Abstract
Purpose
To compare clinical parameters and the tear levels of inflammatory cytokines between pterygium surgery using sutures or fibrin glue.
Methods
Fifty-six patients with primary pterygium were divided into the suture group and the glue group, in which the autograft was secured with 10–0 Vicryl sutures and fibrin glue, respectively. A questionnaire, slit-lamp examination, Schirmer test, and visual acuity test were performed in all participants. Real-time quantitative PCR (q-PCR) was used to analyze the expression of genes in pterygium and healthy conjunctival tissues. Based on the qPCR results and literature reports, five inflammatory cytokines, including hepatocyte growth factor (HGF), fibroblast growth factor 2 (FGF2), transforming growth factor-β1 (TGF-β1), matrix metalloproteinase 2 (MMP2), and tumor necrosis factor-α (TNF-α), were selected, and their protein levels were measured with enzyme-linked immunosorbent assay (ELISA) in patient tears before surgery as well as at postoperative day 1, 7, and 30.
Results
There are 28 patients in either the suture or the glue group. The average duration of surgery was 20.17 ± 3.23 min for the glue group and 32.42 ± 4.47 min for the suture group (p = 0.000). Visual acuity in both groups was improved (p = 0.002) after the surgical procedures. There were more symptoms in the suture group than in the glue group at postoperative day 7 (p = 0.002). Postoperative symptoms disappeared in both groups at 1 month after surgery. Recurrence was observed in one case in the glue group and in two cases in the suture group at the 6 month postoperative follow-up (p = 0.714). In comparison to the preoperative levels (4.33 ± 0.43 ng/ml for the suture group; 4.20 ± 0.26 ng/ml for the glue group), the levels of TNF-α in tears increased in the suture group (5.02 ± 0.49 ng/ml, p = 0.016) and decreased in the glue group (3.84 ± 0.35 ng/ml, p = 0.052) on postoperative day 1. The glue treatment induced higher HGF production (4.78 ± 1.25 ng/ml) than the suture treatment (3.04 ± 1.18 ng/ml) at postoperative day 1 (p = 0.020). Higher levels of TGF-β1 in the glue group were detected at postoperative day 1 (3.71 ± 0.18 ng/ml) and postoperative day 30 (4.50 ± 0.51 ng/ml), compared to those in the suture group, respectively (2.74 ± 0.21 ng/ml, p = 0.000 for day 1; 3.36 ± 0.96 ng/ml, p = 0.017 for postoperative day 30).
Conclusions
Fibrin glue is effective and safe for attaching conjunctival autografts with an easy surgical procedure, shortened operating time, and less postoperative discomfort. In the early postoperative period, the protein expression of inflammatory cytokines implicates that fibrin glue may induce accelerated healing and subdued inflammation on the ocular surface compared to sutures.
Introduction
Pterygium is a common and degenerative ocular surface disorder characterized by the invasion of fibrovascular tissue from the bulbar conjunctiva onto the cornea. Pterygium causes chronic ocular irritation, tear film disturbance, induced astigmatism, and deteriorated vision secondary to growth over the visual axis [1]. The main treatment for pterygium is surgical excision. Conjunctival autografting has been shown to be the best surgical technique with low rates of recurrence and complication, and is usually reserved for patients with recurrence or at an advanced stage of disease to preserve the remaining healthy conjunctiva [2]. Therefore, conjunctival autografting is emerging as a superior procedure compared to other pterygium surgeries, such as amniotic membrane transplantation [2]. The conjunctival autograft is a critical part in surgical management of pterygium. The graft can be secured with either sutures or fibrin glue. Clinical studies have compared the outcomes of using sutures with those in which fibrin glue is used during surgery [3]. The evaluation parameters include the duration of the surgery, postoperative discomfort, and recurrence rate.
However, to the best of our knowledge, no human studies have compared inflammatory responses on the ocular surface following conjunctival autografting in pterygium surgery entailing Vicryl sutures or fibrin glue. Therefore, in this study we determined the inflammatory factors in tears, aiming to investigate the molecular mechanisms underlying the clinical parameters measured after conjunctival autografting pterygium surgery using Vicryl sutures or fibrin glue. In addition, recent studies have reported that stem cell–related factors, such as vascular endothelial growth factor (VEGF), are expressed in the pterygial epithelium [4] and play important roles in inflammation and angiogenesis. Therefore, in addition to the inflammatory cytokines, we compared changes in the expression of stem cell–related factors in tears that might affect the healing process following pterygium excision.
Methods
Subjects
This prospective study recruited 56 primary pterygium patients admitted to Tianjin Medical University Eye Hospital (Tianjin, China) between February 2014 and March 2015. In the study, pterygium size and morphology were assessed by the same surgeon. Pterygium size was measured as the longest diameter from the limbus to the head of the pterygium. The inclusion criterion was primary pterygium confined between the corneal limbus and the papillary margin. The minimum size of the pterygium was 1.50 mm and the maximum 3.20 mm. Patients with a history of ocular trauma, ocular surgery, dry eye and keratitis, recurrent pterygium, and symblepharon or history suggestive of any hypersensitivity to human blood products were excluded. Written informed consent was obtained from the participants. Comprehensive information about the participants was collected, including age, sex, and medical and ocular history. Conventional ocular examinations, such as visual acuity, slit-lamp examination, and anterior segment photography, were performed. The patients were then randomly divided into two groups, in which pterygium excision and conjunctival autografting were performed by the same surgeon following standard procedures. In the suture group (n = 28), the autograft was secured with 10–0 Vicryl sutures; whereas in the glue group (n = 28), the autograft was secured with fibrin glue. All the procedures and the informed consent form in this study were approved by the institutional review board at Tianjin Medical University Eye Hospital, Tianjin, China. All performed procedures adhered to the tenets of the Declaration of Helsinki and the ARVO statement on human subject.
