Abstract
Background:
Development of country plans for prevention of mother-to-child HIV transmission (PMTCT), including expansion of comprehensive, integrated services, was key to Global Plan achievements.
Approaches:
Use of the PMTCT cascade, an evolving series of sequential steps needed to maximize the health of women and HIV-free survival of infants, was critical for development and implementation of PMTCT plans. Regular review of cascade data at national/subnational levels was a tool for evidence-based decision making, identifying areas of greatest need at each level, and targeting program interventions to address specific gaps. Resulting improvements in PMTCT service delivery contributed to success. Populating the cascade highlighted limitations in data availability and quality that focused attention on improving national health information systems.
Limitations:
Use of aggregate, cross-sectional data in the PMTCT cascade presents challenges in settings with high mobility and weak systems to track women and children across services. Poor postnatal follow-up and losses at each step of the cascade have limited use of the cascade approach to measure maternal and child health outcomes beyond the early postnatal period.
Lessons Learned:
A cascade approach was an effective means for countries to measure progress, identify suboptimal performance areas, and be held accountable for progress toward achievement of Global Plan goals. Using the cascade requires investment of time and effort to identify the type, source, and quality of data needed as programs evolve. Ongoing review of cascade data, with interventions to address discontinuities in the continuum of care, can translate across health areas to improve health care quality and outcomes.
Key Words: PMTCT cascade, global plan, HIV, prevention
BACKGROUND
The Global Plan used the 4 prongs of prevention of mother-to-child transmission (PMTCT), each with ambitious targets, as the framework to achieve the goals for the elimination of new HIV infections among children and keeping their mothers alive.1 All 22 priority countries endorsed the Global Plan and agreed to be held accountable for achieving elimination of mother-to-child transmission. The Global Plan emphasized the expansion of comprehensive, integrated, and efficacious interventions for PMTCT within a maternal and child health (MCH) framework, to achieve targets by 2015.1,2
Designated Global Plan country focal points were tasked with overseeing the development of detailed country plans and reporting on their progress. Although all 4 prongs of the implementation strategy were important, immediate gains in preventing pediatric infections and keeping mothers alive were expected to be achieved through rapid expansion of programs addressing prong 3 (antiretroviral medication access) and prong 4 (care, treatment, and support). Many countries adopted a cascade approach, collecting and analyzing data at care points along the service delivery pathway, as a tool for advancing program implementation and monitoring progress toward Global Plan targets.
This article reviews the evolution of the PMTCT cascade and examines its use, contributions, and limitations in achieving Global Plan goals. Country examples illustrate key points.
Evolution of the PMTCT Cascade
The PMTCT cascade is a series of key stepwise activities that constitute a critical pathway to successful PMTCT that begins with all pregnant women and ends with the detection of a final HIV status in HIV-exposed infants (HEIs).
The PMTCT cascade, with defined indicators to measure each step, has evolved over time with each advance in the science of PMTCT and the release of revised World Health Organization (WHO) guidelines (Fig. 1).3–11 Before the use of antiretroviral medicines for PMTCT, early WHO guidelines focused on counseling, offering and acceptance of HIV testing, and receiving HIV antibody test results for pregnant women and HEIs at 18 months of age.3 Steps along the cascade expanded as interventions to prevent transmission were introduced and access to antiretroviral therapy improved. Indicators changed as recommendations evolved from single-dose nevirapine prophylaxis to initiation of lifelong antiretroviral therapy for all pregnant and lactating women (Option B+).4–11 Also, development of early infant diagnosis (EID) capacity led to the addition of HIV testing at 6 weeks to the cascade.9
FIGURE 1.
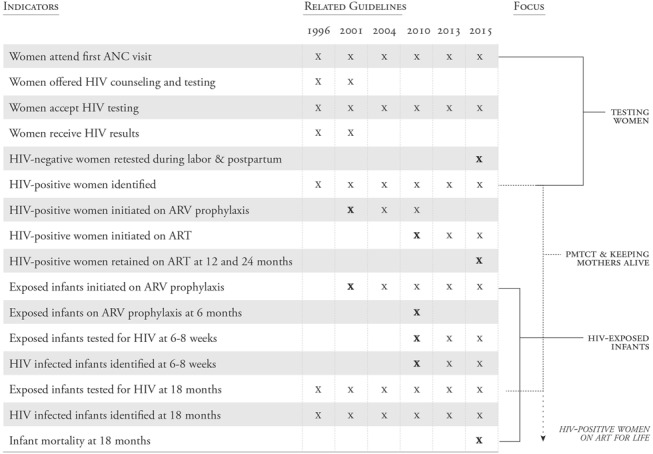
As WHO guidelines have evolved over time, key indicators included in the PMTCT cascade have changed, as shown by X, with bolded X representing the introduction of new indicators.3–11 ART, antiretroviral therapy; ARV, antiretroviral.
