Abstract
Introduction
Acute pancreatitis (AP) is one of the most common diseases involving necrotic inflammation. Bone marrow mesenchymal stem cells (BMMSCs) have the potential of multi-directional differentiation and self-renewal for tissue repair. It remains less clear if granulocyte colony-stimulating factor (G-CSF) can improve the therapeutic effect of BMMSC transplant in AP. Therefore, we explored this issue in a rat model of experimental AP.
Results
Transplanted PKH26-positive BMMSCs were present in the injured pancreatic tissue, with some cells co-expressed pancreatic cellular markers, including Pax-4, Ngn3 and Nkx-6. Pathological, biochemical and serological data suggested an improvement in histological and functional recovery in these animals relative to control. Overall, the AP model rats received BMMSCs and G-CSF co-treatment showed better recovery in terms of tissue regeneration and blood biochemical levels relative to other groups.
Materials and Methods
BMMSCs from donor rats were labeled with the fluorescent dye PKH26 and transfused into recipient rats with AP induced by L-arginine. The animals were divided into a control group, and groups treated with BMMSCs, G-CSF, and BMMSCs together with G-CSF. Therapeutic effects were evaluated histologically with immunohistochemistry and immunofluorescence, together with biochemical measurement of pancreatic markers.
Conclusion
G-CSF therapy with BMMSC transplantation improves histological and functional outcomes in rats with experimental AP.
Keywords: bone marrow stem cell transplant, granulocyte colony-stimulating factor, acute pancreatitis, transdifferentiation
INTRODUCTION
Acute pancreatitis (AP) is a common necrotizing inflammatory disease. AP patients have unbearable abdominal pain, abdominal distention, and in severe cases, concurrent systemic organ failure leading to high the mortality [1]. AP is characterized by inflammation with the activation of digestive proenzymes, including proteases, lipase, and amylase, that results in autodigestive destruction of the pancreatic parenchyma and the peripancreatic adipose tissue [2]. Additionally, AP is associated with pancreatic edema, hemorrhage and necrosis [3, 4]. Its pathogenic mechanism is still not very clear, while a common view is that the interaction of proinflammatory cytokines such as interleukin (IL)-1, tumor necrosis factor (TNF), IL-6, IL-8 and IL-4 with components of the anti-inflammatory response system, such as IL-10, causes severe complications of AP including pancreatic necrosis [5].
Despite various experimental and clinical tests of potential drugs, only a few pharmacologic options are currently available for the treatment of AP [6]. In recent years, stem cell therapy for AP has emerged as a new option [7–9]. Mesenchymal stem cells (MSCs) have the potential to differentiate into bone, cartilage, or adipose tissue under suitable conditions [10, 11]. These non-hematopoietic stromal progenitor cells have self-renewal ability and high plasticity. Evidence suggests that bone marrow mesenchymal stem cells (BMMSCs) can differentiate into pancreatic stem cells, which may mitigate pancreatic injury [12–17]. However, it appears that the effect of BMMSC therapy may be not sufficient as, for instance, the amount of transplanted BMMSCs is limited, or inflammatory mediators affect the survival and differentiation of the cells after transplantation [18]. Granulocyte colony-stimulating factor (G-CSF) can mobilize BMMSCs in vivo and has been used in the treatment of neutropenia and for bone marrow reconstitution and stem cell mobilization [19, 20]. The multi-directional differentiation potential of BMMSCs and the mobilization effect of G-CSF form a tangible theoretical basis for the treatment of AP. Therefore, we attempted to explore a synergetic therapeutic benefit of combining BMMSC transplantation and G-CSF administration in a rat model of AP.
RESULTS
Evaluation of L-arginine induced pancreatic injury
On microscopic examination, the structure of the pancreas was intact in the normal rats, showing complete glandular and lobular organization and distinct pancreatic cytology, without interstitial leakage (Figure 1A). In contrast, in H.E. preparation of the pancreas from the AP model rats, there was apparent structural disruption, with large areas of the tissue showing necrosis, hemorrhage and inflammatory cell infiltration (Figure 1B).
Figure 1.
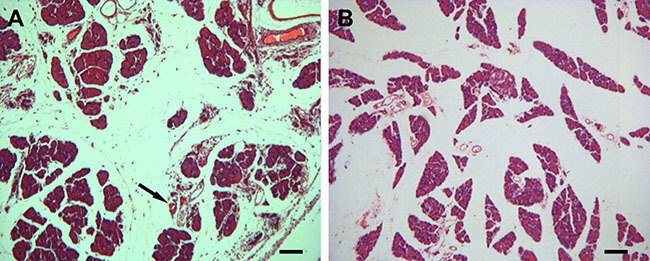
Histological evaluation of pancreatic lesions (A) after L-arginine administration with hematoxylin and eosin (H.E) stain relative to normal control (B) (10×). (A) In the normal rats, the microscopic structure of the pancreas is clear; the glandular lobule is complete, without interstitial leakage. (B) Injured pancreas shows interstitial edema, inflammatory cell infiltration, and necrosis. Scale bar = 100 μm.