Surgical procedures
The eye to be operated on was prepared according to standard sterile ophthalmic procedures, and 0.5% proparacaine hydrochloride drops (Alcon, Mississauga, Canada) were topically applied. The eye was exposed using a lid speculum. The surgery was performed under an operating microscope. The area to be excised was marked, and 1% lidocaine hydrochloride with 1:100,000 epinephrine (AstraZeneca, Mississauga, Canada) was injected subconjunctivally beneath the pterygium body to prevent excessive bleeding. The pterygium head was excised from the cornea with a sharp blade, and the pterygium was separated from the underlying sclera and the surrounding conjunctiva with blunt and sharp dissections. The pterygium body along with the underlying tendons were excised with scissors. The wound bed was scraped to clean the cornea and the sclera, and bleeding vessels were cauterized using a cauterization device. The area of the conjunctival defect was measured with a caliper. A conjunctival autograft of the same size was carefully obtained from the superior bulbar conjunctiva without generating buttonholes. The conjunctival graft was placed after the pterygium excision to cover the bare sclera, with the epithelium side facing up and the limbal edge toward the limbus.
In the suture group, the conjunctival graft was secured with eight interrupted 10/0 nylon sutures; in the glue group, the autograft was attached to the sclera with fibrin glue (Fibrin Sealant (Human), Shanghai, China) as described by Kornayi and associates [5]. The duration of the surgery was recorded from the marking of the pterygium to the removal of the lid speculum.
Tobradex (tobramycin and dexamethasone ophthalmic suspension) ointment (S.A. Alcon-Couvreur N.V., Puurs, Belgium) was applied after surgery in both groups; the eye was closed with gauze. All patients were treated with fluorometholone eye drops (Santen Inc., Osaka, Japan) 4 times daily for 1 week, and then the eye drop dosage was tapered over the next 3 weeks. In addition, levofloxacin (Santen Inc) and pranoprofen (Senju Pharmaceutical Ltd., Osaka, Japan) were used for 1 month. The sutures were removed at 7–10 days following the surgery.
Sample collection for gene expression analyses
The 56 pterygium samples were collected with informed consent from both groups of patients. The conjunctiva samples were harvested with informed consent from the upper bulbar conjunctiva of seven patients with cataracts who did not have pterygium and pinguecula and had undergone extracapsular cataract extraction surgery. The age and sex ratio of these seven patients did not differ significantly from those of the patients with pterygium. The conjunctival samples were 2.5 × 2.5 mm2. All the collected samples were stored at −80 °C.
Evaluation of the ocular surface
All patients were examined by the same ophthalmologist before and on postoperative day 1, 7, 30, and 180. During each follow-up examination, the patients’ discomfort was assessed with a questionnaire; the autograft and the ocular surface were examined under a slit-lamp. Schirmer and visual acuity tests were performed. At the final follow-up at 6 months after surgery, the incidence of recurrence was recorded.
Tear sample collection
Before surgery and on postoperative day 1, 7, and 30, 20 μl basal tear samples were collected with 2-μl microcapillaries (Drummond Scientific Co, Broomall, PA) from the inferior meniscus without anesthesia. The collected tears were immediately transferred to a 0.6-ml microcentrifuge tube and stored at −80 °C until further analysis.
RNA extraction and real-time quantitative PCR
RNA was extracted, and real-time quantitative PCR (qPCR) was performed as previously described [6-8]. Briefly, total RNA was extracted from the pterygium and conjunctival samples collected during the surgeries using TRIzol reagent (Life Technologies, Carlsbad, CA), and 1 μg of the total RNA was reverse transcribed using SuperScript II Reverse Transcriptase (Life Technologies) and oligo d(T)18 primer. The gene-specific primers were selected according to the literature to analyze the expression of 17 inflammatory cytokine genes, including IL-2, IL-4, IL-10, VEGF, IL-1β, TGF-β1, TNF-α, IL-6, MMP2, FGF2, HGF, ICAM-1, IL1-α, MMP9, IL-8, IL-37, and IL-38; the GAPDH gene served as the internal standard (Table 1). qPCR was performed in a final volume of 8 μl containing 2 μl cDNA template, 2 μl target gene-specific primers, and 4 μl SYBR Green 2X Master Mix (Roche, Branford, CT) in a HT7900 Real-Time PCR System (Applied Biosystem, Foster City, CA). The standard curves served as positive controls. All primers for the 17 inflammatory cytokines demonstrated similar priming efficiencies to those of the internal standard GAPDH gene. The reactions using water as templates served as negative controls. The program was composed of 2 min preincubation at 50 °C, 10 min denaturation at 95 °C, followed by 40 cycles of 15 s denaturation at 95 °C and 1 min annealing and extension at 60 °C. A dissociation stage was added to check the amplicon specificity. The relative expression levels of the inflammatory cytokines were analyzed using a comparative threshold cycle (2−∆∆Ct) method. The experiment was repeated three times.
Table 1. The primers used for gene expression analyses in this study.
