Abstract
Background
Long-acting injectable antipsychotics are treatment options for acute and long-term treatment of patients with schizophrenia. In a previously published 12-week randomized, double-blind, placebo-controlled clinical trial of patients with schizophrenia experiencing an acute psychotic episode, aripiprazole once-monthly 400 mg (AOM 400) produced significantly greater improvement than placebo on the primary endpoint, Positive and Negative Syndrome Scale (PANSS) total score at week 10.
Methods
To examine the efficacy of AOM 400 across a broader representation of schizophrenia symptoms, including agitation, a post hoc analysis of this trial was carried out to assess the change in PANSS Marder factor domains (positive symptoms, negative symptoms, disorganized thought, uncontrolled hostility/excitement, and anxiety/depression) and the PANSS excited component (equivalent to Marder factor domain uncontrolled hostility/excitement plus the tension item) by comparing differences in change from baseline between AOM 400 and placebo using a mixed model for repeated measures.
Results
The differences between treatment and placebo for all factors were statistically significant, with improvements seen as early as week 1 or 2, and maintained through week 12. Thus, AOM 400, supplemented with oral aripiprazole in the first 2 weeks, showed significantly greater efficacy versus placebo in acutely ill patients with schizophrenia in all 5 Marder illness domains, as well as in agitation as conceptualized by the PANSS excited component score.
Conclusions
These findings indicate that AOM 400 is efficacious across the spectrum of schizophrenia symptoms in acutely ill patients, with implications for both short-term and, by extension, long-term patient outcomes.
Key Words: aripiprazole once-monthly, schizophrenia, PANSS Marder factors
Schizophrenia is a chronic, disabling illness, with most patients experiencing multiple relapses over time. Relapse is characterized by acute illness exacerbations and may have serious social and biological consequences.1 Relapse is associated with nonadherence to medication,2 decreased response to medication,3,4 increased time to response,3 and failure to recover to the same degree as before the most recent episode.5 Impairments in instrumental activities of daily living, capacity to make treatment decisions, and number of recent psychiatric admissions predict longer hospital length of stay.6 In addition, relapse duration was associated with reduced brain volume, especially in the frontal lobe.7
Although immediate symptom control is the main treatment goal of an acute exacerbation of psychotic symptoms, acute treatment plans should also encompass the longer-term treatment goals of preventing relapse and improving patient functioning, quality of life, and adherence to maintenance treatment.8,9 The variability observed in patient outcomes can be at least partially attributed to modifiable factors, including nonadherence or partial adherence to treatment.10
Long-acting injectable antipsychotics have been shown in naturalistic studies to improve symptoms while increasing adherence and reducing rates of relapse and hospitalization compared with oral antipsychotic formulations.10,11 The efficacy, safety, and tolerability of aripiprazole once-monthly 400 mg (AOM 400) for the treatment of acute exacerbation of psychotic symptoms in adult patients with schizophrenia were demonstrated previously.12 Aripiprazole once-monthly 400 mg was superior to placebo based on change from baseline to week 10 in Positive and Negative Syndrome Scale (PANSS) total score, as well as PANSS positive and negative subscale scores.12
Given the heterogeneous nature of schizophrenia,13 a 5-factor model (referred to as Marder factors) was developed to evaluate the spectrum of clinically relevant schizophrenia symptoms: positive symptoms, negative symptoms, disorganized thought, uncontrolled hostility/excitement, and anxiety/depression.14,15 This model, which uses patient responses on the PANSS items, has demonstrated high internal consistency and reliability in both acute and stable patients.14,16 Given the role of these symptoms in the readaptation of patients with schizophrenia, the 5-factor model is vital for determining the longer-term value of antipsychotics.15–17 A PANSS excited component (PEC) was also developed and validated, allowing for assessment of agitation symptoms.18
This analysis is based on a trial that focused on PANSS total score, as is common in registration studies. However, as treatment goals should include improvement across a wide set of symptoms to maximize short- and long-term outcomes, analysis of Marder factors is important and clinically informative. To further characterize the clinical profile of AOM 400 in the acute treatment of schizophrenia, we conducted a post hoc analysis of data from a 12-week randomized, placebo-controlled trial12 to assess changes in PANSS Marder factor and PEC scores.
