Abstract
Previously, we created an eight hour limited-access dual bottle drinking paradigm to deliver methylphenidate (MP) to rats at two dosages that result in a pharmacokinetic profile similar to patients treated for Attention Deficit Hyperactivity Disorder. Chronic treatment resulted in altered behavior, with some effects persisting beyond treatment. In the current study, adolescent male Sprague-Dawley rats were split into three groups at four weeks of age: control (water), low dose MP (LD), and high dose MP (HD). Briefly, 4 mg/kg (low dose; LD) or 30 mg/kg (high dose; HD) MP was consumed during the first hour, and 10 mg/kg (LD) or 60 mg/kg (HD) MP during hours two through eight. Following three months of treatment, half of the rats in each group (n=8–9/group) were euthanized, and remaining rats went through a one month abstinence period, then euthanized. In vitro receptor autoradiography was performed to quantify binding levels of dopamine transporter (DAT), dopamine type 1 (D1R) -like receptors, and dopamine type 2 (D2R) -like receptors using [3H] WIN35,428, [3H] SCH23390, and [3H] Spiperone, respectively. Immediately following treatment, HD MP treated rats had increased DAT and D1R-like binding in several subregions of the basal ganglia, particularly more caudal portions of the caudate putamen, which correlated with some previously-reported behavioral changes. There were no differences between treatment groups in any measure following abstinence. These findings suggest that chronic treatment with a clinically relevant high dose of MP results in reversible changes in dopamine neurochemistry, which may underlie some effects on behavior.
Keywords: Methylphenidate; Psychostimulant; Dopamine; Transporter; Reward Deficiency Syndrome, reward, addiction
1. Introduction
The psychostimulant methylphenidate (MP) is one of the most frequently prescribed medications used to treat Attention Deficit Hyperactivity Disorder (ADHD) (Greenhill et al. 2002). The prevalence rate of ADHD has jumped to 11% of school-aged children, up 41% over the last decade (Visser et al. 2014). Two-thirds of children diagnosed with ADHD are treated with psychostimulants such as MP, which are also used illegally as cognitive enhancers among adolescents and adults and abused recreationally (McCabe et al. 2006; Wilens et al. 2008). In the United States, studies have found that up to 30% of college students surveyed report using MP illicitly (Bogle and Smith 2009; McCabe et al. 2005; Teter et al. 2006). These striking statistics regarding the increasing use and abuse of MP present concern, as the brain is particularly susceptible to the effects of drugs during adolescence (Dahl 2004; Spear 2000) due to the sprouting and pruning of synapses and changes in neurotransmitter concentrations and receptor levels in several regions such as the prefrontal cortex, hippocampus, and limbic system (Giedd et al. 2008; Rice and Barone 2000; Spear 2000).
MP’s mechanism of action is partly mediated by its ability to block dopamine transporters (DAT) and increase levels of extracellular dopamine (DA) in the prefrontal cortex (PFC) and striatum, while also increasing norepinephrine in the PFC and hippocampus (Bymaster et al. 2002; Kuczenski and Segal 2002; Volkow et al. 2002; Volkow et al. 2001). Therefore, determining the effects of long-term MP treatment on catecholamine systems is of great interest. Preclinical studies investigating the effects of chronic exposure to MP during development have shown changes in the catecholamine systems in the medial PFC (m PFC), hippocampus, striatum, and hypothalamus (Gray et al., 2007). Specifically, tyrosine hydroxylase-immunoreactivity was increased in the m PFC and decreased in the medial striatum following exposure, while norepinephrine transporter density was decreased in the m PFC and hippocampal dentate gyrus (Gray et al., 2007). In addition, there was an increase in Nissl-stained cells in the m PFC and neuropeptide Y- immunoreactivity in the hypothalamus (Gray et al., 2007). Moreover, chronic MP treatment in rats has resulted in changes in DAT and dopamine receptor levels (Gray et al. 2007; Izenwasser et al. 1999; Randall and Hannigan 1999; Thanos et al. 2007).
Most previous studies on the effects of MP treatment on neurochemistry, however, are confounded by the fact that they have utilized an MP dosing regimen that does not mimic the clinical scenario. In ADHD treatment, oral doses of 0.25–1 mg/kg MP are prescribed, resulting in plasma concentrations of 8–40 ng/mL. Most previous studies on MP have injected the drug either subcutaneously or intraperitoneally, which differs significantly from oral administration, specifically with respect to time to peak serum concentration, half-life, and rate of elimination (Kuczenski and Segal 2005), as well as absolute magnitude and time course of increases in extracellular DA and locomotor responses (Gerasimov et al. 2000; Kuczenski and Segal 2001). In these studies, doses were used that have been shown to produce plasma concentrations at the very highest end of the spectrum for clinical relevance (Kuczenski and Segal 2005). Nearly constant dosing would be necessary to maintain clinically-relevant plasma concentrations due to the faster metabolism and shorter half-life of MP in rats compared to humans, whereas previous studies in rodents have generally dosed animals only once or twice per day (Kuczenski and Segal 2005). Moreover, most studies included treatment lengths lasting only a few days to a few weeks. With an estimated 50–60% of ADHD cases with symptoms persisting into adulthood (Mick et al. 2004), it is necessary to determine more long-term effects of MP, which may persist long after the cessation of treatment.
Previously, we developed a dual-bottle eight hour limited-access drinking paradigm that allowed MP to be consumed voluntarily in the rats’ drinking water (Thanos et al. 2015). Two dosing regimens were created: 4 mg/kg MP (low dose; LD) or 30 mg/kg MP (high dose; HD) during the first hour, and 10 mg/kg (LD) or 60 mg/kg (HD) MP from hours 2 through 8. In rats, this dosing produces plasma MP concentrations stably within the clinical pharmacokinetic range (peaking at ~8 ng/mL for the LD and ~30 ng/mL for the HD) (Thanos et al. 2015). A control group was provided with water only for the entire 8 hour drinking period. We previously examined the developmental and behavioral effects of these treatments from adolescence to young adulthood in male Sprague Dawley rats, both during the 3 month treatment and following a 1 month abstinence period, to investigate long-lasting consequences of MP use. While LD MP treatment had few effects, HD MP treatment resulted in changes in food intake, body weight, open field activity, and circadian locomotor activity, with some effects persisting through the abstinence period. It remains to be determined, however, the effects of chronic MP exposure on dopamine neurochemistry, which may underlie some of these long-lasting changes in behavior. Assessing MP-induced changes to the dopaminergic system is of great interest, as MP acts on this system, and dopamine is also involved in behaviors previously known to be affected (e.g. feeding and locomotor behavior). Effects of MP on the dopaminergic system also have implications on the drug’s abuse potential and ability to alter susceptibility to other drugs later in life. Therefore, the current study aims to determine the effects of chronic oral MP treatment, with and without abstinence, on DAT binding, as well as D1R-like and D2R-like receptor levels, in relevant regions of the brain.