| Gene symbol | Gene ID | OMIM | Forward primers | Reverse primers |
|---|---|---|---|---|
| IL-2 |
3558 |
147680 |
GCCCAAGAAGGCCACAGAA |
GCACTTCCTCCAGAGGTTTGAG |
| IL-4 |
3565 |
147780 |
TGGGTCTCACCTCCCAACTG |
GCCGGCACATGCTAGCA |
| IL-10 |
3586 |
124092 |
GAGGCTACGGCGCTGTCA |
TCCACGGCCTTGCTCTTG |
| VEGF |
7422 |
192240 |
CCCACTGAGGAGTCCAACATC |
CTTGCCCCACTTCCCAAA |
| IL-1β |
3553 |
147720 |
ACGATGCACCTGTACGATCACT |
CACCAAGCTTTTTTGCTGTGAGT |
| TGF-β1 |
7040 |
190180 |
GCAGGCACTGGAGGATATTCA |
GCTTTGCCTGCCTTGATGTT |
| TNF-α |
7124 |
191160 |
TGCTCCTCACCCACACCAT |
GGAGGTTGACCTTGGTCTGGTA |
| IL-6 |
3569 |
147620 |
GCTGCAGGCACAGAACCA |
GCTGCGCAGAATGAGATGAG |
| GAPDH |
2597 |
138400 |
ATGGAAATCCCATCACCATCTT |
CGCCCCACTTGATTTTGG |
| MMP2 |
4313 |
120360 |
CGTCTGTCCCAGGATGACATC |
ATGTCAGGAGAGGCCCCATA |
| FGF2 |
2247 |
134920 |
TGGTATGTGGCACTGAAACGA |
GCCCAGGTCCTGTTTTGGAT |
| HGF |
3082 |
142409 |
TCCACGGAAGAGGAGATGAGA |
GGCCATATACCAGCTGGGAAA |
| ICAM-1 |
3383 |
147840 |
GCTCCTGCCTGGGAACAA |
TGGCTATCTTCTTGCACATTGC |
| IL1-α |
3552 |
147760 |
TTGCCCATCCAAACTTGTTTATT |
CCCCCCTGCCAAGCA |
| MMP9 |
4318 |
120361 |
CGCCAGTCCACCCTTGTG |
CAGCTGCCTGTCGGTGAGA |
| IL-8 |
3576 |
146930 |
CTGGCCGTGGCTCTCTTG |
CTTGGCAAAACTGCACCTTCA |
| IL-37 |
27178 |
605510 |
CACCCCGGATGGTTCATC |
GTCACCCCAACAGGCTCATT |
| IL-38 | 84639 | 615296 | CCCTACAGCTGGAGGATGTGA | GAAGCGTGTGGCCTCTTCA |
Measurement of inflammatory cytokine levels in tears
Based on the qPCR results and literature reports, five inflammatory factors, including HGF, FGF2, TGF-β1, MMP2 and TNF-α, were selected for further analysis of protein levels in tear samples as previously described [9]. The samples were diluted tenfold with assay diluent. The protein concentrations of the five inflammatory cytokines were determined with enzyme-linked immunosorbent assay (ELISA) kits purchased from R&D Systems (Minneapolis, MN; HGF: Cat# DHG00; FGF2: Cat# DFB50; TGF-β1: Cat# DB11B; MMP2: Cat# MMP200; TNF-α: Cat# DTA00C) according to the manufacturer’s protocols. Briefly, a monoclonal antibody specific to the target protein was precoated on a 96-well microplate. After the target protein in the samples was bound to the precoated antibody on the plate, an enzyme-linked polyclonal (for detecting HGF, TGF-β1, MMP2, and TNF-α) or monoclonal (for detecting FGF2) antibody was added to sandwich the immobilized target protein. Then the colorimetric substrates were used for measurement and quantification. The standard curves served as the positive controls for the ELISAs, and the diluent was included as the negative control for each assay. The optical density was measured at 450 nm with correction at 540 nm with an Infinite 200 PRO Multimode Microplate Reader (Tecan Group Ltd., Männedorf, Switzerland). The final concentrations of the target proteins were calculated based on the standard curves and adjusted by the dilution factor. The measurement of the protein levels of each inflammatory cytokine in tears was repeated three times.
Statistical analysis
Statistical analyses were performed using Statistical Program for Social Sciences 19.0 (IBM SPSS Inc., New York, NY). All data were expressed as the mean ± standard error of the mean (SEM) and examined with the D’Agostino-Pearson omnibus normality test. The data with a Gaussian distribution were examined with the Levene test to confirm homogeneity of variance. The qPCR data were analyzed with the two-tailed Student t test; the protein levels of the inflammatory cytokines in the tears were analyzed with two-way ANOVA followed by Tukey’s post hoc test. The data with nonparametric distribution were analyzed with the Wilcoxon rank sum test. P values of less than 0.05 were considered statistically significant.
Results
Patient demographics
The suture group and the glue group each contains 28 patients. There were 15 men (53.57%) and 13 women (46.43%) in the suture group. The average age of the patients in this group was 58.32 ± 9.19 years. The average growth period of pterygium was 12.33 ± 12.60 years. The average size of the pterygium was 2.21 ± 0.58 mm (Table 2). In the glue group, there were 16 men (57.14%) and 12 women (42.86%). The average age of the patients was 60.67 ± 9.49 years. The average growth period of the pterygium was 11.78 ± 14.32 years. The average size of the pterygium was 2.37 ± 0.68 mm (Table 2). The patients’ age (p = 0.351), sex ratio (p = 0.793), and growth period of pterygium (p = 0.901) and pterygium size (p = 0.348) did not differ statistically significantly between the two groups.
Table 2. Age, gender of the patients, and pterygium growth time in the study groups.
| Demographics | Suture (n=28) | Glue (n=28) | P | |
|---|---|---|---|---|
| Age (y) |
58.32±9.19 |
60.67±9.49 |
0.351 |
|
| Gender (M/F) |
15 / 13 |
16 / 12 |
0.793 |
|
| Pterygium growth time (m) |
12.33±12.60 |
11.78±14.32 |
0.901 |
|
| Pterygium size (mm) | 2.21±0.58 | 2.37±0.68 | 0.348 | |
Note: The patients’ age, pterygium growth time and size were expressed as Mean ± SD.