The introduction of Option B+ and the interrelationship between PMTCT and antiretroviral therapy services has highlighted important gaps in the PMTCT cascade.12,13 Although the final HIV outcome in children cannot be determined until the end of exposure through breastfeeding, the antenatal and early infant follow-up (6–8 weeks) steps of the cascade have received more attention than the postnatal period. The 2015 WHO Consolidated Strategic Information Guidelines outlines the programmatic changes needed to monitor the full cascade to achieve the 4 prong targets and demonstrates the need for continued evolution of the PMTCT cascade to include additional outcomes related to HIV and maternal, newborn and child health (MNCH).13 An example of a more comprehensive cascade that focuses on both maternal and child outcomes—including key indicators such as retention in care, retests to detect incident HIV infections in women, infant HIV-free survival, and viral suppression—is shown in Figure 2.
FIGURE 2.
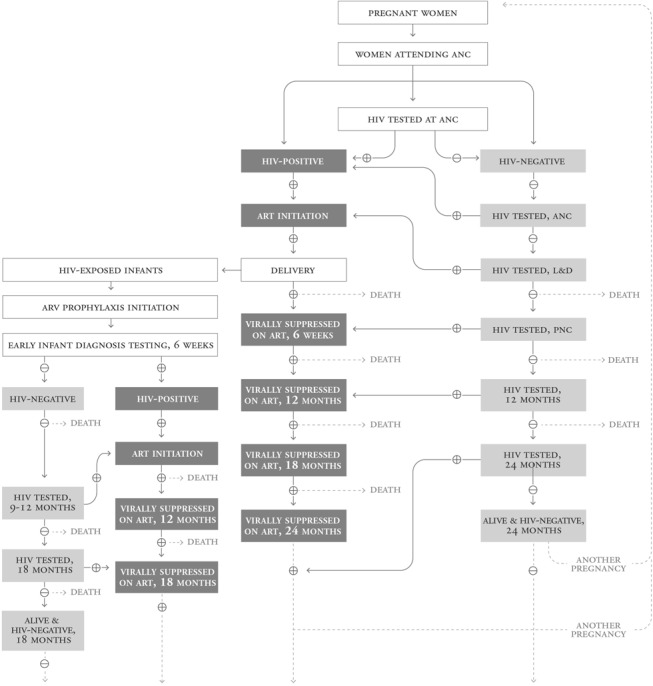
An expanded PMTCT cascade is required to measure progress toward Global Plan goals for elimination of HIV infection in children and keeping mothers alive as in this illustrative example. Parallel but linked maternal and child cascades that begin with all pregnant women and include data on HIV infection, mortality, and HIV treatment endpoints at 18–24 months would enable more comprehensive understanding of the progress and gaps in achieving PMTCT and MCH goals. ART, antiretroviral therapy; ARV, antiretroviral; L&D, labor and delivery; PNC, postnatal care.
Learning From Early PMTCT Cascade Use
As PMTCT programs were introduced and scaled up across resource-limited settings, reporting on the number of women and infants at each step of the cascade enabled governments and donors to monitor the progress of program expansion. Cascade analysis revealed “leaks” at each step, with only a limited number of mother–infant pairs successfully completing the entire cascade.14–16 As the science and evolving guidelines focused on longer and more efficacious combination regimens, cascade analysis by Barker et al16 demonstrated that new HIV infections among children are driven by high transmission rates from women who never entered services or who fell out of them (Fig. 3). The full impact of introducing more efficacious regimens would not be realized until >90% of women entered and received services at each step of the cascade (Fig. 3). Although access to antiretroviral medicines is critical, this observation focused attention on the structure and function of the PMTCT programs and the value of the cascade approach to identify and address leaks to improve service delivery.
FIGURE 3.
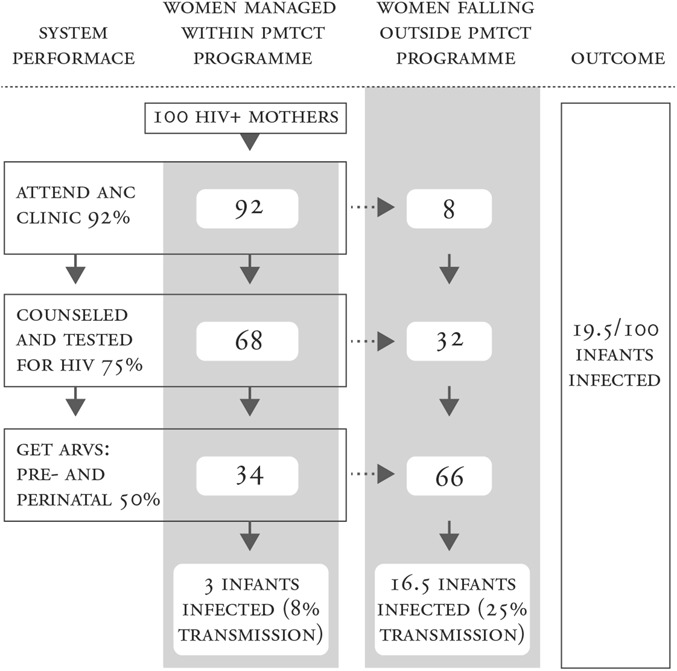
Barker's et al16 cascade analysis illustrates that HIV transmission rates are driven by mother–infant pairs who fall out of the cascade and do not receive the necessary services. This demonstrates the importance of improving PMTCT service delivery, not only implementing the PMTCT regimen change to antiretroviral (ARV) therapy for all women, in reaching Global Plan goals for elimination of new infections in children. Reprinted with permission from J Acquir Immune Defic Syndr. 2011;56:e45–e48.16 Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of the copyright in the translation or adaptation.
