Abstract
Caffeine, as an oocyte aging inhibitor, was used in many different species to control or delay oocyte aging. However, the safety of caffeine and developmental competence of aged oocytes inhibited by caffeine has not been studied systematically. So we detected the spindle morphology, distribution of cortical granules, zona pellucida hardening and pronucleus formation to assess oocyte quality of caffeine treated oocytes. We found that aged oocytes treated by caffeine maintained weak susceptibility to activating stimuli and regained normal competent after aged further 6 hr. Caffeine maintained the spindle morphology, changed cortical granules distribution of aged oocytes and could not prevent zona pellucida hardening. Furthermore, caffeine increased pronucleus formation of aged oocytes and decreased fragmentation after fertilization. These results suggested that caffeine could maintain the quality of aged oocytes safely in mouse.
Keywords: oocyte aging, caffeine, mouse, oocyte quality, Gerotarget
INTRODUCTION
The quality of oocyte influences the embryo's developmental potential after fertilization [1]. It has been proved that the optimal window for fertilization in mouse was 8-12 hr after ovulation. Oocyte became aged and the quality of oocyte would decrease if fertilization did not finish during this window [2]. Fertilized oocytes from B6D2F1 and ICR mouse lost their full-term developmental potential by 14 hr and 18 hr after ovulation [3]. An intact meiotic spindle is critically important for accurate distribution of chromosomes to the dividing blastomeres, thus ensuring accurate embryo development. The spindles in aged oocytes became smaller and could be bi- or multipolar, which would block chromosome segregation and result in abnormalities [2]. The main reason was that oocyte aging induced a loss of centrosome structure at the meiotic poles, which was associated with loss of microtubule integrity and chromosome maintenance at the metaphase plate. Oocyte aging accompanied with the failure of fertilization [4], increased susceptibility to be activated [5], zona pellucida (ZP) hardening [6] and abnormal development of embryos [7]. However, the most important change during oocyte aging was the decrease of maturation-promoting factor (MPF) activity [5] and Kikuchi et al (2000) found that caffeine could maintain higher activity of MPF and inhibit oocyte aging in pig [8, 9]. However, evaluation of developmental competence of aged oocytes inhibited by caffeine was not completed systematically.
Caffeine is a central nervous system stimulant and acts through adenosine receptors and monoamine neurotransmitters. Many labs have tried to use caffeine to inhibit oocyte aging in different species. In pig, 5 mM caffeine decreased fragmentation rate after electrical stimulation, however, it could not increase blastocyst formation and the numbers of total cells in blastocysts. When somatic cell nuclei were injected into aged oocytes treated by caffeine, it promoted nuclear remodeling and could not prevent abnormal development of cloned embryos caused by oocyte aging [10]. Our lab found that caffeine could restore centrosome integrity and maintain spindle cytoskeleton in aged porcine oocytes [11]. In ovine, caffeine not only prevented age-related changes, such as the decline in MPF and MAPK activities [12], but also increased blastocyst formation rates of both somatic cell nuclear transfer (SCNT) embryos and Intracytoplasmic sperm injection (ICSI) embryos, reduced polyspermy and increased the glutathione after aged oocytes treated by caffeine [13, 14]. In mouse, caffeine prevented the reduced function of IP(3)R1 and live offspring from aged oocytes could be obtained after they were treated by caffeine [3, 15]. In golden hamster, caffeine delayed spontaneous oocyte parthenogenetic activation for at least 5 hr, however, it accelerated the ZP hardening [16].
Controlling oocyte aging is very important not only for health reproduction, but also for extending the time for oocyte manipulation. For example, more and more children are produced by in vitro fertilization (IVF). The average fertilization rate after standard IVF is about 60-70% [17], which required to re-inseminate aged unfertilized oocytes failing in IVF cycles by ICSI. Studies showed that only 38% oocytes aged for 1 day that could be fertilized using ICSI and this rate was much lower than that of fresh oocytes after standard ICSI (64.2%). Even if these aged oocytes could form pronucleus (PN), their implantation and developmental potential were very low [18]. In agricultural fields, animal embryo engineering need more oocyte to make in vitro produced embryos, such as IVF embryos or cloned embryo. However, the time for oocyte manipulation is limited. So controlling or delaying oocyte aging might provide more time for these procedures.
It was reported that mouse oocytes lost their developmental potential by 14-18 hr after ovulation and caffeine extended this time to 16-22 hr [3]. However, how and what aspects caffeine inhibited oocyte aging had not been investigated systematically. It showed that spindle analysis, distribution of cortical granules (CGs), zona pellucida (ZP) hardening and PN formation after parthenogenetic activation or fertilization were used to assess oocyte quality [1]. In this study, we used these indicators to evaluate aged oocytes treated by caffeine for 24 hr (an ultimate time for oocyte aging in mouse). This study would provide more evidence to show the safety of using caffeine in both human assisted reproduction technologies (ART) and agricultural animal embryo engineering.
