Abstract
Acetyl tributyl citrate (ATBC), is a phthalate substitute used in food and medical plastics, cosmetics, and toys. Although systemically safe up to 1000 mg/kg/day, its ability to cause reproductive toxicity in females at levels below 50 mg/kg/day has not been examined. This study evaluated the effects of lower ATBC exposures on female reproduction using mice. Adult CD-1 females (n=7–8/treatment) were dosed orally with tocopherol-stripped corn oil (vehicle), 5 or 10 mg/kg/day ATBC daily for 15 days, and then bred with a proven breeder male. ATBC exposure did not alter body weights, estrous cyclicity, and gestational and litter parameters. Relative spleen weight was slightly increased in the 5 mg/kg/day group. ATBC at 10 mg/kg/day targeted ovarian follicles and decreased the number of primordial, primary, and secondary follicles present in the ovary. These findings suggest that low levels of ATBC may be detrimental to ovarian function, thus, more information is needed to better understand the impact of ATBC on female reproduction.
Keywords: acetyl tributyl citrate, phthalate substitute, plasticizer, fertility, female, ovary, ovarian follicle
1. Introduction
Plasticizers are usually low-melting solids or high-boiling liquids which are used to impart flexibility and durability to plastic products (CPSC, 2010). Worldwide, more than six million tons of plasticizers are produced and consumed each year (Lioy et al., 2015). Phthalates are widely used plasticizers which are found in many consumer products such as beauty products (nail polish, perfume, and soaps), oral medications, medical devices (disposable gloves, blood bags), food and beverage containers, and children’s care products and toys (Centers for Disease Control and Prevention., 2005; Hernandez-Diaz et al., 2009; NTP, 1998; Kavlock et al., 2002a; Kavlock et al., 2002b). The idea that humans are continually exposed to phthalates has been supported by biomonitoring studies reporting detectable levels of phthalates in more than 75% of spot urine samples tested (Centers for Disease Control and Prevention, 2005). Furthermore, these plasticizers have been associated with reproductive and developmental toxicity (reviewed in Kay et al., 2013). Therefore, it has become necessary to seek safer alternative chemicals for use in consumer products.
As a result of the Consumer Product Safety Improvement Act of 2008, a Chronic Hazard Advisory Panel was tasked with the evaluation of compounds currently under approved use in consumer products and which could replace phthalates (CPSC, 2010). One such compound is acetyl tributyl citrate (ATBC), a commercially available FDA-approved plasticizer commonly used in polyvinyl resins (including pharmaceutical coatings and extracorporeal tubing) and permitted as a food additive and food contact substance (CPSC, 2010). ATBC has been shown to migrate from food packaging material into cheese (6.1 mg/kg), wrapped cake (3.2 mg/kg), microwaved soup (0.4 mg/kg), and microwaved peanut-containing cookies (79.8 mg/kg; Sheftel, 2000; CPSC, 2010). Most importantly, when evaluating leaching rate from medical equipment, ATBC was found to leach ten times more rapidly than the potent endocrine disruptor di-2-ethylhexyl phthalate (Welle et al., 2005; SCENIHR, 2008; CPSC, 2010). Thus, humans are exposed to ATBC via ingestion of contaminated foods, ATBC-coated medications, and via extracorporeal tubing. Various studies have employed dosages ranging from 50–1000 mg/kg/day to examine the systemic, reproductive and developmental toxicity of ATBC in animal models. Based on these studies, ATBC is considered a safe alternative to phthalates given the relatively high no observed adverse effect levels (NOAELs) assigned for systemic (up to 100 mg/kg/day) and reproductive toxicity (250–1000 mg/kg/day; CPSC, 2010; SCENIHR., 2008). Although biomonitoring data on ATBC is lacking, levels in humans are assumed to be lower than the ranges tested in these high dose studies (Fromme et al., 2016). Therefore, there is a need to examine the consequences of exposure to ATBC at lower dosages which more closely mimic human exposure, as to better inform risk assessment and determine whether ATBC causes toxicity at levels similar to those at which endocrine disruption has been observed with other chemicals.
The purpose of the present study was to determine whether lower dosages of ATBC disrupt reproductive function in female mice. We selected two concentrations of ATBC not previously included in reproductive toxicity testing and determined the effects of ATBC exposure on body weights, estrous cyclicity, gestational and litter parameters, and ovarian follicle numbers. Our observations show that ATBC might produce detrimental effects on the ovary and suggest that further studies at environmentally-relevant levels are needed to understand the impact of this chemical on female reproduction.