USING THE PMTCT CASCADE AT COUNTRY LEVEL TO ACCOMPLISH GLOBAL PLAN GOALS
Early uses of the PMTCT cascade focused primarily on monitoring PMTCT programs. The advent of the Global Plan in 2010 required rapid acceleration of more effective PMTCT service delivery and expanded population coverage in high-burden countries. Countries had to understand where to concentrate resources and efforts to address weaknesses (geographical, structural, and social). The PMTCT cascade became a critical tool for accomplishing this and measuring progress toward elimination goals. Although the Global Plan document did not highlight the PMTCT cascade as a key tool, at the programmatic level the PMTCT cascade evolved to help countries achieve national and Global Plan targets.
Collection of Quality Cascade Data for the Development and Monitoring of National PMTCT Plans
The Democratic Republic of the Congo provides an example of the use of PMTCT cascade data to inform the development of the country's PMTCT plan. Attempts to populate the cascade in 2013 revealed a lack of reliable data at the national and provincial levels; indicator definitions and data collected varied by partner and donor, highlighting an unreliable strategic information management system. To address this, the Ministry of Health focused on harmonizing indicators and developing strategies to improve the quality of data in the routine system. Once this was done, analysis of the cascade revealed troubling gaps in testing and antiretroviral coverage that disproportionately affected rural areas. In 2012, PMTCT services were available in only 4% of health facilities, with significant gaps along the cascade; only 13% of HIV-positive pregnant women accessed antiretroviral medications, only 18% of eligible pregnant women received antiretroviral therapy, and overall rates of retention of mother–infant pairs were low.17
Access to PMTCT cascade data at the provincial level enabled the country to mobilize and focus resources on strategic activities in the 3 provinces that were contributing the most infections in children. This resulted in significant improvement in national cascade results in 2015, with 67% of pregnant women receiving antiretroviral medications and 52% of eligible women on antiretroviral therapy. It did, however, demonstrate limited progress in the postnatal component, with only 17% of infants receiving EID.18
Kenya made PMTCT cascade analysis a key part of a comprehensive and iterative process of monitoring data that helped to inform evidence-based decision making to optimize PMTCT service delivery and improve outcomes. The launch of Option B+ in 2014 by the Kenya Ministry of Health was expected to address low coverage of antiretroviral medicines for pregnant women and to accelerate PMTCT progress. At a national PMTCT stocktaking meeting in April 2015, however, the largest drop-off in the cascade, which was populated with the 2014 national-level and county-level district health information system (DHIS 2) data, remained the number of HIV-positive women who received antiretroviral medicines. Fifteen of the 47 Kenyan counties contributed 75% of the 17,432 missed opportunities that were identified.
In response, the Ministry of Health conducted a Bring Back the Women and Children campaign in June 2015 to find the missed women and strengthen the ownership and accountability of the PMTCT response through data use at the facility level. Review of 9752 (56%) of the missed opportunities revealed that 6935 (71%) were data transcription errors between primary tools and DHIS 2, and 1093 (6%) had received antiretroviral therapy but documentation was limited to pharmacy records. Programmatic changes in Kenya in response to weaknesses in the cascade included mentorship for all staff (from the national level through to the facility level) in the collection and use of the PMTCT indicators along the cascade, prioritization based on the largest gaps for each level, data quality audits, and community engagement with health facilities and outreach to missed women.
The 2015 DHIS 2 data subsequently identified 63,970 HIV-positive pregnant women, of whom 58,058 (91%) received antiretroviral therapy and 5912 (9.2%) were missed. This performance, although not sufficient to achieve the 2015 PMTCT targets, did show significant improvement. Similar to the results in the Democratic Republic of the Congo, this achievement also demonstrated the importance of ensuring the accuracy of the data used to populate the cascade.
Gap and Bottleneck Analyses Identify Priority Programmatic Interventions to Improve Program Performance
The Global Plan stimulated country PMTCT gap and bottleneck analyses for critical review of data at each step of the cascade. This was done to identify discontinuities in the continuum of care, determine their underlying causes, and prioritize actions and interventions to address them.