RESULTS
Caffeine inhibited the separation of cumulus cells from oocytes during aging
Cumulus cells accelerated aging of mouse oocytes as we previously reported [19]. To avoid the interference of cumulus cells on oocyte aging, we observed whether caffeine would keep the attachment of cumulus cells on oocytes during aging. It was shown that all cumulus cells were attached in fresh COCs (Figure 1A) and most cumulus cells were attached in COCs treated by caffeine (Figure 1B). However, most cumulus cells were separated in COCs aged for 24 hr (Figure 1C). So we ruled out the possibility that the separation of cumulus cells from oocytes contributed to the aging inhibition by caffeine.
Figure 1. The effects of caffeine on the cumulus cells attaching and parthenogenetic activation during oocyte aging.
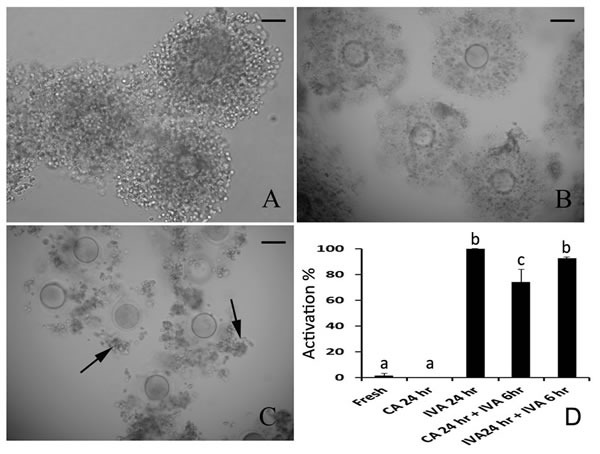
A. Fresh oocytes with full cumulus cells attached. B. Caffeine-treated aged oocytes for 24 hr with most cumulus cells attached. C. Oocytes aged for 24 hr in vitro with few cumulus cells attached. D. The activation of fresh oocytes, caffeine treated oocytes and oocytes aged in vitro after parthenogenetic activation. All graphs show mean ± s.e.m. Abbreviations used in this and all subsequent figures: CA, caffeine treated; IVA, in vitro aging. a-c: Values without a common letter in their superscripts differ significantly (P < 0.05). The black arrows indicate the unattached cumulus cells in aged COCs. Bar, 80 μm.
Effects of caffeine on the activation of aged oocytes treated by caffeine
It has been proved that caffeine could inhibit oocyte aging effectively in mouse [3]. We found that aged oocytes treated by caffeine for 24 hr were hardly activated by weak stimulation like fresh oocytes. However, if these treated oocytes were aged for further 6 hr in CZB, there were 74.2% treated aged oocytes could be activated by weak stimulation again and this activation percentage was very close to that of oocytes aged for 24 hr and 30 hr (Figure 1D). So we concluded that aged oocytes treated by caffeine still could have competence to be activated.
Caffeine maintained the spindle morphology and changed CGs distribution of aged oocytes
Intact spindles displayed bipolar spindles with focused poles in oocytes. There are 92.4% oocytes showed the intact spindle morphology in fresh oocytes. However, if oocytes were aged for 24 hr in vitro, microtubules become gradually lost from the spindle, with preferential loss in the central spindle area near the chromosomes. Astral fibers radiated out from the polar centrosomes into the cytoplasm and astral microtubules in the cytoplasm became gradually depolymerized. There were only 15.7% oocytes showed intact spindle morphology in aged oocytes (Figure 2A). However, 79.5% aged oocytes showed intact spindle morphology after treated by caffeine for 24 hr. So spindle morphology could be recovered by caffeine in aged oocytes (Figure 2B).
Figure 2. The effects of caffeine on the spindle morphology during oocyte aging.