Characterization of cultured BMMSCs in vitro
On the first day of culture, a small amount of cells stuck to the bottom of the bottle, while the majority remained floating in the medium (Figure 2A). A large amount of spindle cells were seen at the bottom on the fifth day (Figure 2B). Following PKH26 labeling, a great amount of cells exhibited bright red fluorescence in vitro for 2 weeks (Figure 2C).
Figure 2. Morphological feature of cultured bone marrow mesenchymal stem cells (BMMSCs) harvested from donor rats.
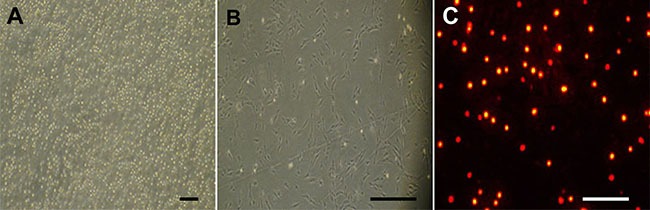
(A) On the first day cultured cells largely remained floating with a few cells adherent to bottle wall. (B) On the fifth day, a large number of spindle cells were attached to the bottle wall. (C) The cells were tagged with the red fluorescent dye PKH26 after 2 weeks. Scale bar = 100 μm.
FACS assays confirmed that 97.58% of the isolated cells expressed CD29 (Figure 3A), 0.69% expressed CD34 (a myeloid progenitor cell antigen also presented in endothelial cells and some fibroblasts, Figure 3B); 66.88% expressed CD44 (a stem cell marker, Figure 3C), 2.24% expressed CD45 (a hematopoietic and leukocyte marker, Figure 3D) [21]; and 63.80% of the cells expressed CD90 (Figure 3E). Thus, the cells showed the antigenic profile of BMMSCs [22].
Figure 3. Fluorescence-activated cell sorting analysis with markers of bone marrow mesenchymal stem cells (BMMSCs).
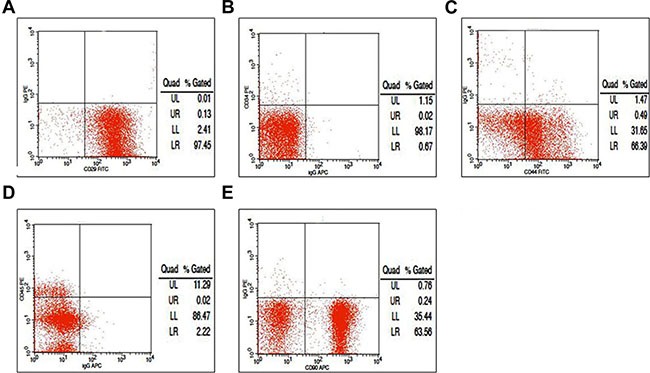
(A) We found that 97.58% of the isolated cells expressed CD29; (B) 0.69% expressed CD34; (C) 66.88% expressed CD44; (D) 2.24% expressed CD45; and (E) 63.80% expressed CD90.
Colocalization of transplanted BMMSCs in the pancreas of recipient rats
A panel of pancreatic cellular markers was used to explore in situ differentiation of the transplanted BMMSCs in recipient rats, including Nkx6 (NK6 homeobox 1), Ngn3 (neurogenin 3) and Pax4 (paired box 4) (Figure 4). In animals surviving 3 weeks after BMMSC transplantation and G-CSF administration, PKH26+ cells were colabeled with Ngn3 (Figure 4A–4D) at a rate of 45% ± 8.0% in the BMMSC group and 86% ± 6.0% in the BMMSC+G-CSF group (Figure 4M). In the control group and G-CSF group, there were no fluorescent cells (data not shown). The rate of Nkx-6 co-expression was 45% ± 7.0% in the BMMSC group and 89% ± 5.0% in the BMMSC+G-CSF group (Figure 4E–4H, 4N). The rate of Pax-4 co-expression was 44% ± 8.0% in the BMMSC group and 84% ± 6.0% in the BMMSC+G-CSF group (Figure 4I–4L, 4O).
Figure 4. Fluorescent microscopic images showing potential in situ proliferation of transplanted BMMSCs in recipient rats with L-arginine–induced acute pancreatitis.