Gap Analyses
Countries conducted gap analyses combining population and program data in the cascade to identify deficiencies and demonstrate the effect that coverage of antenatal care (ANC) and PMTCT services had on final outcomes. This expanded on earlier cascade analyses by providing important information about the relative contributions of population access versus service delivery gaps to pinpoint areas of missed opportunities.16
Figure 4 illustrates one example of how gap analyses conducted at sites supported by the Elizabeth Glaser Pediatric AIDS Foundation in the United Republic of Tanzania in 2010 and 2015 addressed some key postnatal components. The numbers of mother–infant pairs who received the services at each step of the cascade were documented similar to a traditional cascade. However, gap analyses included the entire population of pregnant women. Data were analyzed for all women who did not receive the services, including the numbers of women who did not attend clinics, those who attended clinics but did not receive the available services, and those who attended clinics when services were not available. Missed HIV testing and receipt of antiretroviral medications were important gaps for both women and HEIs. The integration of full antiretroviral therapy services within MNCH clinics in 2013, development of a card to be used to identify HEI that was linked to the mother's antiretroviral therapy card, and mother–baby pair cohort monitoring led to significant improvements in women and children receiving services by 2015 (Fig. 4).
FIGURE 4.
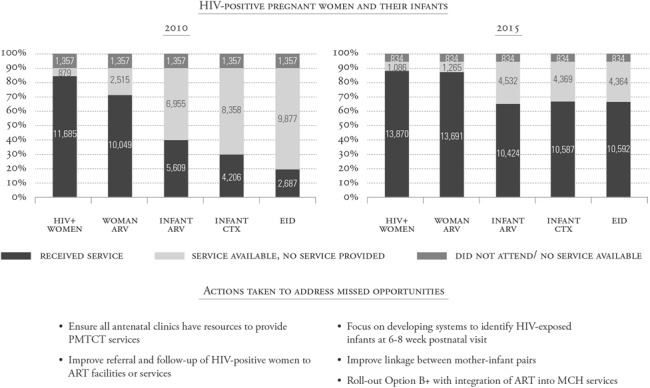
Gap analysis using the cascade can identify missed opportunities for prevention and treatment, and the steps that programs can take to address them. Data from the Elizabeth Glaser Pediatric AIDS Foundation-supported programs in the United Republic of Tanzania in 2010 and 2015 are shown as an example of gap analyses. Some actions taken in response to gaps identified in 2010 are listed with resultant improvements demonstrated in 2015. ART, antiretroviral therapy; ARV, antiretroviral; CTX, cotrimoxazole.
Gap analyses provided the impetus for countries to develop differentiated strategies that addressed specific areas of weakness. Gimbel et al19,20 developed and evaluated a simple cascade analysis tool to help facility staff identify gaps and prioritize clinic-level service delivery changes, which resulted in improvements in some PMTCT outcomes such as antiretroviral coverage and HEI testing.
Bottleneck Analyses
With support from the United Nations Children's Fund and the Interagency Task Team on Prevention and Treatment of HIV Infection in Pregnant Women, Mothers and their Children, countries conducted bottleneck analyses to identify causes of significant gaps found in the cascade analysis.21 Six determinants of health system bottlenecks were evaluated: (1) availability of essential health commodities, (2) availability of trained human resources, (3) physical accessibility of service delivery points, (4) initial use, (5) continuous use, and (6) quality.22 Participatory causality analysis was done to identify underlying factors and root causes of the observed bottlenecks while supply-side analysis looked at health systems processes and managerial competencies. Analysis of demand-side bottlenecks assessed factors such as acceptability, perceived value, and trust from the perspectives of clients and communities. Interventions to address identified causes of bottlenecks were implemented, and PMTCT cascade data were reviewed to measure program improvements (Boxes 1 and 2).
BOX 1. Equity-Focused Bottleneck Analysis.
Uganda October 2011–June 2012.
Cascade gap identified: poor uptake of EID.
Bottleneck causal analysis: lack of data sharing across MNCH service delivery points; poor mother–baby linkage.
Actions taken: development of national dashboards; initiation of mother–baby care points; coordinated mother–baby tracking system.
BOX 2. Ghana Bottleneck Analysis.
New Juaben Municipality, 2012.
Cascade gap identified: low (35%) maternal antiretroviral medicine coverage in ANC.
Bottleneck causal analysis: policy that antiretroviral medicines are only administered by doctors; limited antiretroviral medicine access in lower-level facilities.
Actions taken: 2013 policy revision to allow midwives to provide antiretroviral medicines.
2014 maternal antiretroviral coverage 78%.23
Decentralization of the Use of the PMTCT Cascade for Evidence-Based Decision Making at the District and Facility Levels
In the Republic of Chad, 36 district health teams used the cascade to review program performance and develop decentralized PMTCT action plans.24 The evidence-informed and iterative process used standardized tools to identify geographic areas with the greatest unmet need and to assess the main bottlenecks to service use and uptake. District action plans were implemented and monitored through review of cascade data every 6 months.
