Abstract
Primary gastric squamous cell carcinoma (SCC) is an exceedingly rare disease. We increased the understanding of gastric SCC and evaluated prognostic factors of gastric SCC.
In this large-population cohort study, we retrospectively collected 163 primary gastric SCC and 66,209 primary gastric adenocarcinoma cases from the surveillance, epidemiology, and end results program (SEER) database from 1988 to 2012. The Chi-squared test demonstrated the distributed differences. Cox proportional hazards regression model was used to evaluate the prognostic factors.
Gastric SCC accounted for 0.2% of all the primary gastric cancer cases. The mean age of patients with gastric SCC was 69.6 years old, and the man-to-woman ratio was 2.3:1. The proportion of black was higher in gastric SCC than gastric adenocarcinoma (P < 0.001). Almost half of the gastric SCCs were diagnosed in stage IV and more than half were poorly differentiated. In gastric SCC, the median survival was 8.0 months and the 5-year overall survival (OS) was 32.7%; in gastric adenocarcinoma the median survival rate was 19.0 months and the 5-year OS was 35.4%. The multivariate analysis showed that number of primary lesions, tumor location, grade, and stage were independent prognostic factors in gastric SCC. The tumor stage was the most important prognostic factor.
Primary gastric SCC is exceedingly rare. Compared with gastric adenocarcinoma, gastric SCC was more frequent in black patients and was usually diagnosed when it was poorly differentiated and at a later stage. On the whole, gastric SCC has a poorer outcome. Disease stage is likely a key determinant in survival.
Keywords: clinical characteristics, gastric adenocarcinoma, gastric squamous cell carcinoma, prognosis
1. Introduction
Primary gastric carcinoma is the second leading cause of death worldwide. Glandular epithelium covers the gastric mucosa; therefore, the most common subtype of gastric carcinoma is adenocarcinoma, accounting for more than 90% of gastric tumors.[1] Primary gastric squamous cell carcinoma (SCC) is an exceedingly rare disease, accounting for only 0.04% to 0.09% of all primary gastric cancers.[2,3] Most of the previous articles on gastric SCC have been case reports.[4–10]
Boswell and Helwig[11] defined 4 histopathological criteria to diagnose a primary gastric SCC: keratinized cell masses forming keratin pearls, a mosaic pattern of cell arrangement, intracellular bridges, and a high concentrations of sulphydryl or disulphide bonds.[6,12] Parks[13] proposed that SCC occurring in the cardia of the stomach or in esophagogastric junction should not be considered a primary gastric SCC because it may arise from the distal esophagus or from misplaced islands of squamous cells in the cardia. Therefore, diagnosis of gastric SCC must exclude the invasion of SCC of the lower esophageus or cardia, also required the exclusion of SCC of other organs such as the lung, bronchus, or cervical metastasis to the stomach. Moreover, diagnosis of primary gastric SCC requires normal gastric mucosa between the esophagogastric junction and gastric SCC.[7]
Since limited research is available, we know little about the clinicopathologic features and prognosis of gastric SCC. The rareness of gastric SCC is the major impediment to large-scale clinical trials and the development of accepted guidelines for its management. In clinical practice, primary gastric SCC is often diagnosed at a late stage. Treatment of gastric SCC is based on the approach used for treating gastric adenocarcinoma. The prognosis of primary gastric SCC had been controversial. Some previous studies have indicated a poor prognosis for this disease because the majority of lesions are detected at an advanced stage when there is marked infiltrative growth. However, other studies have reported that this type of cancer trends to grow slowly as polypoid exophytic growths and has a better prognosis than that for gastric adenocarcinoma.
2. Materials and methods
2.1. Origins of materials
The surveillance, epidemiology, and end results program (SEER) registry, sponsored by the National Cancer Institute, collects information of various kinds of cancer. The current SEER database submitted from 18 SEER cancer registries that represent approximately 27.8% of United States population. We obtained permission to access the research data (Reference Number: 10904-Nov 2014). The study was approved by the review board of the Second Affiliated Hospital, Zhejiang University School of Medicine. The SEER. Stat software was used to identify patients with primary gastric cancer, primary gastric SCC, and primary gastric adenocarcinoma.
2.2. Inclusion and exclusion criteria
The specific inclusion criteria for primary gastric cancer were as follows: year of diagnosis ranged from 1988 to 2012; site record ICD-O-3 was limited to the stomach; and diagnostic confirmation was limited to microscopically confirmed.
The specific inclusion criteria for primary gastric SCC were as follows: year of diagnosis ranged from 1988 to 2012; site record ICD-O-3 was limited to the stomach; histological type ICD-O-3 was limited to 8050–8089 (squamous cell neoplasms); and diagnostic confirmation was limited to microscopically confirmed. The exclusion criteria were as follows: originated in the gastric cardia; histological type of adenosquamous carcinoma; absence of basic patient information such as race, age, or sex; or absence of information regarding tumor stage or survival.