PATIENTS AND METHODS
The randomized, double-blind, placebo-controlled trial of AOM 400 for the acute treatment of schizophrenia (ClinicalTrials.gov NCT01663532) was previously described.12 Briefly, adults with a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision diagnosis of schizophrenia currently experiencing acute exacerbation of psychotic symptoms at screening and baseline were eligible if they had a PANSS total score of greater than or equal to 80 and a score greater than 4 on each of the specific items of conceptual disorganization, hallucinatory behavior, suspiciousness/persecution, and unusual thought content. Written informed consent was provided by all patients or a guardian or legal representative. Those with no previous exposure took oral aripiprazole for 3 days to establish tolerability before starting study treatment.12
Patients were randomized 1:1 at baseline to receive intragluteal AOM 400 or placebo once every 4 weeks for 12 weeks.12 Patients were hospitalized during screening and for the first 2 weeks of acute treatment. They received concomitant oral aripiprazole or placebo for the first 14 days. During screening and treatment, benzodiazepine use (oral or intramuscular, ≤6 mg/d lorazepam or equivalent) was permitted for agitation, aggression, and/or insomnia, except within 12 hours of an efficacy or safety assessment. Positive and Negative Syndrome Scale was assessed at weeks 1, 2, 4, 6, 8, 10, and 12/final visit. Trial procedures were implemented in accordance with ethical standards of the institutional review board/independent ethics committee of each site.
This post hoc analysis evaluated the efficacy of AOM 400 versus placebo in improving the PANSS Marder factors, as analyzed in previous studies of aripiprazole in patients with acute exacerbations of schizophrenia or schizoaffective disorder,14 as well as the PEC.18 The PEC conceptualizes agitation and is equivalent to the Marder factor domain “uncontrolled hostility/excitement” with the addition of tension.18
Changes from baseline and percentage changes at weeks 1, 2, 4, 6, 8, 10, and 12 in all 5 Marder factors and PEC were summarized by treatment group for all randomized and treated patients having a baseline assessment and at least 1 postbaseline assessment. Least squares mean changes from baseline were derived from a mixed model for repeated measures (MMRM) analysis (with the assumption of missing at random) with fixed effects of treatment, region (pooled centers), week, and treatment-by-week and baseline-by-week interactions as covariates. An unstructured covariance structure was used for within-patient observations. No adjustments were made for multiple comparisons. Last