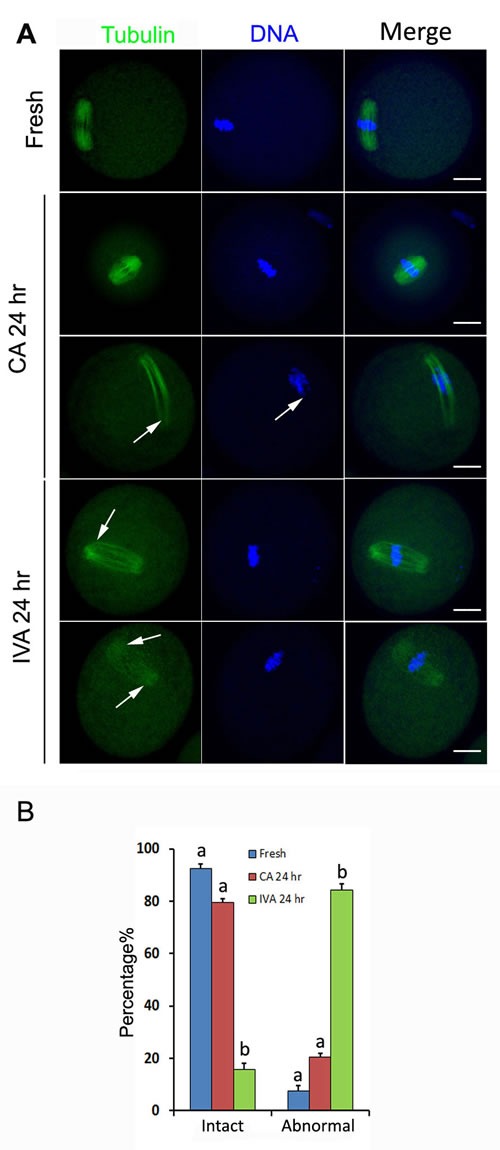
A. Spindle morphology of fresh oocytes, caffeine-treated oocytes and oocytes aged in vitro. B. Percentage of oocytes with intact and abnormal spindles in fresh oocytes, caffeine-treated oocytes and oocytes aged in vitro. All graphs show mean ± s.e.m. Abbreviations used in this and all subsequent figures: CA, caffeine treated; IVA, in vitro aging. a-c: Values without a common letter in their superscripts differ significantly (P < 0.05). Spindle (green) and chromosomes (blue). The white arrows indicate the spindle defects and chromosome misalignment. Bar, 20 μm.
As we knew, CGs were released into the perivitelline space (PVS) and combined with ZP when oocytes were fertilized by sperm, which was called as cortical reaction. During oocyte aging, we found four types of CGs distribution in the oocytes. Type I: cortical granules were densely populated in a line just beneath the oolemma, with typical CG-free domains. Type II: CGs moved to all the cortex of oocytes without CG-free domains. Type III: CGs moved to CG-free domains near the chromosomes with partial exocytosis. Type IV: full exocytosis without CGs under the oolemma (Figure 3A). All oocytes showed Type I in fresh oocytes. If oocyte were aged for 24 hr, CGs distribution showed three kinds of types: Type II (36.1%), Type III (44.3%) and Type IV (19.6%). However, caffeine could inhibit partial exocytosis if oocytes were treated by caffeine for 24 hr. Very few treated oocytes (1.9%) underwent full exocytosis and 39.0% treated oocytes could maintain CGs distribution like fresh oocytes (Figure 3B). These findings indicated that caffeine could maintain spindle morphology and inhibit partial exocytosis in aged oocytes.
Figure 3. The effects of caffeine on the CGs distribution during oocyte aging.
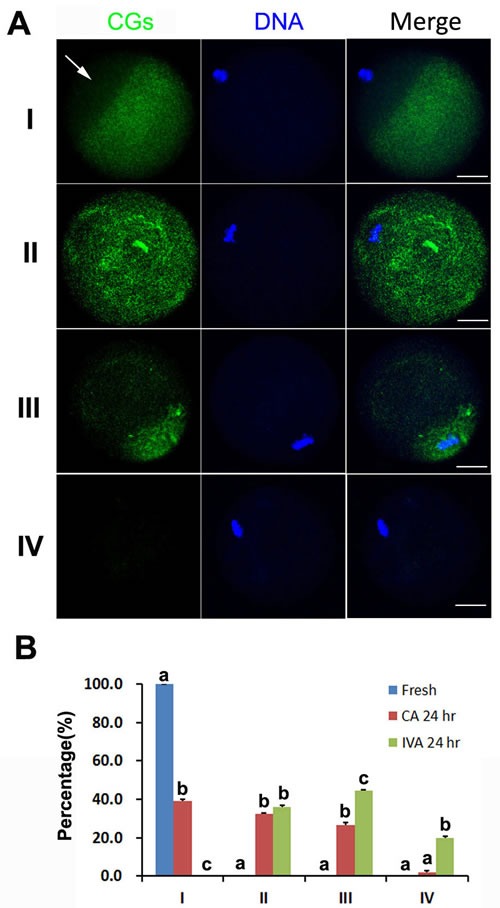
A. Four types of CGs distribution are shown in different oocytes. B. Percentage of oocytes with different CGs distribution in fresh oocytes, caffeine-treated oocytes and oocytes aged in vitro. All graphs show mean ± s.e.m. Abbreviations used in this and all subsequent figures: CA, caffeine treated; IVA, in vitro aging. a-c: Values without a common letter in their superscripts differ significantly (P < 0.05). CGs (green) and chromosomes (blue). The white arrow indicates CG free domain in fresh oocyte. Bar, 20 μm.