The specific inclusion criteria for primary gastric adenocarcinoma were as follows: year of diagnosis ranged from 1988 to 2012; site record ICD-O-3 was limited to the stomach; histological type ICD-O-3 was limited to 8140–8389 (adenomas and adenocarcinomas); and diagnostic confirmation was limited to microscopically confirmed. The exclusion criteria were as follows: histological type of linitis plastic, diffuse type carcinoma, cribriform carcinoma, malignant carcinoid tumor, and neuroendocrine carcinoma; absence of basic patient information such as race, age, or sex; or absence of information regarding tumor stage or survival.
2.3. Statistical analyses
The following factors were retrieved from the SEER database: year and age at diagnosis, sex, race, number of primary lesions, site recorded, differentiated grade, T-classification, N-classification, M-classification and stage according to the Tumor Lymph Node Metastasis (TNM) staging system, whether the patient underwent radiotherapy or surgery, survival months and cause of death. Race was divided into white, black, and others. Age was classified into young (≤70 years old) and old (>70 years old) groups. Tumor site was coded as the cardia, body of the stomach, fundus of the stomach, gastric antrum, pylorus, overlapping lesion of the stomach, and stomach without specific position. All cases were regrouped according to the 7th American Joint Committee on Cancer TNM staging system. Cancer-specific overall survival (OS) was calculated from the date of diagnosis to the date of death from cancer. Death attributed to other causes was defined as a censored observation.
The Chi-squared test demonstrated the distributed differences between gastric SCC and gastric adenocarcinoma. Survival curves were generated using the Kaplan–Meier methods, and the log-rank test was performed to evaluate the differences in survival. We used Cox proportional hazards regression model to evaluate the prognostic factors and adjusted hazard ratios (HRs) and 95% confidence intervals (CI) were calculated using the Cox model. When the two-sided P-value was less than 0.05, the difference was considered statistically significant. SPSS 20.0 (SPSS Chicago IL) software was used for data analysis.
3. Results
From 1988 to 2012, 110,365 primary gastric cancer cases were registered in the SEER database, of which 264 were primary gastric SCC. Primary gastric SCC accounted for 0.2% of all the cases of primary gastric cancer.
From the abundant data of the SEER database, we selected 163 cases of primary gastric SCC and 66,209 cases of primary gastric adenocarcinoma that had relatively complete data for analysis. The cut-off date of follow up was November 2015.
3.1. Characteristics of gastric SCC and gastric adenocarcinoma
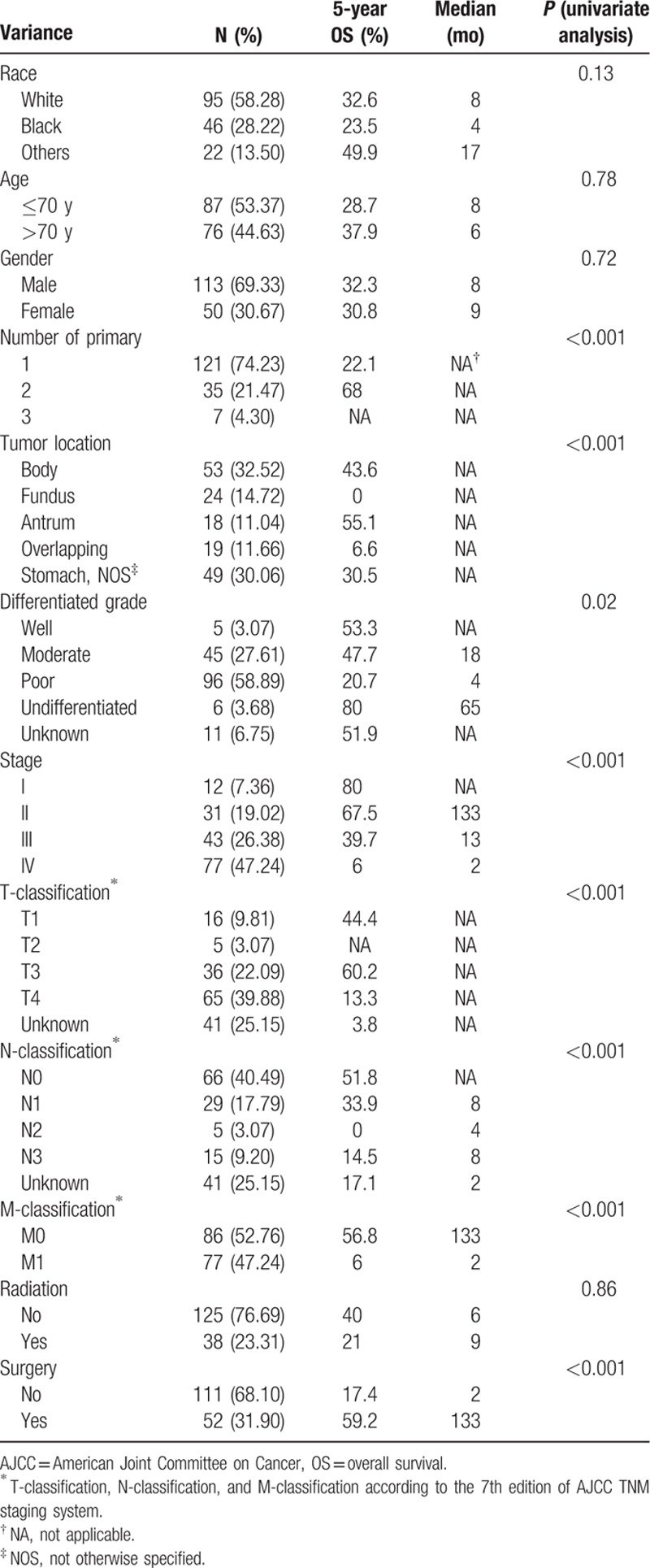
The cohort contained 163 patients with primary gastric SCC. The mean age was 69.6 years old (range, 33–93 years old). Primary gastric SCC was more common in men; the man-to-woman ratio was 2.3:1. The most common site of gastric SCC was the body of the stomach. Almost half of the patients were diagnosed with gastric SCC in stage IV. The detailed characteristics of the patients are provided in Table 1.
