Abstract
The molecular determinants of pathogenic leukocyte migration across the blood-nerve barrier (BNB) in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) are unknown. Specific disease modifying therapies for CIDP are also lacking. Fibronectin connecting segment-1 (FNCS1), an alternatively spliced fibronectin variant expressed by microvascular endothelial cells at sites of inflammation in vitro and in situ, is a counterligand for leukocyte α4 integrin (also known as CD49d) implicated in pathogenic leukocyte trafficking in multiple sclerosis and inflammatory bowel disease. We sought to determine the role of FNCS1 in CIDP patient leukocyte trafficking across the BNB in vitro and in severe chronic demyelinating neuritis in vivo using a representative spontaneous murine CIDP model. Peripheral blood mononuclear leukocytes from 7 untreated CIDP patients were independently infused into a cytokine-treated, flow-dependent in vitro BNB model system. Time-lapse digital video microscopy was performed to visualize and quantify leukocyte trafficking, comparing FNCS1 peptide blockade to relevant controls. Fifty 24-week old female B7-2 deficient non-obese diabetic mice with spontaneous autoimmune peripheral polyneuropathy (SAPP) were treated daily with 2 mg/kg FNCS1 peptide for 5 days via intraperitoneal injection with appropriate controls. Neurobehavioral measures of disease severity, motor nerve electrophysiology assessments and histopathological quantification of inflammation and morphometric assessment of demyelination were performed to determine in vivo efficacy. The biological relevance of FNCS1 and CD49d in CIDP was evaluated by immunohistochemical detection in affected patient sural nerve biopsies. 25 μM FNCS1 peptide maximally inhibited CIDP leukocyte trafficking at the human BNB in vitro. FNCS1 peptide treatment resulted in significant improvements in disease severity, motor electrophysiological parameters of demyelination and histological measures of inflammatory demyelination. Microvessels demonstrating FNCS1 expression and CD49d+ leukocytes were seen within the endoneurium of patient nerve biopsies. Taken together, these results imply a role for FNCS1 in pathogenic leukocyte trafficking in CIDP, providing a potential target for therapeutic modulation.
Keywords: blood-nerve barrier, chronic inflammatory demyelinating polyradiculoneuropathy, fibronectin connecting segment-1, integrin, leukocyte trafficking, spontaneous autoimmune peripheral polyneuropathy
INTRODUCTION
CIDP is a clinically heterogeneous immune-mediated disorder affecting peripheral nerve and nerve roots with maximum severity attained after 8 weeks following symptom onset. CIDP has an estimated annual incidence of 1–2/100,000 and prevalence as high as 9/100,000 (Dalakas and Medscape, 2011; Laughlin et al., 2009). CIDP is often under-recognized and may account for about 14% of chronic disability in adults above the age of 65 (Chia et al., 1996). Its clinical course may be described as relapsing-remitting, steady progressive or step-wise progressive, with albuminocytologic dissociation commonly observed on cerebrospinal fluid analysis. Different clinical variants exist based on the pattern of nerve and nerve root involvement observed on electrodiagnostic studies. Pathologically, CIDP is characterized by infiltration of predominantly monocytes/macrophages and less commonly T lymphocytes into peripheral nerve and nerve root endoneurium with macrophage-mediated demyelination. However, inflammatory infiltrates may be sparse with prominent evidence of demyelination with or without remyelination seen in nerve biopsies. Onion bulb formation, indicative of repetitive demyelination and remyelination may be observed in more chronic cases (Dalakas, 2015; Mathey et al., 2015; Ubogu, 2015). Several diagnostic criteria exist for clinical and research purposes. Consensus statements have been published by consortia of experts to aid clinicians diagnose patients early and institute therapy (Dalakas, 2015; EFNS/PNS, 2010; Mathey et al., 2015)
Current treatments for CIDP include corticosteroids, intravenous immunoglobulin (IVIg) and plasma exchange based on randomized controlled trials, and immunosuppressant drugs such as azathioprine, mycophenolate, cyclosporine and cyclophosphamide based on small case series, case reports or specialist physician experience (Brannagan, 2009). Despite significant advances in understanding the molecular pathogenesis of immune-mediated disorders and the development of disease-specific therapies for disorders such as rheumatoid arthritis, psoriasis, inflammatory bowel disease and multiple sclerosis over the last 20 years, specific disease-modifying therapies for CIDP do not currently exist. CIDP heterogeneity and clinical recognition provide challenges; however lack of patient nerve and nerve root biopsies for extensive exploratory studies, dearth of in vitro human BNB models to study pathogenic inflammatory mechanisms and the limitations of representative animal models needed to ascertain pathogenic mechanisms and test potential drugs in vivo before clinical trials are planned have adversely hampered translational strategies needed for novel therapeutic development in CIDP (Meyer zu Horste et al., 2007; Ubogu, 2015).
Inflammatory leukocyte migration across microvessels is a sequential, coordinated process (i.e. multistep paradigm) involving specific selectins, chemokines and adhesion molecules on endothelial cells, and selectin counterligands, chemokine receptors, integrins and matrix metalloproteases expressed by leukocytes (Man et al., 2007; Muller, 2011; Simon and Green, 2005; Ubogu, 2015; Yonekawa and Harlan, 2005). Direct evidence of pathogenic leukocyte trafficking at the human BNB via the paracellular route has been demonstrated in vitro and in situ (Dong et al., 2016; Yosef and Ubogu, 2012). Observational studies evaluating CIDP patient nerve biopsies, cerebrospinal fluid, plasma and sera have shown increased expression of specific pro-inflammatory cytokines, chemokines and chemokine receptors, adhesion molecules, matrix metalloproteinases and other inflammatory mediators (Dalakas and Medscape, 2011; Mathey et al., 2015; Ubogu, 2015). The molecular determinants and signaling mechanisms of pathogenic leukocyte trafficking at the BNB in CIDP patients are unknown. Targeting pathogenic leukocyte trafficking at the BNB is a plausible approach to limit inflammatory demyelination and improve patient outcomes, as seen with natalizumab (humanized mouse anti-human α4 integrin monoclonal antibody) in relapsing-remitting multiple sclerosis (Pucci et al., 2011). Interestingly, there is some conflicting evidence on the efficacy of natalizumab in medically refractory CIDP based on a single case report showing no benefit and a small three-patient case series showing clinical effect (Vallat et al., 2015; Wolf et al., 2010).
Fibronectin, a major extracellular matrix macromolecule, consists of an alternatively spliced type III connecting segment that generates a high affinity binding domain for leukocyte α4 integrin. This binding domain, called fibronectin connecting segment-1 (FNCS1) has a conserved peptide sequence, leucine-aspartate-valine (LDV), critical for integrin binding (Humphries et al., 1987; Mould et al., 1994). FNCS1 may be involved in the pathogenesis of chronic inflammatory conditions such as rheumatoid arthritis, allergic contact dermatitis and multiple sclerosis (Elices et al., 1994; Man et al., 2009; Martín et al., 2003; Müller-Ladner et al., 1997; Ubogu et al., 2006). FNCS1 expression has been demonstrated on human endoneurial endothelial cells that form the BNB in vitro, with increased relative expression of splice variants that contain the LDV sequence and time-dependent protein expression observed following physiological cytokine stimulus (Yosef and Ubogu, 2012; Yosef et al., 2010). Vascular cell adhesion molecule-1 (VCAM-1) is also a counterligand for α4 integrin implicated in lymphocyte migration during infection and inflammation, as well as lymphocyte bone marrow retention and homing into lymph nodes via high endothelial venules during normal immune processes (Boscacci et al., 2010; Faveeuw et al., 2000; Koni et al., 2001; Papayannopoulou and Craddock, 1997; Xu et al., 2003). Competitive antagonism of FNCS1-α4 integrin binding with small molecular antagonists that target the LDV peptide sequence could result in a specific disease modifying therapy for CIDP without interfering with α4 integrin-VCAM-1 binding implicated in normal immunity (Haworth et al., 1999; Jackson, 2002; Liu et al., 2015; Man et al., 2009; Ubogu et al., 2006).
METHODS
CIDP patient leukocytes and sural nerve biopsies
Peripheral blood mononuclear leukocytes (PBMLs) were obtained from whole heparinized blood donated by seven untreated adult patients with clinical, electrophysiological and supportive cerebrospinal fluid or histopathological evidence of CIDP based on the Inflammatory Neuropathy Cause and Treatment criteria. PBMLs were isolated using density gradient centrifugation and cryopreserved in liquid nitrogen using a controlled rate freezing process. This was performed due to donor unpredictability and to ensure experiments were performed concurrently to limit experimental variation. Leukocyte viability was >99% following reconstitution in warmed medium at 37°C using the 0.4% trypan blue exclusion test. Duration of storage had no significant effect on PBML viability or composition provided experiments were performed within 6 months of preservation, as previously published (Yosef and Ubogu, 2012). Archived frozen sural nerve biopsies from four untreated adult CIDP patients and two normal controls stored in optimum cutting temperature compound at −80°C were obtained from the Shin J. Oh Muscle and Nerve Histopathology Laboratory, Department of Neurology, University of Alabama at Birmingham. The study was approved by the Institutional Review Board, with an exemption obtained to use archived pathological specimens for research. Written informed consent was obtained from each CIDP patient blood donor.