2. Materials and Methods
2.1. Chemicals
All chemicals were obtained from Fisher Scientific (Hanover Park, IL) unless otherwise indicated. Acetyl tributyl citrate (ATBC, CAS 77-90-7), phosphate buffered saline (PBS), and sodium hydroxide (NaOH) were obtained from Sigma-Aldrich (St. Louis, MO). Tocopherol-stripped corn oil was obtained from MP Biomedicals (vehicle, Solon, OH).
2.2. Animals
Animals were obtained from Charles River Laboratories (Charles River, CA). Animals were housed in single-use BPA and phthalate-free Econo-Cage® Disposable Rodent Caging (Lab Products Inc., Seaford, DE, USA) at the University of Arizona Central Animal Care Facility. Animals were subjected to 12L:12D cycles, water and food were provided ad libitum, and temperature was maintained at 22±1°C. Animals were allowed to acclimate for at least 24 h before handling. All experiments and methods involving animals were approved by The Institutional Animal Care and Use Committee (IACUC) at the University of Arizona and conformed to established guidelines (Institute of Laboratory Animal Resources, 1996). Figure 1 shows a schematic of the experimental design used in this study.
Figure 1. Experimental Design.
Body weight and estrous cyclicity were monitored in female CD-1 mice for 20 days prior to dosing. Animals were then separated into vehicle, 5, and 10 mg/kg/day ATBC and dosed prior to housing with a proven breeder male as described in Section 2.2. On the day of parturition, the dams and pups were euthanized and litter data collected. Ovaries and major organs were excised, weighed, and their gross morphology evaluated. Ovaries were processed for histological evaluation as described in Section 2.7.
Cycling female CD-1 mice (n=22, age 60 days) were weighed daily and their estrous cycles monitored by vaginal cytology for 20 days prior to ATBC dosing. On postnatal day 88, animals were randomly assigned to receive tocopherol-stripped corn oil (vehicle) or ATBC (dissolved in vehicle) at 5 or 10 mg/kg/day. Animals were weighed and dosed daily for 15 consecutive days. All doses were administered orally by placing a pipette tip containing the dosing solution into the mouth past the incisors and into the cheek pouch (Hannon et al., 2014; Wang et al., 2014; Sen et al., 2015). In the absence of biomonitoring data in adult women, we began characterizing the effects of ATBC by exposing animals to levels that were 10–100 times lower than those previously tested in standard studies (CPSC, 2010). Findings using this approach will better inform future studies aimed at expanding the dose range to include lower and higher doses. Future use of lower and higher doses will enhance our ability to evaluate exposure levels relevant to humans and compare our findings to classical high-dose studies.
After 15 days of daily oral dosing, animals were placed with a proven breeder male and checked daily for presence of a sperm plug. Dams were considered pregnant following the identification of a sperm plug and their weight was monitored daily to track progression of pregnancy. Dams were housed with their male until day 18 of gestation when they were housed individually. Dams were euthanized within 24 h of parturition by decapitation following isoflurane sedation. Litter size, pup sex, and litter weights were recorded for each dam and the uterus, ovaries, kidneys, adrenals, spleen, and liver of the dam were collected.
2.4. Body Weights
Daily body weight was monitored throughout the experiment and classified as pre-dosing, dosing, and gestational. Specifically, body weight was obtained daily in grams and normalized to baseline to obtain a percent change. Body weight percent change was used to monitor progression through pregnancy and overall animal health.
2.5. Estrous Cyclicity
Estrous cyclicity was monitored by daily vaginal cytology starting on postnatal day 60 and throughout the study (Figure 1). Vaginal cytology was assessed as previously reviewed (Cooper et al., 1993), with minor modifications. Briefly, animals were restrained gently and 20 μL of sterile-filtered PBS was used to perform a vaginal washing. Vaginal washings were placed on microscope slides and evaluated unstained under an inverted microscope without knowledge of treatment. Average cycle length and percentage of days in proestrus, estrus and metestrus/diestrus were determined. Time spent in each stage was obtained by dividing the total number of days spent in each stage by the total number of days in the study period and multiplying that number by 100 (Hannon et al., 2014; Sen et al., 2015).