Table 1.
The characteristics of 163 patients with gastric squamous cell carcinoma.
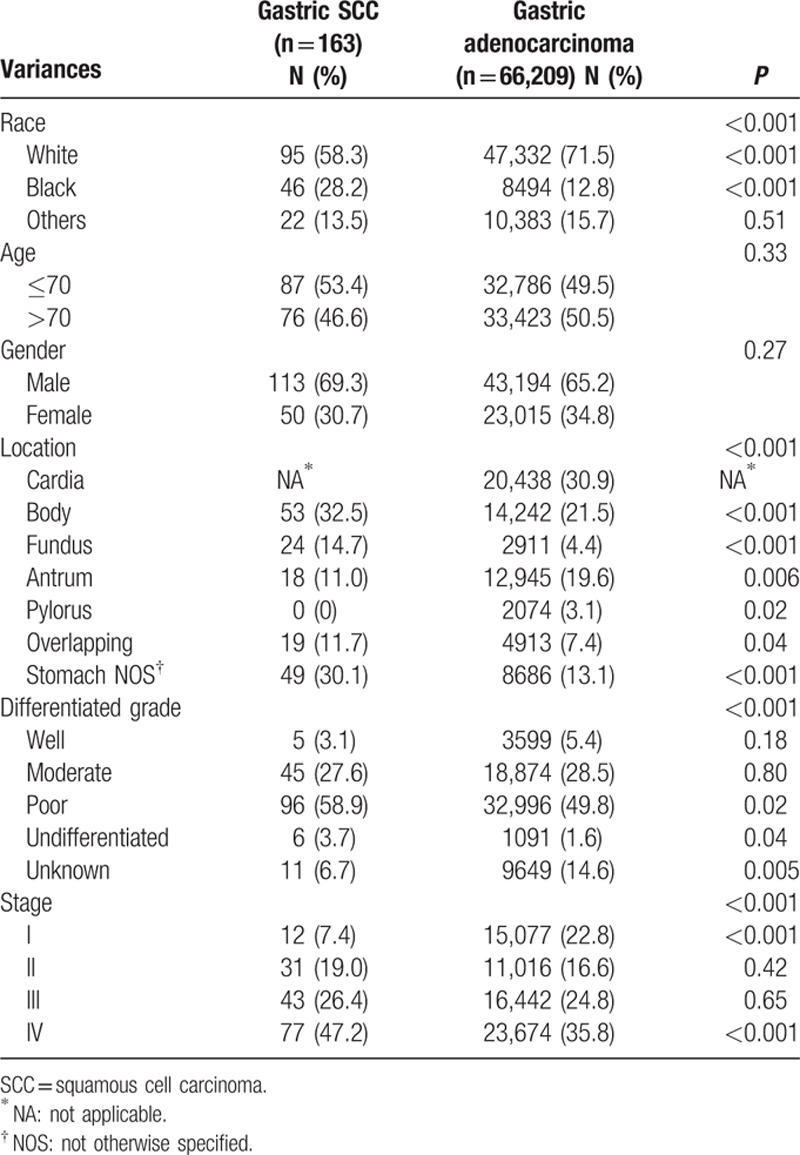
Over the same period, 66,209 patients with primary gastric adenocarcinoma were registered in the SEER database with relatively complete data. The mean age was 69.0 years old. The man-to-woman ratio was 1.8:1. The most common site of gastric SCC was the cardia, followed by body of the stomach and antrum. The detailed characteristics of the patients are provided in Table 2.