2. Material and Methods
2.1 Animals
Four week old male Sprague Dawley rats (Taconic, Hudson, New York USA) were individually housed in a controlled room (22 ± 2°C and 40–60% humidity) with a 12h reverse light-dark cycle (lights off 0800h). Rats were split into three groups: control (drinking only water), low dose MP (LD), or high dose MP (HD). Rats received respective treatment using a previously established dual-bottle eight-hour limited access drinking paradigm, which allowed for an MP pharmacokinetic profile similar to that observed in patients treated with MP (peaking at about 8–10 ng/mL for the LD group and ~25 ng/mL for the HD group) (Thanos et al. 2015). This method also allows for voluntary drinking, reducing stress induced by the gavage method. Rats received 4 mg/kg MP (LD) or 30 mg/kg MP (HD) during the first hour (09:00–10:00), and 10 mg/kg (LD) or 60 mg/kg MP (HD) for the remaining seven hours (10:00–17:00). A lower dose is given in the first bottle, as rats usually consume a larger volume quickly following overnight fluid restriction, bringing plasma concentrations to target range. The second higher dose produces a fairly stable plasma concentration in the target range via intermittent drinking of smaller volumes throughout the rest of the limited access period.
Following a three-month treatment period, half of the rats in each group (n=8/group) were euthanized. Remaining rats (n=9/group for water treated, n=8/group for LD and HD MP treated) underwent a one month abstinence period, during which they were given only water to drink for the entire eight hour limited access drinking period daily, then euthanized. Purina laboratory rat chow was available ad libitum for the entire experiment. All experiments were conducted in conformity with the National Academy of Sciences Guide for Care and Use of Laboratory Animals (National Academy of Sciences NRC, 1996) and approved by the University Institutional Animal Care and Use Committee.
2.2 Drugs
Methylphenidate hydrochloride (Sigma Aldrich, St. Louis, MO) was dissolved in distilled water to produce the 4, 10, 30, and 60 mg/kg solutions. Individual rats’ drinking bottles were made fresh daily based on body weight and the average of their last three days’ fluid intake to achieve desired dosages.
2.3 In vitro Receptor Autoradiography
2.3.1 Tissue preparation
Twenty-four hours following the respective treatment or abstinence period, rats were euthanized under deep isoflurane anesthesia (~3.0%). Brains were harvested, flash frozen, stored at −80°C until cryosectioned at 14 μm, and mounted on glass microscope slides. Tissue sections were stored at −80°C until in-vitro receptor autoradiography (ARG) was performed.
2.3.2 Dopamine Transporter (DAT) Binding
Protocol for dopamine transporter (DAT) binding was adapted from previous studies (Coulter et al. 1997; Wilson et al. 1994). Briefly, slides were pre-incubated for 10 minutes at 4°C in 30mM sodium phosphate buffer (pH = 7.4). Slides were incubated for 90 minutes at 4°C in 30mM sodium phosphate buffer solution with 0.32 M sucrose (pH = 7.4) and in the presence of 6.5 nM [3H] WIN 35, 428 (specific activity = 76 Ci/mmol). Non-specific binding was determined in the presence of 60 μM cocaine. Slides were dipped then washed 2 × 1 minutes in pre-incubation buffer and dipped in dH2O, all at 4°C.
2.3.3 Dopamine Type 1 Receptor (D1R)-like Binding
Protocol for dopamine type 1-like receptor (D1R) binding was adapted from previous studies (Tarazi et al. 1998; Tarazi et al. 1997; Tarazi et al. 1999). Briefly, slides were pre-incubated for 60 minutes at room temperature in 50mM Tris HCl buffer (120 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, pH = 7.4). Slides were incubated for 60 minutes at room temperature in pre-incubation buffer in the presence of 2.5 nM [3H] SCH 23390 (SA=85 Ci/mmol) and 40 nM ketanserin. Non-specific binding was determined in the presence of 1 μM flupenthixol. Slides were washed 2 × 5 minutes at 4°C in pre-incubation buffer followed by a dip at 4°C in dH2O.
2.3.4 Dopamine Type 2 Receptor (D2R)-like Binding
Protocol for dopamine type 2-like receptor (D2R) binding was adapted from a previous study (Tarazi et al. 1997). Briefly, slides were pre-incubated for 60 minutes at room temperature in 50mM Tris HCl buffer (120 mM NaCl, 5 mM KCl, 2 mM CaCl2,1 mM MgCl2, pH = 7.4). Slides were incubated for 60 minutes at room temperature in pre-incubation buffer in the presence of 0.5 nM [3H] Spiperone (specific activity = 16.2 Ci/mmol) and 40 nM ketanserin. Non-specific binding was determined in the presence of 10 μM sulpride. Slides were washed 2 × 5 minutes at 4°C in pre-incubation buffer followed by a dip at 4°C in dH2O.
2.4 Autoradiography Analysis
Slides were dried and apposed to Kodak MR Film (DAT: 12 weeks, D1: 4 weeks, D2: 10 weeks). Film was scanned at 1200 dpi, and images were quantified using Image J software (NIH). Figure 1 shows the regions of interest (ROI’s) analyzed, including the dorsolateral (dl PFC) and medial (m PFC) prefrontal cortex, olfactory tubercle (OT), nucleus accumbens [split into pole of the core (Pole AcbC), pole of the shell (Pole AcbS), core (AcbC) and shell (AcbS)], ventral pallidum (VP), rostral and middle caudate putamen [each split into four quadrants: dorsolateral (dI CPu), dorsomedial (dm CPu), ventrolateral (vl CPu), and ventromedial (vm CPu)], and tail of the caudate putamen [split in half into dorsal (d CPu tail) and ventral (v CPu tail) portions]. Binding in each ROI was measured in uCi/g.
Fig 1.