observation carried forward (LOCF) and observed case (OC) analyses were also conducted for changes from baseline. Significance was set at P < 0.05. Average daily doses of concomitant benzodiazepines were calculated by summing daily doses and dividing by the acute treatment phase duration. Doses were converted to 1-mg lorazepam equivalents (conversion factors: alprazolam, 0.5 mg; clonazepam, 0.5 mg; oxazepam, 15 mg).
RESULTS
Of the 340 randomized patients (AOM 400, n = 168; placebo, n = 172), 167 received AOM 400 and 172 received placebo. Demographic and baseline characteristics were similar between groups.12 A total of 162/168 (96.4%) patients in the AOM 400 group and 167/172 (97.1%) in the placebo group had a baseline and at least 1 postbaseline assessment and were included in the MMRM post hoc analyses. Of these, 99/162 (61.1%) patients receiving AOM 400 and 68/167 (40.7%) receiving placebo had data available at week 12 (OCs data set).
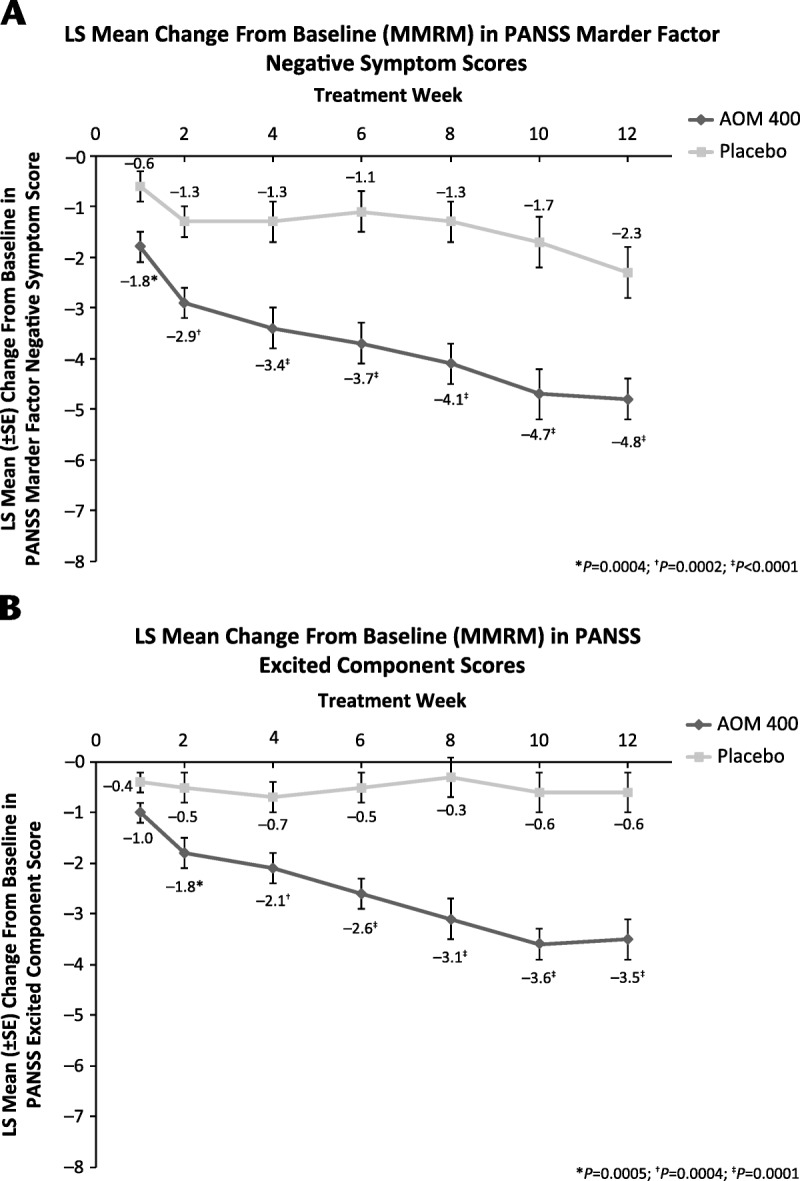
There were no significant differences in mean baseline values for the 5 Marder factor and PEC scores, indicating that the treatment groups were comparable at study start (Table 1). For all Marder factors (representative graph in Fig. 1A) and PEC scores (Fig. 1B), improvements from baseline with AOM 400 were achieved early and maintained. Significant improvements across factor scores with AOM 400 versus placebo were observed with MMRM analyses by week 1 for all but the Marder anxiety/depression and PEC scores, for which significant differences were seen by week 2. Last observation carried forward and OC analyses were consistent with these MMRM results (data not shown).
TABLE 1.
Mean Baseline PANSS Marder Factor and PEC Scores
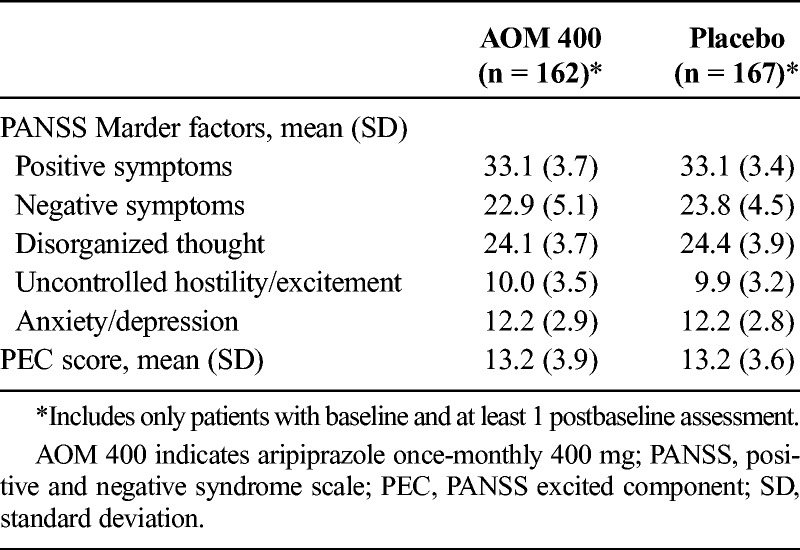
FIGURE 1.
Least squares (LS) mean change from baseline (from MMRM analysis) in (A) PANSS Negative Symptom Score and (B) PEC score. Error bars show standard error. Aripiprazole once-monthly 400 mg group, n = 162; placebo group, n = 167.
We also calculated the mean percentage change by averaging the percentage change from baseline across patients in each treatment group. Mean percentage change from baseline to last visit in the AOM 400 group improved for all 5 Marder factors [25.5% (positive symptoms), 13.8% (negative symptoms), 23.0% (disorganized thought), 9.3% (uncontrolled hostility/excitement), and 23.3% (anxiety/depression)] and for PEC scores (14.1%). In contrast, improvements were not observed with placebo for the uncontrolled hostility/excitement score (worsening of 10.5%) or PEC (worsening of 2.3%), and improvements for the other factors ranged from 5.3% (negative symptoms) to 13.2% (positive symptoms).