2.6. Fertility, gestation, and pup count
Fertility was determined through the ability to become pregnant and carry to term. Gestation data included days to conception, uterine implantation sites, and gestation length. Days to conception were determined by subtracting plug date from days accompanied by male. Gestation length was determined as the time from plug date to parturition. Implantation sites were determined using a 2% NaOH stain which allowed easy identification of uterine implantation sites. Live pup count and litter data were collected for all litters. Pup count is number of live pups born. Litter weight is the total weight of all pups.
2.7. Follicle and corpora lutea counts
Following euthanasia, ovaries were fixed and processed for histological classification and enumeration of ovarian follicles and corpora lutea. Briefly, ovaries were fixed in Bouin’s solution (2 h), transferred to 70% ethanol, and embedded in paraffin. Paraffin-embedded ovaries were serially sectioned at 5 μm thickness, mounted on glass slides, and processed for hematoxylin and eosin staining. Oocyte containing follicles with visible nuclear material and corpora lutea were counted on every 20th section without knowledge of treatment by two experienced individuals using criteria previously described (Sen et al., 2015). Specifically, follicles were classified as primordial if they consisted of a single oocyte surrounded by a single layer of squamous granulosa cells, primary if the oocyte was surrounded by a single layer comprised of ≥50% cuboidal granulosa cells, secondary if the oocyte was surrounded by two or more layers of cuboidal granulosa cells and a theca layer, and antral if the oocyte was surrounded by multiple layers of cuboidal granulosa cells, theca cells and contained an antrum. Atretic follicles were further classified as (a) pyknotic if six of more pyknotic bodies were present, and (b) degenerating if no pyknotic bodies were observed but the follicle showed morphological features of advanced atresia (oocyte fragmentation, disorganized or collapsed granulosa layer, and hypertrophied theca layer).
2.8. Gross organ morphology and organ weight
Gross organ morphology consisted of visual examination of the uterus, kidneys, adrenals, liver, spleen, and ovary following euthanasia. Organs were then weighed and normalized to body weight and compared as a relative weight.
2.9. Statistical analysis
All data were compared using SPSS Statistics 22 software (IBM, Chicago, IL). Body weight (pre-dosing, dosing, and gestational), and pre- versus dosing estrous cyclicity data were compared using a General Linear Model Repeated Measures test. Organ weights, gestational and litter parameters, and follicle and CL count data were analyzed using One-way ANOVA followed by Dunnett’s or Fisher’s LSD post hoc tests as appropriate. For all comparisons, statistical significance was assigned at p ≤ 0.05 and marked using asterisks (*) on their respective tables or graphs.
3. Results
3.1. Effect of oral exposure to ATBC on body weight
Body weight percent change was tracked before dosing, during dosing, and during gestation to monitor animal health and progression through pregnancy. There were no differences in absolute body weight and body weight gain between animals pre-selected to be controls and ATBC-treated mice during the period preceding ATBC dosing (data not shown). ATBC dosing for 15 days did not affect body weight gain (Figure 2A). Interestingly, animals treated with ATBC at 10 mg/kg/day showed decreased body weight gain at specific times (days 3, 5, 12, and 13) during gestation; however, these differences were sporadic and ultimately absent by the time of parturition (Figure 2B).
Figure 2. Effect of oral exposure to ATBC on body weight.
CD-1 female mice were exposed to ATBC as described in Section 2.2. Individual daily body weight values were normalized to baseline (first day of dosing or gestation as applicable) and averaged per treatment to obtain mean body weight percent change ± SEM (n= 7–8 mice per treatment) during dosing (A) and during gestation (B). Data were analyzed using General Linear Model Repeated Measures test with significance set at p≤0.05. Asterisks (*) indicate significantly different from control.
3.2. Effect of oral exposure to ATBC on estrous cyclicity
To determine if oral ATBC exposure disrupts estrous cyclicity, we analyzed vaginal cytology daily prior and during ATBC dosing. Cytology data were used to compare average estrous cycle length as well as the average percentage of time that animals spent in each stage of the estrous cycle (proestrus, estrus, and diestrus) between treatments. Estrous cycle length was not affected in ATBC-treated mice when compared to vehicle controls before or during dosing; however, cycle length was statistically reduced in the 5 mg/kg/day ATBC group when their dosing average was compared to pre-dosing values (Figure 3). Further analysis by stage of the cycle revealed no statistically significant differences in time spent in proestrus, estrus, and diestrus between treatments before (Figure 4A) and during ATBC dosing (Figure 4B). A visual trend for decreased time in estrus at the 10 mg/kg/day ATBC level was observed but was not statistically significant (Figure 4B). No statistically significant differences were observed when the percentage of time in each stage was compared within treatments before and during dosing.