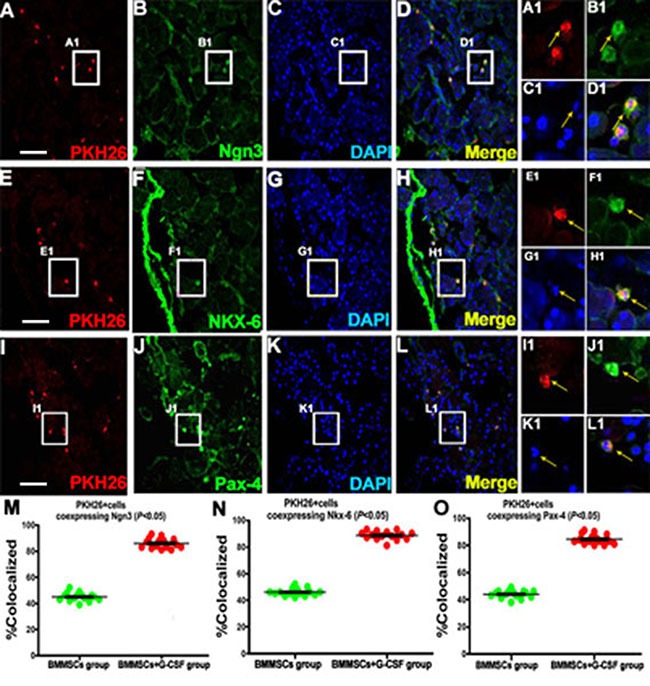
(A, E, I) The left panels show PHK26-positive BMMSCs in the pancreatic tissue in the BMMSC+G-CSF group at 3 weeks after transplantation. These cells show partial colocalization with the molecular markers of pancreatic stem cells or precursors, including (A, B, C, D) Ngn3, (E, F, G, H) Nkx-6 , and (I, J, K, L) Pax-4, in the BMMSC+G-CSF group. Colabeled cells are indicated by arrow. (M, N, O) Plots of the mean percentage ratios of PKH26-positive cells coexpressing Ngn3, Nkx- 6, and Pax-4 relative to the total population of PKH26-positive cells in individual animals with AP survived for 4 weeks after BMMSC transplantation and G-CSF administration. Scale bar = 100 μm for low magnification images, equivalent to 25 μm for enlarged inserts.
Analysis of proliferative mRNA expression in the pancreas of recipient rats
The mRNA levels of proliferative markers were determined using RT-PCR in the pancreatic tissues at 3 weeks after transplantation. The grey value ratio between Ngn3 and β-actin was 1.38 in the BMMSC+G-CSF group, compared to 0.24 in the control group, 0.99 in the BMMSC group and 1.02 in the G-CSF group. The Ngn3 expression in the treatment groups was higher than that in the control group, with the highest level in the BMMSC+G-CSF group (P < 0.05; Figure 5A, 5B). Similarly, there was a statistically significant increase in the expression levels of Pax-4 and Nkx-6 in each group after transplantation treatment (P < 0.05; Figure 5A, 5C, 5D).
Figure 5. Effect of BMMSC transplantation and G-SCF therapy on pancreatic mRNA expression following L-arginine–induced injury in rats.
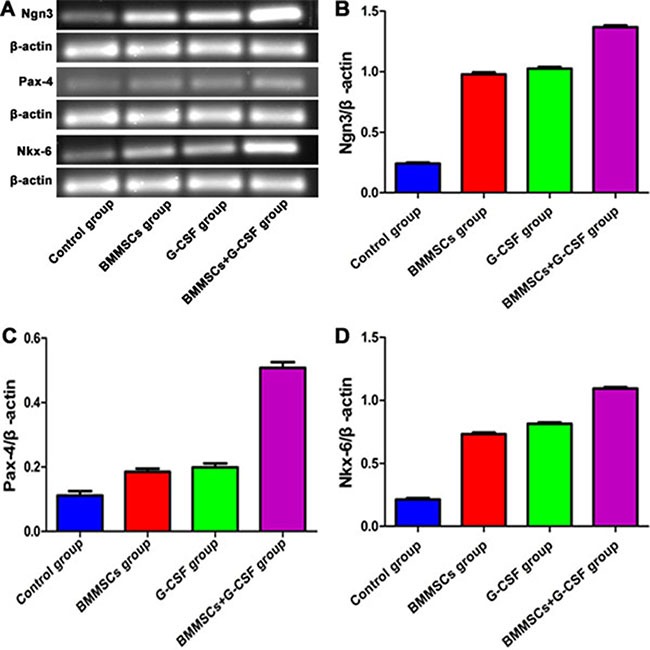
(A) The gene expression levels of Nkx-6, Ngn3, and Pax-4 were determined using RT-PCR analysis at 3 weeks after BMMSC and G-SCF administration. (B) Ngn3 expression was higher in the BMMSC+G-SCF group than in the BMMSC group and G-CSF group (P < 0.05 vs. the control group). (C) The grey value ratio between Pax-4 and β-actin was 0.51 in the BMMSC+G-CSF group, as compared to 0.11 in the control group, 0.18 in the BMMSC group, and 0.192 in the G-CSF group (P < 0.05). (D) The grey value ratio between Nkx-6 and β-actin was 1.10 in the BMMSC+G-CSF group, compared to 0.21 in the control group, 0.73 in the BMMSC group, and 0.82 in the G-CSF group (P < 0.05).