Safety and tolerability results, including treatment-emergent adverse events (previously reported12) were consistent with the established AOM 400 safety/tolerability profile. During acute treatment, concomitant benzodiazepine use was similar between groups (83.2% AOM 400; 83.7% placebo), with respective mean ± SD daily doses of 2.24 ± 1.23 mg and 2.40 ± 1.35 mg (lorazepam equivalents). Median (range) duration of benzodiazepine use was also comparable between AOM 400 and placebo [22.0 (1.0–119.0) and 19.0 (1.0–91.0) days, respectively].
DISCUSSION
The results of this post hoc analysis demonstrated that AOM 400 treatment in patients with schizophrenia experiencing an acute exacerbation of psychotic symptoms provided significant improvements from baseline compared with placebo across all 5 PANSS Marder factors and PEC. Because patients were comparable at study start across symptom domains, these results suggest that AOM 400 effectively treated the continuum of schizophrenia symptoms, including agitation, regardless of the patient's baseline status.
In acute psychiatric settings, the primary goals are to treat positive symptoms and obtain rapid control of disruptive behaviors, such as hostility and agitation. In this analysis, AOM 400 demonstrated a statistically significant improvement in the positive Marder factor domain compared with placebo as early as week 1 that was maintained throughout the study. Aripiprazole once-monthly 400 mg achieved and maintained statistically significant improvements versus placebo in the uncontrolled hostility/excitement Marder factor at week 1. While placebo-treated patients showed negligible score reductions, AOM 400-treated patients had steady improvements from baseline, with the largest differences between treatment groups occurring at weeks 10 and 12.
At the first time point (week 1 postbaseline), significant benefits of AOM 400 with concomitant oral aripiprazole were observed. A 5-day head-to-head study comparing orally administered aripiprazole and olanzapine in acutely psychotic patients gives insight into the speed of response in acute settings, where improvements must be attained within hours or days.19 Daily mean improvement in PEC scores (the primary outcome measure) was seen in both treatment groups at all visits, with no significant differences between groups. By study end, more than half the patients in both treatment groups were considered responders (≥40% reduction in PEC scores). Despite having different activities at the dopamine D2 receptor (aripiprazole: partial agonism; olanzapine: antagonism), aripiprazole showed similar efficacy to olanzapine in treating agitation and positive symptoms in acutely ill patients. This earlier study is of particular relevance because pharmacokinetic data indicate that the first injection of AOM 400 should be accompanied by an overlap with an oral antipsychotic.20 In the acute study reported here, patients in the AOM 400 group took oral aripiprazole for the first 2 weeks of the study; thus, the influence of oral aripiprazole on the rapid effects observed in this post hoc analysis must be considered.14
Aripiprazole has low sedative potential, and its use in schizophrenia is consistent with the principle of managing agitation and other positive symptoms by calming patients rather than sedating them.21 Throughout acute treatment in the current trial, benzodiazepine use was comparable between treatment groups, suggesting that the greater efficacy of AOM 400 versus placebo was not related to sedative effects. A low incidence of sedation-related adverse events was observed in this study in both AOM 400- and placebo-treated patients,12 which further supports the conclusion that psychotic symptoms can be controlled independently from sedation. It should be noted that agitation improvement was observed despite a higher incidence of akathisia with AOM 400 compared with placebo.
These findings are also consistent with those of a pooled post hoc analysis of 5 double-blind trials of oral aripiprazole, 4 to 6 weeks in duration, conducted in patients hospitalized for acute exacerbations of schizophrenia or schizoaffective disorder.14 In that analysis, oral aripiprazole achieved significantly greater improvements from baseline versus placebo in all 5 PANSS Marder factors, and significant differences were seen by week 1 in all factors except depression/anxiety, for which significant differences were seen from week 2.
For patients with schizophrenia experiencing an acute psychotic episode, it is necessary to consider long-term outcomes in addition to short-term treatment goals from the time of the acute treatment phase. Once positive symptoms and agitation have been controlled, clinical focus turns to symptoms that have an ongoing detrimental effect on daily life. Negative symptoms affect long-term functional outcomes22,23 and have an unfavorable impact on self-esteem.24 Several atypical antipsychotics (eg, clozapine, risperidone, olanzapine) have shown significant improvements in negative symptoms compared with placebo and haloperidol. In this study, significant improvements with AOM 400 versus placebo were seen on the negative symptom Marder factor scores from week 1 through the end of the study, with notable score reductions at week 12.