Comparison of ocular discomfort before and after surgery
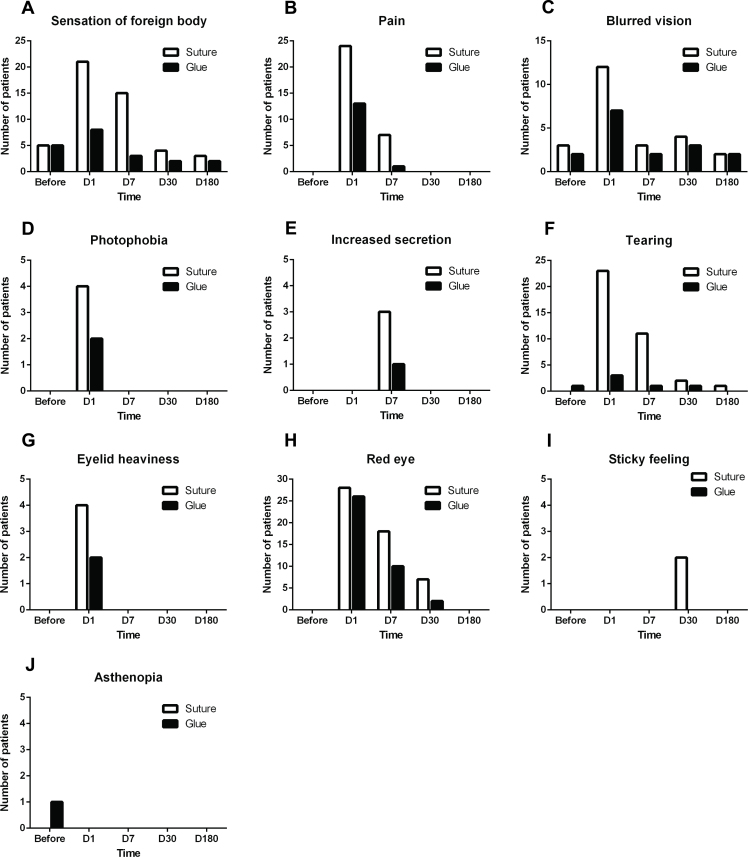
Ocular discomfort before surgery was similar between the two groups; however, discomfort was present in all patients at all time points after surgery (Figure 1). On postoperative day 1, the sensation of a foreign body was reported by 21 (75.00%) patients, and pain was present in 24 (85.71%) patients in the suture group; whereas in the glue group, only eight (28.57%) patients reported the sensation of a foreign body, and 13 patients (46.43%) felt pain (the suture group vs the glue group, p = 0.000 for sensation of a foreign body; p = 0.002 for pain (Figure 1A,B). In addition, at this time point, blurred vision was reported by seven (25.00%) patients in the glue group and 12 (42.86%) patients in the suture group (p = 0.164 the suture group vs the glue group; Figure 1C). On postoperative day 7, the sensation of a foreign body was still a major complaint for 15 (53.57%) patients in the suture group, whereas only three (10.71%) patients in the glue group (p = 0.002, the suture group versus the glue group, Figure 1A). As for the number of symptoms, on postoperative day 1, six and seven symptoms were observed in the suture and glue groups, respectively (p = 0.106). On postoperative day 7, more symptoms were observed in the suture group than in the glue group (p = 0.002). On postoperative day 30, the number of symptoms decreased to (p = 0.187 for the suture group; p = 1.000 for the glue group; Figure 1).
Figure 1.
The discomfort reported by the patients in the glue and suture groups before and after conjunctival autografting pterygium surgery. The number of patients in both groups with (A) foreign body sensation, (B) pain, (C) blurred vision, (D) photophobia, (E) increased secretion, (F) tearing, (G) eyelid heaviness, (H) red eye, (I) sticky feeling, and (J) asthenopia is shown.
The average duration of surgery was 32.42 ± 4.47 min for the suture group and 20.17 ± 3.23 min for the glue group (Table 3). This difference was statistically significant (p = 0.000). The use of fibrin glue statistically significantly reduced the duration of the surgery as reported in the literature [3]. Visual acuity in both groups after surgery was statistically significantly improved compared to that before surgery (after surgery versus before surgery, p = 0.002 for the suture group; p = 0.003 for the glue group, Table 3). The results of the Schirmer test, an indicator of tear secretion, did not change statistically significantly within either group (after surgery versus before surgery, p = 0.794 for the suture group; p = 0.727 for the glue group, Table 3) before and after surgery. Moreover, recurrence of the pterygium was recorded in these patients. One case of recurrence was noted in the glue group, whereas two were found in the suture group at the end of the 6-month postoperative follow-up period (p = 0.714, the suture group versus the glue group, Table 3). Dellen of the graft was noted in one case in the glue group but in two cases in the suture group (p = 0.714, the suture group versus the glue group, Table 3). There was no graft loss in either group.
Table 3. The clinical parameters of suture and glue groups during follow-up.
| Clinical parameters | Suture (n=28) | Glue (n=28) | P |
|---|---|---|---|
| Surgery time (min) |
32.42±4.47 |
20.17±3.23 |
0.000 |
| Visual acuity |
|
|
|
| Before surgery |
0.66±0.28 |
0.57±0.25 |
0.210 |
| D 30 post surgery |
0. 89±0.26 |
0.78±0.26 |
0.133 |
| Schirmer test (mm) |
|
|
|
| Before surgery |
13.75±2.50 |
13.77±1.93 |
0.973 |
| D 30 post surgery |
13.92±2.34 |
13.95±1.91 |
0.958 |
| Recurrence |
2 (7.14%) |
1 (3.6%) |
0.714 |
| Dellen | 2 (7.14%) | 1 (3.6%) | 0.714 |
Note: The surgical time, patients’ vision, and the results of Schirmer test were expressed as Mean ± SD.
Expression of inflammatory cytokine genes in conjunctival and pterygium tissues
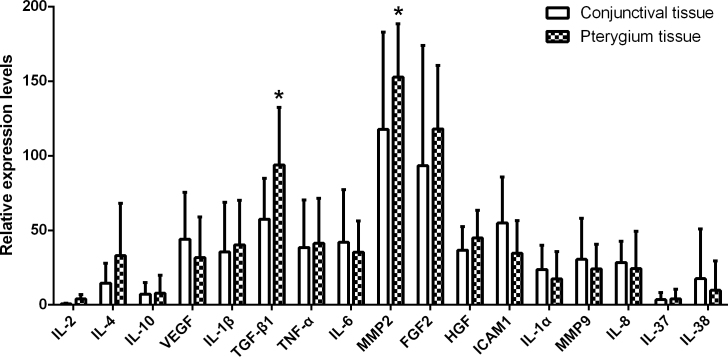
The expression of 17 inflammatory cytokine genes, including IL-2, IL-4, IL-10, VEGF, IL-1β, TGF-β1, TNF-α, IL-6, MMP2, FGF2, HGF, ICAM-1, IL1-α, MMP9, IL-8, IL-37, and IL-38, was examined with qPCR in the pterygium (n = 56) and healthy conjunctival (n = 7) tissues collected during surgery. The results showed that the mRNA levels of TGF-β1 and MMP2 were statistically significantly increased in the pterygia compared to those in the healthy conjunctiva (p = 0.005 for TGF-β1; p = 0.037 for MMP2; Figure 2), suggesting the important roles of these two inflammatory cytokines in pterygium pathology. However, no statistically significant difference in the transcript levels of other inflammatory cytokines was found between the pterygium and conjunctival tissues (all p > 0.05; Figure 2). Therefore, TGF-β1 and MMP2 were selected for further analyses of protein levels in the patients’ tears. In addition, TNF-α, HGF, and FGF2, although their gene expression was not significantly upregulated in the pterygia, have been reported to be closely associated with the initiation and progression of this ocular surface disorder. Thus, these three inflammatory cytokines were included in the following lacrimal protein analyses.