Improvement in serological and histological parameters after transplantation
We withdrew blood samples from the eyeball in each group (n = 3) at different time points (2 d, 3 d, 5 d, 7 d, 2 wk), and determined serological changes in serum amylase and lipase. The data showed that there was no significant difference between the different groups at 2 d (P > 0.05). However, compared to the control group, the treatment groups showed significantly reduced serum amylase and lipase levels at 3 d, 5 d, 7 d, and 2 wk, with the levels in the BMMSC+G-CSF group being the lowest (P < 0.05; Figure 6A, 6B).
Figure 6. Improvement in blood amylase and serum lipase and overall histological parameters after transplantation.

There was no significant difference in serum amylase and serum lipase between the different groups at 2 d (P > 0.05). (A, B) Compared to the control group, the treatment groups showed obviously decreased serum amylase and serum lipase levels at 3 d, 5 d, 7 d, and 2 wk after transplantation, and the levels were lowest in BMMSC+G-SCF group (P < 0.05). (C) The macroscopic evaluation score was significantly lower in the BMMSC+G-SCF group than in the other two treatment groups (P < 0.05).
To determine whether the transplantation treatment was beneficial for pancreatic and overall histological recovery, we used the scoring system as established by Wacke et al. [28] to report histological integrity of the pancreas. The score of the macroscopic evaluation in the BMMSC+G-CSF group was significantly lower than that in the other two treatment groups (P < 0.05; Figure 6C).
DISCUSSION
BMMSCs have potential therapeutic value in repairing tissues or organs in disease conditions. Research has suggested that BMMSC therapy facilitates tissue regeneration and improves functional recovery in a number of central and peripheral systems after acute or chronic injury [18–24, 37–38]. BMMSC transplantation has been considered for the treatment of pancreatitis. This treatment appears to promote the formation of pancreatic stellate cells [24]. Some studies show that transplanted BMMSCs may develop into pancreatic cells in situ [13–17]. Moreover, transplanted BMMSCs can reduce inflammation via downregulation of cytokines in experimental model of AP [17].
Consistent with literature [21, 25], rats treated with l-arginine show the gross anatomical appearance and histological changes of AP in the present study, including inflammatory cell infiltration, acinar necrosis, vacuolization and cavitation. To explore the therapeutic potential of BMMSC transplantation in our experimental AP model, BMMSCs were isolated from adult SD rats and expanded in vitro. The hematopoietic nature of the cultured cells was confirmed with a panel of specific membrane markers of BMMSCs, including CD29, CD34, CD44, CD45 and CD90 [22]. The in vitro expanded BMMSCs were efficiently labeled with PKH26 in order to track these cells in vivo for a few weeks. Following transplantation, the tagged BMMSCs migrate into the damaged tissue of the pancreas in the rat AP model, mostly present in the acini and ducts. These cells were colocalized with Pax-4. Thus, they appear to home in but proliferate in situ in the injured pancreatic tissue [13–17, 25, 26].
The administration of G-CSF is known to mobilize hematopoietic stem cells from the bone marrow into the peripheral blood [27–30]. This soluble factor can mobilize BMMSCs to home in tissue following myocardial or craniocerebral injuries and promote their differentiation towards target tissue cells [19, 20]. Likewise, G-CSF can enhance the proliferation of hepatic progenitor cells in both rodents and humans [31, 32]. G-CSF has been widely used in the treatment of leukemia [33]. The G-CSF receptor is a membrane protein, and Wexler et al. have proved that G-CSF promotes the proliferation of BMMSCs because these cells have G-CSF receptors [34]. G-CSF can alter cytokine expression in the liver, so hepatic stem cells proliferate under the influence of G-CSF [35]. Together, these experiments provide an important theoretical basis for the clinical treatment of AP with G-CSF for the mobilization of stem cells. In our previous experimental study, G-CSF was found to promote BMMSC homing to acutely injured livers in mice [36]. We further demonstrate here that G-CSF could promote BMMSC homing to the acutely injured pancreas to participate in pancreatic regeneration.
It is worth noting that in animals that have not infused with PKH26-tagged BMMSCs, no any fluorescently labeled cells can be found in tissue sections. The overall retention of transplanted BMMSCs in the pancreatic tissue, as assessed microscopically, appears not abundant. Given that the colabled cells are small in number, it is likely that the beneficial effect of BMMSCs may also involve potential soluble factors from the transplantation. This is consistent with the finding that addition of G-CSF improves the overall effect of the stem cell therapy in histological, biochemical and serological perspectives. Therefore, our experiment provides a theoretical basis for utilizing BMMSC transplantation and G-CSF administration in the clinical treatment of AP.
MATERIALS AND METHODS
Animals and main reagents
Male Sprague–Dawley (SD) rats weighing ∼200 g each were used for model preparation, while those weighing 80–100 g were used for extraction of BMMSCs. The animals were obtained from the animal facility of the Second Affiliated Hospital of Harbin Medical University, housed at constant temperature and humidity, with a 12/12 h light/dark illumination cycle and free access to food chow and water. All experimental procedures were approved by the Harbin Medical University Administrative Panel on Laboratory Animal Care. The reagents and medications used are shown below (Table 1).