Flow-dependent human in vitro blood-nerve barrier trafficking assay
Untreated CIDP patient PBML trafficking across the human BNB in vitro was studied in real time using a flow-dependent leukocyte trafficking assay. A parallel plate flow chamber was attached to basal and cytokine-treated confluent primary human endoneurial endothelial cells (that form the BNB) cultured on rat tail collagen-coated CellBIND® Petri dishes, coupled to time-lapse video microscopy as previously described (Greathouse et al., 2016; Ubogu, 2013; Yosef and Ubogu, 2012). CIDP patient PBMLs were incubated with FNCS1 peptide EILDVPST (GenScript, Piscataway, NJ; 0–100 μM), a scrambled control peptide DELPQLVTL (designated as FNCS1C, GenScript, 25 μM: negative control), human IVIg (Carimune® nanofiltered, CSL Behring AG, Bern, Switzerland, 5 mg/mL; current CIDP treatment: positive control) and 6-alpha methylprednisolone (6αMP; Sigma Aldrich, St. Louis, MO; 200 μg/mL: current CIDP treatment: positive control) for 10 mins at 37°C before performing the leukocyte-BNB trafficking assay with the drug in solution. Each experiment lasted 30 minutes. FNCS1 peptide was compared to current CIDP treatments, 6αMP and human IVIg, to determine equivalence or superiority in modulating leukocyte trafficking at the BNB in vitro. FNCS1 peptide is being evaluated as a competitive antagonist occupying the critical α4 integrin binding site on PBMLs. This is hypothesized to prevent leukocyte adhesion to endothelial cell-expressed FNCS1 during the trafficking cascade (Man et al., 2009). Leukocytes from healthy controls or patients with other neurological disorders were not used in this study as the aim was to determine comparative drug efficacy in CIDP patients, with each patient serving as his/her own control.
Videos were generated by merging digital photomicrographs generated by an Axiocam MRc 5 digital camera (Carl Zeiss Microscopy, Jena, Germany) using the National Institutes of Health Image J software (field of view 870 μm long × 650 μm wide) or directly using an Eclipse Ci-S Upright epifluorescent microscope with a D5-Qi2 camera (Nikon Instruments Inc., Melville, NY) coupled to the Nikon NIS-Elements AR software program (field of view 1420 μm long × 950 μm wide). Automated quantification of firmly adherent and migrated PBMLs was performed following software module validation and concurrence with manual counts. The total number of adherent and migrated PBMLs was counted in three non-adjacent fields and averaged per experiment to account for observed regional variations in leukocyte trafficking. The adhesion/migration index (AMI) was calculated by dividing the mean numbers of adherent/migrated PBMLs for each patient and experimental condition following trafficking after physiological BNB cytokine treatment by numbers obtained from the same patient using the untreated (basal) BNB. The effect of FNCS1 peptide inhibition on CIDP patient PBML trafficking at the BNB in vitro was ascertained by comparing the mean AMI to untreated, FNCS1C peptide, IVIg and 6αMP-treated conditions.
Spontaneous autoimmune peripheral polyneuropathy (SAPP)
SAPP is a chronic demyelinating neuritis that occurs in female B7-2 (CD86)-deficient non-obese diabetic mice (NOD.129S4-Cd86tm1Shr /JbsJ; initially purchased from the Jackson Laboratory [Bar Harbor, ME]), with features that recapitulate a severe form of progressive CIDP with subsequent secondary axonal loss. Disease onset is at 20 weeks of age with 100% of females reaching maximum severity by 32 weeks old (Salomon et al., 2001; Ubogu et al., 2012). A continuous colony of these mice has been maintained for over 7 years in the specific pathogen-free Transgenic Mouse Facility (a barrier level 3 facility) at Baylor College of Medicine, Houston, Texas with transfer to an equivalent pathogen-free animal facility room located in the Research Services Building, University of Alabama at Birmingham, Birmingham, Alabama. Mice were kept in micro-isolator cages with chow and water provided ad libitum, maintaining a 12 hour light–dark cycle with standard environmental enrichment without restrictions to free movement.
To determine the effect of FNCS1 peptide inhibition on chronic demyelinating neuritis in vivo, 4 independent experiments were performed using a total of fifty 24-week old female, non-pregnant, SAPP-affected mice with discernible involvement for 4 weeks, as ascertained using a published 6-point semiquantitative Neuromuscular Severity Scale (NMSS) score (Xia et al., 2010a; Yuan et al., 2014). The score is as follows: 0: no weakness, 1: tail weakness, 2: mild-to-moderate hind or forelimb weakness, 3: severe hind or forelimb weakness, 4: mild-to-moderate hind and forelimb weakness, 5: severe hind and forelimb weakness. Intermediate scores were given when variable degrees of weakness were observed between limbs. Mice from different cages were evaluated to determine degree of severity and grouped as new littermates with equivalent mean NMSS scores between experimental groups prior to drug administration. Mice with NMSS scores >4 at the onset were not included in this study in order to exclude mice with significant, potentially irreversible early axonal degeneration.
SAPP mice were treated daily for 5 days via intraperitoneal injection with FNCS1 peptide (2 mg/kg, 12 mice), FNCS1C peptide (2 mg/kg, 12 mice), 400 mg/kg human IVIg or 50 mg/kg 6αMP (13 mice each). Each experimental mouse was weighed and evaluated for neurobehavioral signs of weakness using the NMSS 3 times a week for 30 days after treatment initiation. FNCS1 peptide was compared to current CIDP treatments, 6αMP and human IVIg to determine efficacy equivalence or superiority in vivo, as may be expected in a clinical trial. In another series of experiments, the efficacy of FNCS1 peptide inhibition (2 mg/kg; 3 mice) in SAPP was compared to α4 integrin (CD49d, rat anti-mouse IgG1, 5 mg/kg: 4 mice) and VCAM-1 (rat anti-mouse IgG2b, 10 mg/kg: 4 mice) antibody inhibition, with blockade using FNCS1C peptide (2 mg/kg: 3 mice) and mouse IgG2b isotype antibody (10 mg/kg: 3 mice) serving as negative controls. Research studies received approval from the Institutional Animal Care and Use Committees, and were conducted in accordance with the National Institutes of Health Guide on Humane Care and Use of Laboratory Animals.
Peripheral nerve electrophysiology and sciatic nerve procurement
Bilateral dorsal caudal tail nerve (DCTN) and sciatic nerve motor electrophysiology studies were performed on all experimental mice in the prone position under ketamine-xylazine anesthesia on day 30 post-treatment initiation as previously described (Ubogu et al., 2012; Xia et al., 2010a, b). Distal and proximal compound motor action potential (CMAP) amplitudes (in mV), conduction velocity (in m/s) and distal and proximal CMAP total waveform durations (in ms) were measured or deduced for each nerve studied. Following motor nerve electrophysiology, both sciatic nerves were procured from each experimental mouse following euthanasia under general anesthesia and immediately stored at −80°C for future cryostat sectioning or further processed for plastic embedding, as previously published (Ubogu et al., 2012; Xia et al., 2010a; Yuan et al., 2014).
Indirect immunohistochemistry of peripheral nerves
Indirect fluorescent immunohistochemistry was performed on consecutive 20 μm thick axial and longitudinal cryostat sections of four adult CIDP and two control human sural nerve biopsies to detect and compare endoneurial microvessel FNCS1 and leukocyte CD49d expression. Sections were fixed in 4% paraformaldehyde in 1X phosphate buffered saline (PBS) for 20 minutes, washed with 0.1% bovine serum albumin and air-dried prior to blocking with 3% bovine serum albumin. All primary and secondary antibodies were diluted in 3% bovine serum albumin in 1X PBS. Sections were incubated with 2 μg/mL mouse anti-human FNCS1 IgM antibody (clone 2Q607, Santa Cruz) at 4°C overnight, washed and coincubated with goat anti-mouse IgM-Alexa Fluor® 594 (5 μg/mL, Thermo Fisher Scientific, Waltham, MA) and 25 μg/mL FITC-conjugated Ulex Europaeus Agglutinin I (UEA-1) Lectin [Sigma Aldrich; most sensitive detector of human endothelial cells] (Yosef et al., 2010) for an hour at room temperature in the dark. To detect CD49d+ endoneurial leukocytes, sections were fixed in acetone at −20°C, washed and blocked with 10% normal goat serum as previously described (Dong et al., 2016). Sections were coincubated with mouse anti-human CD45 IgG2a antibody (2 μg/mL, Bio-Rad, Hercules, CA) and mouse anti-human CD49d IgG1 antibody (10 μg/mL, Bio-Rad) in 2% normal goat serum in 1X PBS for an hour at room temperature, washed and incubated with goat anti-mouse IgG2a-Alexa Flour® 488 and goat antimouse IgG1-Alexa Flour® 594 antibodies (both 5 μg/mL, Thermo Fisher Scientific) in the dark for an hour at room temperature.