Figure 3. Effect of oral exposure to ATBC on estrous cycle length.
CD-1 female mice were exposed to ATBC and vaginal smears taken prior to and during dosing as described in Section 2.2. Data are presented as average number of days per cycle ± SEM (n=7–8 mice per treatment) and were analyzed using General Linear Model Repeated Measures test with significance set at p≤0.05. Asterisks (*) indicate significantly different from values prior to the start of dosing.
Figure 4. Effect of oral exposure to ATBC on estrous cyclicity.
CD-1 female mice were exposed to ATBC and vaginal smears taken prior to and during dosing as described in Section 2.2. Data are presented as mean percentage of time spent in each stage of proestrus, estrus, and diestrus ± SEM (n=7–8 mice per treatment) prior (A) and during ATBC dosing (B) and were analyzed using General Linear Model Repeated Measures test with significance set at p≤0.05.
3.3. Effect of oral exposure to ATBC on fertility
On the last day of dosing, we placed treated mice with proven breeder males to determine whether ATBC exposure affects female fertility. Data on days to conception, gestation length, litter size and weight, implantation sites, and corpora lutea were used in our assessment of fertility. There were no differences in the time to conception and the duration of gestation between ATBC- and vehicle-treated mice (Table 1). We used corpora lutea counts, implantation sites in the post-partum uterus, and litter size to calculate reproductive indices as previously described (Parker, 2012). No differences in implantation index and pre- and post-implantation loss were observed between ATBC- and vehicle-treated mice (Table 2). There were also no differences in the ratio of males to females between treatments (data not shown).
Table 1.
Effect of ATBC Exposure on Conception, Gestation, and Litter Parameters1
| ATBC Exposure (mg/kg/day) | Time to Conception (days) | Length of Gestation (days) | Live Births | Average Pup Weight (g) |
|---|---|---|---|---|
| 0 | 2.4 ± 0.65 | 19.7 ± 0.18 | 12.3 ± 0.84 | 1.71 ± 0.05 |
| 5 | 2.0 ± 0.33 | 20.0 ± 0.19 | 10.9 ± 1.46 | 1.78 ± 0.04 |
| 10 | 2.5 ± 0.22 | 20.0 ± 0.00 | 12.7 ± 0.78 | 1.67 ± 0.02 |
All data obtained from 7–8 mice per treatment and presented as mean ± SEM.
Table 2.
Effect of ATBC Exposure on Reproductive Indices1
| ATBC Exposure (mg/kg/day) | Reproductive Indices | ||
|---|---|---|---|
|
| |||
| Implantation Index (%) | Pre-Implantation Loss (%) | Post-Implantation Loss (%) | |
|
|
|||
| 0 | 93.4 ± 3.6 | 8.7 ± 3.2 | 7.6 ± 3.4 |
| 5 | 94.0 ± 2.3 | 5.9 ± 2.3 | 5.3 ± 2.0 |
| 10 | 100 ± 0.0 | 0 ± 0.0 | 5.2 ± 3.6 |
All data obtained from 7–8 mice per treatment and presented as mean ± SEM.
