury: new insights. Chest 2003; 124:2341–2351. [DOI] [PubMed] [Google Scholar]
- 24.Petrov MS, Shanbhag S, Chakraborty M, et al. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139:813–820. [DOI] [PubMed] [Google Scholar]
- 25.Funnell IC, Bornman PC, Weakley SP, et al. Obesity: an important prognostic factor in acute pancreatitis. Br J Surg 1993; 80:484–486. [DOI] [PubMed] [Google Scholar]
- 26.Gloor B, Muller CA, Worni M, et al. Late mortality in patients with severe acute pancreatitis. Br J Surg 2001; 88:975–979. [DOI] [PubMed] [Google Scholar]
- 27.Taguchi M, Kubo T, Yamamoto M, et al. Body mass index influences the outcome of acute pancreatitis: an analysis based on the Japanese administrative database. Pancreas 2014; 43:863–866. [DOI] [PubMed] [Google Scholar]
- 28.Schirmer BD, Winters KL, Edlich RF. Cholelithiasis and cholecystitis. J Long Term Eff Med Implants 2005; 15:329–338. [DOI] [PubMed] [Google Scholar]
- 29.Zhu AJ, Shi JS, Sun XJ. Risk factors influencing mortality of patients with severe acute pancreatitis within 24 hours after admission. Hepatobiliary Pancreat Dis Int 2003; 2:453–457. [PubMed] [Google Scholar]
- 30.Isenmann R, Rau B, Beger HG. Early severe acute pancreatitis: characteristics of a new subgroup. Pancreas 2001; 22:274–278. [DOI] [PubMed] [Google Scholar]
- 31.Tumer AR, Dener C. Diagnostic dilemma of sudden deaths due to acute hemorrhagic pancreatitis. J Forensic Sci 2007; 52:180–182. [DOI] [PubMed] [Google Scholar]
- 32.Browne GW, Pitchumoni CS. Pathophysiology of pulmonary complications of acute pancreatitis. World J Gastroenterol 2006; 12:7087–7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu AJ, Shi JS, Sun XJ. Organ failure associated with severe acute pancreatitis. World J Gastroenterol 2003; 9:2570–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffman WH, Burek CL, Waller JL, et al. Cytokine response to diabetic ketoacidosis and its treatment. Clin Immunol 2003; 108:175–181. [DOI] [PubMed] [Google Scholar]
- 35.Silliman CC, McLaughlin NJ. Transfusion-related acute lung injury. Blood Rev 2006; 20:139–159. [DOI] [PubMed] [Google Scholar]