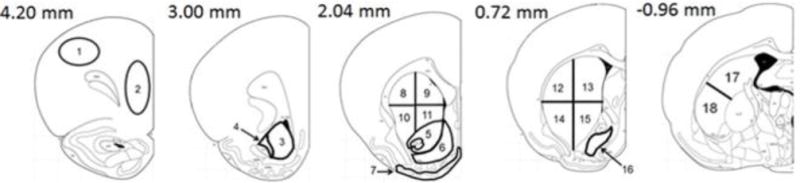
Adapted schematic of coronal brain sections showing regions of interest analyzed (Paxinos and Watson 2006): 1 dorsolateral prefrontal cortex (dl PFC), 2 medial prefrontal cortex (m PFC), 3 pole of the nucleus accumbens shell (Pole AcbS), 4 pole of the nucleus accumbens core (Pole AcbC), 5 nucleus accumbens core (AcbC), 6 nucleus accumbens shell (AcbS), 7 olfactory tubercle (OT), 8 rostral dorsolateral caudate putamen (rost dl CPu), 9 rostral dorsomedial. caudate putamen (rost dm CPu), 10 rostral ventrolateral caudate putamen (rost vl CPu), 11 rostral ventromedial caudate putamen (rost vm CPu), 12 middle dorsolateral caudate putamen (mid dl CPu), 13 middle dorsomedial caudate putamen (mid dm CPu), 14 middle ventrolateral caudate putamen (mid vl CPu), 15 middle ventromedial caudate putamen (mid vm CPu), 16 ventral pallidum (VP), 17 dorsal tail of the caudate putamen (d CPu tail), 18 ventral tail of the caudate putamen (v CPu tail). Measures on the top left of each section indicates approximate distance from bregma.
2.5 Statistical Analysis
Three way repeated measures ANOVAs [between-subjects factors: treatment (water, LD: 4/10 mg/kg MP, or HD: 30/60 mg/kg MP) and abstinence (rats euthanized immediately following treatment or following an abstinence period); within-subjects factor: region of interest (ROI)] were used to analyze autoradiography data by ligand separately. ANOVAs were followed by multiple pairwise comparisons (Fisher method). Pearson correlations were run between D1R-like, D2R-like, and DAT binding within ROI’s, as well as autoradiography binding measures and food intake, body weight, and dark cycle circadian locomotor activity, for rats euthanized immediately following treatment and abstinence separately. Food intake, body weight, and dark cycle circadian locomotor activity data used for correlations were previously reported (Thanos et al., 2015). Statistical significance was set at α=0.05 for ANOVA main effects and interactions, and α=0.01 for ANOVA pairwise comparisons and correlations to control for multiple tests. All statistical tests were run using SigmaStat 3.5 software.
3. Results
3.1 In vitro Autoradiography for Dopamine Transporter Binding
A three way repeated measures ANOVA was performed to determine the effects of MP treatment, with and without abstinence, on dopamine transporter binding in each region of interest (Figure 2). The main effects of abstinence [F(1,43)=6.046, p=0.018] and ROI [F(17,731)=97.016, p<0.001] were significant, as were the treatment × ROI [F(34,731)=1.892, p=0.002], abstinence × ROI [F(17,731)=4.523, p<0.001], and treatment × abstinence × ROI [F(34,731)=1.726, p=0.007] interactions. The main effect of treatment [F(2,43)=1.871, p=0.166] and the treatment × abstinence interaction [F(2,43)=1.056, p=0.357] were not significant. Pairwise comparison testing of the treatment × abstinence × ROI interaction found that HD MP treatment increased DAT binding in several ROI’s in rats euthanized immediately following treatment. HD MP rats had greater binding in the mid dl CPu, mid dm CPu, mid vl CPu, mid vm CPu, and v CPu tail compared to water treated rats, and in the mid dl CPu, mid dm CPu, mid vl CPu, and mid vm CPu compared to LD MP treated rats (p≤0.01 for all). LD MP rats did not differ from water treated rats in any ROI measured, and there were no differences between treatment groups in rats euthanized following an abstinence period.
Figure 2.
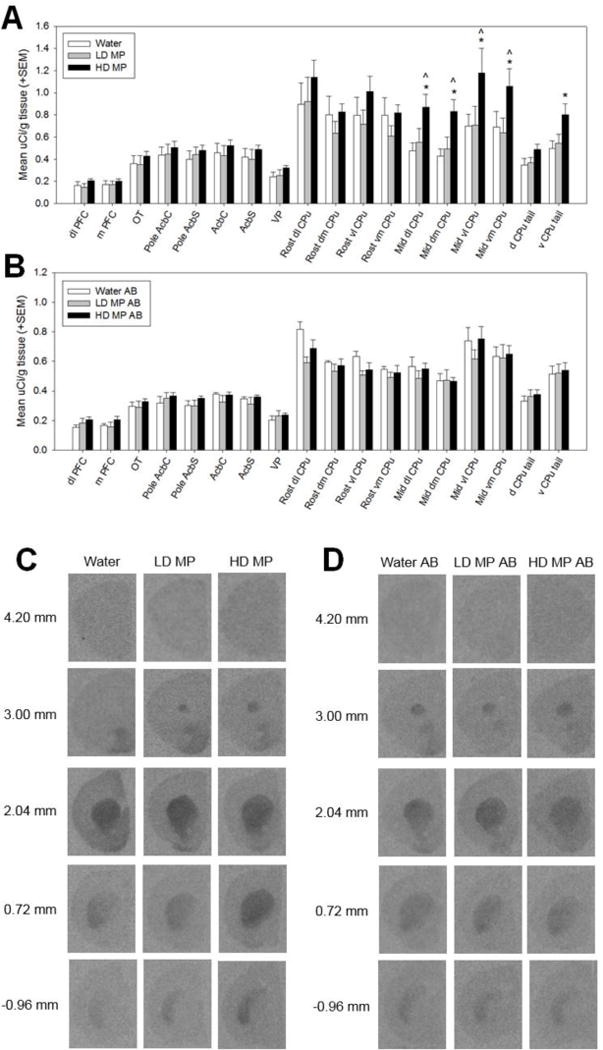
Dopamine transporter (DAT) binding following chronic oral methylphenidate treatment (A&C) and abstinence (AB) (B&D). (A) High dose (HD MP) treated rats had increased DAT binding in the middle dorsolateral caudate putamen (mid dl CPu), middle dorsomedial caudate putamen (mid dm CPu), middle ventrolateral caudate putamen (mid vl CPu), middle ventromedial caudate putamen (mid vm CPu), and ventral tail of the caudate putamen (v CPu tail) compared to water treated rats, and in the mid dl CPu, mid dm CPu, mid vl CPu, and mid vm CPu compared to low dose (LD MP) treated rats (p≤0.01 for all). *HD MP vs. water, ∧HD MP vs. LD MP. (B) There were no significant differences in DAT binding between treatment groups in rats euthanized after an abstinence period. (C&D) Autoradiographic images of dopamine transporter binding with [3H] WIN35,428. Images represent brain sections from rats treated with water, low dose methylphenidate (LD MP), and high dose methylphenidate (HD MP) and euthanized immediately following treatment (C) or after an abstinence (AB) period (D). Measures on left show approximate distance from Bregma.