The district approach to PMTCT cascade data collection and review resulted in a notable expansion in PMTCT coverage. The number of sites offering PMTCT services increased from 120 in 2011 to 463 in 2014. This in turn contributed to the improvement in the national PMTCT coverage rate, which rose from 11% to 40% over the same period of time. The rate of HIV testing in ANC increased from 10% to 54% (176,339–326,553), and the percentage of pregnant women living with HIV receiving antiretroviral medicine for PMTCT increased from 12% in 2011 to 35% in 2014.24,25
Using the PMTCT Cascade to Reduce Missed Opportunities for Prevention in South Africa
Analysis of the individual steps in the PMTCT cascade to identify and address service delivery gaps began before the Global Plan, but it advanced with the Global Plan's focus on accelerating progress toward PMTCT.26,27 In South Africa, although the National Strategic Plan for HIV and AIDS specified process and outcome-related goals, no reliable cascade data existed to track national mother-to-child HIV transmission (MTCT) rates as a measure of PMTCT program effectiveness.28,29 This prompted nationally and provincially representative annual surveillance of all infants receiving their 6-week immunizations, between 2010 and 2013.27,30 Infant dried blood spot analyses and maternal interviews were undertaken among a representative sample of women attending 6-week immunization services, regardless of their HIV status. Infants of self-reported HIV-positive women and all HIV antibody-positive infants underwent DNA polymerase chain reaction testing.31 The PMTCT cascade drawn from this surveillance showed a reduction in the number of women receiving CD4 cell count tests and maternal antiretroviral prophylaxis between 2010 and 2013, and a concomitant increase in maternal antiretroviral therapy associated with the policy shift to PMTCT Option A in 2010 (Fig. 5).31
FIGURE 5.
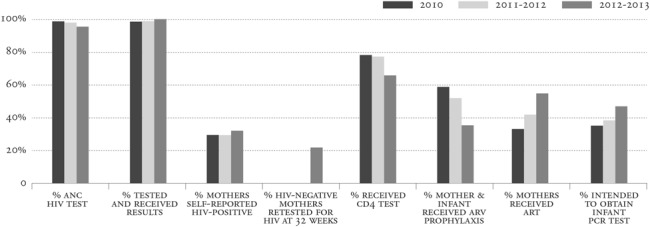
PMTCT cascade results from South African surveillance activities in 2010–2013 reflect an increase in access to antiretroviral therapy for pregnant women after implementation of the 2010 WHO guidelines and gaps in the PMTCT cascade for EID and retesting of HIV-negative women (only measured beginning in 2012). ART, antiretroviral therapy; ARV, antiretroviral; PCR, polymerase chain reaction. Reprinted with permission from: Goga et al.31 Available at: http://www.mrc.ac.za/healthsystems/SAPMTCTEReport2012.pdf. Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
A retrospective review of services received along the cascade for HIV-positive mothers was conducted to pinpoint key missed opportunities for prevention that needed to be addressed to achieve Global Plan targets.16 The 2010 surveillance data revealed that 34.9% of women dropped out of the cascade at some point.27 Analyses of 4 key PMTCT cascade steps reported by mothers of HIV-infected infants (knowledge of maternal HIV status, knowledge of maternal CD4 cell count, maternal access to antiretroviral medicines, and infants' access to nevirapine) demonstrated that missing 1 or more cascade steps explained 33.8% of early infant HIV transmissions. Missing maternal HIV diagnoses from women who either were not tested (2.4%) or who self-reported as HIV-negative (8.6%) explained 15.3% of early MTCT, indicating that eliminating missed opportunities from undiagnosed maternal infection could reduce early infant MTCT to 2.8%.27
The data highlighted the importance of retaining HIV-negative women in the PMTCT cascade through the end of breast-feeding to identify and address incident infections (Fig. 2). In the South African 2010 evaluation, 4.1% of self-reported HIV-negative women had HIV antibody-positive infants; this dropped to 2.6% in the 2012–2013 surveillance: new HIV infections were undetected due to low rates of retesting of HIV-negative women.30,31 The 2013 PMTCT guidelines thus included new steps in the PMTCT cascade with repeat HIV testing after 12 weeks, at 32 weeks gestation, in the labor ward, and at 6 weeks and 3, 6, and 12 months during breastfeeding to identify any new maternal HIV infections and to facilitate immediate care.32,33 Additional cascade steps were added with the 2015 introduction of birth testing of HEIs to reduce missed opportunities to identify HIV-positive infants before 6 weeks of age.
LIMITATIONS OF THE CURRENT CASCADE APPROACH IN THE CONTEXT OF THE GOALS OF THE GLOBAL PLAN
Although the Global Plan stimulated use of the PMTCT cascade as a platform for the development of both national PMTCT plans and data-driven programmatic interventions, the approach has limitations:
The cascade does not include PMTCT prongs 1 or 21
Problems with data reliability arise from generating the cascade using aggregate cross-sectional data from routine reporting systems with poor tracking of mother–infant pairs.13,34 The cross-sectional approach does not account for numbers of deaths and client movement among health facilities, which leads to inaccurate reporting of some indicators. The inability to determine accurate denominators, starting with the population of pregnant women, and inconsistencies in denominator definitions in reported percentages along the cascade, make results difficult to interpret and compare across settings.35
Despite the goals of the Global Plan to eliminate new HIV infections among children and keep their mothers alive, in most Global Plan settings, the current PMTCT cascade data do not accurately measure either outcome.