Indirect immunohistochemistry was also performed on serial 10 μm thick axial cryostat sections of six representative mouse sciatic nerves per experimental group to detect CD45 as required to quantify total endoneurial leukocyte infiltration, as well as qualitatively compare demyelination and axonal loss between experimental groups, using published protocols (Ubogu et al., 2012; Xia et al., 2010a; Yuan et al., 2014). Serial sections of the distal sciatic nerve were obtained at 30–50 μm intervals. Sections were fixed in acetone at −20°C, washed and air-dried prior to blocking with 10% normal goat serum in 1X PBS. All primary and secondary antibodies were diluted in 2% normal goat serum in 1X PBS. The following primary antibodies were used: Rat anti-mouse CD45 IgG2b antibody (Pan-leukocyte marker, Clone: YW62.3, Bio-Rad: 10 μg/mL) polyclonal rabbit anti-mouse S100β IgG (Schwann cell marker; DAKO North America Inc. Carpinteria, CA: 20 μg/mL), polyclonal rabbit anti-mouse neurofilament-H IgG (NF-H; axonal marker, Santa Cruz Biotechnology, Santa Cruz, CA: 4 μg/mL). The following secondary antibodies were used: goat anti-rat IgG (H+L)-TXRD (SouthernBiotech, Birmingham, AL: 2 μg/mL), and goat anti-mouse IgG (H + L) Alexa Fluor® 488 and 594 conjugates (Life technologies, Carlsbad, CA: 5 μg/mL).
All sections (human and mouse nerves) were stained with 0.45 μM 4′, 6-diamidino-2-phenylindole (DAPI) for 5 minutes to detect nuclei and mounted with ProLong® Gold antifade mounting medium (Life technologies). Photomicrographs were taken using an Eclipse Ci-S Upright epifluorescent microscope with a D5-Qi2 camera (Nikon). Automated quantification of mouse CD45+ leukocytes per section was performed on merged photomicrographs using the NIS-Elements AR software program (Nikon). A total of 110 sections were quantified (FNCS1 26, FNCS1C 28, IVIg 27 and 6αMP 29). Data were expressed as number of leukocytes/section to eliminate errors in cross-sectional area that may occur with tissue processing ex vivo and variable increases in endoneurial area that may occur with edema associated with inflammation, and compared between experimental groups.
Sciatic nerve morphometric analysis
The degree of demyelination (% total demyelinated area per section) in SAPP-affected sciatic nerves was ascertained using glutaraldehyde-fixed, osmium tetroxide post-fixed, epoxy resin embedded, semi-thin sections stained with 1% toluidine blue, as previously published (Dong et al., 2016; Ubogu et al., 2012; Xia et al., 2010a; Yuan et al., 2014). A total of 52 sections of the distal sciatic nerve separated by 40–100 μm were analyzed (FNCS1 12, FNCS1C 12, IVIg 12, 6αMP 16). Representative color photomicrographs were subsequently obtained using an Axioskop epifluorescent microscope equipped with an Axiocam MRc 5 digital camera (Carl Zeiss Microscopy, Jena, Germany).
Statistical analyses
Investigator-blinded data analyses were performed for all parameters evaluated using the GraphPad Prism® 6 statistical program (GraphPad Software, Inc., La Jolla, CA). Mann-Whitney U-test or the Wilcoxon-Kruskall’s Rank Sum Test was used to determine statistically significant differences between non-parametric variables while one- or two-tailed unpaired Student’s/Welch’s t-test was used for parametric variables based on the Shapiro-Wilk test of normality (including measures of skew and kurtosis). Holm-Sidak’s test was used for multiple parametric variable comparisons. Means are displayed, with variations of the mean depicted as standard errors. Statistical significance is defined as a p-value < 0.05.
RESULTS
FNCS1 peptide inhibits CIDP patient PBML trafficking at the BNB in vitro
The characteristics of the untreated CIDP patients that participated in this study are shown in Table 1. Although patient E.P. reported a symptom onset of 9 days, the electrodiagnostic study clearly showed evidence of chronic reinnervation changes on needle electromyography consistent with CIDP. FNCS1 peptide induced a dose-dependent reduction in PBML AMI in the first three CIDP patients studied, with a minimum concentration of 25 μM maximally inhibiting PBML trafficking down to approximate basal levels (data not shown). This concentration was used in subsequent experiments. Physiological endothelial cytokine treatment induced a mean 2.13-fold increase in CIDP PBML AMI (range 1.23–2.84; Figure 1), implying an important role of local inflammatory cascades (e.g. upregulation in cytokines, chemokines, selectins and cell adhesion molecules) (Dalakas and Medscape, 2011; Lindenlaub and Sommer, 2003; Mathey et al., 1999; Press et al., 2003; Ubogu, 2015) in driving pathogenic CIDP patient PBML trafficking at the BNB in vitro, as demonstrated with Guillain-Barré syndrome patients (Yosef and Ubogu, 2012).
Table 1. Untreated CIDP patient characteristics.
Basic demographic, clinical, electrodiagnostic and supportive laboratory diagnostic information is provided on the cohort of untreated patients providing PBMLs for the flow-dependent human blood-nerve barrier assay to determine the effect of FNCS1 antagonism on pathogenic leukocyte trafficking in vitro. All patients were evaluated by a board-certified neuromuscular and electrodiagnostic medicine specialist and met the Inflammatory Neuropathy Cause and Treatment diagnostic criteria for CIDP
| PATIENT | GENDER | AGE (years) | RACE | SYMPTOM DURATION | ELECTRODIAGNOSTIC STUDY SUMMARY | CEREBROSPINAL FLUID/SURAL NERVE BIOPSY |
|---|---|---|---|---|---|---|
| M.A.C. | F | 54 | Caucasian | 8 months | Chronic sensorimotor demyelinating polyradiculoneuropathy | CSF Protein 167.0 mg/dL CSF white cell count 2/μL |
| E.P. | M | 56 | African-American | 9 days | Motor predominant chronic demyelinating polyradiculoneuropathy with conduction block | CSF Protein 247.7 mg/dL CSF white cell count 1/μL |
| K.S. | F | 53 | Caucasian | > 10 years (worse in last 18 months) | Chronic sensorimotor demyelinating polyradiculoneuropathy with multifocal temporal dispersion and sural sparing | CSF Protein 66.0 mg/dL CSF white cell count 0/μL |
| G.H. | F | 66 | Caucasian | > 6 years | Chronic sensorimotor demyelinating polyradiculoneuropathy with secondary axonal involvement. Multifocal non-length dependent inexcitable motor nerves | CSF Protein 218.0 mg/dL CSF white cell count 0/μL |
| J.B. | M | 45 | African-American | >20 years | Multifocal Acquired Demyelinating Sensory and Motor Neuropathy | Demyelinating neuropathy with axonal loss |
| M.S. | F | 71 | Caucasian | 3 months | Multifocal Acquired Demyelinating Sensory and Motor Neuropathy | CSF Protein 82.0 mg/dL CSF white cell count 2/μL |
| P.G. | F | 64 | Caucasian | 14 months | Subacute-to-chronic sensorimotor demyelinating polyradiculoneuropathy | CSF Protein 168.1 mg/dL CSF white cell count 0/μL |
Figure 1. Effect of FNCS1 antagonism on CIDP patient PBML trafficking at the blood-nerve barrier in vitro.
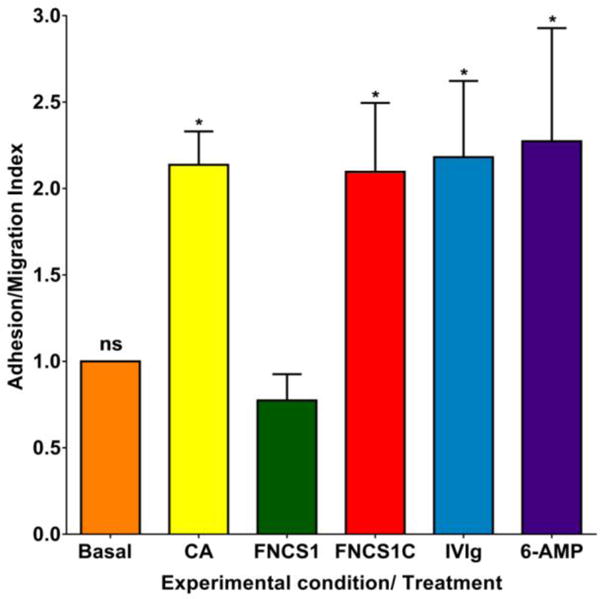
25 μM of FNCS1 peptide EILDVPST significantly reduced the flow-dependent untreated CIDP patient PBML adhesion and transmigration following endothelial cytokine activation (CA) to basal levels, while a control peptide that lacks the LDV sequence (FNCS1C: DELPQLVTL) demonstrated no effect. Neither human IVIg nor 6-α methylprednisolone (6-AMP) modulated CIDP PBML trafficking at the human BNB relative to uninhibited conditions in vitro. N=7 (ns: not significant, * indicates p<0.05)
25 μM FNCS1 peptide maximally inhibited CIDP PBML trafficking in vitro in this cohort, with a mean AMI of 0.77 (range 0.41–1.39), compared to FNCS1C peptide (AMI 2.09, range 0.81–3.67). Neither human IVIg (mean AMI 2.18, range 1.00–4.55) nor 6αMP (mean AMI 2.27, range 0.45–5.00) at estimated human serum concentrations when administered at 400 mg/kg and 50 mg/kg respectively, significantly altered CIDP PBML trafficking in this model system (Figure 1). These data support an important role of FNCS1 in untreated CIDP patient leukocyte trafficking at the human BNB at the adhesion and transmigration phase under conditions mimicking estimated in vivo microvascular hemodynamics in peripheral nerves. Representative videos depicting a single representative untreated CIDP patient’s PBML trafficking under basal, and BNB endothelial cytokine stimulated conditions, with and without FNCS1 or FNCS1C peptide inhibition are shown to illustrate the efficacy of FNCS1 peptide antagonism (Supplementary videos 1–4).