3.2 In vitro Autoradiography for Dopamine Type 1 Receptor (D1R)-like Binding
A three way repeated measures ANOVA was performed to determine the effects of MP treatment, with and without abstinence, on dopamine type 1 receptor (D1R)-like binding in each region of interest (Figure 3). The main effect of ROI [F(17,731)=993.03, p<0.001] and the treatment × ROI [F(34,731)=1.92, p=0.001] and treatment × abstinence × ROI [F(34,731)=1.68, p=0.010] interactions were significant. The main effects of treatment [F(2,43)=0.86, p=0.432] and abstinence [F(1,43)=2.06, p=0.158], and the treatment × abstinence [F(2,43)=2.30, p=0.112] and abstinence × ROI [F(17,731)=0.89, p=0.586] interactions were not significant. Pairwise comparison testing of the treatment × abstinence × ROI interaction found that HD MP treatment increased D1R binding in several ROI’s in rats euthanized immediately following treatment. HD MP rats had greater binding in the OT, mid dl CPu, mid dm CPu, mid vm CPu, and d CPu tail compared to water treated rats, and in the OT, vm CPu, and d CPu tail compared to LD MP treated rats (p<0.01 for all). LD MP rats did not differ from water treated rats in any ROI measured, and there were no differences between treatment groups in rats euthanized following an abstinence period.
Figure 3.
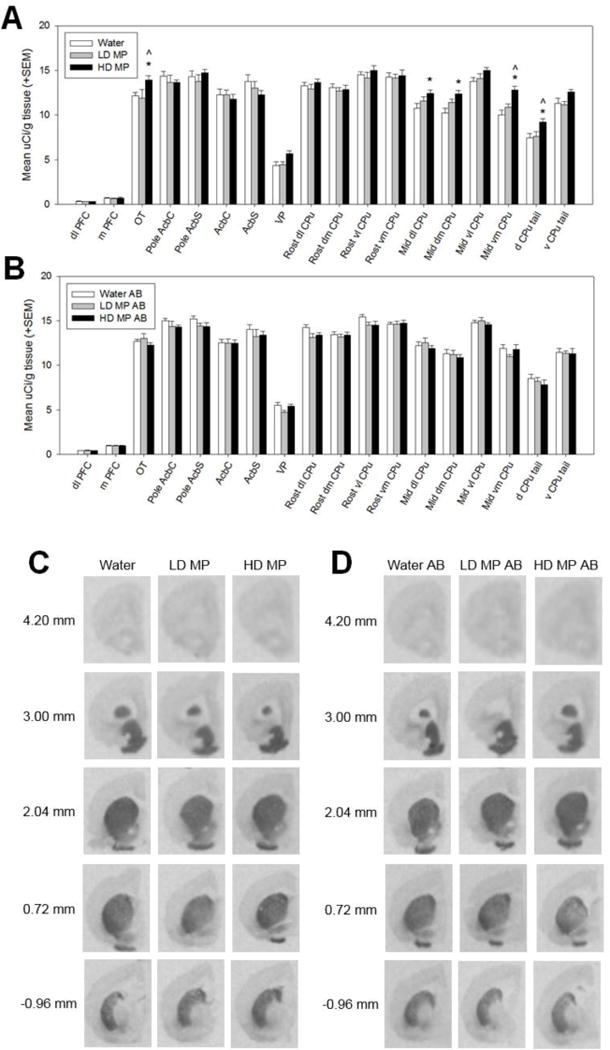
Dopamine type 1-like receptor (D1R) binding following chronic oral methylphenidate treatment (A&C) and abstinence (AB) (B&D). (A) High dose (HD MP) treated rats had increased D1R-like binding in the olfactory tubercle (OT), middle dorsolateral caudate putamen (mid dl CPu), middle dorsomedial caudate putamen (mid dm CPu), middle ventromedial caudate putamen (mid vm CPu), and dorsal tail of the caudate putamen (d CPu tail) compared to water treated rats, and in the OT, vm CPu, and d CPu tail compared to LD MP treated rats (p≤0.01 for all). *HD MP vs. water, ∧HD MP vs. LD MP. (B) There were no significant differences in D1R-like binding between treatment groups in rats euthanized after an abstinence period. (C&D) Autoradiographic images of dopamine type 1-like receptor binding with [3H] SCH23390. Images represent brain sections from rats treated with water, low dose methylphenidate (LD MP), and high dose methylphenidate (HD MP) and euthanized immediately following treatment (C) or after an abstinence (AB) period (D). Measures on left show approximate distance from Bregma.
3.3 In vitro Autoradiography for Dopamine Type 2 Receptor (D2R)-like Binding
A three way repeated measures ANOVA was performed to determine the effects of MP treatment, with and without abstinence, on dopamine type 2 receptor (D2R)-like binding in each region of interest (Figure 4). Only the main effect of ROI [F(17,731)=182.43, p<0.001] and the abstinence × ROI interaction [F(17,731)=16.151, p<0.001] were significant. There was no significant main effect of treatment [F(2,43)=0.956, p=0.392], nor was there any significant interaction of treatment with any of the other factors: treatment × abstinence [F(2,43)=0.061, p=0.941], treatment × ROI [F(34,731)=0.748, p=0.851], treatment × abstinence × ROI [F(34,731)=0.461, p=0.997]. The main effect of abstinence was also not significant [F(1,43)=0.087, p=0.770].
Figure 4.
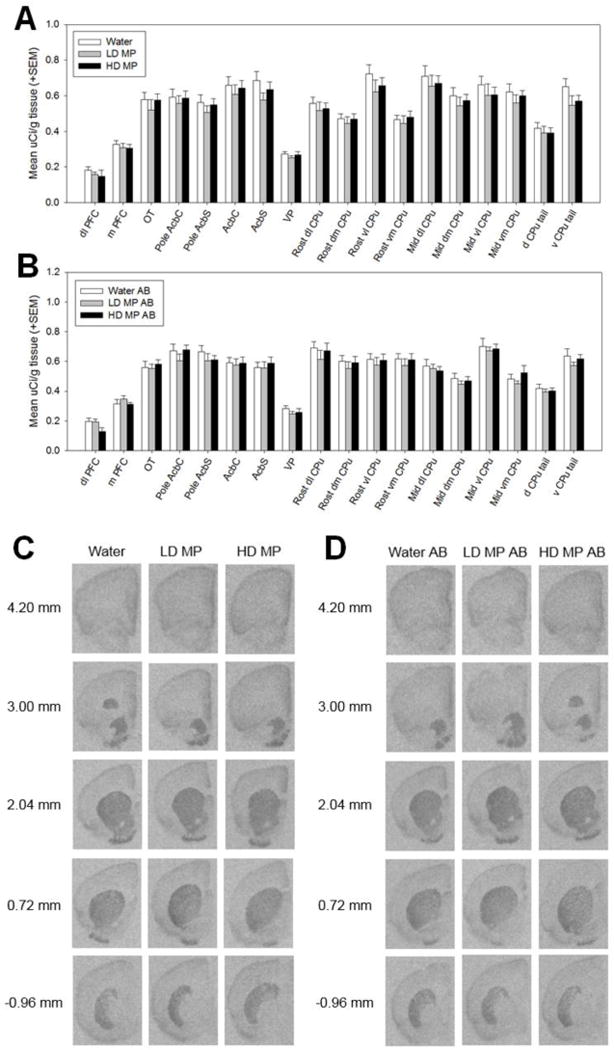
Dopamine type 2-like receptor (D1R) binding following chronic oral methylphenidate treatment (A&C) and abstinence (AB) (B&D). There were no significant differences in D2R-like binding between treatment groups in rats euthanized immediately following abstinence or after an abstinence period. (C&D) Autoradiographic images of dopamine type 2-like receptor binding with [3H] Spiperone. Images represent brain sections from rats treated with water, low dose methylphenidate (LD MP), and high dose methylphenidate (HD MP) and euthanized immediately following treatment (C) or after an abstinence (AB) period (D). Measures on left show approximate distance from Bregma.