3.4. Effect of oral exposure to ATBC on ovarian morphology
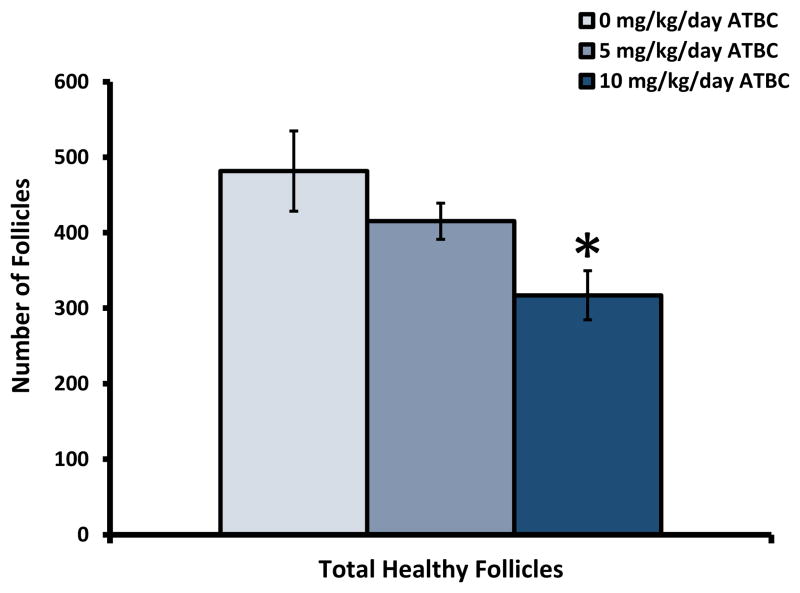
To determine whether ATBC exposure has an impact on ovarian morphology, we evaluated ovarian sections from vehicle and ATBC-treated mice to classify and count ovarian follicle numbers present. The number of corpora lutea present were also counted as an indication of ovulation for the calculation of reproductive indices as shown in Section 2.7 (Table 2). Animals exposed to ATBC at the 10 mg/kg/day level had significantly fewer ovarian follicles in their ovaries (Figure 5). Specifically, ATBC exposure resulted in a significant decrease in the number of primordial, primary, and secondary follicles when compared to numbers in the ovaries of vehicle controls (Figure 6A). We also counted atretic follicles and classified them as pyknotic or degenerating based on their morphological features as described in Section 3.3. ATBC exposure did not affect the number of atretic follicles present at any of the dosages tested (Figure 6B).
Figure 5. Effect of oral exposure to ATBC on total follicle numbers.
CD-1 female mice were exposed to ATBC as described in Section 2.2. Ovaries were dissected and processed for histological evaluation as described in Section 2.7. Data are presented as mean number of follicles ± SEM (n=7–8 mice per treatment) and were analyzed using One-Way ANOVA followed by Fisher’s LSD post hoc test with significance set at p≤0.05. Asterisks (*) indicate significantly different from control.
Figure 6. Effect of oral exposure to ATBC on the number of ovarian follicles in the different developmental stages.
CD-1 female mice were exposed to ATBC as described in Section 2.2. Ovaries were dissected and processed for histological evaluation as described in Section 2.7. Data are presented as mean number of follicles ± SEM (n=7–8 mice per treatment) for healthy (A) and atretic (B) follicles separately and were analyzed using One-Way ANOVA followed by Dunnett’s post hoc test with significance set at p≤0.05. Asterisks (*) indicate significantly different from control.
3.5. Effect of oral exposure to ATBC on the relative weight of major organs
During necropsy, we examined major organ morphology and relative weight to assess whether exposure to ATBC resulted in overall toxicity. No differences in overall gross organ appearance were observed at any of the ATBC concentrations tested. When compared to vehicle-treated controls, no significant differences in relative weight of the uterus, kidneys, adrenals, liver, and ovary were observed in ATBC-treated mice (Table 3). There was; however, a statistically significant but small increase in relative spleen weight in animals treated with ATBC at the 5 mg/kg/day level (p≤0.05; Table 3).
Table 3.
Effect of ATBC Exposure on Relative Organ Weights1
| ATBC Exposure (mg/kg/day) | Ovary (% b.w.) | Uterus (% b.w.) | Adrenals (% b.w.) | Spleen (% b.w.) | Kidneys (% b.w.) | Liver (% b.w.) |
|---|---|---|---|---|---|---|
| 0 | 0.07 ± 0.01 | 2.43 ± 0.13 | 0.02 ± 0.002 | 0.35 ± 0.01 | 0.95 ± 0.03 | 5.34 ± 0.24 |
| 5 | 0.07 ± 0.01 | 2.39 ± 0.18 | 0.02 ± 0.003 | 0.45 ± 0.03* | 1.04 ± 0.04 | 5.60 ± 0.22 |
All data obtained from 7–8 mice per treatment and presented as mean ± SEM
p≤0.05 versus vehicle control.
4. Discussion
Using an in vivo oral dosing study we examined the effects of lower ATBC exposure levels (5 and 10 mg/kg/day) compared to those previously used by others (≥ 50 mg/kg/day). Here we show that while ATBC exposure at 5 and 10 mg/kg/day did not cause overall toxicity or affect estrus cyclicity and fertility, it did decrease the number of ovarian follicles present in the ovary and caused a slight increase in the relative weight of the spleen in some animals. Although considered safe at exposure levels from 50–1000 mg/kg/day based on previous studies, our findings highlight the need to further investigate whether ATBC acts as an ovarian endocrine disruptor at low exposure levels such as those potentially present in women of reproductive age.