FNCS1 peptide reduces murine SAPP disease severity
Prior to drug administration, there was no significant difference in mean NMSS scores between experimental groups (mean NMSS score 2.8, range 1–4), as shown in Figure 2. FNCS1 peptide antagonism resulted in significant improvement in mean NMSS compared to FNCS1C peptide from day 8 that persisted for the duration of the experiment, with mean NMSS score of 1.4 (range 0–4) vs. 3.6 (range 3–5) on day 15 and 2.0 (range 0–4) vs. 4.2 (range 3.5–5) on day 30. FNCS1 peptide treatment also resulted in significantly reduced mean NMSS in comparison to both human IVIg and 6αMP treatment from day 11, with mean NMSS of 3.7 (range 2.5–5) on day 15 and 4.3 (range 4–5) on day 30 for human IVIg and mean NMSS of 3.7 (range 2–5) on day 15 and 4.3 (range 4–5) on day 30 for 6αMP (Figure 2). There was no significant difference in daily weights between the experimental groups (data not shown). Mice were not studied beyond 30 days, so the maximum FNCS1 peptide efficacy duration was undetermined. These data demonstrate the potent efficacy of FNCS1 peptide inhibition at a low micromolar concentration during the most rapidly progressive SAPP disease effector phase. Neither human IVIg nor 6αMP significantly modulated SAPP disease severity compared to the negative control, FNCS1C peptide in this mouse cohort based on NMSS scores.
Figure 2. Effect of FNCS1 antagonism on neuromuscular severity scores (NMSS) in murine SAPP.
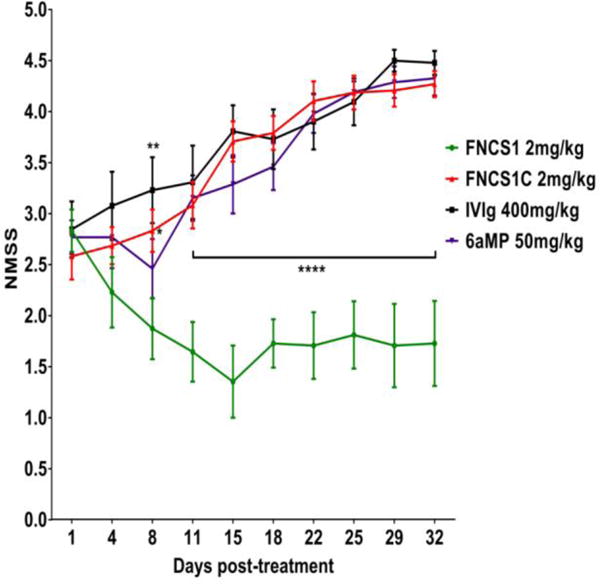
2 mg/kg FNCS1 peptide administered daily for 5 days significantly reduced disease severity with improvement in motor function compared to control FNCS1C peptide and human IVIg-treated mice 8 days after initial treatment. Significant reductions in mean NMSS were observed between FNCS1 peptide treatment and all control drugs from day 11 post-treatment that persisted throughout the experimental course. N=50, with 4 independent cohorts studied. * p<0.05, ** p < 0.01, **** p<0.0001 relative to FNCS1 peptide treatment
FNCS1 peptide improves motor electrophysiological measures of demyelination in murine SAPP
CMAP amplitude is a measure of axonal integrity, conduction velocity measures myelination status of the fastest firing axons and total waveform duration measures the degree of synchrony in myelinated axon conduction (Xia et al., 2010b). The latter two are commonly considered measures of demyelination, although distal conduction block may cause CMAP amplitude reduction. FNCS1 peptide antagonism resulted in a significant increase in mean conduction velocity and decrease in total distal waveform duration for the DCTN (Figure 3a–b) and sciatic nerves (Figure 3d–e) compared to FNCS1C, with no significant difference in CMAP amplitudes (Figure 3c and 3f). These data support FNCS1 peptide efficacy in treating early pathogenic demyelination in SAPP. Compared to human IVIg and 6αMP, FNCS1 peptide treatment demonstrated a significant increase in DCTN and sciatic nerve mean conduction velocities (Figure 3a and 3d) without a significant difference in total waveform durations (Figure 3b and 3e) or CMAP amplitudes (Figure 3c and 3f), implying relative protection of the fastestfiring, large myelinated motor axons in this model.
Figure 3. Effect of FNCS1 peptide antagonism on motor electrophysiology in murine SAPP.
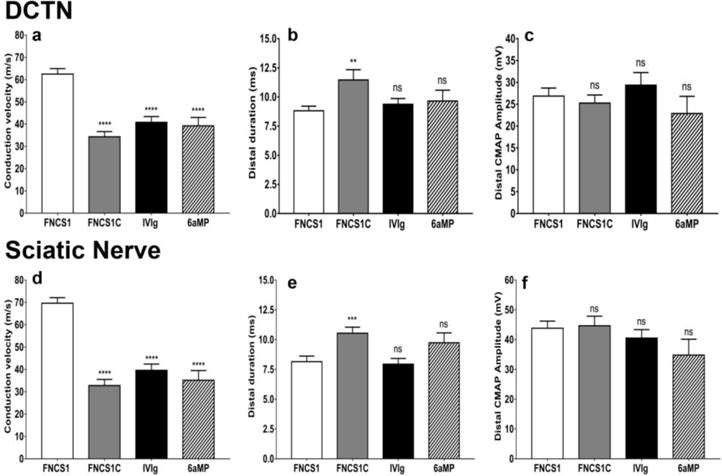
Bar histograms of mean motor electrophysiology parameters at day 30 post-treatment from the dorsal caudal tail nerves (DCTN, a–c) and the sciatic nerves (d–f) comparing FNCS1 peptide treatment with control FNCS1C peptide-, human IVIg-and 6-α methylprednisolone (6aMP)-treated mice is shown. Significantly increased conduction velocities (a and d) were seen following FNCS1 peptide treatment compared the other experimental treatments, with reduction in total distal waveform duration relative to FNCS1C peptide treatment only (b and e). There were no differences in distal compound motor unit action potential (CMAP) amplitudes between the experimental groups (c and f). These data imply FNCS1 peptide efficacy against demyelination of the fastest firing large myelinated axons in SAPP. N= 50, with 4 independent cohorts studied. ** p<0.01, *** p<0.001, **** p<0.0001 and ns = not significant relative to FNCS1 peptide treatment
FNCS1 peptide improves histopathological features of demyelination in murine SAPP
Qualitative analyses imply more retained myelination (inferred based more preserved honeycomb and less disorganized endoneurial S100β expression expected due to Schwann cell expression on myelinated axons) with relatively intact axonal density in the sciatic nerves following FNCS1 peptide antagonism compared to FNCS1C peptide-, human IVIg- and 6αMP -treated mice (Figure 4). As stated in the methods, serial sections were initially stained to detect leukocytes (CD45+) prior to S100β and NFH staining. The changes seen in S100β expression and axonal density within the endoneurium were more uniform in FNCS1 and FNCS1C peptide-treated SAPP mice (Figure 4a–d), while variability in demyelination and axonal loss associated with mononuclear cell infiltration were observed in human IVIg- (Figure 4e–h) and 6αMP -treated (Figure 4i–l) mice. These data suggest potential efficacy of FNCS1 peptide against inflammatory demyelination in SAPP. Persistent diffuse or multifocal disease is seen in FNCS1C peptide and IVIg/6αMP -treated mice respectively.
Figure 4. Qualitative effect of FNCS1 antagonism on inflammatory demyelination in murine SAPP.

Representative digital indirect fluorescent photomicrographs from the sciatic nerves of SAPP-affected mice 30 days after treatment initiation show the normal honeycomb appearance of myelinated axons with S100β staining [green]) following FNCS1 treatment (a) in contrast to multiple foci of inflammatory cells (blue) associated with endoneurial architecture disruption (altered honeycomb appearance) consistent with demyelination in FNCS1C peptide-treated mice (b). Normal axonal density (NF-H staining [red]) with rare mononuclear cells is shown in a FNCS1 peptide-treated mouse (c), in contrast to the intense diffuse inflammatory infiltrate associated with focal axonal loss in a FNCS1C peptide-treated mouse (d). The variability in demyelination and focal axonal loss associated with endoneurial mononuclear cell infiltration following IVIg- (e–h) and 6αMP- (i– l) treated SAPP-affected mice is also shown. These images were obtained from serial sections containing CD45+ leukocytes. Scale bars = 50 μm
Quantitative morphometric evaluation of demyelination performed on semi-thin plastic-embedded sciatic nerve sections demonstrated significant reductions in mean % demyelinated area per section following FNCS1 peptide treatment compared to FNCS1C peptide-, human IVIg-and 6αMP-treated mice (2.7% vs. 21.3%, 7.2% and 7.9% respectively) (Figure 5a). The more diffuse or multifocal demyelination seen with FNCS1C peptide and human IVIg/6αMP-treated SAPP mice as suggested by immunohistochemistry was also observed. Representative photomicrographs demonstrating normal myelinated axons in FNCS1 peptide-treated mice (Figure 5b) and foci of infiltrated mononuclear cells associated with demyelination in FNCS1C peptide-, human IVIg- and 6αMP-treated SAPP mice respectively (Figure 5c–e) are shown.