Caffeine could not inhibit ZP hardening of aged oocytes
The most reason of aged oocytes failed to be fertilized was that ZP became harden in aged oocytes. We found that caffeine could inhibit CGs release partially, so we tested ZP harden by the ZP hardening assay. The results showed that the half-time (T50) for chymotrypsin-mediated dissolution of the ZP increased significantly in the ZP from both aged oocyte with or without caffeine treatment (Figure 4A), which suggested that caffeine could not inhibit CGs release completely and partial CGs were enough to induce cortical reaction and make ZP harden.
Figure 4. The effects of caffeine on the ZP hardening and fertilization by ICSI during oocyte aging.
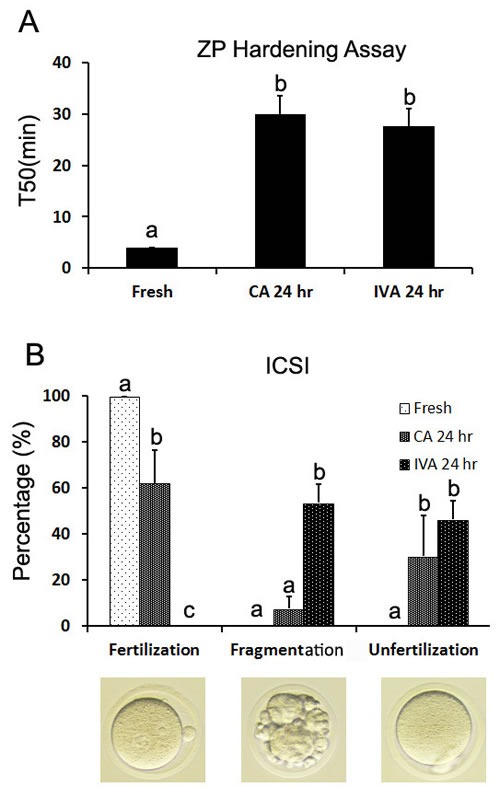
A. Changes in chymotrypsin digestion time of ZP (T50 is the time at which 50% of the ZPs per group were completely digested) of fresh oocytes, caffeine-treated oocytes and oocytes aged in vitro. B. The fertilization of fresh oocytes, caffeine treated oocytes and oocytes aged in vitro after Intracytoplasmic sperm injection (ICSI). Abbreviations used in this and all subsequent figures: CA, caffeine treated; IVA, in vitro aging. a-c: Values without a common letter in their superscripts differ significantly (P < 0.05).
Caffeine increased the fertilization rate of aged oocytes and decreased fragmentation by intracytoplasmic sperm injection
Aged oocytes were hardly fertilized by in vitro fertilization (IVF) because of hardened ZP. So we used ICSI as methods to test the fertilization competence of aged oocytes. We found that oocytes aged for 24 hr lost their fertilization competence. None pronucleus (PN) was formed in aged oocytes and 53.6% oocytes became fragmented. However, there were 62.2% aged oocytes treated by caffeine were fertilized normally with PN formation and only 7.5% treated oocytes became fragmentation (Figure 4B).
DISCUSSION
Kikuchi et al. (2000) found that maturation/M-phase promoting factor (MPF) was a regulator of aging in porcine oocytes and our previous studies proved that cumulus cells accelerated oocyte aging by regulating the activity of MPF in mouse oocyte [9, 19]. Smythe et al (1992) found that caffeine could maintain higher MPF activity by inhibiting Myt1/Wee1 activity [20]. Therefore, caffeine was used to suppress Myt1/Wee1 kinase in aged oocytes and increase MPF activity, which induced low susceptibility to activating stimuli and a lower percentage of fragmentation in aged oocytes. It has been reported that caffeine was used to inhibit oocyte aging in pig, ovine, mouse and golden hamster [3, 10–12, 14, 16]. However, the safety of caffeine used to delay oocyte aging has not been investigated systematically. In this study, we used mouse oocytes as model to evaluate the safety of caffeine to inhibit oocyte aging and developmental competence of aged oocytes treated by caffeine. We employed spindle morphology analysis, distribution of CGs, ZP hardening and pronucleus (PN) formation after parthenogenetic activation or fertilization to assess their quality.