Table 1. Primary antibodies and main reagents used in the present study.
| Antibody | Source | Product # | Dilution |
|---|---|---|---|
| PE anti-rat CD34 | Santa Cruz | SC-7324 | 1:200 |
| FITC anti-rat CD29 | Biolegend | 555005 | 1:100 |
| APC anti-rat CD90 | Biolegend | 561409 | 1:100 |
| FITC anti-rat CD44 | Biolegend | 550947 | 1:100 |
| PE anti-rat CD45 | Biolegend | 554878 | 1:100 |
| Pax-4 (rabbit IgG) | Santa Cruz | sc-98942 | 1:200 |
| Nkx-6 (goat IgG) | Santa Cruz | sc-15030 | 1:200 |
| Neurogenin3 (goat IgG) | Santa Cruz | Sc-23832 | 1:200 |
PE, phycoerythrin; FITC, fluoroisothiocyanate; APC, allophycocyanin.
AP model preparation and grouping
The experimental model of AP was established via l-arginine administration in adult rats [21]. Animals were fasted for 12 h (allowed to drink) before model preparation, and then intraperitoneally injected with l-arginine (1.5 g/kg in 0.9% normal saline) two times a day, at 1 h interval, for 3 consecutive days. Four l-arginine treated rats were killed after being anesthetized with pentobarbital (50 mg/kg, i.p.) to evaluate the establishment of the model. The remaining animals (n = 60) were divided into four groups: (1) BMMSC transplantation and G-CSF treatment (BMMSC+G-CSF group, n = 15); (2) BMMSC transplantation only (BMMSC group, n = 15) and (3) G-CSF treatment only (G-CSF group, n = 15); and (4) vehicle (saline) infusion (control group, n = 15). Histological, serological and functional data were obtained following animal survival to different time points (Figure 7).
Figure 7. Experimental design.
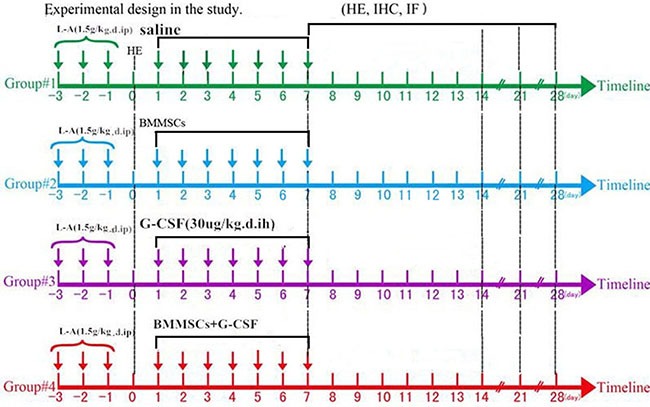
L-Arginine was administrated on days –3 to –1, to animals, and pancreatic tissue was removed and examined visually and using hematoxylin and eosin (H&E) staining on day 0. In the next week (days 1–7), the rats in the different groups were intravenously injected with saline, BMMSCs, G-CSF, or BMMSC+G-CSF through tail vein. Histological, RT-PCR, and serological analyses were carried out from samples from animals surviving for 7, 14, 21, and 28 days.
BMMSC isolation, cultivation, and fluorescent pre-labeling
BMMSC isolation, cultivation and fluorescent pre-labeling were carried out as described previously [24]. Briefly, the femoral bones were removed from anesthetized (sodium pentobarbital, 50 mg/kg, i.p.) animals (n = 4). The medulla ossium was bathed twice with phosphate-buffered saline (PBS); the bone marrow was aspirated and suspended in a sterile centrifuge tube, centrifuged at 1500 rpm for 5 min at 25°C. The supernatant was discarded, and the cells were suspended in complete culture solution, and transferred into a sterile culture bottle. The cultured cells were pre-labeled with the red fluorescent lipophilic dye PKH26 (Sigma-Aldrich, St. Louis, US) [37]. After being blended with 15% complete culture solution and centrifuged at 1500 rpm for 2 min at 25°C, the PKH26-labeled cell suspension was adjusted to a concentration of approximately 3 × 107 cells/mL. Subsequently, the cells with viability greater than 95%, as measured using trypan blue exclusion, were used for cell transplantation.
BMMSC identification by fluorescence-activated cell sorting analysis
Fluorescence-activated cell sorting (FACS) was used to determine the expression of signature antigen markers of BMMSCs. Approximately 1 × 107 cells were incubated in 2% fetal bovine serum in 0.01 M PBS (pH = 7.2) at 4°C for 20 min, and then supplemented with 1.0 μL of monoclonal antibodies to CD29, CD34, CD44, CD45 and CD90, with negative control incubated in fetal bovine serum without primary antibodies. The immunofluorescent signal was monitored and evaluated using the FACS Calibur with CellQuest software (Becton Dickinson, Franklin Lakes, NJ, USA).