Measurement of HIV-free survival in children after the risk of HIV transmission through breast-feeding is not possible due to limited data on the postnatal components of the cascade. This is not an inherent limitation in the cascade approach, but rather a result of lack of attention to addressing the challenges of poor postnatal follow-up for both women and infants.36 In the absence of data on the HIV status of HIV-exposed children at 18–24 months of age, countries have relied on the EID cascade step at 6–8 weeks to monitor HIV transmission outcomes.17,18
The PMTCT cascade has primarily focused on the reduction of infections in children and not HIV-related maternal mortality. Maternal indicators such as antiretroviral therapy adherence, retention in care, and mortality are critical to PMTCT, but they are not systematically collected and evaluated as part of the PMTCT cascade.
REMAINING CHALLENGES
Although significant progress has been made through the Global Plan and has focused attention on the PMTCT cascade, there is considerable work to be done before the ambitious Global Plan targets are reached and countries can be certified to have reached MTCT elimination.18,37,38 With the evolution of PMTCT guidelines to lifelong antiretroviral therapy for all HIV-positive pregnant women, there are now 2 intersecting cascades: one for the prevention of HIV infection in children and another for the long-term care and treatment of mothers (Fig. 2).12,13 Efforts to improve collection of accurate data on the continuum of care across service delivery sites with strong linkages of mother–infant pairs must be strengthened. The PMTCT cascade must continue to evolve to measure more comprehensive endpoints such as HIV-free survival in children, viral suppression in HIV-positive women, and HIV incidence in pregnant and lactating women who were initially HIV negative.
Improving the follow-up of both HIV-positive and HIV-negative mother–infant pairs until 2 years post-delivery is critical to reaching elimination of mother-to-child transmission and healthy MCH outcomes. Collection of data required in the postpartum period remains a considerable challenge, which limits our understanding of program effectiveness in breast-feeding populations.35 Maternal and infant outcomes are still largely modeled, due to the limited availability of 18–24 month data. Thus, estimates of global numbers of HIV-positive children are quite broad and have recently undergone a significant adjustment.13,39
Considerable efforts are needed to get more pregnant women into ANC and thus to enter the PMTCT cascade, to keep women in care throughout the antenatal and postnatal periods, and to quickly identify women and children who fall out of the cascade (to return them to care). As delivery of comprehensive PMTCT services is strengthened, an increasing number of new infections in children are attributable to women dropping out of care along the cascade; this includes incident infections missed in initially HIV-negative women.40
LESSONS FOR THE FUTURE
The Global Plan focused attention on use of the evolving cascade as an operational framework for accelerating more effective implementation of programs to eliminate new HIV infections among children and to keep their mothers alive. Within the context of the Global Plan, the cascade proved valuable for measuring progress, identifying key areas that require attention, and holding countries accountable for demonstrating improved program outcomes.
The cascade has evolved organically over time, but cascade development entails investing time and effort in (1) identifying the type and quality of data needed to populate the cascade, (2) enabling mechanisms to link records of mother–infant pairs and track them across service delivery sites (both community and facility), and (3) maintaining mother–infant pairs in long-term follow-up to ascertain final health outcomes for both. Use of mother–infant pair cohort monitoring (as in the United Republic of Tanzania), implementation of a retention dashboard (as in Uganda), and use of patient-level electronic databases by national programs are examples of some ways that more accurate and complete cascade data could be collected.34,41,42
A careful review of the cascade is in order. Starting the cascade with all pregnant women, monitoring mother–infant pairs, and ending with outcomes of all mothers (not just HIV-positive mothers) and their children would shift the focus of the cascade from MTCT to broader MCH concerns.
Effective use of the PMTCT cascade has fostered the development of other cascades to measure the continuum of care, such as the EID, HIV treatment, and HIV and tuberculosis cascades.34,35,43–45 As HIV programs evolve to a test-and-treat model, close attention to the cascade—from HIV testing, through initiation and adherence to treatment, to overall health outcomes—will be critical.45 These programs will benefit from adapting the lessons learned from the Global Plan and the roll-out of Option B+ (Box 3). For countries to achieve the ambitious United Nations 90–90–90 goals for HIV testing, treatment, and viral suppression, the focus must be on identifying and defining each step needed along the continuum of care.13,34,46 As with the Global Plan, there must be a clear strategy for quality data collection and regular analysis of the treatment cascade from the national to facility levels to inform program performance and accelerate progress toward these goals.
BOX 3. Lessons Learned.
The key steps in the cascade need to be clearly identified, with clear indicator definitions at each step.
To achieve and measure outcomes, all steps of the cascade need attention, not just the early ones.
Cascade analysis is a tool for improving service delivery, not just for monitoring.
For more effective action planning to address program gaps, cascade data should be disaggregated to the facility level.
The cascade approach is insufficient for measuring outcomes if there are major leaks at every step.
Active tracing of people who enter the cascade is critical to ensuring that they are retained.
Tracking mother–infant pairs across service delivery sites is critical for an accurate cascade (this requires use of unique identifiers).
Use of cohort monitoring data rather than cross-sectional data may provide more accurate information in a cascade.
Facility–community linkages are essential, both for ensuring and for measuring the full continuum of care.