There are several morphological, cellular and molecular predictors of oocytes to evaluate the oocyte quality, including of cumulus-oocyte complex morphology, spindle morphology analysis, distribution of CGs, and developmental potential [1]. Our previous studies has proved that cumulus cells accelerated the aging progression of both in vivo-matured and in vitro-matured mouse oocytes. Further studies found that soluble Fas ligand (sFasL) secreted by cumulus cells could activate Fas on the oocyte by increasing reactive oxygen species (ROS) and glucose metabolism in cumulus cells prevented oocyte aging by producing pyruvate and NADPH through glycolysis and pentose phosphate pathway (PPP) [21–23]. To exclude the interrelationship between cumulus cells and caffeine, we found that caffeine prevented cumulus cells from separating in COCs during oocyte aging in vitro (Figure 1B). So caffeine inhibited oocyte aging by MPF activity without disturbance by cumulus cells. Spindle analysis is a good criterion to assess oocyte quality [24]. An intact spindle is necessary for accurate chromosome segregation, thus ensuring normal embryo development. In aged oocytes, spindles became shorter, smaller, would be bi- or multipolar. Some microtubules radiated towards the cell periphery and formed additional microtubule asters in the cytoplasm. Some centrosome structure lost at the meiotic poles [25]. We previously reported that centrosomes were absent and spindles became abnormal and disorganized in porcine oocytes aged for 48 hr, however, caffeine prevented these changes and restored centrosome integrity in the meiotic spindle poles and displayed similar meiotic spindles as those seen in fresh oocytes [11]. Our data showed that most of aged oocytes showed intact spindle morphology after treated by caffeine for 24 hr in mouse, which provided a precondition for chromosome segregation in aged oocytes (Figure 2).
Cortical granules (CGs) distribution was another important criterion to evaluate oocyte quality and they migrated to the cortex and formed a continuous layer under the oolemma [26]. In fresh oocytes, cortical reaction was triggered by sperm or artificial activation. However, it was easily triggered spontaneously without fertilization in aged oocytes. CGs become displaced and underwent partial exocytosis [27]. It was showed that caffeine accelerated CGs release in aged oocytes and normal CG distribution significantly decreased after aged for 6 hr [28]. We have reported that there were two CG distributions in aged oocytes: one was that a ring of CGs beneath the oolemma; another was that a cap of higher density CGs located above the chromosome area [29]. In our studies, we found that partial or full exocytosis occurred in oocytes aged for 24 hr. However, caffeine inhibited CGs release and full exocytosis. About 40% oocytes showed CGs distribution as seen in fresh oocytes and only 1.9% oocytes had full exocytosis (Figure 3). ZP hardening was the main reason to block fertilization, so we detected ZP hardening in oocytes treated by caffeine. Our data showed that caffeine cannot block ZP hardening. We speculate that caffeine could not inhibit CGs release completely during oocyte aging. Few CGs were released to induce cortical reaction and make ZP harden (Figure 4A). Schroeder et al. has reported that fetuin could inhibit ZP hardening and conversion of ZP2 to ZP2f during oocyte maturation in vitro in mouse [30], which provided an impossible way to inhibit oocyte aging using caffeine and fetuin together.
To evaluate the developmental potential of oocytes treated by caffeine, we employed weak artificial activation and ICSI to active oocytes and observe their PN formation. Fresh oocytes have low sensibility to artificial activation and are not easily activated [19]. In our studies, both fresh oocytes and aged oocytes treated by caffeine for 24 hr were hardly activated by ethanol and 6-DMAP, however, oocytes aged for 24 hr in vitro were easily activated and the activation percentage reached almost 100%. We further tested that the recoverability of oocytes treated by caffeine. Caffeine-treated oocytes were aged for 6 hr without caffeine and they were activated by ethanol and 6-DMAP. We found that 74.2% caffeine-treated oocytes were activated and formed PN, which proved that caffeine-treated oocytes could be aged again and still had potential to be activated. We also used ICSI to test the activation potential of caffeine-treated oocytes. Activation-induced fragmentation frequently occurred in aged oocytes. Our data showed that 53.6% aged oocytes became fragmentation and none of them were fertilized after sperm injection. However, oocytes treated by caffeine had a high fertilization (62.2%) and low fragmentation (7.5%) (Figure 4B). These suggested that aged oocytes treated by caffeine had a good potential to be activated.
In summary, we found that caffeine was a good oocyte aging inhibitor and most aged oocytes treated by caffeine had a developmental competence. With the development of modern ART, more matured oocytes are widely used in advanced reproductive technologies such as IVF and ICSI in vitro. However, the success rates of ART technologies are frequently impacted by oocyte aging, control of oocyte aging would offer a significant advantage in allowing sufficient manipulation time and selection of oocytes of the highest quality. Therefore, establishment of methods for aging control might enhance progress in ART technologies. Our data provided important information that caffeine could safely be used to control oocyte aging in related animal or clinical assisted reproductive technology.