BMMSC transplantation and G-SCF administration
BMMSC transplantation was carried out via caudal vein injection [22, 38]. Thus, a total of 1.0 mL of the stem cell suspension (0.9% saline solution suspension containing 1 × 107 cells), or 0.9% normal saline, were injected into a tail vein of a recipient rat at a constant slow speed. G-CSF was administered for 5 days via subcutaneous injection at a dosage of 30 μg/kg/ day.
Pancreatic tissue preparation
Animals were perfused with PBS (pH = 7.4) via the ascending aorta after receiving an overdose of sodium pentobarbital (100 mg/kg, i.p.). The entire pancreas was removed from the abdominal cavity for visual inspection and tissue sampling. The degree of pancreatic injury was scored according to the method described by Wacke et al. [26]. Thus, score + (1) was defined if the pancreas was edematous, swollen, but no visible necrotic areas, calcification or discoloration; score ++ (2) if it was edematous with small solitary necrotic areas and a small number of fine points of calcification; and score +++ (3) if patches of calcification and necrosis were seen on the pancreas as extensive grayish-white areas.
Each pancreas was then divided into three parts. One part was rinsed in cold PBS several times and then immersed in 4% paraformaldehyde for 24 h. This sample was embedded in paraffin, sectioned at a thickness of 3 μm, and used for histology and immunohistochemistry. The second part was also fixed in 4% paraformaldehyde, treated with 10%, 20% and 30% sucrose (every 6 h), sectioned in a cryostat at 4 μm and used for immunofluorescence. The last part was snap-frozen in liquid nitrogen for polymerase chain reaction (PCR) assays. Blood was collected from the rat eyeball and centrifuged at 2000 rpm for 10 min (4°C), with the resultant serum used for biochemical studies.
Histological and immunohistochemical processing and image analysis
Pancreatic sections were stained with hematoxylin and eosin (H.E.). For immunofluorescence, dewaxed or cryostat sections were preincubated for 30 min in PBS containing 5% normal donkey serum, followed by incubations with rabbit anti-Pax-5, goat anti-Nkx-6 or goat anti-Ngn3 antibody, at 4°C overnight. The sections were washed with PBS, and then, incubated with Alexa Fluor® 488 conjugated goat anti-rabbit IgG or rabbit anti-goat IgG (1:200, Invitrogen, USA) for 2 h. The sections were counterstained with DAPI (4′,6-diamidino-2-phenylindole), and mounted with an anti-fading medium before microscopic examination.
Cells with and without colabeling were counted in confocal immunofluorescent images. For each type of double labeling, at least 10 sets of images were obtained from the laminar region of each pancreas tissue sample at 10×. At least 200 cells were analyzed to determine the rate of colocalization between two given markers in each animal.
Reverse transcription PCR
RNA was extracted using the TRIzol reagent (Sangon Biotech, Shanghai) according to the manufacturer's instructions and stored at −70°C until use. Next, cDNAs were synthesized by reverse transcription (RT) using a commercial kit (Sangon Biotech, Shanghai). The following forward (F) and reverse (R) primers were used for RT-PCR amplifications.
Nkx-6 (293 bp), 5′-AAGGGGACTTCGGAGAA TGA-3′ (F) and 5′-CCGCCACAATTTCTAGGTTAA-3′ (R); Pax-4 (293 bp), 5′-GCCCTCTGCTCCTGAGTGAA-3′ (F) and 5′-GCCAACTGGCAAACTGAAAA-3′ (R); Ngn3 (170 bp), 5′-CACTCAGCAAACAGCGAAGA-3′ (F) and 5′-GATGTAGTTGTGGGCGAAGC-3′ (R); and rat β-actin (157 bp), 5′-TTTTCCAGCCTTCCTTCTTG-3′ (F) and 5′-CAATGCCTGGGTACATGGTGGTACC-3′ (R).
Statistical analysis
Data were expressed as mean ± SEM. Statistical analyses were performed using two-way analysis of variance (ANOVA) for repeated measures (Prism GraphPad 4.1, San Diego, CA). P < 0.05 was considered statistically significant. Figures were assembled with Photoshop 7.1 and saved as TIFF files.
CONCLUSIONS
The present study shows that in vitro expanded BMMSCs may relocate to and proliferate locally in injured pancreas in a rat model of acute pancreatitis. While BMMSC transplantation alone shows a certain degree of therapeutic effect on tissue repair, co-administration of G-CSF has a synergetic benefit by improving the histopathological, serological and functional recovery of pancreas following the experimental injury.
Abbreviations
- AP
Acute pancreatitis
- BMMSCs
Bone marrow mesenchymal stem cells
- G-CSF
granulocyte colony-stimulating factor
- MSCs
mesenchymal stem cells
- PBS
phosphate-buffered saline
- FACS
fluorescence-activated cell sorting
Footnotes
CONFLICTS OF INTERESTS
The authors declare that they have no competing interests.
FUNDING
This study was supported by no foundation.