Figure 5. Quantitative effect of FNCS1 antagonism on demyelination in murine SAPP.
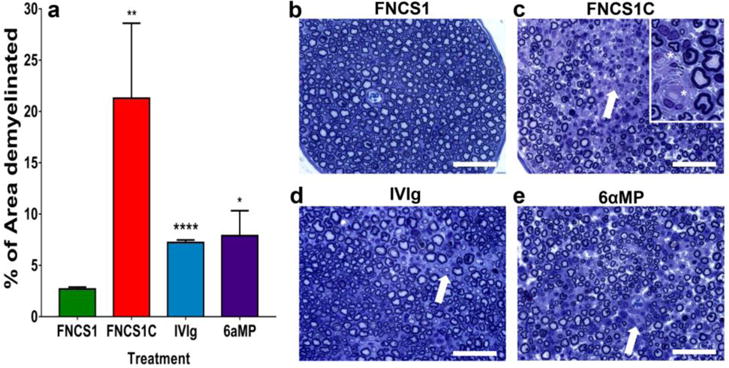
Bar histograms indicating the mean demyelinated area per plastic-embedded, toluidine-blue-stained, semithin sciatic nerve sections from SAPP-affected mice 30 days after treatment initiation show a significant reduction following FNCS1 peptide treatment compared with FNCS1C peptide-, IVIg- and 6-alpha methylprednisolone (6aMP)-treated mice (a), supporting the motor electrophysiological data. A total of 52 sections were analyzed. * p<0.05, ** p<0.01 and *** p<0.0001 relative to FNCS1 peptide treatment. Representative digital light photomicrographs show relative paucity of demyelination in a FNCS1 peptide-treated SAPP-affected mouse (b), in contrast to regions of mononuclear leukocyte infiltration with demyelination and axonal loss in FNCS1C peptide- (c), human IVIg- (d) and 6αMP- (e) treated mice (white arrows). More diffuse inflammatory demyelination was seen with FNCS1C peptide treatment. The insert in (c) demonstrates completed demyelinated axons (white asterisk) associated with mononuclear cells (300% magnification relative to initial photomicrograph). Scale bars = 50 μm
FNCS1 peptide reduces mononuclear leukocyte infiltration in murine SAPP
FNCS1 peptide antagonism significantly reduced the mean number of CD45+ leukocytes per sciatic nerve section in SAPP-affected mice compared to FNCS1C peptide-, human IVIg- and 6αMP-treated mice (Figure 6a). FNCS1 peptide blockade resulted in mean reductions in total leukocyte infiltration into the sciatic nerves in SAPP of 88%, 76% and 83% relative to FNCS1C peptide-, human IVIg- and 6αMP- treated mice respectively. This implies potent efficacy against mononuclear leukocyte infiltration in SAPP, attesting to a crucial anti-inflammatory effect in chronic demyelinating neuritis in vivo. Representative photomicrographs depicting differences in sciatic nerve inflammation between FNCS1 peptide antagonism and FNCS1C peptide-, human IVIg- and 6αMP-treated mice are shown in Figure 6b–g. The variability in leukocyte infiltration seen in human IVIg- and 6aαMP-treated SAPP mice is consistent with the multifocal progressive demyelination pattern described previously. The similarity in clinical course despite the observed differences in % area demyelinated and leukocyte counts between FNCS1C peptide- and human IVIg- and 6αMP-treated mice suggests a minimum threshold above which inflammatory demyelination would occur similarly in SAPP, as seen in a severe murine Guillain-Barré syndrome model (Dong et al., 2016; Yuan et al., 2014). Due to the multifocal disease observed, it is also possible that more proximal sciatic nerve segments had more demyelination, leukocyte infiltration or both than the distal segments evaluated.
Figure 6. Effect of FNCS1 antagonism on inflammation in murine SAPP.
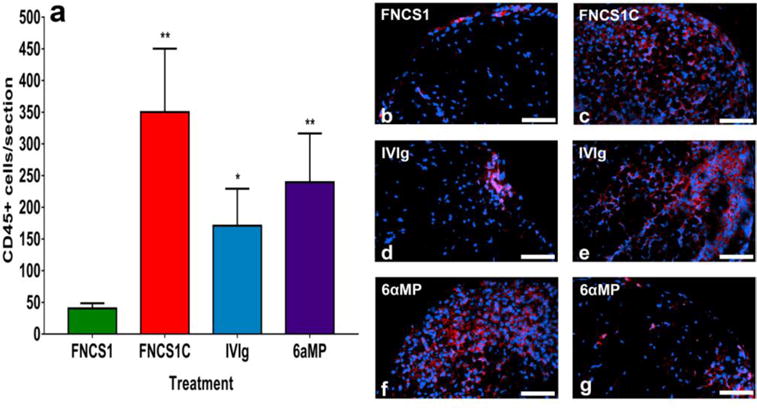
Bar histograms of the mean numbers of sciatic nerve endoneurial CD45+ leukocytes of SAPP-affected mice 30 days after treatment initiation are shown (a). Significant reductions in CD45+ leukocytes were observed following FNCS1 peptide treatment compared to mice treated with FNCS1C peptide, human IVIg and 6-alpha methylprednisolone (6aMP). These data imply a robust inhibitory effect of FNCS1 peptide on hematogenous leukocyte infiltration into the endoneurium in SAPP. A total of 110 sections were analyzed. * p <0.05 and ** p<0.01 relative to FNCS1 peptide treatment. Representative digital indirect fluorescent photomicrographs show the paucity of infiltrated CD45+ leukocytes (red) in a FNCS1 peptide-treated mouse (b) compared to diffuse endoneurial infiltration seen following FNCS1C peptide treatment (c). The variability in leukocyte infiltration following human IVIg (d and e) and 6αMP (f and g) treatment in SAPP is also demonstrated. Scale bars = 50 μm
FNCS1 peptide antagonism is as efficacious as α4-integrin and VCAM-1 blockade in murine SAPP
FNCS1 peptide blockade resulted in an equivalent reduction in NMSS when compared to monoclonal antibody blockade of α4 integrin (CD49d) and VCAM-1 in SAPP. There was a decline in mean NMSS to 1.6 seen by day 15 for both FNCS1 peptide and VCAM-1 antibody inhibition (range 0–3) that did not significantly worsen for the duration of the experiment. Direct α4 integrin blockade resulted in a reduction in mean NMSS to 1.6 (range 0–2.5) by day 10, implying more rapid therapeutic efficacy in SAPP, with no further decline observed for the duration of the experiment. These values are in contrast to a mean NMSS of 3.5 (range 3–5) with FNCS1C peptide and 3.7 (range 3.5–5) with mouse isotype IgG2b antibody treatment on day 15 that gradually increased during the experiment. No significant differences were observed in the motor electrophysiological or histopathological parameters of chronic inflammatory demyelination between FNCS1 peptide, α4 integrin and VCAM-1 antibody blockade in this model on day 30 based on this small experimental cohort (data not shown), implying equivalent therapeutic potency in modulating chronic demyelinating neuritis in vivo.
FNCS1 peptide modulates pathogenic leukocyte trafficking in vitro and in vivo
The CIDP patient PBML- human BNB in vitro trafficking assay, coupled with the in vivo neurobehavioral, electrophysiological and histopathological data in SAPP support a functional role of FNCS1 in chronic pathogenic leukocyte trafficking at the BNB into peripheral nerves that is amenable to therapeutic blockade. Intravital microscopy techniques to visualize leukocyte trafficking across the BNB in mice peripheral nerves have not been developed (Greathouse et al., 2016), so time-point assessment of hematogenously-derived leukocyte infiltration into peripheral nerve endoneurium provides an acceptable surrogate measure of trafficking across the mouse BNB in vivo. FNCS1 peptide significantly reduced CIDP PBML firm adhesion and subsequent transmigration to slightly below basal levels, consistent with blockade of activated leukocyte α4 integrin binding to surface expressed endoneurial endothelial cell FNCS1 in vitro, as hypothesized during the multistep paradigm (see Supplementary videos). FNCS1 peptide efficacy was similar to direct α4 integrin and VCAM-1 antibody blockade in vivo, implying that α4 integrin-FNCS1 interactions are potentially relevant to pathogenic leukocyte trafficking in CIDP. However, it is unknown whether endoneurial microvessels express FNCS1 in normal and pathologic human nerves in situ.