Although various studies have estimated the migration of ATBC from plastics into foods, biomonitoring data on phthalate alternatives is scarce and, to our knowledge, limited to a study evaluating levels in children attending daycare centers in Germany (Fromme et al., 2016). Although that study evaluated levels of various phthalate substitutes in air and dust, including ATBC, only levels of one substitute (DINCH) were also determined in the urine of children attending the tested facilities. Median levels of ATBC in settled dust were estimated at 24 mg/kg and used in back-calculations to obtain a median estimated intake from dust in children of 0.1 μg/kg of body weight (Fromme et al., 2016). Still, the levels of ATBC present in adults and, more specifically, women remain to be determined.
In agreement with previous high dose studies (reviewed in CPSC, 2010), here we observed that oral dosing with ATBC does not cause overall toxicity as shown by comparable weight gain during dosing and gestation, as well as, relative major organ weights between vehicle-treated and ATBC-treated mice. Interestingly, we observed that mice treated with ATBC at 5 mg/kg/day for 15 days experienced an increase in the relative size of their spleen (28% versus control). Because no obvious signs of distress were observed in these animals, it is possible that this increase in size may result in no biological impact to the animal’s health. Due to the focus of this study on reproductive function, future studies will be needed to further characterize this effect of ATBC on relative spleen weight and its significance.
Using data from previous high dose studies, it was concluded that the NOAEL for reproductive toxicity for ATBC is 1000 mg/kg/day (CPSC, 2010). Specifically, Sprague Dawley rats were treated with ATBC at 0, 100, 300, and 1000 mg/kg/day three weeks prior to mating, during mating, gestation, and lactation and experienced no reproductive or developmental toxicity. Furthermore, the F1 from that group of animals experienced ten weeks of exposure to ATBC and showed no toxicity (Robbins, 1994). A similar study using Han Wistar rats reported no effects on estrous cycles, mating performance, fertility, and gestation (Chase and Willoughby, 2002). Likewise, the low level exposures employed in the present study did not disrupt fertility, gestation length, and litter size in CD-1 mice. Furthermore, ATBC exposure did not affect ovarian or uterine weight and did not alter estrous cyclicity in our study. A decrease in average estrous cycle length was observed in mice treated with ATBC at the 5 mg/kg/day level when pre-dosing and during dosing values were compared. Although, statistically significant this change was not detrimental to fertility in the present study.
Finally, we evaluated whether ATBC causes alterations in folliculogenesis by classifying and counting follicles present in the ovaries of vehicle and ATBC-treated mice. To our surprise, ATBC-treated animals (10 mg/kg/day) had fewer total ovarian follicles when compared to controls. Our findings suggest that ATBC targeted the primordial, primary, and secondary populations causing this effect. ATBC exposure did not alter the number of atretic follicles in the ovaries of treated mice. This observation is interesting, since an increase in atretic follicles would explain lower follicle number in ATBC-treated mice. Previous studies focused on cigarette smoke exposures have demonstrated that ovarian follicles can undergo programmed cell death via autophagy, an alternative mechanism to apoptosis (Gannon et al., 2012). It is reasonable to suggest that the lack of effect on atretic follicles despite a decrease in preantral follicles could be due to the fact that our assessment of atretic follicles does not account for follicles undergoing autophagy. Further studies are necessary to determine whether ATBC interacts with autophagy regulation in the ovary.
Mechanistic information on ATBC is limited to studies assessing its effects on cell viability and ability to activate nuclear receptors. Mochida et al. (1996) and Ekwall et al. (1982) published work showing that ATBC can inhibit growth of various mammalian cell lines including human KB and HeLa cells, monkey Vero cells, and dog MDCK cells. Another study aimed at determining the estrogenic potential of phthalate substitutes for use in tissue conditioners in the lining of dentures reported that ATBC does not produce estrogenic responses in MCF-7 cells (Nishijima et al., 2002). More recently, Takeshita et al. (2011) demonstrated that ATBC induces steroid and xenobiotic sensing nuclear receptor (SXR; a.k.a. pregnane X-receptor, PXR) activity and increases the expression of members of the CYP3A family of xenobiotic-metabolizing enzymes in human and rat intestinal cells. Although no such work has been completed using ovarian cells, PXR receptor and its transcriptional target are expressed in the ovary (Masuyama et al., 2001). CYP3A enzymes are responsible for the oxidation of endogenous steroids, thus, it is possible that ATBC exposure leads to increased PXR-mediated expression of CYP3A in PXR-expressing tissues, thus leading to an imbalance in steroid levels. Future studies will focus on understanding the involvement, if any, of this pathway in the ovarian effects of ATBC reported here.