Disorganized thinking may be one of the most debilitating aspects of schizophrenia,25 and cognitive function is often severely affected.26 Cognitive deficits, anxiety, and depression each compound negative symptoms, contributing to amotivation, social withdrawal, and poor functional outcomes.27 In this post hoc analysis, patients achieved significantly better results with AOM 400 versus placebo in disorganized thought and anxiety/depression factors throughout the study.
This analysis was based on data from a robustly designed, large, randomized, placebo-controlled, double-blind study. Although week 10 (primary endpoint) completion rates were higher in the AOM 400 (64%) versus the placebo (49%) groups,12 there was concordance between the MMRM, LOCF, and OC analyses, suggesting that the differences in discontinuation rates did not bias the results. Discontinuations due to lack of efficacy, which can lead to efficacy assessment biases, were far greater in the placebo than the AOM 400 group.12
Limitations include (1) the post hoc nature of the analyses reported here, because the study was not powered to detect differences on Marder factors or the PEC, and (2) the lack of correction for multiple comparisons. However, since all P values were less than 0.001 at weeks 10 and 12, the chance of type 1 error was minimal. Also, the comparator was placebo rather than an active comparator; greater symptom improvements are expected when an antipsychotic agent is compared with placebo.
In patients experiencing acute schizophrenia exacerbation, treatment with AOM 400 and concomitant oral aripiprazole in the first 2 weeks was efficacious and demonstrated a rapid onset of action across all domains of the illness conceptualized by the PANSS Marder factors and the PEC scores. Aripiprazole once-monthly 400 mg provided efficacy across a range of symptom domains in patients receiving once-monthly dosing, which has important implications for both short- and long-term outcomes.
ACKNOWLEDGMENT
Editorial support for development of this article was provided by C4 MedSolutions, LLC (Yardley, Pa), a CHC Group company, and funded by Otsuka Pharmaceutical Commercialization & Development, Inc, and H. Lundbeck A/S. The authors wish to acknowledge Dr David Tano at the University of Calgary Cumming School of Medicine and Veronique Littmann at Otsuka Canada for assistance with initiating and addressing conceptual issues with the article.
AUTHOR DISCLOSURE INFORMATION
Funding for this study and for writing assistance was provided by Otsuka Pharmaceutical Development & Commercialization, Inc, and H. Lundbeck A/S.
Ross Baker, Na Jin, Pamela Perry, Timothy Peters-Strickland, Raymond Sanchez, and Robert D. McQuade are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. Anna Eramo is an employee of Lundbeck LLC. Peter Hertel is an employee of H. Lundbeck A/S. Maia Miguelez is an employee of Otsuka Canada. Zahinoor Ismail has received honoraria for lectures by The Canadian Psychiatric Association, Calgary Foothills Primary Care Network, Calgary West Central Primary Care Network, Canadian Conference on Dementia, Alberta College of Family Physicians, and the University of British Columbia; and for consultancy for Janssen, Lundbeck, Otsuka, Pfizer, and Sunovion. John Kane has received honoraria for lectures and/or consulting from Alkermes, Amgen, Bristol-Myers Squibb, Cephalon, Eisai, Boehringer Ingelheim, Eli Lilly, Forrest, Genentech, Intracellular Therapeutics, Janssen, Johnson & Johnson, Lundbeck, Merck, Novartis, Otsuka, Pfizer, Pierre Fabre, Proteus, Reviva, Roche, Sunovion, and Targacept; and is a shareholder of MedAvante.