Endoneurial microvessel FN CS1 and leukocyte CD49d expression in CIDP patient sural nerves
The characteristics of the CIDP patients whose biopsies were examined in this study are shown in Table 2. FNCS1 was expressed on endoneurial microvessels within focal regions of inflammation in untreated CIDP patient sural nerve biopsy sections (Figure 7a–d). Expression was not detected in normal nerve biopsy sections (not shown). Clusters of CD49d+ endoneurial leukocytes were also observed in the untreated CIDP patient nerves (Figure 7e–j) with trafficking and perivascular CD49d+ leukocytes seen occasionally (Figure 7k–l respectively). Endoneurial CD49d+ leukocytes were rarely seen in normal patient nerves (Figure 7m). These observations, coupled with the in vitro leukocyte-BNB trafficking assay and the in vivo murine SAPP studies, suggest potential relevance of CD49d-FNCS1 in CIDP pathogenesis during leukocyte trafficking at the BNB, with FNCS1 peptide serving as a plausible disease-modifying small molecular antagonist for this disorder.
Table 2. Clinical characteristics of biopsied CIDP patients.
Basic demographic, clinical, electrodiagnostic and supportive laboratory diagnostic information is provided on the CIDP patients whose sural nerve biopsies were examined to determine FNCS1 and CD49d expression in situ is shown based on available medical record review.
| PATIENT | GENDER | AGE (years) | RACE | SYMPTOM DURATION | CLINICAL FEATURES | ELECTRODIAGNOSTIC STUDY SUMMARY | CEREBROSPINAL FLUID/LEFT SURAL NERVE BIOPSY |
|---|---|---|---|---|---|---|---|
| J.L. | M | 77 | Caucasian | >3 years | Bilateral foot drops with progressive lower extremity weakness and atrophy. Bilateral asymmetrical lower extremity numbness below knees. Trace knee jerks and absent ankles jerks. | Chronic diffuse sensorimotor demyelinating polyneuropathy with predominant lower extremity involvement. | CSF analysis: None Biopsy: Marked loss of myelinated axons with many thinly myelinated axons |
| J.H. | M | 75 | Caucasian | >4 years | Progressive bilateral lower extremity weakness resulting in wheelchair dependency. Clinical examination not documented | Chronic diffuse sensorimotor demyelinating polyneuropathy. | CSF analysis: None Biopsy: Marked loss of myelinated axons with many thinly myelinated axons in one fascicle |
| D.H. | F | 49 | Caucasian | > 40 years | Progressive bilateral distal lower extremity weakness and pain refractory/intolerant to IVIg. Bilateral reduced sensation below knees with areflexia at the knees and ankles. | Chronic diffuse sensorimotor demyelinating polyneuropathy. | CSF analysis: Protein 54.0 mg/dL, white cell count 0/μL Biopsy: Minimal decrease in myelinated axon population with thinly myelinated axons and few onion-bulb formation |
| B.V. | M | 68 | Caucasian | > 4 months | Progressive bilateral lower > Upper extremity weakness with right eyelid ptosis following chronic Epstein-Barr infection. Refractory to IVIg and prednisone. No discernible sensory loss with right cranial nerve III paresis. Generalized hypo- and areflexia. | Chronic sensorimotor polyneuropathy with demyelinating features in a single motor nerve. | CSF analysis: Protein 95.0 mg/dL, white cell count 10/μL (100% lymphocytes) Biopsy: Moderate loss of myelinated axons with myelin debris. Many thinly myelinated axons with few frankly demyelinated axons |
Figure 7. FNCS1 and α4 integrin (CD49d) expression in CIDP patient nerve biopsies.
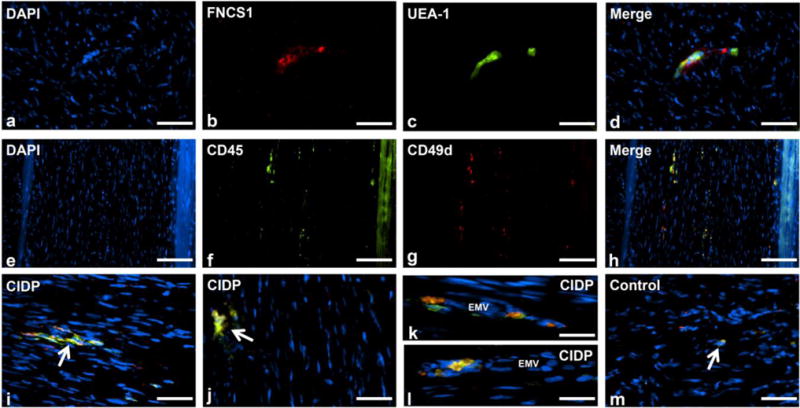
Digital indirect fluorescent photomicrographs of an axial section from the sural nerve biopsy of an untreated CIDP patient demonstrates a cluster of round mononuclear cells (a: DAPI, blue) associated with a FNCS1 expressing (b: red) endoneurial microvessel (c: UEA-1, green), verified by co-localization in the merged image (d: yellow). Longitudinal sural nerve biopsy sections show multiple small foci of endoneurial CD49d+ CD45+ leukocytes at lower magnification (e–h). Higher magnification photomicrographs from different untreated CIDP patients show clusters of endoneurial CD49d+ CD45+ leukocytes (i and j: white arrows). Extravasating and perivascular CD49d+ CD45+ leukocytes are also seen (k and l). CD49d+ CD45+ leukocytes were rarely seen within the endoneurium of normal control sural nerve biopsies (m: white arrow). EMV= endoneurial microvessel. Scale bars = 50 μm (a–d), 100 μm (e–h), 40 μm (i, j and m) and 20 μm (k and l)
DISCUSSION
In this study, we provide evidence supporting a role for FNCS1-α4 integrin (CD49d) in CIDP patient mononuclear leukocyte trafficking, with proof-of-principle data attesting to the therapeutic efficacy of FNCS1 peptide blockade in this disorder based on representative in vitro and in vivo experimental models. Although CD49+ leukocytes had been previously reported in a CIDP patient nerve biopsy (Wolf et al., 2010), we demonstrated endoneurial microvessel FNCS1 expression in areas associated with inflammation in CIDP, in addition to CD49d+ leukocytes in clusters within the endoneurium, including perivascular locations and cells undergoing transmigration in situ. We propose that FNCS1 peptide prevents demyelination by inhibiting pro-inflammatory leukocyte trafficking across the blood-nerve barrier via competitive antagonism of CD49-mediated leukocyte adhesion to FNCS1 expressed by chronically inflamed endoneurial microvessels in CIDP.
Pathogenic leukocyte trafficking into peripheral nerves in CIDP has been implied based on several observational studies; however the precise mechanisms are unknown. Pro-inflammatory cytokine expression (TNF-α, IFN- γ, interleukin-1, interleukin-2 and interleukin-6) in active CIDP patient sural nerves localizing to the innermost layer of the perineurium, epineurial and endoneurial blood vessels, infiltrating inflammatory cells and possibly Schwann cells has been described relative to control patient nerves (providing rationale for physiological cytokine stimulation of the in vitro BNB leukocyte trafficking model) (Lindenlaub and Sommer, 2003; Mathey et al., 1999; Van Rhijn et al., 2000). Endoneurial monocytes/macrophages (the most common leukocyte subset), CD4+ and CD8+ T-lymphocytes are seen at higher numbers in CIDP nerves compared to controls (Krendel et al., 1989; Schmidt et al., 1996; Ubogu, 2015). Interestingly, expression of E-selectin (upregulated on inflamed microvessels and implicated in leukocyte rolling) was observed on epineurial but not endoneurial vessels in a subset of CIDP sural nerves with cells positive for Sialyl Lewis × (a counterligand) adherent to their lumens (Oka et al., 1994).
Increased expression of chemokine CXCL10 (implicated in the migration of CD3+ CD4+ CXCR3 + T- helper 1 lymphocytes) has been observed in CIDP nerve biopsies, as well as CCR1 and CCR5 expression on endoneurial macrophages, with CCR2, CCR4 and CXCR3 expression on infiltrating T lymphocytes (Kieseier et al., 2002). This implies that chemoattraction of specific leukocyte subsets (a prerequisite for integrin activation, firm adhesion and subsequent transmigration) is pathogenically relevant to CIDP. Increased levels of cerebrospinal fluid CCL2, CCL7, CCL27, CXCL9, CXCL10, CXCL12, ICAM-1 and VCAM-1 have been described in CIDP compared to controls, further suggesting potential pathogenic relevance of chemokines and cell adhesion molecules in CIDP (Mei et al., 2005; Nagai et al., 2000; Sainaghi et al., 2010; Sainaghi et al., 2008). Although the direct relationship between leukocytes in nerve root endoneurium and cerebrospinal fluid is unknown, markedly elevated IFN-γ+ interleukin-4− CD4+ T cell percentages in CSF were observed in CIDP patients with a significant increase of intracellular IFN-γ/interleukin-4 ratio in the absence of pleocytosis. That study provided suggestive evidence of T helper 1 shift over T helper 2 phenotype (Mei et al., 2005); further emphasizing the migration of specific hematogenously derived pathogenic T-lymphocytes in CIDP.