Figure 2.
The expression of inflammatory cytokines in pterygium and healthy conjunctival tissues. The expression of 17 inflammatory cytokine genes, including IL-2, IL-4, IL-10, VEGF, IL-1β, TGF-β1, TNF-α, IL-6, MMP2, FGF2, HGF, ICAM-1, IL1-α, MMP9, IL-8, IL-37, and IL-38, was examined with real-time quantitative PCR (q-PCR) in the pterygia (n = 56) and healthy conjunctiva (n = 7). The experiment was repeated three times. The data were presented as the mean ± standard error of the mean (SEM). p < 0.05, conjunctival tissue vs pterygium tissue.
Protein levels of the selected inflammatory cytokines in tear fluid
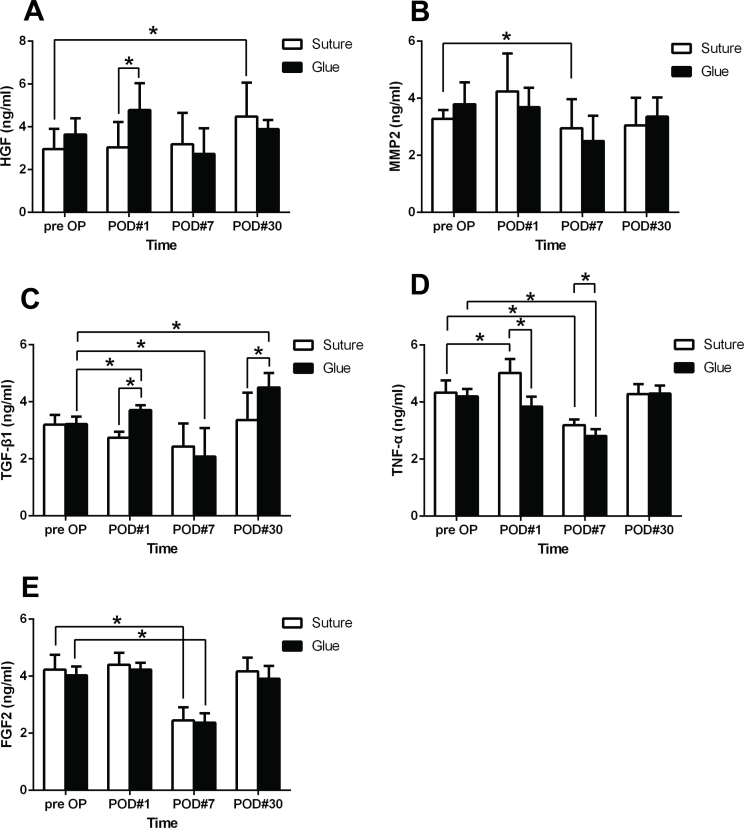
In this study, the protein levels of five inflammatory cytokines, including HGF, MMP2, TGF-β1, TNF-α, and FGF2, were measured in the tears of the patients in both groups (n = 28 for each group). No statistical differences were found between the glue and suture groups in the protein levels of inflammatory cytokines before surgery (all p>0.05, the suture group versus the glue group, Figure 3).
Figure 3.
The protein levels of five inflammatory factors in patient tears were measured before and after the conjunctival autografting pterygium surgery. The protein levels of (A) HGF, (B) MMP2, (C) TGF-β1, (D) TNF-α, and (E) FGF2 were measured with enzyme-linked immunosorbent assay (ELISA) in the tears of patients in the suture group (n = 28) and the glue group (n = 28) before surgery and at postoperative day 1, 7, and 30. Each experiment was repeated three times. The data were presented as the mean ± standard error of the mean (SEM). * p<0.05.
On postoperative day 1, the lacrimal HGF level was similar to the preoperative level in the suture group (p = 0.635, postoperative day 1 versus before surgery, Figure 3A); whereas the level was statistically significantly higher than the preoperative level in the glue group (p = 0.020, postoperative day 1 versus before surgery, Figure 3A). On postoperative day 30, the lacrimal HGF level was statistically significantly elevated compared to the other time points in the suture group (p = 0.000 for postoperative day 30 vs preoperative; p = 0.001 for postoperative day 30 vs postoperative day 1; p = 0.003 for postoperative day 30 vs postoperative day 7, Figure 3A), and the lacrimal HGF level was higher than that in the glue group (p = 0.070, the suture group vs the glue group; Figure 3A).
Compared to the preoperative level, the protein level of MMP2 in the suture group statistically significantly increased at postoperative day 1 (p = 0.015) and decreased at postoperative day 7 (p = 0.009, Figure 3B). The levels of MMP2 in the glue group exhibited similar dynamics but without statistical significance (p = 0.630 postoperative day 1 vs preoperative; p = 0.078 postoperative day 7 vs preoperative). The MMP2 levels in both groups returned to the preoperative levels at postoperative day 30 (p = 0.265 for the suture group; p = 0.464 for the glue group, Figure 3B).
At postoperative day 1, the lacrimal TGF-β1 levels decreased in the suture group (p = 0.026) but increased in the glue group (p = 0.001, Figure 3C), compared to the respective preoperative levels. A statistically significant difference was found between these two groups (p = 0.000) at this time point. At postoperative day 7, the lacrimal TGF-β1 levels decreased in the suture group (p>0.05) and in the glue group (p = 0.023). The lacrimal TGF-β1 levels in these two groups were still higher at postoperative day 30 than the preoperative levels (p = 0.139 for the suture group; p = 0.000 for the glue group). The lacrimal TGF-β1 level in the glue group was statistically significantly higher than that in the suture group at postoperative day 30 (p = 0.017, Figure 3C).