MATERIALS AND METHODS
Animals and chemicals
Mice (Kunming breed) were kept in a room with 12 hr/12 hr light-dark cycles, with the dark starting from 7 PM. The mice were handled in accordance with the rules stipulated by the Animal Care and Use Committee of Huazhong Agriculture University (HZAUMO-2015-018). All chemicals were purchased from Sigma Chemical unless otherwise indicated.
Recovery of oocytes, in vitro aging and caffeine treatment
To induce superovulation, female mice, 6 to 8 wk old, were given an ip treatment of 10 IU PMSG (Ningbo Hormone Product Co., Ltd., P.R. China) followed 48 hr later by ip treatment of 10 IU hCG (Ningbo Hormone Product Co., Ltd., P.R. China). Three superovulated mice in each group were killed 13 hr after hCG injection and the oviductal ampullae were broken to release the cumulus-oocyte complexes (COCs). The COCs were denuded of cumulus cells by pipetting in M2 medium (Sigma, M7167), containing 0.1% hyaluronidase (Sigma, H3506). For in vitro aging, the COCs were cultured in wells (25-35 oocytes per well) of a 96-well culture plate containing 200 ml of Chatot- Ziomek-Bavister (CZB) medium [31] and covered with mineral oil at 37.5°C under 5% CO2 in humidified air for 24 hr. For Caffeine treatments, the COCs were incubated with CZB supplemented with 5 mM caffeine (Sigma, C0750) for 24 hr.
Oocyte activation and assessment
Oocytes were activated with ethanol and 6-DMAP in combination as described by Miao et al., [19]. Oocytes were first treated with 5% (v/v) ethanol in M2 medium for 5 min at room temperature, then washed three times and cultured in CZB containing 2 mM 6-DMAP for 6 hr. At the end of culture, oocytes were observed under a microscope for activation. Only those oocytes with one pronucleus or two pronuclei, or two cells each having a nucleus, were considered activated.
Fluorescence and immunofluorescence microscopy
For spindle staining, oocytes were fixed in 4% (wt/vol) paraformaldehyde, washed in blocking buffer (PBS containing 0.3% BSA, 0.01% Tween-20, and 0.01% NaN3), and then permeabilized in PBS containing 0.3% BSA, 0.1% Triton X-100, and 0.01% NaN3. The oocytes were incubated in primary anti-α-tubulin antibody (Sigma, F2168) diluted 1:100 in blocking buffer. For CG staining, zonae pellucidae were removed by brief incubation in acid Tyrode's solution. The oocytes were fixed in 4% (wt/vol) paraformaldehyde in PBS, blocked in PBS containing 0.3% BSA and 100 mM glycine, and then permeabilized in PBS containing 0.1% Triton X-100. CGs were labeled in 100 μg/mL FITC-conjugated peanut agglutinin in PBS. The oocytes were washed in blocking buffer and mounted as above. Slides were scanned by using a Zeiss confocal microscope (Zeiss LSM 510 UV).
Assay for zona pellucida hardening
The assay for zona pellucida (ZP) hardening was performed as described by Gulyas and Yuan [32] with minor modifications. Briefly, 20 cumulus-free oocytes were treated with 1 mg/ml achymotrypsin (Sigma C4129) contained in a 100 ml drop of PBS covered with mineral oil at 30°C. Oocytes were monitored every 2 min during the first 30 min of the treatment and then every 5 min until the end of the treatment (3 hr). The time at which 50% of the ZP underwent a complete dissolution (with denuded oocytes stuck on the bottom of the dish) was assessed as T50 for ZP dissolution.
Intracytoplasmic sperm injection
Cauda epididymal sperm were collected from Kunming males into HTF medium (Millipore, MR-070-D). A single sperm head was microinjected into an MII oocyte by using a NIKON Ti-S inverted microscope equipped with a Narishige Micromanipulator system and an Eppendorf Piezo drill. Only those oocytes with two pronuclei were considered as fertilization and oocytes showed like cleavage without pronuclei were considered as fragmentation (Figure 4B).
Data analysis
For each treatment, three replicates were run. Statistical analyses were carried out by analysis of variance. Differences between groups treated were evaluated with the Duncan multiple comparison test. Data are expressed as mean ± SEM and P < 0.05 is considered significant.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant No.31471350), “The Recruitment Program for Young Professionals” of “The Thousand Talents Plan” (Grant No.159905) and Starting Fund for New Recruitment of Huazhong Agricultural University (Grant No.14009).
Footnotes
CONFLICTS OF INTERESTS
The authors declare no competing financial interests.