3.4 Correlations
3.4.1 Correlations in rats euthanized following treatment
Correlations were run between D1R-like, D2R-like, and DAT binding, as well as autoradiography binding measures and behavior, for rats euthanized immediately following treatment (Table 1). Positive correlations between DAT and D1R-like binding levels approached significance (p≤0.07) in the mid dm CPu, mid vl CPu, mid vm CPu, and OT.
Table 1.
Correlations between dopamine-related binding measures and behaviors in rats euthanized immediately following treatment with methylphenidate. Correlations with p≤0.1 are shown, and significant correlations with p≤0.01 are bolded, underlined, and have an asterisk (*).
| Body weight | Dark cycle circadian | Mid dm CPu - DAT | Mid vl CPu - DAT | Mid vm CPu - DAT | OT - DAT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | |
| Mid dl CPu - DAT | 0.55* | 0.01 | ||||||||||
| Mid dm CPu - DAT | −0.38 | 0.07 | 0.59* | 0.00 | ||||||||
| Mid vl CPu - DAT | 0.56* | 0.01 | ||||||||||
| Mid vm CPu - DAT | 0.52* | 0.01 | ||||||||||
| d CPu tail - DAT | 0.41 | 0.04 | ||||||||||
| v CPu tail - DAT | 0.55* | 0.01 | ||||||||||
| Mid dl CPu - D1 | −0.41 | 0.04 | 0.36 | 0.09 | ||||||||
| Mid dm CPu - D1 | −0.52* | 0.01 | 0.47 | 0.02 | 0.41 | 0.05 | ||||||
| Mid vl CPu - D1 | 0.35 | 0.1 | 0.38 | 0.07 | ||||||||
| Mid vm CPu - D1 | −0.42 | 0.04 | 0.61* | 0.00 | 0.50 | 0.02 | ||||||
| d CPu tail - D1 | −0.55* | 0.01 | 0.45 | 0.03 | ||||||||
| OT - D1 | 0.43 | 0.04 | 0.43 | 0.04 | ||||||||
| Body weight | −0.54* | 0.01 | ||||||||||
| Food intake | 0.48 | 0.02 | ||||||||||
Dark cycle circadian locomotor activity was positively correlated with D1R-like binding in the middle vm CPu, and this association approached significance in the OT, mid dI CPu, mid dm CPu, mid vl CPu, and d CPu tail. Dark cycle circadian activity was also positively correlated with DAT binding in all quadrants of the middle CPu, as well as the v CPu tail, and this correlation approached significance in the d CPu tail.
Body weight was negatively correlated with D1R-like binding in the mid dm CPu and d CPu tail, and this association approached significance in the mid dl CPu and mid vm CPu. The negative association between body weight and DAT binding in the middle dm CPu approached significance.
Correlations were also run between behavioral measures. There was no correlation between food intake and body weight; however, there was a significant negative correlation between body weight and dark cycle circadian activity. There was also a positive correlation between dark cycle circadian activity and food intake that approached significance.
3.4.2 Correlations in rats euthanized following abstinence
Correlations found to be significant in rats euthanized immediately following treatment were run between D1R-like, D2R-like, and DAT binding, as well as autoradiography binding measures and behavior, for rats euthanized following abstinence. Significant correlations between binding measures and behavior observed in rats euthanized immediately following treatment were not significant in rats euthanized immediately following abstinence. The positive correlation between dark cycle circadian activity and food intake approached significance, r(22)=0.362, p=0.075. Despite not being significant in rats euthanized immediately following treatment, there was a positive correlation between food intake and body weight that approached significance, r(22)=0.486, p=0.014, in rats euthanized following abstinence.
4. Discussion
Chronic treatment with a clinically-relevant high dose (HD) of methylphenidate (MP) resulted in increased DAT binding compared to water treated controls in the middle dorsolateral (89%), dorsomedial (106%), ventrolateral (78%), and ventromedial (54%) caudate putamen (CPu), as well as the ventral CPu tail (69%). HD MP treatment also increased D1R-like binding in the olfactory tubercle (14%) and middle dorsolateral (15%), dorsomedial (21%), and ventromedial (28%) CPu, as well as the dorsal CPu tail (24%). Following an abstinence period, DAT and D1R-like binding levels in rats previously treated with either dose of MP were not significantly different from water treated controls. Neither dose of MP treatment affected D2R-like binding in rats euthanized immediately following treatment or abstinence. These findings suggest that chronic oral treatment with clinically-relevant high doses of MP results in reversible increases in DAT and D1R in several subregions of the basal ganglia, with more caudal portions of the caudate putamen seemingly being particularly vulnerable to these effects. Increased binding in many of these regions following treatment was associated with previously-reported developmental and behavioral effects of this drug treatment (attenuated body weight and increased dark cycle circadian locomotor activity) (Thanos et al. 2015), and these correlations were no longer significant following abstinence.