In comparison to the preoperative level, the lacrimal TNF-α level was increased in the suture group (p = 0.016) but decreased in the glue group (p = 0.005) at postoperative day 1 (Figure 3D). The difference between the two groups was statistically significant (p = 0.000) at this time point. The lacrimal TNF-α levels were statistically significantly decreased in both groups at postoperative day 7 (p = 0.006 for the suture group; p = 0.001 for the glue group) compared to the preoperative level and recovered at 1 month after surgery (p = 0.671 for the suture group; p = 0.384 for the glue group; Figure 3D).
The glue and suture procedures resulted in a slight increase in the lacrimal FGF2 levels at postoperative day 1 and then in an obvious decrease at postoperative day 7 (both p = 0.000, Figure 3E). At 1 month after surgery, the lacrimal FGF2 levels in both groups returned to the preoperative levels (p = 0.641 for the suture group; p = 0.485 for the glue group). There was no significant difference in the lacrimal FGF2 levels between the two groups at all the time points examined (all p > 0.05; Figure 3E).
Discussion
Pterygium is a common ophthalmic disorder that is easy to diagnose but difficult to tackle. Multiple surgical techniques and modifications have been developed to manage this disorder to prevent recurrence [10]. Conjunctival autografting is widely used in the management of pterygium. Pterygium excision with conjunctival autografting significantly reduces recurrence rates and elicits fewer complications, and therefore, has become the surgical procedure of choice [11].
In the current study, the use of fibrin glue markedly shortened the duration of the surgery (Table 3). Moreover, although foreign body sensation was present in most patients on postoperative day 1, the patients in the glue group were more comfortable than those in the suture group on the subsequent days (Figure 1). However, there was no statistically significant difference in the recurrence rates between the two groups at the end of the 6-month postoperative follow-up period (p = 0.714, Table 3). This result is consistent with those in other studies that compared sutures versus fibrin glue for conjunctival autografting [12,13]. Therefore, the benefits of fibrin glue demonstrated in this study include shorter duration of surgery (Table 1), lower surgical skill, and less postoperative discomfort (Figure 1).
Previous reports have shown that pterygium and its surgical treatment can elicit inflammatory responses [14-16], which might be involved in the postoperative wound healing process. This study, for the first time, explored the expression of inflammatory factor proteins in tears in conjunctival autografting pterygium surgery using Vicryl sutures or fibrin glue.
HGF has been shown to regulate epithelial and stromal wound healing in the cornea [17] and mediate the disruption of cell–cell junctions, including desmosomes, hemidesmosomes [18], and gap junctions [19], in the corneal epithelium. In addition, HGF stimulates the migration of corneal epithelial cells in cultures [20]. These findings suggest that HGF is capable of enhancing reepithelialization after corneal damage. The present results showed that glue treatment induced higher HGF production than suture treatment at postoperative day 1 (Figure 3A), implicating a greater potential to recover in the conjunctival epithelia of the patients treated with fibrin glue.
Recently, several studies have suggested the possible role of proteolytic metalloproteinase (MMP) in pathogenesis of pterygium [21-23]. Under a tissue degenerative condition, pterygium cells produce MMPs and display tumor-like features, such as proliferation, invasion, and angiogenesis [24]. However, a previous study suggested that the upregulated expression of MMPs may promote the migration and growth of pterygium, but the upregulation of MMP is not likely a pathogenic factor of this ocular surface disorder [25]. Moreover, the abundant expression of MMPs in pterygia might be responsible for the extensive collagen remodeling observed in pterygium, as few MMPs were detected in the healthy conjunctiva [26].
TGF-β1 is a multifunctional protein, regulating migration, proliferation, differentiation, and apoptosis of several cell types, including corneal epithelial cells, stromal cells, and endothelial cells [27]. TGF-β1 also regulates the extracellular matrix deposition and expression of integrin family adhesion molecules [27]. In addition, TGF-β1 has been reported to promote cutaneous wound healing processes [28,29]. TGF-β1 is detected in tear [30] and corneal tissue [31], and TGF-β1 receptors are expressed in corneal cells [32]. The present experiments showed higher levels of TGF-β1 in the glue group than in the suture group at postoperative day 1 and postoperative day 30 (Figure 3C), indicating that there might be a more amenable microenvironment for healing in the corneas treated with glue than in those treated with sutures.
As a proinflammatory cytokine, TNF-α is expressed by corneal epithelia and inflammatory cells, and is considered to participate in corneal wound healing [33]. It has been reported that TNF-α levels are elevated in the tears of patients with dry eye syndrome [34]. Moreover, Vesaluoma et al. [35] detected a transient and slight elevation in the TNF-α level in the cornea after photorefractive keratectomy. The present study showed that the TNF-α level was increased in the suture group but decreased in the glue group on postoperative day 1 (Figure 3D), indicating a milder inflammatory response in the glue group at this time point.
Few studies have investigated the effects of FGF2 on corneal wound healing except one that reported that FGF2 accelerates the rate of reepithelialization in vivo in a dose-dependent manner [36]. In the present study, the FGF2 levels did not alter statistically significantly in either group during the time course except a dramatic reduction in both groups at postoperative day 7 (Figure 3E). This result suggests that the ocular surface changes caused by surgery using either glue or sutures may not be great enough to induce statistically significant upregulation in FGF2 expression.