Authors’ contributions
BQ performed the experiments and wrote the manuscript with the help of YJC, FZ, BBW. TL and BY contributed to isolate and characterize BMMSCs. YJC and FZ contributed to histological procedures. BQ, BBW and TL interpreted the data. SZJ conceived and designed the research. JSZ revised the manuscript critically for important intellectual content. All authors read and approved the manuscript.
REFERENCES
- 1.Capurso G, Marignani M, Piciucchi M, Merola E, Delle FG. Probiotics and severe acute pancreatitis. Jop Journal of the Pancreas. 2008;7:92–93. [Google Scholar]
- 2.Schneider DT, Nürnberger W, Stannigel H, Bönig H, Göbel U. Adjuvant treatment of severe acute pancreatitis with C1 esterase inhibitor concentrate after haematopoietic stem cell transplantation. Gut. 1999;45:733–736. doi: 10.1136/gut.45.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xue XJ, Tu XH, Chen SQ. Progress in the tretment of acute pancreatitis with stem cells. Basic & Clinical Medicine. 2010;30:1109–1111. [Google Scholar]
- 4.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Biology Physics Humanities. 2005;24:45–51. doi: 10.1097/01.shk.0000191413.94461.b0. [DOI] [PubMed] [Google Scholar]
- 6.Lankisch PG, Lerch MM. Pharmacological prevention and treatment of acute pancreatitis: where are we now? Dig Dis. 2006;24:148–159. doi: 10.1159/000090318. [DOI] [PubMed] [Google Scholar]
- 7.Cui HF, Bai ZL. Protective effects of transplanted andmobilized bonemarrowstem cells on mice with severe acute pancreatitis. World J Gastroenterol. 2003;9:2274–2277. doi: 10.3748/wjg.v9.i10.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider G, Saur D. Mesenchymal stem cells: therapeutic potential for acute pancreatitis. Gastroenterology. 2011;140:779–782. doi: 10.1053/j.gastro.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Brisinda G, Vanella S, Crocco A, Mazzari A, Tomaiuolo P, Grossi U, Cructti A. Severe acute pancreatitis: advances and insights in assessment of severity and management. Eur J Gastroenterol Hepatol. 2011;23:541–5451. doi: 10.1097/MEG.0b013e328346e21e. [DOI] [PubMed] [Google Scholar]
- 10.Marrache F, Pendyala S, Bhagat G, Betz KS, Song Z, Wang TC. Role of bone marrow-derived cells in experimental chronic pancreatitis. Gut. 2008;57:1113–1120. doi: 10.1136/gut.2007.143271. [DOI] [PubMed] [Google Scholar]
- 11.Jongpaiboonkit L, King WJ, Murphy WL. Screening for 3D environments that support human mesenchymal stem cell viability using hydrogel arrays. Tissue Eng Part A. 2009;15:343–353. doi: 10.1089/ten.tea.2008.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higuchi K, Umegaki E, Watanabe T, Yoda Y, Morita E, Murano M, Tokioka S, Arakawa T. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44:879–888. doi: 10.1007/s00535-009-0102-2. [DOI] [PubMed] [Google Scholar]
- 13.Tu XH, Song JX, Xue XJ, Guo XW, Ma YX, Chen ZY, Zou ZD, Wang L. Role of bone marrow-derived mesenchymal stem cells in a rat model of severe acute pancreatitis. World J Gastroenterol. 2012;18:2270–2279. doi: 10.3748/wjg.v18.i18.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin G, Hu G, Wan R, Yu G, Cang X, Ni J, Xiong J, Hu Y, Xing M, Fan Y, Xiao W, Qiu L, Wang S, Wang X. Role of bone marrow mesenchymal stem cells in L-arg-induced acute pancreatitis: effects and possible mechanisms. Int J Clin Exp Pathol. 2015;8:4457–4468. [PMC free article] [PubMed] [Google Scholar]
- 15.Qian D, Gong J, He Z, Hua J, Lin S, Xu C, Meng H, Song Z. Bone Marrow-Derived Mesenchymal Stem Cells Repair Necrotic Pancreatic Tissue and Promote Angiogenesis by Secreting Cellular Growth Factors Involved in the SDF-1 α /CXCR4 Axis in Rats. Stem Cells Int. 2015;2015:3945–3948. doi: 10.1155/2015/306836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Q, Wang F, Hou Y, Chen S, Wang B, Lu F, Huang H, Chen Y. The effect of allogenetic bone marrow-derived mesenchymal stem cell transplantation on lung aquaporin-1 and -5 in a rat model of severe acute pancreatitis. Hepatogastroenterology. 2012;59:965–976. doi: 10.5754/hge12094. [DOI] [PubMed] [Google Scholar]
- 17.Jung KH, Yi T, Son MK, Song SU, Hong SS. Therapeutic effect of human clonal bone marrow-derived mesenchymal stem cells in severe acute pancreatitis. Arch Pharm Res. 2015;38:742–751. doi: 10.1007/s12272-014-0465-7. [DOI] [PubMed] [Google Scholar]