Author contributions
X.Z., X.L, L.C., D.Y.W., Z.W.N., Y.Y.G., conducted the experiments; X.Z. and Y.L.M. analyzed the data, designed the experiments and wrote the manuscript. All authors reviewed the manuscript.
REFERENCES
- 1.Wang Q, Sun QY. Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reproduction, fertility, and development. 2007;19:1–12. doi: 10.1071/rd06103. [DOI] [PubMed] [Google Scholar]
- 2.Austin CR. In: In “concepts of development “. Whittaher J R, editor. Sinauer Associates Inc; 1974. Publisher. [Google Scholar]
- 3.Ono T, Mizutani E, Li C, Yamagata K, Wakayama T. Offspring from intracytoplasmic sperm injection of aged mouse oocytes treated with caffeine or MG132. Genesis. 2011;49:460–471. doi: 10.1002/dvg.20756. [DOI] [PubMed] [Google Scholar]
- 4.Goud P, Goud A, Van Oostveldt P, Van der Elst J, Dhont M. Fertilization abnormalities and pronucleus size asynchrony after intracytoplasmic sperm injection are related to oocyte postmaturity. Fertil Steril. 1999;72:245–252. doi: 10.1016/s0015-0282(99)00231-9. [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi K, Izaike Y, Noguchi J, Furukawa T, Daen FP, Naito K, Toyoda Y. Decrease of histone H1 kinase activity in relation to parthenogenetic activation of pig follicular oocytes matured and aged in vitro. Journal of reproduction and fertility. 1995;105:325–330. doi: 10.1530/jrf.0.1050325. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T. Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-trisphosphate sensitivity. Biology of reproduction. 1997;57:743–750. doi: 10.1095/biolreprod57.4.743. [DOI] [PubMed] [Google Scholar]
- 7.Wilcox AJ, Weinberg CR, Baird DD. Post-ovulatory ageing of the human oocyte and embryo failure. Hum Reprod. 1998;13:394–397. doi: 10.1093/humrep/13.2.394. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi K, Naito K, Noguchi J, Kaneko H, Tojo H. Maturation/M-phase promoting factor regulates aging of porcine oocytes matured in vitro. Cloning Stem Cells. 2002;4:211–222. doi: 10.1089/15362300260339494. [DOI] [PubMed] [Google Scholar]
- 9.Kikuchi K, Naito K, Noguchi J, Shimada A, Kaneko H, Yamashita M, Aoki F, Tojo H, Toyoda Y. Maturation/M-phase promoting factor: a regulator of aging in porcine oocytes. Biology of reproduction. 2000;63:715–722. doi: 10.1095/biolreprod63.3.715. [DOI] [PubMed] [Google Scholar]
- 10.Iwamoto M, Onishi A, Fuchimoto D, Somfai T, Suzuki S, Yazaki S, Hashimoto M, Takeda K, Tagami T, Hanada H, Noguchi J, Kaneko H, Nagai T, et al. Effects of caffeine treatment on aged porcine oocytes: parthenogenetic activation ability, chromosome condensation and development to the blastocyst stage after somatic cell nuclear transfer. Zygote. 2005;13:335–345. doi: 10.1017/S0967199405003370. [DOI] [PubMed] [Google Scholar]
- 11.Miao YL, Sun QY, Zhang X, Zhao JG, Zhao MT, Spate L, Prather RS, Schatten H. Centrosome abnormalities during porcine oocyte aging. Environmental and molecular mutagenesis. 2009;50:666–671. doi: 10.1002/em.20506. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Campbell KH. Caffeine treatment prevents age-related changes in ovine oocytes and increases cell numbers in blastocysts produced by somatic cell nuclear transfer. Cloning Stem Cells. 2008;10:381–390. doi: 10.1089/clo.2007.0091. [DOI] [PubMed] [Google Scholar]
- 13.Maalouf WE, Lee JH, Campbell KH. Effects of caffeine, cumulus cell removal and aging on polyspermy and embryo development on in vitro matured and fertilized ovine oocytes. Theriogenology. 2009;71:1083–1092. doi: 10.1016/j.theriogenology.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Ye XF, Chen SB, Wang LQ, Zhao YC, Lv XF, Liu MJ, Huang JC. Caffeine and dithiothreitol delay ovine oocyte ageing. Reproduction, fertility, and development. 2010;22:1254–1261. doi: 10.1071/RD10062. [DOI] [PubMed] [Google Scholar]