Table 2.
The distribution differences between gastric SCC and gastric adenocarcinoma.
3.2. Differences in distribution and survival between gastric SCC and gastric adenocarcinoma
The proportion of black was higher in gastric SCC than gastric adenocarcinoma. There were no significant differences between the two groups in terms of sex and age course. In the gastric SCC subgroup, only 7.4% of patients were diagnosed at stage I and 47.2% were diagnosed at stage IV. In the adenocarcinoma subgroup, the percentages were 22.8% and 35.8%, respectively, with significantly statistical significance (P < 0.001). In conclusion, compared with gastric adenocarcinoma, gastric SCC was more frequent in black patients and was usually diagnosed when it was poorly differentiated and at a later stage (Table 2).
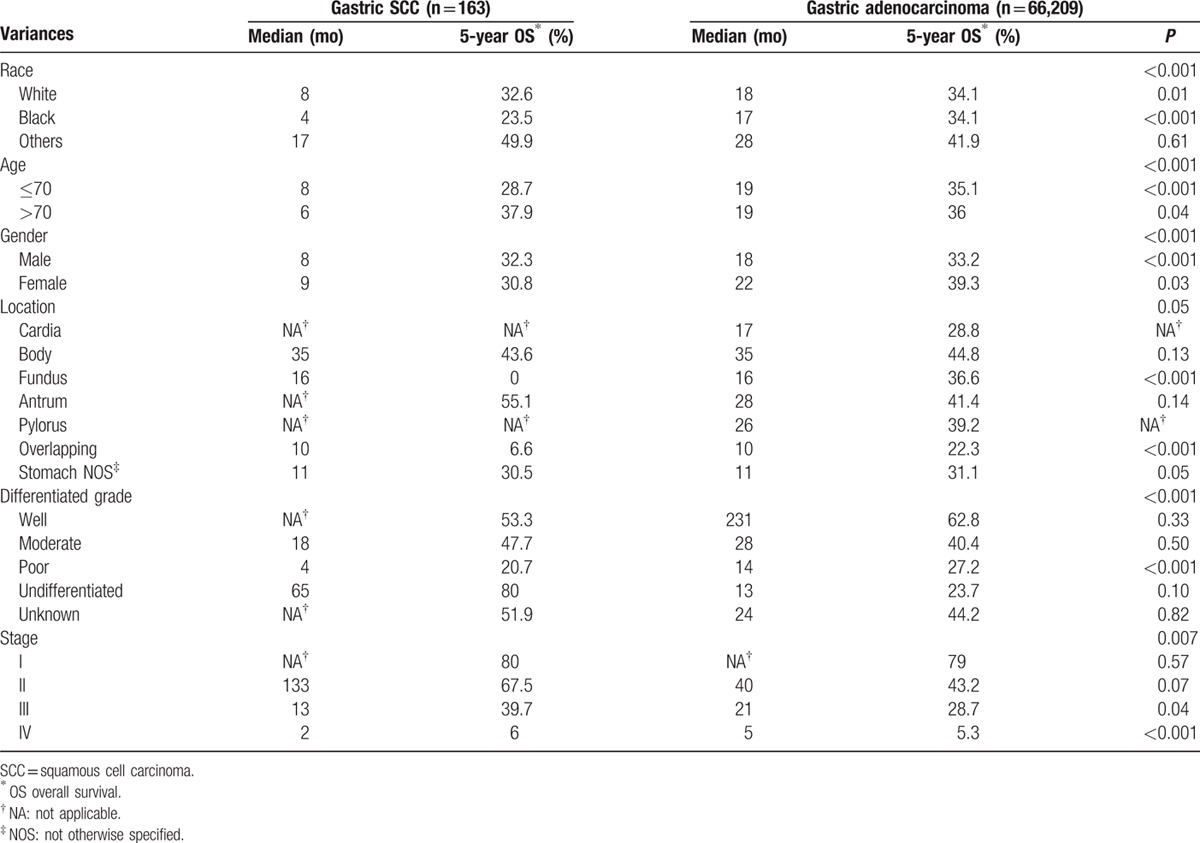
In gastric SCC, the median survival was 8.0 months and the 5-year OS was 32.7%; in gastric adenocarcinoma the median survival rate was 19.0 months and the 5-year OS was 35.4%, demonstrating that although gastric SCC had a poorer outcome on the whole. The 5-year OS were 80.0%, 67.5%, 39.7%, and 6.0% in the stage I, II, III, and IV subgroups for primary gastric SCC, respectively, and 79.0%, 43.2%, 28.7%, and 5.3% in the stage I, II, III, and IV subgroups for primary gastric adenocarcinoma, respectively (Fig. 1A, Table 3).
Figure 1.