These findings on dopamine transporter levels are in agreement with a recent clinical study, which found that long-term (12 months) of treatment with MP significantly increases DAT availability in the caudate and putamen, but not in the ventral striatum (Wang et al. 2013). The present findings can also be compared to those from studies on the neurochemical effects of other psychostimulants like cocaine, which has the same mechanism of action as MP (blocking of DAT) (Gatley et al. 1999; Volkow et al. 1997; Volkow et al. 2014). In humans and non-human primates, chronic psychostimulant administration is associated with increased striatal levels of DAT (Letchworth et al. 2001; Little et al. 1998; Mash et al. 2002), while prolonged abstinence from cocaine normalizes previously-elevated DAT binding levels in the striatum (Beveridge et al. 2009). Increased DAT levels observed following HD MP treatment may represent an adaptive response to maintain homeostatic levels of synaptic DA. Previous studies suggest that MP administration increases extracellular DA, as measured by in vivo microdialysis in rats (Gerasimov et al. 2000), and as a reduction in Bmax/Kd (measure of D2 receptor availability) in humans (Volkow et al. 2001). Therefore, higher DAT levels would be necessary to clear excess DA from the synapse (Zahniser and Doolen 2001). Oral MP at therapeutic doses of 0.25 to 1.0 mg/kg (resulting in plasma concentration of 8–40 ng/mL) dose-dependently block 50–75% of DAT (Volkow et al. 2014), while an oral dose of ~0.8 mg/kg MP (resulting in plasma concentrations of ~30 ng/mL of the pharmacologically active D-enantiomer) significantly increases extracellular DA in the striatum (Volkow et al. 2001). It has been shown that repeated treatment with MP and other psychostimulants results in increased midbrain DA neuron firing, while abstinence from these drugs results in reduced DA neuron activity (Brandon et al. 2003; Imperato et al. 1992; Imperato et al. 1996; Parsons et al. 1991; Robertson et al. 1991; Segal and Kuczenski 1992; Zhang et al. 2001). These effects could contribute to the changes in DAT density following treatment and abstinence observed in the current study. LD MP treatment had no effect on any binding measure in any region assessed. In mice, acute administration of an MP dose producing similar plasma levels (6–10 ng/mL) to our LD does not increase striatal DA levels. Assessment of these dosing regimens’ ability to elicit regional increases in extracellular DA is needed to further explore this possible relationship.
This study also found that while neither dose of MP influenced D2R-like binding, HD MP increased D1R-like binding in the OT and caudal portions of the caudate putamen, an effect that was no longer apparent in rats undergoing an abstinence period following treatment. While one methodologically different study found no effect of MP on D1R levels (Randall and Hannigan 1999), another found that the highest dose tested (8 mg/kg subcutaneous) increased D1R receptors (Gill et al. 2012). Similar to our findings, chronic cocaine self-administration in mice increases D1R in caudal portions of the CPu, with no reported effect in the rostral CPu or nucleus accumbens (Schlussman et al. 2003). In non-human primates, chronic cocaine administration is also associated with increased striatal levels of D1R (Nader et al. 2002), while prolonged abstinence from cocaine normalizes previously-elevated D1R binding levels in the striatum (Beveridge et al. 2009). The lack of MP-induced changes in D2R levels is in agreement with a prior study of chronic MP treatment in rats (Randall and Hannigan 1999) and cocaine administration in non-human primates (Beveridge et al. 2009). Our lab previously found that two months of oral MP treatment results in decreases in striatal D2R availability, while eight months of treatment enhances striatal D2R availability (Thanos et al., 2007). This study, however, differed from the current study in a number of ways, including MP dose, length of treatment, and unlike this study measured in-vivo D2R availability (using 11C raclopride microPET), which measures availability of D2R in the presence of endogenous dopamine (DA).
D1R-like receptors are coupled to stimulatory G proteins and D1R agonists promote locomotor activity, while D2R-like receptors are coupled to inhibitory G proteins and D2R agonists attenuate locomotor activity (Eilam et al. 1991; Halberda et al. 1997). Increased D1R-like binding with no change in D2R-like binding in several brain regions in HD MP rats would consequently alter the ratio of D1R:D2R signaling towards the D1R. It has been suggested that repeated administration of other psychostimulants alters the D1R:D2R ratio, favoring D1R, and that this effect mediates behavioral sensitization to the drug (Thompson et al. 2010). Previously, we found that the same chronic oral HD MP treatment resulted in marked hyperactivity that was greater during later weeks of treatment (Thanos et al. 2015); therefore, it is plausible that this apparent locomotor sensitization to the drug is mediated by an altered ratio of D1R:D2R signaling caused by an increase in D1R receptors. Sensitization is believed to underlie some aspects of addictive behavior (Robinson and Berridge 1993; Steketee and Kalivas 2011), creating concerns regarding the drug’s possible addictive properties and potential as a “gateway drug” at these doses, especially since epidemiological studies have shown that ADHD patients are at a significantly higher risk of substance abuse disorders (Elkins et al. 2007; Grant and Dawson 1997). A major concern about treating children and adolescents with psychostimulants is that prolonged treatment could alter reward-related neurochemistry, predisposing individuals to substance abuse later in life. While animal studies have shown mixed results on the effects of MP on reactivity and vulnerability to other drugs (Brandon et al. 2001; Carlezon Jr et al. 2003; Thanos et al. 2007), clinical studies have shown that psychostimulant treatment either has no effect (Biederman et al. 2008; Mannuzza et al. 2008) or reduces the risk of later substance abuse (Wilens et al. 2003), possibly by attenuating risk-taking and disinhibited behavior. Further investigation is necessary to determine the abuse liability of MP and its ability to alter predisposition to other drugs later in life.
Previously, we found that this chronic HD MP treatment resulted in significantly enhanced dark cycle circadian locomotor activity and an attenuation of body weight that does not appear to be directly linked to changes in food intake (Thanos et al. 2015). During abstinence, hyperactivity was markedly attenuated, and body weight increased but did not return to control levels. LD MP treatment did not produce the hyperactivity observed in HD rats (Thanos et al. 2015). Given that dopaminergic signaling in the basal ganglia plays a role in motor behavior (DeLong and Georgopoulos 2011; Takakusaki et al. 2004), and that behavioral changes seem to parallel the pattern of changes to the dopaminergic system, the link between MP-induced changes in DA neurochemistry and behavior were assessed. Several correlations were found to be significant in rats euthanized immediately following MP treatment. Dark cycle circadian locomotor activity was positively correlated with D1R-like binding in the OT, middle CPu, and dorsal tail of the CPu. Dark cycle circadian activity was also positively correlated with DAT binding in the middle CPu and caudal tail of the CPu. Body weight was negatively correlated with D1R-like binding in the middle CPu, as well as the dorsal tail of the CPu. Body weight was negatively correlated with dark cycle circadian locomotor activity, but surprisingly was not correlated with food intake. Food intake was, however, positively correlated with dark cycle circadian activity. Taken together, these results suggest that MP-induced changes in DA signaling may drive increases in locomotor behavior that appear to reduce body weight while enhancing food intake to maintain energy balance. Following abstinence, none of the aforementioned correlations were significant; however, there was a positive correlation between food intake and body weight as would have been expected.