One of the limitations of this study is that we did not identify a battery of inflammatory factors unambiguously responsible for the differences in the clinical parameters following the two surgical procedures, although the expression trends of HGF, TGF-β1, and TNF-α at postoperative day 1 did suggest their contributions (Figure 3A,C,D). This result could be due to the topical application of corticosteroid-containing agents during the 1st week following surgery. Applications of corticosteroids may dampen inflammatory responses on the ocular surface [37,38], as shown by the universal trend of reduced expression of these inflammatory factors at postoperative day 7 (Figure 3), and might also blunt the dramatic differences in the expression of the inflammatory factors between the two treatment groups. Therefore, it would be interesting in the future to perform conjunctival autografting surgery using sutures or fibrin glue in rabbit eyes, which are mammalian eyes that are human size. Then changes in the ocular surface and lacrimal levels of inflammatory factors could be closely monitored without applications of anti-inflammatory agents; thus, the molecular mechanisms underlying the inflammatory response and the healing process following fibrin glue– or Vicryl suture–mediated conjunctival autografting could be investigated without the interference of corticosteroids. The results of the mechanistic study would facilitate the development of a supplementary approach rendering the ocular surface microenvironment more amenable to wound healing and recovery of visual function.
In summary, this prospective study demonstrated that fibrin glue is effective and safe for attaching a conjunctival autograft during pterygium surgery. Moreover, the operation using glue requires an easier surgical procedure and shorter duration of surgery, and incurs less postoperative discomfort than that using sutures. In the early postoperative period, particularly at postoperative day 1, the higher levels of HGF and TGF-β1, as well as lower levels of TNF-α, in the tears of the patients treated with glue suggest that there may be an accelerated healing process and a subdued inflammatory response on the ocular surface of these patients compared to that of the patients treated with sutures.
Acknowledgments
This research was supported by the grants from National Natural Science Foundation of China (81100646, 81570872, and 31100991), and from Tianjin Municipal Science and Technology Commission (15JCYBJC24900). Dr. Ruihua Wei (braveheart0717@sina.com) and Dr. Lijie Dong (aitaomubang@126.com) are co-corresponding authors for this paper.
References
- 1.Hirst LW. The treatment of pterygium. Surv Ophthalmol. 2003;48:145–80. doi: 10.1016/s0039-6257(02)00463-0. [DOI] [PubMed] [Google Scholar]
- 2.Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997;104:974–85. doi: 10.1016/s0161-6420(97)30197-3. [DOI] [PubMed] [Google Scholar]
- 3.Vichare N, Choudhary T, Arora P. A comparison between fibrin sealant and sutures for attaching conjunctival autograft after pterygium excision. Med J Armed Forces India. 2013;69:151–5. doi: 10.1016/j.mjafi.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcovich AL, Morad Y, Sandbank J, Huszar M, Rosner M, Pollack A, Herbert M, Bar-Dayan Y. Angiogenesis in pterygium: morphometric and immunohistochemical study. Curr Eye Res. 2002;25:17–22. doi: 10.1076/ceyr.25.1.17.9959. [DOI] [PubMed] [Google Scholar]
- 5.Koranyi G, Seregard S, Kopp ED. The cut-and-paste method for primary pterygium surgery: long-term follow-up. Acta Ophthalmol Scand. 2005;83:298–301. doi: 10.1111/j.1600-0420.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 6.Tian F, Dong L, Zhou Y, Shao Y, Li W, Zhang H, Wang F. Rapamycin-Induced apoptosis in HGF-stimulated lens epithelial cells by AKT/mTOR, ERK and JAK2/STAT3 pathways. Int J Mol Sci. 2014;15:13833–48. doi: 10.3390/ijms150813833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Dong L, Liu X, Jiang Y, Zhang L, Zhang X, Li X, Zhang Y. alpha-Melanocyte-stimulating hormone protects retinal vascular endothelial cells from oxidative stress and apoptosis in a rat model of diabetes. PLoS One. 2014;9:e93433. doi: 10.1371/journal.pone.0093433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Bo Q, Wu W, Xu C, Yu G, Ma S, Yang Q, Cao Y, Han Q, Ru Y, Liu X, Hua WR, Wang FE, Zhang X, Li X. alpha-Melanocyte-stimulating hormone prevents glutamate excitotoxicity in developing chicken retina via MC4R-mediated down-regulation of microRNA-194. Sci Rep. 2015;5:15812. doi: 10.1038/srep15812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong L, Nian H, Shao Y, Zhang Y, Li Q, Yi Y, Tian F, Li W, Zhang H, Zhang X, Wang F, Li X. PTB-associated splicing factor inhibits IGF-1-induced VEGF upregulation in a mouse model of oxygen-induced retinopathy. Cell Tissue Res. 2015;360:233–43. doi: 10.1007/s00441-014-2104-5. [DOI] [PubMed] [Google Scholar]
- 10.Katircioglu YA, Altiparmak UE, Duman S. Comparison of three methods for the treatment of pterygium: amniotic membrane graft, conjunctival autograft and conjunctival autograft plus mitomycin C. Orbit. 2007;26:5–13. doi: 10.1080/01676830600972724. [DOI] [PubMed] [Google Scholar]
- 11.Janson BJ, Sikder S. Surgical management of pterygium. Ocul Surf. 2014;12:112–9. doi: 10.1016/j.jtos.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Sati A, Shankar S, Jha A, Kalra D, Mishra S, Gurunadh VS. Comparison of efficacy of three surgical methods of conjunctival autograft fixation in the treatment of pterygium. Int Ophthalmol. 2014;34:1233–9. doi: 10.1007/s10792-014-0013-y. [DOI] [PubMed] [Google Scholar]