Ovarian endocrine disruption has been reported in various studies evaluating phthalate plasticizers for which ATBC represents a safer alternative. For example, the idea of the ovary as a target for DEHP and DBP-mediated toxicity has been well documented in mouse studies reporting their ability to cause ovarian follicle toxicity in vitro, as well as, leading to disruptions in folliculogenesis and steroidogenesis in vivo (Hannon et al., 2014; Wang et al., 2012; Gupta et al., 2010; Craig et al., 2013; Hannon et al., 2015; Sen et al., 2015). Most importantly, most of these studies have employed environmentally-relevant exposures when evaluating the toxicity to these phthalates and, thus, detected ovarian endocrine disruption at levels not previously predicted by high dose studies. Here we tested whether ATBC may act as an ovarian toxicant at low doses by evaluating two exposure levels not previously tested. However, it is important to acknowledge that our findings represent the first step towards this goal and, thus, more studies should be undertaken prior to concluding this as a complete risk assessment of low exposures to ATBC.
Acknowledgments
5. Funding
This research was supported by National Institutes of Health grant R00ES021467 and the Southwest Environmental Health Sciences Center’s Career Development Core (P30 ES006694).
References
- Centers for Disease Control and Prevention. Third National Report on Human Exposure to Environmental Chemicals. 2005 NCEH Pub. No. 05-0570. [Google Scholar]
- Chase K, Willoughby C. Huntington Life Sciences. 2002. Citroflex A-4 toxicity study by dietary administration to Han Wistar rats for 13 weeks with an in uteru exposure phase followed by a 4-week recovery period. MOX 002/013180: As cited in U.S. EPA (2008a) [Google Scholar]
- Cooper R, Goldman J, Stoker T. Monitoring the estrous cycle in the laboratory rodent by vaginal lavage. In: Heindel J, Chapin R, editors. Female Reproductive Toxicology. Academic Press; San Diego, CA: 1993. pp. 45–56. [Google Scholar]
- CPSC. Review of Exposure and Toxicity Data for Phthalate Substitutes. 2010. [Google Scholar]
- Craig Z, Hannon P, Wang W, Ziv-Gal A, Flaws J. Di-n-butyl phthalate disrupts the expression of genes involved in cell cycle and apoptotic pathways in mouse ovarian antral follicles. Biol Reprod. 2013;88:23. doi: 10.1095/biolreprod.112.105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwall B, Nordensten C, Albanus L. Toxicity of 29 plasticizers to HeLa cells in the MIT-24 system. Toxicology. 1982;24:199–210. doi: 10.1016/0300-483x(82)90002-6. [DOI] [PubMed] [Google Scholar]
- Fromme H, Schütze A, Lahrz T, Kraft M, Fembacher L, Siewering S, Burkardt R, Dietrich S, Koch H, Volkel W. Non-phthalate plasticizers in German daycare centers and human biomonitoring of DINCH metabolites in children attending the centers (LUPE 3) Int J Hyg Environ Health. 2016;219:33–39. doi: 10.1016/j.ijheh.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Gannon AM, Stampfli MR, Foster WG. Cigarette smoke exposure leads to follicle loss via an alternative ovarian cell death pathway in a mouse model. Toxicol Sci. 2012;125:274–284. doi: 10.1093/toxsci/kfr279. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Singh JM, Leslie TC, Meachum S, Flaws JA, Yao HH. Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol Appl Pharmacol. 2010;242:224–230. doi: 10.1016/j.taap.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P, Brannick K, Wang W, Flaws J. Mono(2-ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles. Biol Reprod. 2015;92:120. doi: 10.1095/biolreprod.115.129148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P, Peretz J, Flaws J. Daily exposure to di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol Reprod. 2014;90:1–11. doi: 10.1095/biolreprod.114.119032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Diaz S, Mitchell AA, Kelley KE, Calafat AM, Hauser R. Medications as a potential source of exposure to phthalates in the U.S. population. Environ Health Perspect. 2009;117:185–189. doi: 10.1289/ehp.11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. National Academies Press; Washington, DC: 1996. [Google Scholar]
- Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacoba S, Tyl R, Williams P, Zacharewski T. NTP Center for the evaluation of risks to human reproduction; Phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl)phthalate. Reprod Toxicol. 2002a;16:529–653. doi: 10.1016/s0890-6238(02)00032-1. [DOI] [PubMed] [Google Scholar]
- Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R, Williams P, Zacharewski T. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di-n-butyl phthalate. Reprod Toxicol. 2002b;16:489–527. doi: 10.1016/s0890-6238(02)00033-3. [DOI] [PubMed] [Google Scholar]
- Kay V, Chambers C, Foster W. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol. 2013;43:200–219. doi: 10.3109/10408444.2013.766149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy PJ, Hauser R, Gennings C, Koch HM, Mirkes PE, Schwetz BA, Kortenkamp A. Assessment of phthalates/phthalate alternatives in children’s toys and childcare articles: Review of the report including conclusions and recommendation of the Chronic Hazard Advisory Panel of the Consumer Product Safety Commission. J Expo Sci Environ Epidemiol. 2015;25:343–353. doi: 10.1038/jes.2015.33. [DOI] [PubMed] [Google Scholar]
- Masuyama H, Hiramatsu Y, Mizutani Y, Inoshita H, Kudo T. The expression of pregnance X receptor and its target gene, cytochrome P450 3A1, in perinatal mouse. Mol Cell Endocrinol. 2001;172:47–56. doi: 10.1016/s0303-7207(00)00395-6. [DOI] [PubMed] [Google Scholar]
- Mochida K, Gomyoda M, Fujita T. Acetyl tributyl citrate and dibutyl sebacate inhibit the growth of cultured mammalian cells. Bull Environ Contam Toxicol. 1996;56:635–637. doi: 10.1007/s001289900092. [DOI] [PubMed] [Google Scholar]
- Nishijima M, Hashimoto Y, Nakamura M. Cytocompatibility of new phthalate ester-free tissue conditioners in vitro. Dent Mater J. 2002;21:118–132. doi: 10.4012/dmj.21.118. [DOI] [PubMed] [Google Scholar]
- NTP. Report on carcinogens. National Toxicology Program; Research Triangle Park, NC: 1998. [Google Scholar]
- Parker R. Reproductive toxicity testing - methodology. In: Hood R, editor. Developmental and Reproductive Toxicology: A Practical Approach. Informal Healthcare; New York, NY: 2012. pp. 184–228. [Google Scholar]
- Robbins M. BIBRA Toxicology International. 1994. A two-generation reproduction study with acetyl tributyl citrate in rats. 1298/1/2/94: As cited in U.S. EPA (2008) [Google Scholar]
- Scientific Committee on Emerging and Newly-Identified Health Risks (SCENIHR) Preliminary report on the safety of medical devices containing DEHP-plasticized PVC or other plasticizers on neonates and other groups possibly at risk. Health & Consumer Protection Directorate-General, European Commission; 2008. http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_014.pdf. [Google Scholar]
- Sen N, Liu X, Craig Z. Short term exposure to di-n-butyl phthalate (DBP) disrupts ovarian function in young CD-1 mice. Reprod Toxicol. 2015;53:15–22. doi: 10.1016/j.reprotox.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheftel V. Indirect Food Additives and Polymers: Migration and Toxicology. Lewis Publishers; Boca Raton, FL: 2000. [Google Scholar]
- Takeshita A, Igarashi-Migitaka J, Nishiyama K, Takahashi H, Takeuchi Y, Koibuchi N. Acetyl tributyl citrate, the most widely used phthalate substitute plasticizer, induces cytochrome p450 3a through steroid and xenobiotic receptor. Toxicol Sci. 2011;123:460–470. doi: 10.1093/toxsci/kfr178. [DOI] [PubMed] [Google Scholar]
- Wang W, Craig Z, Basavarajappa M, Gupta R, Flaws J. Di (2-ethylhexyl) phthalate inhibits growth of mouse antral follicles through an oxidative stress pathway. Toxicol Appl Pharmacol. 2012;258:288–295. doi: 10.1016/j.taap.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Hafner K, Flaws J. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol Appl Pharmacol. 2014;276:157–164. doi: 10.1016/j.taap.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle F, Wolz G, Franz R. Migration of plasticizers from PVC tubes into enteral feeding solutions. Pharma International. 2005;3:17–21. [Google Scholar]