Abstract
Objective
Breastfeeding is linked to lower rates of childhood obesity. Human milk contains cortisol, known to regulate glucose storage and metabolism. We aimed to test the hypothesis that early exposure to cortisol in human breast milk helps to modulate infant BMI trajectories over the first two years of life.
Methods
Growth curve modeling was used to examine whether infant exposure to cortisol in human milk at 3 months predicted changes in child body mass index percentile (BMIP) at 6, 12, and 24 months of age in 51 breastfeeding mother-child pairs.
Results
Infants exposed to higher milk cortisol levels at 3 months were less likely to exhibit BMIP gains over the first 2 years of life, compared to infants exposed to lower milk cortisol. By age 2, infants exposed to higher milk cortisol levels had lower BMIPs then infants exposed to lower milk cortisol. Milk cortisol was a stronger predictor of BMIP change in girls than boys.
Conclusions
Cortisol exposure through human milk may help to program metabolic functioning and childhood obesity risk. Further, because infant formula contains only trace amounts of glucocorticoids, this finding represents a novel biological pathway through which breastfeeding may protect against later obesity.
Keywords: Pediatric Obesity, Glucocorticoids, Breastfeeding, Human Milk, Cortisol
Introduction
Nearly 17% of children in the United States are obese before the age of 9.1 Children with obesity are more likely to experience negative psychological outcomes (e.g. poor body image, depressive symptoms) and are at higher risk for cardiovascular, metabolic, and pulmonary disorders as adults.2 Several meta-analyses have concluded that breastfeeding confers protection against childhood obesity and appears a promising target for prevention.3,4 In a prospective study of over 40,000 Japanese children, for example, infants exclusively breastfed for the first 6 months of life exhibited slower increases in BMI from 1 to 8 years-of-age and had lower BMIs at 8 years-of-age compared to exclusively formula-fed infants.5 Despite the evidence linking breastfeeding to reductions in childhood obesity, the underlying biological mechanisms are not fully understood.3,4
Glucocorticoids in breastmilk may modulate obesity risk. Glucocorticoids play a critical role in maintaining the delicate hormonal equilibrium that controls metabolism in mammals,6 and even slight variations in exposure to glucocorticoids in early development can exert lasting impacts on metabolism throughout the lifespan.7 Prenatal exposure to cortisol, for example, predicts birth weight and weight gain patterns in early life,7 both of which are important determinants of lifetime obesity risk.8 Human milk contains cortisol transferred from plasma.9 Ingested milk glucocorticoids readily cross the intestinal epithelial barrier into neonatal plasma and the brain.10 Further, milk glucocorticoids predict infant growth patterns in rodents and non-human primates.11,12 To test whether early cortisol exposure through human milk modulates child metabolism, we examined the impact of early exposure on children’s Body Mass Index Percentile (BMIP) over the first two years of life.
Methods
Participants included 51 adult breastfeeding mothers and their full-term, singleton infants (25 boys, 26 girls) who were enrolled in a larger longitudinal study investigating early life influences on child development. Eligibility criteria included English fluency and singleton intrauterine pregnancy. The study was approved by the Institutional Review Board at the University of California, Irvine. The current study includes full-term infants with milk cortisol data and child BMIP data from at least two time points (see Table 1 for sample characteristics).
Table 1.