- 13.Hall RC, Logan AJ, Wells AP. Comparison of fibrin glue with sutures for pterygium excision surgery with conjunctival autografts. Clin Experiment Ophthalmol. 2009;37:584–9. doi: 10.1111/j.1442-9071.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- 14.Al-Swailem S, Xu Z, Wu L, Hartsock MJ, Yiu SC, Duh EJ. Induction of endothelial RAGE expression in pterygium. Mol Vis. 2014;20:1740–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Golu T, Mogoanta L, Streba CT, Pirici DN, Malaescu D, Mateescu GO, Mutiu G. Pterygium: histological and immunohistochemical aspects. Rom J Morphol Embryol. 2011;52:153–8. [PubMed] [Google Scholar]
- 16.Awdeh RM, DeStafeno JJ, Blackmon DM, Cummings TJ, Kim T. The presence of T-lymphocyte subpopulations (CD4 and CD8) in pterygia: evaluation of the inflammatory response. Adv Ther. 2008;25:479–87. doi: 10.1007/s12325-008-0056-4. [DOI] [PubMed] [Google Scholar]
- 17.Carrington LM, Boulton M. Hepatocyte growth factor and keratinocyte growth factor regulation of epithelial and stromal corneal wound healing. J Cataract Refract Surg. 2005;31:412–23. doi: 10.1016/j.jcrs.2004.04.072. [DOI] [PubMed] [Google Scholar]
- 18.Grierson I, Heathcote L, Hiscott P, Hogg P, Briggs M, Hagan S. Hepatocyte growth factor/scatter factor in the eye. Prog Retin Eye Res. 2000;19:779–802. doi: 10.1016/s1350-9462(00)00015-x. [DOI] [PubMed] [Google Scholar]
- 19.Moorby CD, Stoker M, Gherardi E. HGF/SF inhibits junctional communication. Exp Cell Res. 1995;219:657–63. doi: 10.1006/excr.1995.1276. [DOI] [PubMed] [Google Scholar]
- 20.Wilson SE, He YG, Weng J, Zieske JD, Jester JV, Schultz GS. Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility and differentiation of human corneal epithelial cells. Exp Eye Res. 1994;59:665–78. doi: 10.1006/exer.1994.1152. [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Lee M, Lee Y, Choi S, Yang J. Chondrocyte-derived extracellular matrix suppresses pathogenesis of human pterygium epithelial cells by blocking the NF-kappaB signaling pathways. Mol Vis. 2016;22:1490–502. [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YH, Jung JC, Jung SY, Kim YI, Lee KW, Park YJ, Cyclosporine A. Downregulates MMP-3 and MMP-13 Expression in Cultured Pterygium Fibroblasts. Cornea. 2015;34:1137–43. doi: 10.1097/ICO.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 23.Seet LF, Tong L, Su R, Wong TT. Involvement of SPARC and MMP-3 in the pathogenesis of human pterygium. Invest Ophthalmol Vis Sci. 2012;53:587–95. doi: 10.1167/iovs.11-7941. [DOI] [PubMed] [Google Scholar]
- 24.Chui J, Coroneo MT, Tat LT, Crouch R, Wakefield D, Di Girolamo N. Ophthalmic pterygium: a stem cell disorder with premalignant features. Am J Pathol. 2011;178:817–27. doi: 10.1016/j.ajpath.2010.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley JC, Yang W, Bradley RH, Reid TW, Schwab IR. The science of pterygia. Br J Ophthalmol. 2010;94:815–20. doi: 10.1136/bjo.2008.151852. [DOI] [PubMed] [Google Scholar]
- 26.Yang SF, Lin CY, Yang PY, Chao SC, Ye YZ, Hu DN. Increased expression of gelatinase (MMP-2 and MMP-9) in pterygia and pterygium fibroblasts with disease progression and activation of protein kinase C. Invest Ophthalmol Vis Sci. 2009;50:4588–96. doi: 10.1167/iovs.08-3147. [DOI] [PubMed] [Google Scholar]
- 27.Miyazono K, Ten DP, Ichijo H, Heldin CH. Receptors for transforming growth factor-beta. Adv Immunol. 1994;55:181–220. [PubMed] [Google Scholar]
- 28.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 29.Vesaluoma M, Teppo AM, Gronhagen-Riska C, Tervo T. Release of TGF-beta 1 and VEGF in tears following photorefractive keratectomy. Curr Eye Res. 1997;16:19–25. doi: 10.1076/ceyr.16.1.19.5119. [DOI] [PubMed] [Google Scholar]
- 30.Roszkowska AM, De Grazia L, Visalli M, Mondello M, Teti D, Venza M, Venza I. Contact lens wearing and chronic cigarette smoking positively correlate with TGF-beta1 and VEGF tear levels and impaired corneal wound healing after photorefractive keratectomy. Curr Eye Res. 2013;38:335–41. doi: 10.3109/02713683.2012.745880. [DOI] [PubMed] [Google Scholar]
- 31.Nishida K, Kinoshita S, Yokoi N, Kaneda M, Hashimoto K, Yamamoto S. Immunohistochemical localization of transforming growth factor-beta 1, -beta 2, and -beta 3 latency-associated peptide in human cornea. Invest Ophthalmol Vis Sci. 1994;35:3289–94. [PubMed] [Google Scholar]
- 32.Obata H, Kaburaki T, Kato M, Yamashita H. Expression of TGF-beta type I and type II receptors in rat eyes. Curr Eye Res. 1996;15:335–40. doi: 10.3109/02713689609007629. [DOI] [PubMed] [Google Scholar]
- 33.Okada Y, Ikeda K, Yamanaka O, Miyamoto T, Kitano A, Kao WW, Saika S. TNFalpha suppression of corneal epithelium migration. Mol Vis. 2007;13:1428–35. [PubMed] [Google Scholar]
- 34.Yoon KC, Jeong IY, Park YG, Yang SY. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea. 2007;26:431–7. doi: 10.1097/ICO.0b013e31803dcda2. [DOI] [PubMed] [Google Scholar]
- 35.Vesaluoma M, Teppo AM, Gronhagen-Riska C, Tervo T. Increased release of tumour necrosis factor-alpha in human tear fluid after excimer laser induced corneal wound. Br J Ophthalmol. 1997;81:145–9. doi: 10.1136/bjo.81.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller LJ, Pels L, Vrensen GF. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995;36:2557–67. [PubMed] [Google Scholar]
- 37.Kim YJ, Ryu JS, Park SY, Lee HJ, Ko JH, Kim MK, Wee WR, Oh JY. Comparison of Topical Application of TSG-6, Cyclosporine, and Prednisolone for Treating Dry Eye. Cornea. 2016;35:536–42. doi: 10.1097/ICO.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 38.Kim YH, Jung JC, Jung SY, Yu S, Lee KW, Park YJ. Comparison of the Efficacy of Fluorometholone With and Without Benzalkonium Chloride in Ocular Surface Disease. Cornea. 2016;35:234–42. doi: 10.1097/ICO.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]