- 18.Piao H, Youn TJ, Kwon JS, Kim YH, Bae JW, DW Bora-Sohn Kim, Lee MM, Park YB. Effects of bone marrow derived mesenchymal stem cells transplantation in acutely infracting myocardium. Eur J Heart Fail. 2005;7:730–738. doi: 10.1016/j.ejheart.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Weaver CH, Buckner CD, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Syngeneic transplantation with peripheral blood mononuclear cells collected after the administration of recombinant human granulocyte colony-stimulating factor. Blood. 1993;82:1981–1984. [PubMed] [Google Scholar]
- 20.Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, Li H. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110:1847–1854. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- 21.Sidhu S, Pandhi P, Malhotra S, Vaiphei K, Khanduja KL. Beneficial Effects of Emblica officinalis in l-Arginine-Induced Acute Pancreatitis in Rats. Journal of medicinal food. 2011;14:147–155. doi: 10.1089/jmf.2010.1108. [DOI] [PubMed] [Google Scholar]
- 22.Ohishi M, Schipani E. Bone marrow mesenchymal stem cells. J Cell Biochem. 2010;109:277–282. doi: 10.1002/jcb.22399. [DOI] [PubMed] [Google Scholar]
- 23.Suh KT, Kim SW, Roh HL, Youn MS, Jung JS. Decreased osteogenic differentiation of mesenchymal stem cells in alcohol-induced osteonecrosis. Clin Orthop Relat Res. 2005;431:220–225. doi: 10.1097/01.blo.0000150568.16133.3c. [DOI] [PubMed] [Google Scholar]
- 24.Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20:5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J, Watanabe M. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011–1017. doi: 10.1038/nm755. [DOI] [PubMed] [Google Scholar]
- 26.Wacke R, Park S, Mundkowski RG, Block N, Kuhn-Thiel A, Drewelow B. The penatration of moxifloxacin into the pancreas of male rats in experimental acute necrotizing pancreatitis. Chemotherapy. 2003;49:167–171. doi: 10.1159/000071140. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Bosch O, Ricart E, Panes J. Review article: stem cell therapies for inflammatory bowel disease - efficacy and safety. Aliment Pharmacol Ther. 2010;32:939–952. doi: 10.1111/j.1365-2036.2010.04439.x. [DOI] [PubMed] [Google Scholar]
- 28.Henseleit KD, Nelson SB, Kuhlbrodt K, Henninqs JC, Ericson J, Sander M. NKX6 transcription factor activity is required fora- and β-cell development in the pancreas. Development. 2005;132:3139–3149. doi: 10.1242/dev.01875. [DOI] [PubMed] [Google Scholar]
- 29.Liang XD, Guo YY, Sun M, Ding Y, Wang N, Yuan L, De W. Streptozotocin-induced expression of Ngn3 and Pax4 in neonatal rat pancreatic α-cells. World J Gastroentrol. 2011;17:2812–2820. doi: 10.3748/wjg.v17.i23.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–2808. [PubMed] [Google Scholar]
- 31.Piscagia AC, SH Shupe TD Oh, Gasbarrini A, Petersen BE. Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats. Gastroenterology. 2007;133:619–631. doi: 10.1053/j.gastro.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spahr L, Lambert JF, Rubbia-Brandt L, Chalandon Y, Frossard JL, Giostra E, Hadenque A. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology. 2008;48:221–229. doi: 10.1002/hep.22317. [DOI] [PubMed] [Google Scholar]
- 33.Konuma T, Ooi J, Uchida N, Ogawa H, Ohashi K, Kanamori H, Aotsuka N, Onishi Y, Yamaguchi H, Kozai Y, Nagamura-Inoue T, Kato K, Suzuki R, Atsuta Y, Kato S, Asano S, Takahash S. Granulocyte colony-stimulating factor combined regimen in cord blood transplantation for acute myeloid leukemia: a nationwide retrospective analysis in Japan. Haematologica. 2014;99:e264–e268. doi: 10.3324/haematol.2014.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wexler SA, Donaldson C, Denning-Kendall P, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal stem cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368–374. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 35.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovasularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodelling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 36.Jin JZ, Meng XW, Sun X, Han MZ, Liu BR, Wang XH, Sun LY, Huang Q, Zhao BB, Ban X, Yu HY, Yu HW. Granulocyte colony-stimulating factor enhances bone marrow mononuclear cell homing to the liver in a mouse model of acute hepatic injury. Dig Dis Sci. 2010;55:2805–2813. doi: 10.1007/s10620-009-1117-5. [DOI] [PubMed] [Google Scholar]
- 37.Qi S, Wu D. Bone marrow-derived mesenchymal stem cells protect against cisplatin-induced acute kidney injury in rats by inhibiting cell apoptosis. Int J Mol Med. 2013;32:1262–1272. doi: 10.3892/ijmm.2013.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Ge S, McNamara G, Hao QL, Crooks GM, Nolta JA. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003;101:4201–4208. doi: 10.1182/blood-2002-05-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]