ACKNOWLEDGMENTS
The authors thank Chris Hudnall and Janet Kim (the Elizabeth Glaser Pediatric AIDS Foundation) for their support in the generation of this article. Also, Elevanie Nyankesha and Dr Claudes Kamenga (the United Nations Children's Fund [UNICEF], West and Central Africa Region); Dr L. K. Senaya (Director of New Juaben Municipality Health Services, Eastern Region, Ghana) and Hari Krishna Banskota (UNICEF Ghana); Dr Bongdene Ngarbaye Hélène (PMTCT Coordinator, Ministry of Health, Chad) and Thomas Munyuzangabo (UNICEF Chad) for their contribution to country bottleneck analysis data.
Footnotes
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.UNAIDS. Countdown to Zero. Global Plan Towards the Elimination of New Infections in Children by 2015 and Keeping Mothers Alive, 2011–2015. Geneva, Switzerland: UNAIDS; 2011. [Google Scholar]
- 2.World Health Organization. PMTCT Strategic Vision 2010–2015: Preventing Mother-to-Child Transmission of HIV to Reach the UNGASS and Millennium Development Goals. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 3.UNAIDS, World Health Organization. Revised recommendations for the selection and use of HIV antibody tests. Wkly Epidemiol Rec. 1997;72:81–88. [PubMed] [Google Scholar]
- 4.World Health Organization. Prevention of Mother-to-Child Transmission of HIV: Selection and Use of Nevirapine. Technical Notes. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 5.World Health Organization. New Data on the Prevention of Mother-to-Child Transmission of HIV and Their Policy Implications: Conclusions and Recommendations. WHO Technical Consultation on Behalf of the UNFPA/UNICEF/WHO/UNAIDS Inter-Agency Task Team on Mother-to-Child Transmission of HIV. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 6.World Health Organization. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. Guidelines on Care, Treatment and Support for Women Living with HIV/AIDS and Their Children in Resource-constrained Settings. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 7.World Health Organization. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. Recommendations for a Public Health Approach. 2006 Rev. Geneva, Switzerland: World Health Organization; 2006. [PubMed] [Google Scholar]
- 8.World Health Organization. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants: Recommendations for a Public Health Approach. 2010 Ver. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 9.World Health Organization. WHO Recommendations on the Diagnosis of HIV Infection in Infants and Children. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 10.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Recommendations for a Public Health Approach. Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 11.World Health Organization. Guideline on When to Start Antiretroviral Therapy and on Pre-exposure Prophylaxis for HIV. Geneva, Switzerland: World Health Organization; 2015. [PubMed] [Google Scholar]
- 12.McNairy ML, Teasdale CA, El-Sadr WM, et al. Mother and child both matter: reconceptualizing the prevention of mother-to-child transmission care continuum. Curr Opin HIV AIDS. 2015;10:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Consolidated Strategic Information Guidelines for HIV in the Health Sector. Geneva: Switzerland: World Health Organization; 2015. [PubMed] [Google Scholar]
- 14.Stringer EM, Ekouevi DK, Coetzee D, et al. Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in 4 African countries. JAMA. 2010;304:293–302. [DOI] [PubMed] [Google Scholar]
- 15.Sibanda E, Weller I, Hakim J, et al. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS. 2013;27:2787–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barker P, Mphatswe W, Rollins N. Antiretroviral drugs in the cupboard are not enough: the impact of health systems' performance on mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2011;56:e45–e48. [DOI] [PubMed] [Google Scholar]
- 17.UNAIDS. 2013 Progress Report on the Global Plan. Geneva, Switzerland: UNAIDS; 2013. [Google Scholar]
- 18.UNAIDS. On the Fast-Track to an AIDS-Free Generation: The Incredible Journey of the Global Plan Towards the Elimination of New HIV Infections Among Children by 2015 and Keeping Their Mothers Alive. Geneva, Switzerland: UNAIDS; 2016. [Google Scholar]
- 19.Gimbel S, Voss J, Mercer MA, et al. The prevention of mother-to-child transmission of HIV cascade analysis tool: supporting health managers to improve facility-level service delivery. BMC Res Notes. 2014;7:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rustagi A, Gimbel S, Nduati R, et al. Impact of a systems engineering intervention on PMTCT service delivery in Cote d'Ivoire, Kenya, and Mozambique: a cluster-randomized trial. J Acquir Immune Defic Syndr. 2016;72:e68–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United Nations Children's Fund. Reaching Universal Health Coverage Through District Health System Strengthening: Using a Modified Tanahashi Model Sub-nationally to Attain Equitable and Effective Coverage. New York, NY: United Nations Children's Fund; 2013. [Google Scholar]
- 22.Tanahashi T. Health service coverage and its evaluation. Bull World Health Organ. 1978;56:295–303. [PMC free article] [PubMed] [Google Scholar]
- 23.Njiraini R, Agongo E, Awoonor-Williams J, et al. Adoption and Use of the Bottleneck Analysis Approach in Ghana's Health Sector. New York, NY: United Nations Children's Fund; 2015. [Google Scholar]
- 24.Chad Ministry of Health. Report of Bottlenecks and Disparities Analysis for National PMTCT Program Chad. N'Djamena, Chad: Ministry of Health; 2011. [Google Scholar]
- 25.Chad Ministry of Health. Progress Report on PMTCT Program in Chad. N'Djamena, Chad: Ministry of Health; 20132014. [Google Scholar]
- 26.Herce ME, Mtande T, Chimbwandira F, et al. Supporting Option B+ scale-up and strengthening the prevention of mother-to-child transmission cascade in central Malawi: results from a serial cross-sectional study. BMC Infect Dis. 2015;15:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woldesenbet S, Jackson D, Lombard C, et al. Missed opportunities along the prevention of mother-to-child transmission services cascade in South Africa: uptake, determinants, and attributable risk (the SAPMTCTE). PLoS One. 2015:10:e0132425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.South African National AIDS Council. HIV & AIDS and STI strategic plan for South Africa 2007–2011. 2007. Available at: http://www.tac.org.za/documents/NSP-Draft10-2007-2011.pdf. Accessed April 1, 2016.