Characteristics of the Sample
| Mean/% | Standard Deviation | Range | |
|---|---|---|---|
| Maternal Characteristics | |||
| Milk Cortisol (μg/dl) | 0.21 | 0.18 | 0.03 – 1.03 |
| Maternal age | 29.91 | 4.94 | 19.15 – 39.90 |
| Education | 2.63 | 1.074 | 0.00 – 4.0 |
| Household Income | $60,000–$70,000 | $30,000 | < $5,000 – >$100,000 |
| Pre-Pregnancy BMI | 25.69 | 6.34 | 18.85 – 47.25 |
| Pregnancy Weight Gain (lbs) | 25.72 | 9.15 | 6.00 – 44.00 |
| Parity | 1.59 | 0.67 | 1 – 4 |
| Breastfeeding Cessation (months) | 10.40 | 6.51 | 3.5–25 |
| % Exclusively Breastfeeding | 65% | ||
| % Married | 73% | ||
| % White | 53% | ||
| % Hispanic | 20% | ||
| % Asian | 13% | ||
| % Multi-Ethnic | 13% | ||
| Infant Characteristics | |||
| % Female Infants | 51% | ||
| Birth Weight (grams) | 3405.45 | 445.23 | 2380 – 4275 |
| Gestational Age at Birth (weeks) | 39.43 | 1.13 | 37.14 – 42.29 |
| BMIP at 3 months | 46% | 25% | 1% – 97% |
| BMIP at 6 months | 53% | 28% | 1% – 97% |
| BMIP at 12 months | 65% | 24% | 1% – 99% |
| BMIP at 24 months | 66% | 25% | 3% – 98% |
Note: Education is coded: 0= less than high school, 1= high school, 2= some college/vocational/AA degree, 3= BA degree, 4= graduate degree
Infant length and weight were measured at birth and at 3, 6, 12, and 24 months of age. Mothers undressed their child and weight was recorded using a digital infant scale (Mid Mark, Versailles, OH). Infant length was measured in the supine position on a pediatric exam table. A standard sliding height rod was used at 2 year. Body mass index (BMI; weight kg/height m2) was standardized using percentiles accounting for age and sex within the LMS model13 for child growth standards provided by the World Health Organization.14 Milk samples were collected at the 3-month visit. Milk cortisol concentrations were determined by chemiluminescent immunoassay (see Supplemental Materials).15 Milk cortisol levels were log transformed to improve distribution normality and z-scored to ease model interpretation.
Random-effects growth curve modeling was used to estimate changes in BMIP, weight, and length for each infant from 3 months to 2 years-of-age (level 1 variable) as a function of milk cortisol exposure at 3 months (level 2 variable). To address potential confounding variables, linear regression tested infant characteristics (sex, birth weight percentile, and gestational age at birth) and maternal characteristics (age, education, income, parity, pre-pregnancy BMI, weight gain in pregnancy, or ethnicity) as predictors of milk cortisol levels (see Table 2). Weight gain during pregnancy inversely predicted milk cortisol (see Table 2), as did time of sample collection (β = −0.27, p = .05), consistent with cortisol’s diurnal rhythm. Both variables were therefore included as covariates in all further analyses.
Table 2.
Associations between Milk Cortisol at 3 Months with Maternal and Infant Characteristics
| Unstandardized βeta | Standard Error | Standardized βeta | P-value | |
|---|---|---|---|---|
| Maternal Age | 0.02 | 0.03 | 0.12 | 0.41 |
| Infant Sex | −0.46 | 0.27 | −0.23 | 0.10 |
| Pre-pregnancy BMI | 0.02 | 0.02 | 0.13 | 0.34 |
| Pregnancy Weight Gain | −0.04 | 0.02 | −0.34 | 0.02 |
| Education Level | −0.12 | 0.14 | −0.13 | 0.42 |
| Household Income | −0.06 | 0.04 | −0.20 | 0.17 |
| Martial Status | 0.07 | 0.09 | 0.11 | 0.47 |
| Parity | 0.24 | 0.21 | 0.16 | 0.26 |
| Gestational Age at Birth | −0.02 | 0.13 | −0.02 | 0.89 |
| Breastfeeding Cessation | −0.01 | 0.02 | −0.04 | 0.82 |
| Exclusively Breastfeeding | −0.12 | 0.31 | −0.06 | 0.70 |
| Birth Weight | 0.01 | 0.01 | 0.07 | 0.61 |
| BMIP at 3 Months | −0.37 | 0.54 | −0.10 | 0.50 |
| BMIP at 6 Months | 0.39 | 0.51 | 0.11 | 0.45 |
| BMIP at 12 Months | −0.36 | 0.59 | −0.09 | 0.53 |
| BMIP at 24 Months | −1.10 | 0.55 | −0.30 | 0.05 |
Note: Linear regression models were run for each factor individually, adjusting for time of milk sample collection. Infant sex was coded as 0 = Male, 1= Female. Marital status was coded as 0 = unmarried, 1 = married.