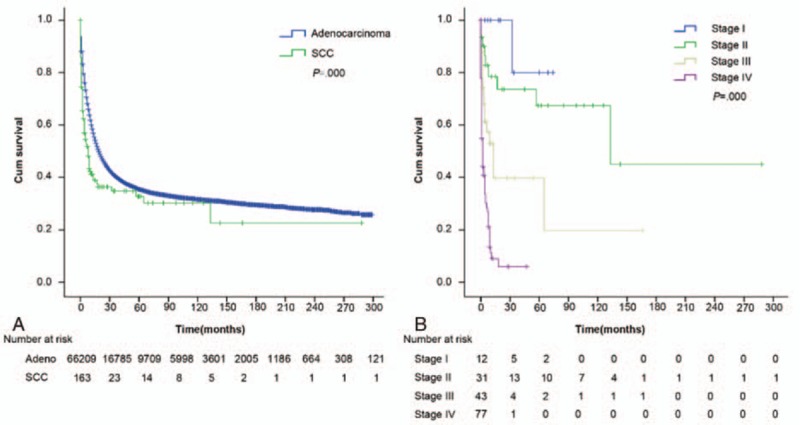
(A) The median survival was 8.0 months and 5-year OS was 32.7% in gastric SCC, 19.0 months and 35.4% in gastric adenocarcinoma. The gastric SCC had a poorer outcome than gastric adenocarcinoma (P < 0.001). (B) The 5-year OS were 80.0%, 67.5%, 39.7%, and 6.0% in the stage I, II, III, and IV subgroups of gastric SCC, respectively, with significant difference (P < 0.001). OS = overall survival, SCC = squamous cell carcinoma.
Table 3.
The survival differences between gastric SCC and gastric adenocarcinoma.
3.3. Univariate predictors of outcome in gastric SCC
The univariate analysis showed that the 5-year OS were 80.0%, 67.5%, 39.7%, and 6.0% in the stage I, II, III, and IV subgroups, respectively, according to the 7th TNM classification system, with significant difference (P < 0.001) (Fig. 1B).
The median survival times were 2.0 and 133.0 months in the surgery and non-surgery subgroups, respectively, with significant differences (P < 0.001). The 5-year OS were 17.4% and 59.2% in the surgery and non-surgery subgroups, respectively.
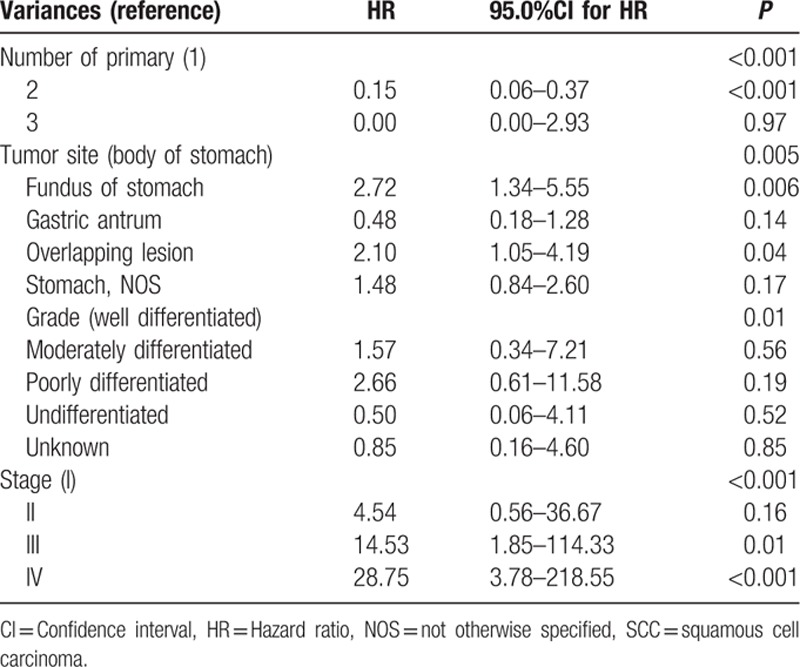
Additionally, number of primary lesions, tumor location, differentiated grade, T-classification, N-classification, and M-classification could predict the outcome (Table 1).
3.4. Multivariate analyses of outcome in gastric SCC
All factors associated with survival based on the univariate analysis were included in the Cox model. The multivariate analysis showed that number of primary lesions, tumor location, tumor grade, and tumor stage were independent prognostic factors. The tumor stage was the most important prognostic factor, with the highest HR. However, undergoing surgery was not an independent prognostic factor (Table 4).
Table 4.
Multivariate analysis (Cox proportional hazard model) of overall survival for 163 patients with gastric SCC.
4. Discussion
Because gastric SCC is rarely encountered clinically, limited research and literature are available. At present, no more than 100 cases of primary gastric SCC have been reported.[5] The mechanism, clinicopathological features, and survival outcome of patients with gastric SCC remain unclear. With the aim of improving our understanding of this disease, we retrospectively evaluated 163 cases of primary gastric SCC with relatively complete data in the SEER database to identify the clinicopathologic features and prognostic factors. Our study of primary gastric SCC has the largest sample size to date.