It should be noted that aspects of the study, in addition to the MP treatment itself, could have influenced the results of the binding assays. Rats in this study were single housed out of necessity to accurately monitor and control treatment dosages. Single housing is acknowledged as a “deprived” rather than “standard” environment, which can impact the dopaminergic system (Fone and Porkess 2008; Isovich et al. 2001; King et al. 2009; Zakharova et al. 2009). Animals reared in socially isolated environments have also been shown to be more sensitive to psychostimulants via dopamine-dependent mechanisms (Howes et al. 2000; Jones et al. 1990; Zakharova et al. 2009). Additionally, animals in this study went through behavioral testing, completed at least three days prior to euthanasia, which also could have influenced dopamine neurochemistry. Behavior and binding assays, however, were performed in the same rats to assess the possible mechanistic relationship between these measures. Although all groups were single housed to control for housing effects and went through the same behavioral testing, it cannot be ruled out that there was an interaction of the drug with either of these factors to create the observed effects. Future studies should address these concerns.
CONCLUSION
Previously, we created an eight hour limited-access dual bottle drinking paradigm to deliver methylphenidate (MP) in a manner that mimics the pharmacokinetic profile of patients treated with this drug for ADHD (Thanos et al. 2015). Three months of treatment with the high dose regimen (30 mg/kg MP for the first hour; 60 mg/kg for the remaining seven hours), which results in plasma concentrations at the high end of the clinical spectrum (~30 ng/mL), was found to increase binding levels of (DAT) and D1R in several subregions of the basal ganglia, particularly the middle and caudal portions of the caudate putamen. These increases were no longer apparent following a one month abstinence period, suggesting the reversibility of these effects. Interestingly, DAT and D1R binding in several of these regions were correlated with previously-reported effects on locomotor activity and body weight in rats euthanized immediately following treatment, suggesting that MP-induced changes in dopamine neurochemistry may contribute to some of these effects.
Acknowledgments
Funding: This work was supported by the New York Research Foundation [Q0942016] and the National Institute of Health [R01HD70888].
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Abstinence from chronic cocaine self-administration alters striatal dopamine systems in rhesus monkeys. Neuropsychopharmacology. 2009;34:1162–1171. doi: 10.1038/npp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Spencer T, Wilens TE, MacPherson HA, Faraone SV. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. American Journal of Psychiatry. 2008;165:597–603. doi: 10.1176/appi.ajp.2007.07091486. [DOI] [PubMed] [Google Scholar]
- Bogle KE, Smith BH. Illicit methylphenidate use: a review of prevalence, availability, pharmacology, and consequences. Curr Drug Abuse Rev. 2009;2:157–176. doi: 10.2174/1874473710902020157. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, White FJ. Adolescent exposure to methylphenidate alters the activity of rat midbrain dopamine neurons. Biological psychiatry. 2003;54:1338–1344. doi: 10.1016/s0006-3223(03)00787-x. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biological Psychiatry. 2003;54:1330–1337. doi: 10.1016/j.biopsych.2003.08.020. doi: http://dx.doi.org/10.1016/i.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Happe HK, Murrin LC. Dopamine transporter development in postnatal rat striatum: an autoradiographic study with [3 H] WIN 35,428 Developmental brain research. 1997;104:55–62. doi: 10.1016/s0165-3806(97)00135-1. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Georgopoulos AP. Comprehensive Physiology. John Wiley & Sons, Inc; 2011. Motor Functions of the Basal Ganglia. [DOI] [Google Scholar]
- Eilam D, Clements KV, Szechtman H. Differential effects of D1 and D2 dopamine agonists on stereotyped locomotion in rats. Behavioural brain research. 1991;45:117–124. doi: 10.1016/s0166-4328(05)80077-4. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of general psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Fone KCF, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents—Relevance to developmental neuropsychiatric disorders. Neuroscience & Biobehavioral Reviews. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. doi: http://dx.doi.org/10.1016/i.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Volkow ND, Gifford AN, Fowler JS, Dewey SL, Ding Y-S, Logan J. Dopamine-transporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology. 1999;146:93–100. doi: 10.1007/s002130051093. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, et al. Comparison between intraperitoneal and oral methylphenidate administration: a microdialysis and locomotor activity study. Journal of Pharmacology and Experimental Therapeutics. 2000;295:51–57. [PubMed] [Google Scholar]
- Giedd JN, et al. Trajectories of anatomic brain development as a phenotype. Novartis Found Symp. 2008;289:101–112. doi: 10.1002/9780470751251.ch9. ; discussion 112–108, 193–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KE, Beveridge T, Porrino LJ. Interaction of environment and chronic methylphenidate on anxiety-like behavior and dopamine receptors in adolescent rodents. The FASEB Journal. 2012;26:844.846. [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of substance abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Gray JD, et al. Methylphenidate administration to juvenile rats alters brain areas involved in cognition, motivated behaviors, appetite, and stress. The Journal of neuroscience. 2007;27:7196–7207. doi: 10.1523/JNEUROSCI.0109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill LL, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41:26S–49S. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- Halberda JP, Middaugh LD, Gard BE, Jackson BP. DAD1-and DAD2-like agonist effects on motor activity of C57 mice. Differences compared to rats Synapse. 1997;26:81–92. doi: 10.1002/(SICI)1098-2396(199705)26:1<81::AID-SYN9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology. 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Imperato A, Mele A, Scrocco MG, Puglisi-Allegra S. Chronic cocaine alters limbic extracellular dopamine. Neurochemical basis for addiction. European journal of pharmacology. 1992;212:299–300. doi: 10.1016/0014-2999(92)90349-9. [DOI] [PubMed] [Google Scholar]
- Imperato A, Obinu MC, Carta G, Mascia MS, Casu MA, Gessa GL. Reduction of dopamine release and synthesis by repeated amphetamine treatment: role in behavioral sensitization. European journal of pharmacology. 1996;317:231–237. doi: 10.1016/s0014-2999(96)00742-x. [DOI] [PubMed] [Google Scholar]
- Isovich E, Engelmann M, Landgraf R, Fuchs E. Social isolation after a single defeat reduces striatal dopamine transporter binding in rats. European Journal of Neuroscience. 2001;13:1254–1256. doi: 10.1046/j.0953-816x.2001.01492.x. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Coy AE, Ladenheim B, Loeloff RJ, Cadet JL, French D. Chronic methylphenidate alters locomotor activity and dopamine transporters differently from cocaine. European Journal of Pharmacology. 1999;373:187–193. doi: 10.1016/s0014-2999(99)00274-5. doi: http://dx.doi.org/10.1016/S0014-2999(99)00274-5. [DOI] [PubMed] [Google Scholar]