Results
Consistent with infant growth patterns in the US,1 infant BMIP increased from 3 months to 2 years-of-age (intercept: Coef = 0.509, SE = 0.033, t = 15.51, p < .001; Slope: Coef = 0.009, SE = 0.002, t = 5.05, p < .001; see Table 1). Infants exposed to higher milk cortisol levels at three-months postpartum exhibited significantly less BMIP gains over the first 2 years of life compared to infants exposed to lower milk cortisol levels (Coef = −0.004, SE = 0.002, t = − 2.05, p = 0.046) (see Figure 1). Further, by 24 months, infants exposed to higher levels of milk cortisol had lower BMIPs than infants exposed to lower levels of milk cortisol (Coef = −.09, SE = .04, t = −2.41, p = .020). Milk cortisol was unrelated to infant BMIP at 3 months.
Figure 1. Milk Cortisol Exposure at 3 months and Changes in Body Mass Index Percentile (BMIP) Across the First Two Years of Life for Male and Female Infants.
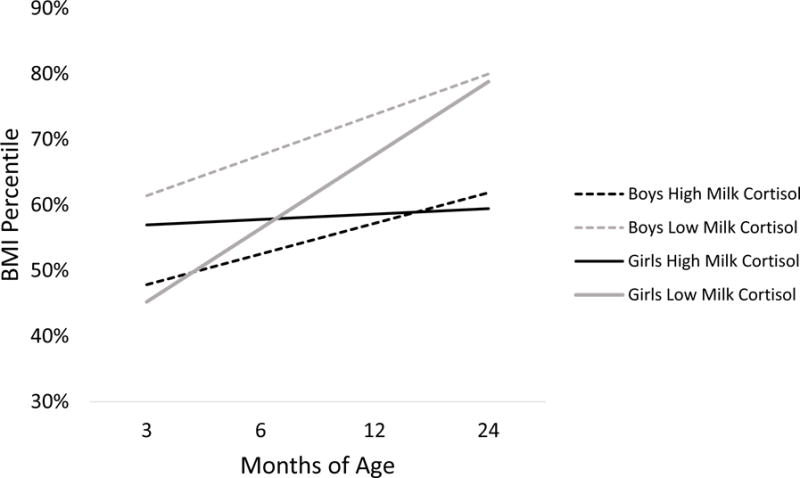
Girls exposed to higher milk cortisol levels at three-months of age exhibited significantly less BMIP gains over the first 2 years of life compared to girls exposed to lower milk cortisol levels. By 24 months of age, infants, regardless of sex, exposed to higher levels of milk cortisol had lower BMIPs than infants exposed to lower levels of milk cortisol. For graphing purposes, high and low cortisol levels were estimated for milk cortisol levels at one standard deviation above and below the mean, respectively. The model estimates are adjusted for maternal weight gain in pregnancy and the time of day of the milk cortisol collection. Infant age was modeled as a continuous variable (in months).
Infant sex moderated the effect of milk cortisol exposure on changes in BMIP trajectories (Coef = − 0.009. SE = 0.004, t = −2.23, p = 0.031; See Figure 1). Girls exposed to higher milk cortisol exhibited more stable BMIPs over the first 2 years of life, whereas girls exposed to lower milk cortisol exhibited clear BMIP gains (Coef = −0.007, SE = 0.003, t = −2.54, p = 0.018). In boys, milk cortisol exposure at 3 months did not significantly predict changes in BMIP from 3–24 months.