- 15.Zhang N, Wakai T, Fissore RA. Caffeine alleviates the deterioration of Ca(2+) release mechanisms and fragmentation of in vitro-aged mouse eggs. Molecular reproduction and development. 2011;78:684–701. doi: 10.1002/mrd.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H, Wang C, Guan J, Wang L, Li Z. Changes of spontaneous parthenogenetic activation and development potential of golden hamster oocytes during the aging process. Acta histochemica. 2015;117:104–110. doi: 10.1016/j.acthis.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Kruger TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, van Zyl JA, Smith K. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46:1118–1123. doi: 10.1016/s0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Nagy Z, Joris H, Tournaye H, Smitz J, Camus M, Devroey P, Van Steirteghem A. Analysis of 76 total fertilization failure cycles out of 2732 intracytoplasmic sperm injection cycles. Hum Reprod. 1995;10:2630–2636. [PubMed] [Google Scholar]
- 19.Miao YL, Liu XY, Qiao TW, Miao DQ, Luo MJ, Tan JH. Cumulus cells accelerate aging of mouse oocytes. Biology of reproduction. 2005;73:1025–1031. doi: 10.1095/biolreprod.105.043703. [DOI] [PubMed] [Google Scholar]
- 20.Smythe C, Newport JW. Coupling of mitosis to the completion of S phase in Xenopus occurs via modulation of the tyrosine kinase that phosphorylates p34cdc2. Cell. 1992;68:787–797. doi: 10.1016/0092-8674(92)90153-4. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Miao DQ, Zhou P, Wu YG, Gao D, Wei DL, Cui W, Tan JH. Glucose metabolism in mouse cumulus cells prevents oocyte aging by maintaining both energy supply and the intracellular redox potential. Biology of reproduction. 2011;84:1111–1118. doi: 10.1095/biolreprod.110.089557. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Lin FH, Zhang J, Lin J, Li H, Li YW, Tan XW, Tan JH. The signaling pathways by which the Fas/FasL system accelerates oocyte aging. Aging (Albany NY) 2016;8:291–303. doi: 10.18632/aging.100893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J, Zhang J, Li H, Wang TY, Zhang CX, Luo MJ, Tan JH. Cumulus cells accelerate oocyte aging by releasing soluble Fas ligand in mice. Scientific reports. 2015;5:8683. doi: 10.1038/srep08683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao YL, Shi LH, Lei ZL, Huang JC, Yang JW, Ouyang YC, Sun QY, Chen DY. Effects of caffeine on in vivo and in vitro oocyte maturation in mice. Theriogenology. 2007;68:640–645. doi: 10.1016/j.theriogenology.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 25.George MA, Pickering SJ, Braude PR, Johnson MH. The distribution of alpha- and gamma-tubulin in fresh and aged human and mouse oocytes exposed to cryoprotectant. Molecular human reproduction. 1996;2:445–456. doi: 10.1093/molehr/2.6.445. [DOI] [PubMed] [Google Scholar]
- 26.Liu XY, Mal SF, Miao DQ, Liu DJ, Bao S, Tan JH. Cortical granules behave differently in mouse oocytes matured under different conditions. Hum Reprod. 2005;20:3402–3413. doi: 10.1093/humrep/dei265. [DOI] [PubMed] [Google Scholar]
- 27.Goud AP, Goud PT, Diamond MP, Van Oostveldt P, Hughes MR. Microtubule turnover in ooplasm biopsy reflects ageing phenomena in the parent oocyte. Reproductive biomedicine online. 2005;11:43–52. doi: 10.1016/s1472-6483(10)61297-7. [DOI] [PubMed] [Google Scholar]
- 28.Zheng J, Yin XQ, Ge W, He GF, Qian WP, Ma JY, Shen W, Yin S, Sun QY. Post-ovulatory aging of mouse oocytes in vivo and in vitro: Effects of caffeine on exocytosis and translocation of cortical granules. Animal science journal = Nihon chikusan Gakkaiho. 2016;87:1340–1346. doi: 10.1111/asj.12611. [DOI] [PubMed] [Google Scholar]
- 29.Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Human reproduction update. 2009;15:573–585. doi: 10.1093/humupd/dmp014. [DOI] [PubMed] [Google Scholar]
- 30.Schroeder AC, Schultz RM, Kopf GS, Taylor FR, Becker RB, Eppig JJ. Fetuin inhibits zona pellucida hardening and conversion of ZP2 to ZP2f during spontaneous mouse oocyte maturation in vitro in the absence of serum. Biology of reproduction. 1990;43:891–897. doi: 10.1095/biolreprod43.5.891. [DOI] [PubMed] [Google Scholar]
- 31.Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. Journal of reproduction and fertility. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- 32.Gulyas BJ, Yuan LC. Cortical reaction and zona hardening in mouse oocytes following exposure to ethanol. The Journal of experimental zoology. 1985;233:269–276. doi: 10.1002/jez.1402330215. [DOI] [PubMed] [Google Scholar]