According to the available literature, the common complaints of gastric SCC include melena, epigastralgia, weight loss, abdominal distension, and vomiting. Abdominal computer tomography scan always shows a protruding mass and/or a thickened stomach wall. Upper gastrointestinal endoscopy reveals gray-white and fragile masses or large, infiltrative, and ulcerated lesion (Fig. 2). Generally speaking, gastric SCC is similar to gastric adenocarcinoma in clinical symptoms, imaging findings, endoscopic findings and pathologic gross classification, and characteristics distinguishing the two have not been identified.
Figure 2.
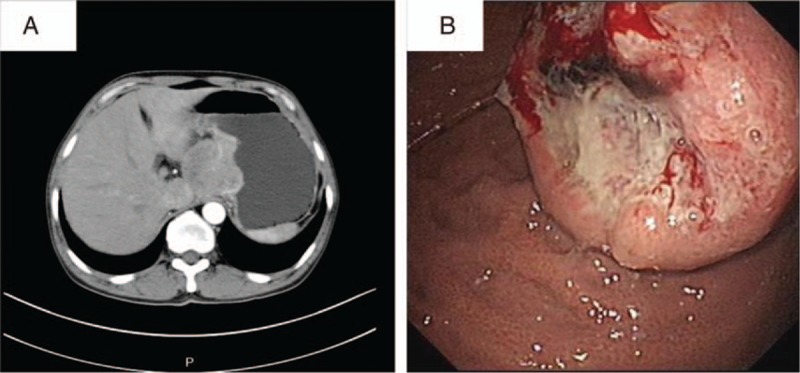
(A) Abdominal enhanced computed tomography (CT) scan images of a 50-year-old male patient with gastric SCC treated in our hospital showed a mass of 5.0 × 4.8 cm in size in the fundus of stomach, protruding to hepatogastric space. (B) Electronic gastroscopy revealed a gray-white and fragile tumor-like bulge of 4.0 × 4.0 cm in size with irregular surrounding mucosa. SCC = squamous cell carcinoma.
According to previous studies, primary gastric SCC accounts for 0.04% to 0.09% of all cases of primary gastric cancer.[2,3] However, in the current study, gastric SCC accounted for a much higher proportion (0.2%) of all cases of primary gastric cancer. Possible reasons for the differences are the higher incidence of gastric SCC and the inaccuracy of the previous data obtained.
The clinical characteristics of gastric SCC include a higher prevalence in men. Studies have reported a man-to-woman ratio of 2–5:1[14] in gastric SCC. Thomas and Sobin[1] found a man-to-woman ratio of 2.6:1, but that study did not exclude tumors originating from the cardia. Hwang et al[7] collected data for 90 patients with gastric SCC from previous studies in the English-language literature and summarized that the incidence of SCC was three times higher in men than in women. The clinicopathological features of 53 Japanese gastric SCC were reviewed, and the man-to-woman ratio was 43:11.[15] In the current study, the man-to-woman ratio was 2.3:1 in gastric SCC and 1.8:1 in gastric adenocarcinoma. Both gastric SCC and gastric adenocarcinoma were more frequent in men; however, there were no significant differences in the sex course between these two subgroups.
The peak incidence is in the 6th decade for gastric SCC according to previously published studies.[6] The mean age of the patients was 59.7 years old in the study by Hwang et al[7] and 64.4 years old in the study by Tokuhara et al,[15] which is obviously younger than our mean age of 69.6 years in the SCC subgroup. Moreover, gastric adenocarcinoma patients have similar mean age of 69.0 years old in the current study.
With respect to staging, only 7.4% of gastric SCC cases could be diagnosed at an early stage, and almost half of the patients were diagnosed with distant metastases. However, according to our data, 22.8% of gastric adenocarcinoma could be diagnosed at stage I and 35.8% at stage IV, which resulted in somewhat earlier detection compared with gastric SCC, as well as a better outcome.