Follow-up analyses tested whether milk cortisol predicted changes in infant weight and length. Milk cortisol did not predict weight gain. However, infants exposed to higher levels of milk cortisol grew taller faster (Coef = .053, SE = .015, t = 3.44, p = .001) and were taller by age 2 (Coef = 1.15, SE = .39, t = 2.97, p = .004) compared to infants exposed to lower milk cortisol. The relation between milk cortisol and changes in infant length was not moderated by infant sex and was independent of the association between milk cortisol and infant BMIP. Follow-up tests also revealed no effects or interactions of infant temperament, breastfeeding exclusivity, time of breastfeeding cessation, or birth weight on any of the aforementioned findings (see Supplemental Materials).
Discussion
As hypothesized, we found evidence that cortisol exposure via human milk contributes to early metabolic programming. We found that higher milk cortisol exposure predicted lower absolute BMIP at 2 years-of-age; previous research shows that rapid BMI increases during infancy and higher BMI at age 2 predict adult obesity.2,8 Further, higher milk cortisol exposure was protective against rapid BMIP gains in girls in our sample. Together, these findings suggest that early milk cortisol exposure may provide protection against later obesity.
The relation between early glucocorticoid exposure and infant metabolism is complex, moderated by factors such as timing of exposure, circulating levels, and sex. Both endogenous and exogenous exposure to excessive levels of cortisol during pregnancy have been previously linked to obesity risk factors (i.e. low birth weight and “catch-up” growth).7,16 Crucially, however, maternal cortisol normally increases across gestation, and typical elevations at the end of pregnancy benefit infant lung development,16 exemplifying a positive developmental role for cortisol. Likewise, breastmilk cortisol ingestion facilitates maturation of the infant’s intestines, lengthening microvilli which allows for greater nutrient absorption.17 Absorbing more nutrients before weaning protects against adult obesity in rodent models, particularly under conditions of later overfeeding.18 Speculatively, therefore, milk cortisol may similarly improve obesity outcomes in humans, in part, by facilitating intestinal maturation.
We found that early milk cortisol exposure predicts taller, leaner phenotypes. This result agrees with animal models suggesting that milk cortisol may trigger heightened investment in somatic growth at the expense of energy storage.11,12 Cortisol exposure early in development may also exerted stronger influences on the phenotypic programming of girls than boys in our sample, similarly echoing patterns observed in previous research. For example, milk cortisol exposure has been observed to predict infant temperament in girls, but not boys15 (although animal studies have found non-sex-specific and male-specific effects).11,12
Methodologically, the prospective design and carefully characterized sample utilized in this study allowed us to rule out important potential confounds (e.g., maternal BMI, maternal prenatal weight gain, birth weight, gestational age at birth). However, as this study was limited by a relatively small sample size, caution is warranted in generalizing to more diverse populations. Moreover, correlational studies cannot establish causality. Other factors (e.g., particular feeding practices) might co-vary with milk cortisol levels and infant BMIP. Finally, we did not have information about breastmilk energy content, whereas primate studies suggest a positive correlation between glucocorticoids and caloric concentration in milk.11 If this relationship holds in humans, then milk calories are unlikely to account for our BMIP findings.
The results of this study underscore the need for further investigation of the determinants of milk cortisol in humans. In this study, maternal weight gain in pregnancy predicted milk cortisol, suggesting that milk cortisol levels could represent one pathway through which maternal energetics influence infant growth patterns. Maternal plasma concentrations of glucocorticoids predict about 35% of the variance in milk glucocorticoid levels.11 Therefore, factors known to influence plasma cortisol levels (e.g., psychological stress) warrant investigation. Further research is also needed to assess the relation between cortisol and other bioactive factors in milk linked to childhood adiposity (e.g., adiponectin, EGF and EGF-R).19
Conclusion
These findings suggest that cortisol in human milk is another avenue through which early glucocorticoid exposure helps shape infant metabolism. Further, because infant formula contains only trace amounts of glucocorticoids,20 milk cortisol may represent a novel biological pathway through which breastfeeding protects against obesity.
Supplementary Material
Bullet Point Summaries.
What is already known about this subject?