- Jones GH, Marsden CA, Robbins TW. Increased sensitivity to amphetamine and reward-related stimuli following social isolation in rats: possible disruption of dopamine-dependent mechanisms of the nucleus accumbens. Psychopharmacology. 1990;102:364–372. doi: 10.1007/BF02244105. [DOI] [PubMed] [Google Scholar]
- King MV, Seeman P, Marsden CA, Fone KCF. Increased dopamine D 2High receptors in rats reared in social isolation. Synapse. 2009;63:476–483. doi: 10.1002/syn.20624. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. Journal of Pharmacology and Experimental Therapeutics. 2001;296:876–883. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. The Journal of neuroscience. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Stimulant actions in rodents: implications for attention-deficit/hyperactivity disorder treatment and potential substance abuse. Biological psychiatry. 2005;57:1391–1396. doi: 10.1016/j.biopsych.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. The Journal of Neuroscience. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little KY, et al. Brain dopamine transporter messenger RNA and binding sites in cocaine users: a postmortem study. Archives of general psychiatry. 1998;55:793–799. doi: 10.1001/archpsyc.55.9.793. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Truong NL, Moulton JL, III, Roizen ER, Howell KH, Castellanos FX. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. American Journal of Psychiatry. 2008 doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, Pablo J, Ouyang Q, Hearn WL, Izenwasser S. Dopamine transport function is elevated in cocaine users. Journal of neurochemistry. 2002;81:292–300. doi: 10.1046/j.1471-4159.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: Prevalence and correlates from a national survey. Addiction. 2005;100:96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use and diversion of prescription stimulant medication. J Psychoactive Drugs. 2006;38:43–56. doi: 10.1080/02791072.2006.10399827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick E, Faraone SV, Biederman J. Age-dependent expression of attention-deficit/hyperactivity disorder symptoms. Psychiatric Clinics of North America. 2004;27:215–224. doi: 10.1016/j.psc.2004.01.003. doi: http://dx.doi.org/10.1016/i.psc.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Nader MA, et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Parsons L, Smith A, Justice J. Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. (6th) 2006 doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Randall S, Hannigan J. In utero alcohol and postnatal methylphenidate: locomotion and dopamine receptors. Neurotoxicology and teratology. 1999;21:587–593. doi: 10.1016/s0892-0362(99)00017-3. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. doi: sc271_5_1835 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MW, Leslie CA, Bennett JP. Apparent synaptic dopamine deficiency induced by withdrawal from chronic cocaine treatment. Brain research. 1991;538:337–339. doi: 10.1016/0006-8993(91)90451-z. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. doi: http://dx.doi.org/10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Kane S, Stewart CL, Ho A, Kreek MJ. Locomotion, stereotypy, and dopamine D1 receptors after chronic “binge” cocaine in C57BL/6J and 129/J mice Pharmacology. Biochemistry and Behavior. 2003;75:123–131. doi: 10.1016/s0091-3057(03)00067-4. doi: http://dx.doi.org/10.1016/S0091-3057(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Repeated cocaine administration induces behavioral sensitization and corresponding decreased extracellular dopamine responses in caudate and accumbens. Brain research. 1992;577:351–355. doi: 10.1016/0006-8993(92)90297-m. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. doi:S0149–7634(00)000142 [pii] [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug Wanting: Behavioral Sensitization and Relapse to Drug-Seeking Behavior. Pharmacological Reviews. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, Saitoh K, Harada H, Kashiwayanagi M. Role of basal ganglia-brainstem pathways in the control of motor behaviors. Neuroscience research. 2004;50:137–151. doi: 10.1016/j.neures.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Campbell A, Yeghiayan SK, Baldessarini RJ. Localization of dopamine receptor subtypes in corpus striatum and nucleus accumbens septi of rat brain: comparison of D1-, D2- and D4-like receptors. Neuroscience. 1998;83:169–176. doi: 10.1016/s0306-4522(97)00386-2. doi: http://dx.doi.org/10.1016/S0306-4522(97)00386-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Florijn WJ, Creese I. Differential regulation of dopamine receptors after chronic typical and atypical antipsychotic drug treatment. Neuroscience. 1997;78:985–996. doi: 10.1016/s0306-4522(96)00631-8. doi: http://dx.doi.org/10.1016/S0306-4522(96)00631-8. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Dev Neurosci. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006;26:1501–1510. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacology Biochemistry and Behavior. 2007;87:426–433. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Thanos PK, et al. A pharmacokinetic model of oral methylphenidate in the rat and effects on behavior. Pharmacology Biochemistry and Behavior. 2015;131:143–153. doi: 10.1016/j.pbb.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D, Martini L, Whistler JL. Altered ratio of D1 and D2 dopamine receptors in mouse striatum is associated with behavioral sensitization to cocaine. PloS one. 2010;5:e11038. doi: 10.1371/journal.pone.0011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser SN, et al. Trends in the Parent-Report of Health Care Provider-Diagnosed and Medicated Attention-Deficit/Hyperactivity Disorder: United States, 2003–2011. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53:34–46.e32. doi: 10.1016/j.jaac.2013.09.001. doi: http://dx.doi.org/10.1016/i.iaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. 1997 doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacol. 2002;12:557–566. doi: 10.1016/s0924-977x(02)00104-9. doi: S0924977X02001049 [pii] [DOI] [PubMed] [Google Scholar]
- Volkow ND, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. 2014 doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Volkow ND, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-J, et al. Long-term stimulant treatment affects brain dopamine transporter level in patients with attention deficit hyperactive disorder. PloS one. 2013;8:e63023. doi: 10.1371/journal.pone.0063023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens T, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Wilson JM, et al. Heterogeneous subregional binding patterns of 3H-WIN 35,428 and 3H-GBR 12,935 are differentially regulated by chronic cocaine self-administration. The Journal of neuroscience. 1994;14:2966–2979. doi: 10.1523/JNEUROSCI.14-05-02966.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, Doolen S. Chronic and acute regulation of Na+/Cl− -dependent neurotransmitter transporters: drugs, substrates, presynaptic receptors, and signaling systems. Pharmacology & therapeutics. 2001;92:21–55. doi: 10.1016/s0163-7258(01)00158-9. doi: S0163-7258(01)00158-9 [pii] [DOI] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009;163:890–897. doi: 10.1016/j.neuroscience.2009.06.068. doi: http://dx.doi.org/10.1016/i.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Loonam TM, NOAILLES PA, Angulo JA. Comparison of Cocaine-and Methamphetamine-Evoked Dopamine and Glutamate Overflow in Somatodendritic and Terminal Field Regions of the Rat Brain during Acute, Chronic, and Early Withdrawal Conditions. Annals of the New York Academy of Sciences. 2001;937:93–120. doi: 10.1111/j.1749-6632.2001.tb03560.x. [DOI] [PubMed] [Google Scholar]