Thomas and Sobin,[1] who also used the SEER database, reported that the 5-year survival rates for all stages were 17.1% in gastric adenocarcinoma and 13.2% in gastric SCC. They concluded that these two types of gastric carcinoma have the same poor outcome. However, there are many different ideas about the outcomes of gastric SCC. Tokuhara et al[15] reported that cases of gastric SCC tend to be diagnosed at a more advanced stage and the prognosis tends to be poorer. Some studies have shown that SCC tends to be locally aggressive; it rarely has distant hematogenous spread of metastasis in the early stages. By contrast, adenocarcinoma tends to have endophytic grow, and it can easily infiltrate the deeper layers and lymphatic vascular space, resulting in lymph node and distant metastasis. The authors of those studies concluded that gastric SCC has a better prognosis than gastric adenocarcinoma. In the current study, the median survival times were 8.0 months and 19.0 months for gastric SCC and gastric adenocarcinoma, respectively. The 5-year OS for all stages were 32.7% and 35.4% in gastric SCC and gastric adenocarcinoma, respectively. The 5-year OS were 80.0%, 67.5%, 39.7%, and 6.0% in the stage I, II, III, and IV subgroups of gastric SCC, and 79.0%, 43.2%, 28.7%, and 5.3% in the stage I, II, III, and IV subgroups of gastric adenocarcinoma. We concluded that gastric SCC had a poorer outcome than gastric adenocarcinoma as a whole, a finding that might be related to the later stage at diagnosis and low sensitivity of gastric SCC to radiotherapy and chemotherapy.
Both univariate and multivariate analyses indicated that tumor stage was an independent factor for the prognosis of gastric SCC. As with gastric adenocarcinoma, the tumor stage was also the most significant prognostic factor. Thomas and Sobin[1] reported 5-year survival rates of 15.5% in the regional metastatic stage and 2.5% in the distant metastatic stage of gastric SCC. The disease stage is likely a key determinant, and early diagnosis is important for improving the prognosis of primary gastric SCC.
As it is for gastric adenocarcinoma, surgical resection is the first-line treatment approach for gastric SCC. Endoscopic treatment, which may be complementary to surgery, is considered the procedure of choice for patients with early SCC. Patients should undergo early and aggressive surgery, as long as their overall condition permit and they lack distant metastases. There are three main surgical procedures: radical resection, palliative resection, and Roux-en-Y gastric bypass. The optimal treatment for primary gastric SCC is radical subtotal gastrectomy with Roux-en-Y reconstruction and D2 lymphadenectomy.[16,17] Adjuvant radiotherapy and/or chemotherapy might help to achieve a better prognosis. However, the role of surgery in stage IV gastric SCC is controversial. In the current study, undergoing surgery was statistically significant in the univariate analysis but not in the multivariate analysis. Based on the above results, we hypothesize that the better outcome for the surgery group may be due to their better physical condition and to selection bias rather than to superiority of the surgical approach.
The sensitivity of gastric SCC to radiotherapy and chemotherapy is lower than that of gastric adenocarcinoma. Therefore, chemotherapy and radiotherapy are rarely used for gastric SCC treatment other than for palliative treatment in late-stage patients. Because the SEER database lacks data on chemotherapy status, we could not verify the impact of chemotherapy on the patients. In the current study, undergoing radiotherapy did not affect the prognosis. However, according to previously published studies, radiation therapy may be to some extent alleviate the symptoms of cardiac obstruction and chronic bleeding from an unresectable tumor. Japanese scholars have reported a case of primary gastric SCC that was treated with neoadjuvant chemotherapy involving low-dose 5-fluorouracil plus cisplatin, having striking effectiveness.[18] Modi et al[19] also reported a patient received neoadjuvant chemotherapy with carboplatin and paclitaxel, which could help influence future chemotherapy regimens.
The pathogenesis of gastric SCC is speculative, 5 main theories have been proposed, including the presence of ectopic squamous cells in gastric mucosa, squamous metaplasia of the gastric mucosa preceding malignant transformation, overgrowth of a squamous epithelium element in a primary adenocarcinoma, totipotent stem cells in the gastric mucosa and gastric vascular endothelial cells.[12,16,20] Takita et al[21] proposed that Epstein-Barr virus infection may be involved in the pathogenesis of gastric SCC. However, in most gastric SCC cases, no Epstein-Barr virus infection were demonstrated and there is no strong proof supporting the hypothesis. No conclusive statement about the pathogenesis of gastric SCC can be made on the basis of the present literature. Physicians need to accumulate more data on primary gastric SCC cases and analyze the pathogenesis of this tumor to improve its diagnosis and treatment, which should in turn improve the prognosis.
5. Conclusion
Primary gastric SCC is exceedingly rare, accounting for 0.2% of all the primary gastric cancers. Compared with gastric adenocarcinoma, gastric SCC occurred more frequently in black patients, and it was usually diagnosed with poorer differentiation and later stage. Gastric SCC has a poorer outcome as a whole. Because the disease stage is likely a key determinant, early diagnosis is important to improving the prognosis of primary gastric SCC. The pathogenesis of SCC remains unclear and further studies are needed.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CI = confidence intervals, CT = computer tomography, HR = hazard ratio, OS = overall survival, SCC = squamous cell carcinoma, SEER = surveillance, epidemiology, and end results program.
CD and MJ are co-first authors and have contributed equally to the work.
Author contributions: YY, CD and MJ made substantial contributions to the conception and design of this study; CD, MJ, YT, YK, ZY, CZ, and DL collected, analyzed, and interpreted the data; CD and MJ drafted the manuscript; YY gave final approval to submit the manuscript for publication; MJ submitted the manuscript for publication.