Breastfeeding is associated with lower rates of obesity in childhood and adulthood.
Exposure to glucocorticoids early in development can modulate metabolism and shape later obesity risk.
What does this study add?
Cortisol exposure via human milk may be another avenue through which early glucocorticoid exposure regulates infant metabolism.
Cortisol in human milk is a novel and plausible biological pathway through which breastfeeding could provide protection against childhood obesity.
Acknowledgments
Funding:
This research was supported by grants from the National Institutes of Health (HD-40967, MH-96889,NS-41298, and Conte Center award MH-96889) and by a grant from the University of California, Irvine (CORCLR to LMG). The breast pumps were donated by Medela.
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniels SR, Arnett DK, Eckel RH, et al. Overweight in children and adolescents pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111(15):1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 3.Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97(12):1019–1026. doi: 10.1136/archdischild-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2005;162(5):397–403. doi: 10.1093/aje/kwi222. [DOI] [PubMed] [Google Scholar]
- 5.Jwa SC, Fujiwara T, Kondo N. Latent protective effects of breastfeeding on late childhood overweight and obesity: a nationwide prospective study. Obesity. 2014;22(6):1527–1537. doi: 10.1002/oby.20735. [DOI] [PubMed] [Google Scholar]
- 6.Rose AJ, Herzig S. Metabolic control through glucocorticoid hormones: an update. Mol Cell Endocrinol. 2013;380(1):65–78. doi: 10.1016/j.mce.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Entringer S. Impact of stress and stress physiology during pregnancy on child metabolic function and obesity risk. Curr Opin Clin Nutr Metab Care. 2013;16(3):320. doi: 10.1097/MCO.0b013e32835e8d80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11(4):496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 9.Hamosh M. Bioactive factors in human milk. Pediatr Clin North Am. 2001;48(1):69–86. doi: 10.1016/s0031-3955(05)70286-8. [DOI] [PubMed] [Google Scholar]
- 10.Angelucci L. A model for later-life effects of perinatal drug exposure: maternal hormone mediation. Neurobehav Toxicol Teratol. 1985 [PubMed] [Google Scholar]
- 11.Hinde K, Skibiel AL, Foster AB, Del Rosso L, Mendoza SP, Capitanio JP. Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behavioral Ecology. 2014:aru186. doi: 10.1093/beheco/aru186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinde K. Lactational programming of infant behavioral phenotype. Springer; 2013. [Google Scholar]
- 13.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11(10):1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 14.De Onis M. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. WHO; 2006. [Google Scholar]
- 15.Grey KR, Davis EP, Sandman CA, Glynn LM. Human milk cortisol is associated with infant temperament. Psychoneuroendocrinology. 2013;38(7):1178–1185. doi: 10.1016/j.psyneuen.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis E, Waffarn F, Uy C, Hobel C, Glynn L, Sandman C. Effect of prenatal glucocorticoid treatment on size at birth among infants born at term gestation. J Perinatol. 2009;29(11):731–737. doi: 10.1038/jp.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheard NF, Walker WA. The role of breast milk in the development of the gastrointestinal tract. Nutr Rev. 1988;46(1):1–8. doi: 10.1111/j.1753-4887.1988.tb05343.x. [DOI] [PubMed] [Google Scholar]
- 18.Mozes S, Sefcikova Z. Functional changes of the small intestine in over-and undernourished suckling rats support the development of obesity risk on a high-energy diet in later life. Physiol Res. 2007;56(2):183. doi: 10.33549/physiolres.930952. [DOI] [PubMed] [Google Scholar]
- 19.Savino F, Liguori SA, Fissore MF, Oggero R. Breast milk hormones and their protective effect on obesity. International journal of pediatric endocrinology. 2009;2009(1):1. doi: 10.1155/2009/327505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cigliana G, Patacchioli F, Casolini P, Angelucci L. Glucocorticoid hormone in human, cow and infant formula milks infant formula milks. Pharmacol Res. 1989;21(5):655–656. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