- 29.South African National AIDS Council. The national strategic plan on HIV, STIs and TB, 2012–2016. 2011. Available at: http://sanac.org.za//wp-content/uploads/2015/11/National-Strategic-Plan-on-HIV-STIs-and-TB.pdf. Accessed June 23, 2015.
- 30.Goga A, Dinh T, Jackson D, et al. First population-level effectiveness evaluation of a national programme to prevent HIV transmission from mother to child, South Africa. J Epidemiol Community Health. 2015;69:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goga A, Jackson D, Lombard C, et al. Early (4–8 Weeks Post-delivery) Population-level Effectiveness of WHO PMTCT Option A, South Africa. Tygerberg, South Africa: South African Medical Research Council and National Department of Health of South Africa; 2015. Available at: http://www.mrc.ac.za/healthsystems/SAPMTCTEReport2012.pdf. Accessed June 30, 2015. [Google Scholar]
- 32.South Africa National Department of Health and South African National AIDS Council. Clinical guidelines: PMTCT (prevention of mother-to-child transmission). 2010. Available at: http://www.fidssa.co.za/Content/Documents/PMTCT_Guidelines.pdf. Accessed June 10, 2015.
- 33.South Africa National Department of Health. The South African antiretroviral treatment guidelines, 2013. 2013. Available at: http://www.sahivsoc.org/Files/2013%20ART%20Treatment%20Guidelines%20Final%2025%20March%202013%20corrected.pdf. Accessed May 2, 2015.
- 34.Colasanti J, Kelly J, Pennisi E, et al. Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clin Infect Dis. 2016;62:648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medland NA, McMahon JH, Chow EPF, et al. The HIV care cascade: a systematic review of data sources, methodology, and comparability. J Int AIDS Soc. 2015;18:20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Psaros C, Remmert J, Bangsberg DR, et al. Adherence to HIV care after pregnancy among women in sub-Saharan Africa: falling off the cliff of the treatment cascade. Curr HIV/AIDS Rep. 2015;12:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adetokunboh O, Oluwasanu M. Eliminating mother-to-child transmission of the human immunodeficiency virus in sub-Saharan Africa: the journey so far and what remains to be done. J Infect Public Health. 2015;9:396–407. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. Global Guidance on the Criteria and Process for Validation: Elimination of Mother-to-Child Transmission (EMTCT) of HIV and Syphilis. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 39.UNAIDS. AIDS by the Numbers, 2016. Geneva, Switzerland: UNAIDS; 2016. [Google Scholar]
- 40.Dinh TH, Delaney KP, Goga A, et al. Impact of maternal HIV sero-conversion during pregnancy on early mother to child transmission of HIV (MTCT) measured at 4–8 weeks postpartum in South Africa 2011–2012: a national population-based evaluation. PLoS One. 2015:10:e0125525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haskew J, Rø G, Turner K, et al. Implementation of a cloud-based electronic medical record to reduce gaps in the HIV treatment continuum in rural Kenya. PLoS One. 2015:10:e0135361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inter-Agency Task Team on the Prevention and Treatment of HIV Infection in Pregnant Women, Mothers and Children. Monitoring and Evaluation Framework for Antiretroviral Treatment for Pregnant and Breastfeeding Women Living With HIV and Their Infants (IATT M&E Option B+ Framework). New York, NY: Centers for Disease Control and Prevention, World Health Organization, and United Nations Children's Fund; 2015. [Google Scholar]
- 43.McNairy ML, Lamb MR, Abrams EJ, et al. Use of a comprehensive HIV care cascade for evaluating HIV program performance: findings from 4 sub-Saharan African countries. J Acquir Immune Defic Syndr. 2015;70:e44–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacCarthy S, Hoffmann M, Ferguson L, et al. The HIV care cascade: models, measures and moving forward. J Int AIDS Soc. 2015;18:19395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kilmarx P, Mutasa-Apollo T. Patching a leaky pipe: the cascade of HIV care. Curr Opin HIV AIDS. 2013;8:59–64. [DOI] [PubMed] [Google Scholar]