All authors declare that they have no competing interests, including financial.
The research was supported by Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2014BAI09B07).
The authors report no conflicts of interest.
References
- 1.Thomas RM, Sobin LH. Gastrointestinal cancer. Cancer 1995; 75 suppl 1:154–170. [DOI] [PubMed] [Google Scholar]
- 2.Ruck P, Wehrmann M, Campbell M, et al. Squamous cell carcinoma of the gastric stump. A case report and review of the literature. Am J Surg Pathol 1989; 13:317–324. [DOI] [PubMed] [Google Scholar]
- 3.Straus R, Heschel S, Fortmann DJ. Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer 1969; 24:985–995. [DOI] [PubMed] [Google Scholar]
- 4.von Waagner W, Wang Z, Picon AI. A rare case of a primary squamous cell carcinoma of the stomach presenting as a submucosal mass. Case Rep Surg 2015; 2015:482342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callacondo D, Ganoza-Salas A, Anicama-Lima W, et al. Primary squamous cell carcinoma of the stomach with paraneoplastic leukocytosis: a case report and review of literature. Hum Pathol 2009; 40:1494–1498. [DOI] [PubMed] [Google Scholar]
- 6.Dursun M, Yaldiz M, Isikdogan A, et al. Primary squamous cell carcinoma of the stomach: a case report and review of the literature. Eur J Gastroenterol Hepatol 2003; 15:329–330. [DOI] [PubMed] [Google Scholar]
- 7.Hwang SH, Lee JH, Kim K, et al. Primary squamous cell carcinoma of the stomach: a case report. Oncol Lett 2014; 8:2122–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karaca G, Pekcici MR, Ozer H, et al. Primary squamous cell carcinoma of the stomach in a 68-years-old man. Geriatr Gerontol Int 2011; 11:119–120. [DOI] [PubMed] [Google Scholar]
- 9.Shi L, Liu FJ, Jia QH, et al. Synchronous squamous cell carcinoma in the esophagus and stomach: a case report. Turk J Gastroenterol 2014; 25 suppl 1:244–245. [DOI] [PubMed] [Google Scholar]
- 10.Sakemi R, So S, Morimitsu Y, et al. Endoscopic submucosal dissection of squamous cell carcinoma in the upper stomach 5 years after chemoradiotherapy for adenocarcinoma. Clin J Gastroenterol 2014; 7:310–315. [DOI] [PubMed] [Google Scholar]
- 11.Boswell JT, Helwig EB. Squamous cell carcinoma and adenoacanthoma of the stomach. A clinicopathologic study. Cancer 1965; 18:181–192. [DOI] [PubMed] [Google Scholar]
- 12.Gao S, Chen D, Huang L, et al. Primary squamous cell carcinoma of the stomach: a case report and literature review. Int J Clin Exp Pathol 2015; 8:9667–9671. [PMC free article] [PubMed] [Google Scholar]
- 13.Parks RE. Squamous neoplasms of the stomach. AJR Am J Roentgenol 1967; 101:447–449. [DOI] [PubMed] [Google Scholar]
- 14.Volpe CM, Hameer HR, Masetti P, et al. Squamous cell carcinoma of the stomach. Am Surg 1995; 61:1076–1078. [PubMed] [Google Scholar]
- 15.Tokuhara K, Nakano T, Inoue K, et al. Primary squamous cell carcinoma in the gastric remnant. Surg Today 2012; 42:666–669. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt C, Schmid A, Luttges JE, et al. Primary squamous cell carcinoma of the stomach. Report of a case and review of literature. Hepatogastroenterology 2001; 48:1033–1036. [PubMed] [Google Scholar]
- 17.Bonnheim DC, Sarac OK, Fett W. Primary squamous cell carcinoma of the stomach. Am J Gastroenterol 1985; 80:91–94. [PubMed] [Google Scholar]
- 18.Marubashi S, Yano H, Monden T, et al. Primary squamous cell carcinoma of the stomach. Gastric Cancer 1999; 2:136–141. [DOI] [PubMed] [Google Scholar]
- 19.Modi Y, Shaaban H, Parikh N, et al. Primary pure squamous cell carcinoma of the stomach treated with neoadjuvant chemotherapy and surgical resection. Ind J Cancer 2015; 52:145. [DOI] [PubMed] [Google Scholar]
- 20.Mori M, Iwashita A, Enjoji M. Squamous cell carcinoma of the stomach: report of three cases. Am J Gastroenterol 1986; 81:339–342. [PubMed] [Google Scholar]
- 21.Takita J, Kato H, Miyazaki T, et al. Primary squamous cell carcinoma of the stomach: a case report with immunohistochemical and molecular biologic studies. Hepatogastroenterology 2005; 52:969–974. [PubMed] [Google Scholar]