REFERENCES
- 1.Kane JM. Treatment strategies to prevent relapse and encourage remission. J Clin Psychiatry. 2007;68(suppl 14):27–30. [PubMed] [Google Scholar]
- 2.Caseiro O, Pérez-Iglesias R, Mata I, et al. Predicting relapse after a first episode of non-affective psychosis: a three-year follow-up study. J Psychiatr Res. 2012;46:1099–1105. [DOI] [PubMed] [Google Scholar]
- 3.Emsley R, Nuamah I, Hough D, et al. Treatment response after relapse in a placebo-controlled maintenance trial in schizophrenia. Schizophr Res. 2012;138:29–34. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman JA, Alvir JM, Koreen A, et al. Psychobiologic correlates of treatment response in schizophrenia. Neuropsychopharmacology. 1996;14:13S–21S. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman JA, Perkins D, Belger A, et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50:884–897. [DOI] [PubMed] [Google Scholar]
- 6.Ismail Z, Arenovich T, Grieve C, et al. Predicting hospital length of stay for geriatric and adult patients with schizophrenia. J Hosp Adm. 2015;4:15–22. [Google Scholar]
- 7.Andreasen NC, Liu D, Ziebell S, et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Addington DE, Mckenzie E, Wang J, et al. Development of a core set of performance measures for evaluating schizophrenia treatment services. Psychiatr Serv. 2012;63:584–591. [DOI] [PubMed] [Google Scholar]
- 9.Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14:2–44. [DOI] [PubMed] [Google Scholar]
- 10.Manchanda R, Chue P, Malla A, et al. Long-acting injectable antipsychotics: evidence of effectiveness and use. Can J Psychiatry. 2013;58:5S–13S. [DOI] [PubMed] [Google Scholar]
- 11.Kane JM, Zhao C, Johnson BR, et al. Hospitalization rates in patients switched from oral anti-psychotics to aripiprazole once-monthly: final efficacy analysis. J Med Econ. 2015;18:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kane JM, Peters-Strickland T, Baker RA, et al. Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75:1254–1260. [DOI] [PubMed] [Google Scholar]
- 13.Lindenmayer JP, Bernstein-Hyman R, Grochowski S. Five-factor model of schizophrenia. Initial validation. J Nerv Ment Dis. 1994;182:631–638. [DOI] [PubMed] [Google Scholar]
- 14.Janicak PG, Glick ID, Marder SR, et al. The acute efficacy of aripiprazole across the symptom spectrum of schizophrenia: a pooled post hoc analysis from 5 short-term studies. J Clin Psychiatry. 2009;70:25–35. [DOI] [PubMed] [Google Scholar]
- 15.Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58:538–546. [DOI] [PubMed] [Google Scholar]
- 16.Lançon C, Auquier P, Nayt G, et al. Stability of the five-factor structure of the Positive and Negative Syndrome Scale (PANSS). Schizophr Res. 2000;42:231–239. [DOI] [PubMed] [Google Scholar]
- 17.Gastpar M, Masiak M, Latif MA, et al. Sustained improvement of clinical outcome with risperidone long-acting injectable in psychotic patients previously treated with olanzapine. J Psychopharmacol. 2005;19:32–38. [DOI] [PubMed] [Google Scholar]
- 18.Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinon BJ, Stauffer VL, Kollack-Walker S, et al. Olanzapine versus aripiprazole for the treatment of agitation in acutely ill patients with schizophrenia. J Clin Psychopharmacol. 2008;28:601–607. [DOI] [PubMed] [Google Scholar]
- 20.Raoufinia A, Baker RA, Eramo A, et al. Initiation of aripiprazole once-monthly in patients with schizophrenia. Curr Med Res Opin. 2015;31:583–592. [DOI] [PubMed] [Google Scholar]
- 21.Cañas F. Management of agitation in the acute psychotic patient—efficacy without excessive sedation. Eur Neuropsychopharmacol. 2007;17(suppl 2):S108–S114. [DOI] [PubMed] [Google Scholar]
- 22.Hwu HG, Chen CH, Hwang TJ, et al. Symptom patterns and subgrouping of schizophrenic patients: significance of negative symptoms assessed on admission. Schizophr Res. 2002;56:105–119. [DOI] [PubMed] [Google Scholar]
- 23.Arango C, Buchanan RW, Kirkpatrick B, et al. The deficit syndrome in schizophrenia: implications for the treatment of negative symptoms. Eur Psychiatry. 2004;19:21–26. [DOI] [PubMed] [Google Scholar]
- 24.Hofer A, Kemmler G, Eder U, et al. Quality of life in schizophrenia: the impact of psychopathology, attitude toward medication, and side effects. J Clin Psychiatry. 2004;65:932–939. [PubMed] [Google Scholar]
- 25.Palmer BW, Heaton RK, Paulsen JS, et al. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11:437–446. [DOI] [PubMed] [Google Scholar]
- 26.Carbon M, Correll CU. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 2014;19(suppl 1):38–52; quiz 35–37, 53. [DOI] [PubMed] [Google Scholar]
- 27.Millan MJ, Fone K, Steckler T, et al. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. 2014;24:645–692. [DOI] [PubMed] [Google Scholar]