CIDP patient leukocyte trafficking at the BNB is hypothesized to be an active coordinated process, supported by the in vitro video microscopy under flow conditions observed in this study. This is consistent with the widely accepted multi-step paradigm of leukocyte migration (Man et al., 2007; Muller, 2011; Simon and Green, 2005; Ubogu, 2015; Yonekawa and Harlan, 2005). We provide evidence that the FNCS1- α4 integrin signaling pathway has pathologic relevance to CIDP and is amenable to therapeutic blockade using a peptide antagonist based on human in situ and in vitro data that are supported by in vivo data using a severe progressive murine CIDP model. The discrepancy seen in therapeutic efficacy of natalizumab in reported cases of medically refractory CIDP may be due clinical heterogeneity, stage of disease (i.e. relapsing-remitting vs. progressive), to differences in dose needed to inhibit pathogenic leukocyte trafficking at the BNB in CIDP compared to the blood-brain and blood-spinal cord barriers with multiple sclerosis and the possibility that natalizumab may inhibit the migration of both pathogenic and regulatory/immunosuppressive leukocyte subpopulations into peripheral nerves/nerve roots and lymphoid organs respectively or facilitate the release of pathogenic lymphoblasts from the bone marrow.
It is also plausible that other integrins and cell adhesion molecules may participate in chronic leukocyte trafficking across the BNB in CIDP. Next generation whole transcriptome sequencing performed using PBMLs from an untreated CIDP patient showed expression of α1, α2, α3, α4, α5, α6, α7, α10, αD, αE, αL, αM and αX, as well as β1, β2, β3, β5 and β7 integrins (unpublished observations). Further studies are planned to determine whether other leukocyte integrins are activated by specific chemokines and participate in leukocyte subpopulation trafficking at the BNB during normal immune surveillance or in pathological conditions such as CIDP. Although monocytes are the most prevalent PBML subpopulation that adheres to and migrates across the human BNB in vitro and within the endoneurium in vivo in the SAPP model (Ubogu et al., 2012; Yosef and Ubogu, 2012), further studies are planned to determine whether alterations in relative leukocyte subpopulation numbers or phenotypic characteristics occur with specific leukocyte trafficking inhibitors.
Despite known limitations of in vitro leukocyte trafficking assays and animal models of CIDP (Greathouse et al., 2016; Man et al., 2009; Meyer zu Horste et al., 2007; Ubogu, 2013, 2015; Yosef and Ubogu, 2012), we propose a pathogenic role for FNCS1-CD49d in CIDP leukocyte BNB trafficking, recognizing that our patient cohort and nerve biopsies may not reflect the entire spectrum of disease. FNCS1 antagonism is a plausible therapeutic approach worth considering for early stage clinical trials based on our proof-of-concept data, with potential translational relevance to patients non-responsive to IVIg or corticosteroids. Modulating pathogenic leukocyte trafficking could occur following systemic drug administration without need for high BNB penetrance, and could potentially spare homeostatic lymphocyte homing into secondary lymphoid organs or retention in the bone marrow that are dependent on CD49d-VCAM-1 interactions. Further work is needed to completely elucidate the molecular determinants and signaling mechanisms involved in CIDP leukocyte trafficking, as well as potential mechanisms by which systemically generated pathogenic autoantibodies may penetrate the BNB in this disorder.
Supplementary Material
HIGHLIGHTS.
Further insights into the pathogenesis of CIDP are described.
FNCS1 peptide inhibits CIDP patient leukocyte trafficking at the blood-nerve barrier in vitro.
FNCS1 peptide ameliorates severe murine chronic demyelinating neuritis in vivo.
FNCS1 and its ligand, α4 integrin are expressed in CIDP patient nerve biopsies.
FNCS1 antagonism is a plausible targeted molecular therapy for CIDP.
Acknowledgments
Special thanks to past and current employees of the Shin J Oh Muscle and Nerve Histopathology Laboratory, University of Alabama at Birmingham for archived peripheral nerves from CIDP patients and controls. This project was supported by National Institutes of Health (NIH) grant [R21 NS073702; 2011–2014] and institutional funds from the University of Alabama at Birmingham. The funding sources had no involvement in the conduct of the research, manuscript preparation, data collection/analyses or decision to submit this work for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The data was presented in part as abstracts at the Inflammatory Neuropathy Consortium 2016 meeting, the 2016 Annual meeting of the American Association of Neuromuscular and Electrodiagnostic Medicine and ANA2016, the 141st Annual Meeting of the American Neurological Association.
POTENTIAL CONFLICTS OF INTEREST
E.E.U. has received royalties from Baylor Licensing Group for simian virus-40 large T-antigen immortalized human endoneurial endothelial cells and from Springer Science + Business Media for an edited book on laboratory protocols that describes the flow-dependent in vitro BNB assay. C.D., K.M.G., R.L.B., S.P.P. and E.S.H. have nothing to disclose.
References
- Boscacci RT, Pfeiffer F, Gollmer K, Sevilla AI, Martin AM, Soriano SF, Natale D, Henrickson S, von Andrian UH, Fukui Y, Mellado M, Deutsch U, Engelhardt B, Stein JV. Comprehensive analysis of lymph node stroma-expressed Ig superfamily members reveals redundant and nonredundant roles for ICAM-1, ICAM-2, and VCAM-1 in lymphocyte homing. Blood. 2010;116:915–925. doi: 10.1182/blood-2009-11-254334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannagan TH., 3rd Current treatments of chronic immune-mediated demyelinating polyneuropathies. Muscle & nerve. 2009;39:563–578. doi: 10.1002/mus.21277. [DOI] [PubMed] [Google Scholar]
- Chia L, Fernandez A, Lacroix C, Adams D, Plante V, Said G. Contribution of nerve biopsy findings to the diagnosis of disabling neuropathy in the elderly. A retrospective review of 100 consecutive patients. Brain : a journal of neurology. 1996;119(Pt 4):1091–1098. doi: 10.1093/brain/119.4.1091. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Pathogenesis of immune-mediated neuropathies. Biochimica et biophysica acta. 2015;1852:658–666. doi: 10.1016/j.bbadis.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Dalakas MC, Medscape Advances in the diagnosis, pathogenesis and treatment of CIDP. Nature reviews. Neurology. 2011;7:507–517. doi: 10.1038/nrneurol.2011.121. [DOI] [PubMed] [Google Scholar]
- Dong C, Palladino SP, Helton ES, Ubogu EE. The pathogenic relevance of alphaM-integrin in Guillain-Barre syndrome. Acta neuropathologica. 2016 doi: 10.1007/s00401-016-1599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFNS/PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society–First Revision. Journal of the peripheral nervous system : JPNS. 2010;15:1–9. doi: 10.1111/j.1529-8027.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- Elices M, Tsai V, Strahl D, Goel A, Tollefson V, Arrhenius T, Wayner E, Gaeta F, Fikes J, Firestein G. Expression and functional significance of alternatively spliced CS1 fibronectin in rheumatoid arthritis microvasculature. J Clin Invest. 1994;93:405–416. doi: 10.1172/JCI116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faveeuw C, Di Mauro ME, Price AA, Ager A. Roles of alpha(4) integrins/VCAM-1 and LFA- 1/ICAM-1 in the binding and transendothelial migration of T lymphocytes and T lymphoblasts across high endothelial venules. International immunology. 2000;12:241–251. doi: 10.1093/intimm/12.3.241. [DOI] [PubMed] [Google Scholar]
- Greathouse KM, Palladino SP, Dong C, Helton ES, Ubogu EE. Modeling leukocyte trafficking at the human blood-nerve barrier in vitro and in vivo geared towards targeted molecular therapies for peripheral neuroinflammation. J Neuroinflammation. 2016;13:3. doi: 10.1186/s12974-015-0469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth D, Rees A, Alcock PJ, Wood LJ, Dutta AS, Gormley JJ, Jones HB, Jamieson A, Reilly CF. Anti-inflammatory activity of c(ILDV-NH(CH2)5CO), a novel, selective, cyclic peptide inhibitor of VLA-4-mediated cell adhesion. British journal of pharmacology. 1999;126:1751–1760. doi: 10.1038/sj.bjp.0702511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M, Komoriya A, Akiyama S, Olden K, Yamada K. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987;262:6886–6892. [PubMed] [Google Scholar]
- Jackson DY. Alpha 4 integrin antagonists. Current pharmaceutical design. 2002;8:1229–1253. doi: 10.2174/1381612023394737. [DOI] [PubMed] [Google Scholar]
- Kieseier BC, Tani M, Mahad D, Oka N, Ho T, Woodroofe N, Griffin JW, Toyka KV, Ransohoff RM, Hartung HP. Chemokines and chemokine receptors in inflammatory demyelinating neuropathies: a central role for IP-10. Brain : a journal of neurology. 2002;125:823–834. doi: 10.1093/brain/awf070. [DOI] [PubMed] [Google Scholar]
- Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. The Journal of experimental medicine. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendel DA, Parks HP, Anthony DC, St Clair MB, Graham DG. Sural nerve biopsy in chronic inflammatory demyelinating polyradiculoneuropathy. Muscle & nerve. 1989;12:257–264. doi: 10.1002/mus.880120402. [DOI] [PubMed] [Google Scholar]
- Laughlin RS, Dyck PJ, Melton LJ, 3rd, Leibson C, Ransom J, Dyck PJ. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology. 2009;73:39–45. doi: 10.1212/WNL.0b013e3181aaea47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenlaub T, Sommer C. Cytokines in sural nerve biopsies from inflammatory and noninflammatory neuropathies. Acta neuropathologica. 2003;105:593–602. doi: 10.1007/s00401-003-0689-y. [DOI] [PubMed] [Google Scholar]
- Liu H, Dolkas J, Hoang K, Angert M, Chernov AV, Remacle AG, Shiryaev SA, Strongin AY, Nishihara T, Shubayev VI. The alternatively spliced fibronectin CS1 isoform regulates IL-17A levels and mechanical allodynia after peripheral nerve injury. J Neuroinflammation. 2015;12:158. doi: 10.1186/s12974-015-0377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S, Tucky B, Bagheri N, Li X, Kochar R, Ransohoff R. alpha4 Integrin/FN-CS1 mediated leukocyte adhesion to brain microvascular endothelial cells under flow conditions. J Neuroimmunol. 2009;210:92–99. doi: 10.1016/j.jneuroim.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S, Ubogu EE, Ransohoff RM. Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain pathology. 2007;17:243–250. doi: 10.1111/j.1750-3639.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín A, Gagliardi J, Baena-Cagnani C, Eberhard Y, Uguccioni M, Gallino N, Mariani A, Serra H. Expression of CS-1 fibronectin precedes monocyte chemoattractant protein-1 production during elicitation of allergic contact dermatitis. Clin Exp Allergy. 2003;33:1118–1124. doi: 10.1046/j.1365-2222.2003.01712.x. [DOI] [PubMed] [Google Scholar]
- Mathey EK, Park SB, Hughes RA, Pollard JD, Armati PJ, Barnett MH, Taylor BV, Dyck PJ, Kiernan MC, Lin CS. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. Journal of neurology, neurosurgery, and psychiatry. 2015 doi: 10.1136/jnnp-2014-309697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey EK, Pollard JD, Armati PJ. TNF alpha, IFN gamma and IL-2 mRNA expression in CIDP sural nerve biopsies. Journal of the neurological sciences. 1999;163:47–52. doi: 10.1016/s0022-510x(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Mei FJ, Ishizu T, Murai H, Osoegawa M, Minohara M, Zhang KN, Kira J. Th1 shift in CIDP versus Th2 shift in vasculitic neuropathy in CSF. Journal of the neurological sciences. 2005;228:75–85. doi: 10.1016/j.jns.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Meyer zu Horste G, Hartung HP, Kieseier BC. From bench to bedside–experimental rationale for immune-specific therapies in the inflamed peripheral nerve. Nature clinical practice. Neurology. 2007;3:198–211. doi: 10.1038/ncpneuro0452. [DOI] [PubMed] [Google Scholar]
- Mould A, Askari J, Craig S, Garratt A, Clements J, Humphries M. Integrin alpha 4 beta 1-mediated melanoma cell adhesion and migration on vascular cell adhesion molecule-1 (VCAM-1) and the alternatively spliced IIICS region of fibronectin. J Biol Chem. 1994;269:27224–27230. [PubMed] [Google Scholar]
- Müller-Ladner U, Elices M, Kriegsmann J, Strahl D, Gay R, Firestein G, Gay S. Alternatively spliced CS-1 fibronectin isoform and its receptor VLA-4 in rheumatoid arthritis synovium. J Rheumatol. 1997;24:1873–1880. [PubMed] [Google Scholar]
- Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai A, Murakawa Y, Terashima M, Shimode K, Umegae N, Takeuchi H, Kobayashi S. Cystatin C and cathepsin B in CSF from patients with inflammatory neurologic diseases. Neurology. 2000;55:1828–1832. doi: 10.1212/wnl.55.12.1828. [DOI] [PubMed] [Google Scholar]
- Oka N, Akiguchi I, Nagao M, Nishio T, Kawasaki T, Kimura J. Expression of endothelial leukocyte adhesion molecule-1 (ELAM-1) in chronic inflammatory demyelinating polyneuropathy. Neurology. 1994;44:946–950. doi: 10.1212/wnl.44.5.946. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T, Craddock C. Homing and trafficking of hemopoietic progenitor cells. Acta haematologica. 1997;97:97–104. doi: 10.1159/000203665. [DOI] [PubMed] [Google Scholar]
- Press R, Pashenkov M, Jin JP, Link H. Aberrated levels of cerebrospinal fluid chemokines in Guillain-Barre syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. Journal of clinical immunology. 2003;23:259–267. doi: 10.1023/a:1024532715775. [DOI] [PubMed] [Google Scholar]
- Pucci E, Giuliani G, Solari A, Simi S, Minozzi S, Di Pietrantonj C, Galea I. Natalizumab for relapsing remitting multiple sclerosis. The Cochrane database of systematic reviews. 2011:CD007621. doi: 10.1002/14651858.CD007621.pub2. [DOI] [PubMed] [Google Scholar]
- Sainaghi PP, Collimedaglia L, Alciato F, Leone MA, Naldi P, Molinari R, Monaco F, Avanzi GC. The expression pattern of inflammatory mediators in cerebrospinal fluid differentiates Guillain-Barre syndrome from chronic inflammatory demyelinating polyneuropathy. Cytokine. 2010;51:138–143. doi: 10.1016/j.cyto.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Sainaghi PP, Collimedaglia L, Alciato F, Leone MA, Puta E, Naldi P, Castello L, Monaco F, Avanzi GC. Elevation of Gas6 protein concentration in cerebrospinal fluid of patients with chronic inflammatory demyelinating polyneuropathy (CIDP) Journal of the neurological sciences. 2008;269:138–142. doi: 10.1016/j.jns.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Salomon B, Rhee L, Bour-Jordan H, Hsin H, Montag A, Soliven B, Arcella J, Girvin AM, Padilla J, Miller SD, Bluestone JA. Development of spontaneous autoimmune peripheral polyneuropathy in B7-2-deficient NOD mice. The Journal of experimental medicine. 2001;194:677–684. doi: 10.1084/jem.194.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Toyka KV, Kiefer R, Full J, Hartung HP, Pollard J. Inflammatory infiltrates in sural nerve biopsies in Guillain-Barre syndrome and chronic inflammatory demyelinating neuropathy. Muscle & nerve. 1996;19:474–487. doi: 10.1002/(SICI)1097-4598(199604)19:4<474::AID-MUS8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng. 2005;7:151–185. doi: 10.1146/annurev.bioeng.7.060804.100423. [DOI] [PubMed] [Google Scholar]
- Ubogu E, Callahan M, Tucky B, Ransohoff R. Determinants of CCL5-driven mononuclear cell migration across the blood-brain barrier. Implications for therapeutically modulating neuroinflammation. J Neuroimmunol. 2006;179:132–144. doi: 10.1016/j.jneuroim.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ubogu EE. Chemokine-dependent signaling pathways in the peripheral nervous system. Methods Mol Biol. 2013;1013:17–30. doi: 10.1007/978-1-62703-426-5_2. [DOI] [PubMed] [Google Scholar]
- Ubogu EE. Inflammatory neuropathies: pathology, molecular markers and targets for specific therapeutic intervention. Acta neuropathologica. 2015;130:445–468. doi: 10.1007/s00401-015-1466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubogu EE, Yosef N, Xia RH, Sheikh KA. Behavioral, electrophysiological, and histopathological characterization of a severe murine chronic demyelinating polyneuritis model. Journal of the peripheral nervous system : JPNS. 2012;17:53–61. doi: 10.1111/j.1529-8027.2012.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallat JM, Mathis S, Ghorab K, Milor MA, Richard L, Magy L. Natalizumab as a Disease-Modifying Therapy in Chronic Inflammatory Demyelinating Polyneuropathy – A Report of Three Cases. European neurology. 2015;73:294–302. doi: 10.1159/000381767. [DOI] [PubMed] [Google Scholar]
- Van Rhijn I, Van den Berg LH, Bosboom WM, Otten HG, Logtenberg T. Expression of accessory molecules for T-cell activation in peripheral nerve of patients with CIDP and vasculitic neuropathy. Brain : a journal of neurology. 2000;123(Pt 10):2020–2029. doi: 10.1093/brain/123.10.2020. [DOI] [PubMed] [Google Scholar]
- Wolf C, Menge T, Stenner MP, Meyer zu Horste G, Saleh A, Hartung HP, Wiendl H, Kieseier BC. Natalizumab treatment in a patient with chronic inflammatory demyelinating polyneuropathy. Archives of neurology. 2010;67:881–883. doi: 10.1001/archneurol.2010.143. [DOI] [PubMed] [Google Scholar]
- Xia RH, Yosef N, Ubogu EE. Clinical, electrophysiological and pathologic correlations in a severe murine experimental autoimmune neuritis model of Guillain-Barre syndrome. Journal of neuroimmunology. 2010a;219:54–63. doi: 10.1016/j.jneuroim.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Xia RH, Yosef N, Ubogu EE. Dorsal caudal tail and sciatic motor nerve conduction studies in adult mice: technical aspects and normative data. Muscle Nerve. 2010b;41:850–856. doi: 10.1002/mus.21588. [DOI] [PubMed] [Google Scholar]
- Xu B, Wagner N, Pham LN, Magno V, Shan Z, Butcher EC, Michie SA. Lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is mediated by L-selectin/PNAd, alpha4beta1 integrin/VCAM-1, and LFA-1 adhesion pathways. The Journal of experimental medicine. 2003;197:1255–1267. doi: 10.1084/jem.20010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekawa K, Harlan JM. Targeting leukocyte integrins in human diseases. J Leukoc Biol. 2005;77:129–140. doi: 10.1189/jlb.0804460. [DOI] [PubMed] [Google Scholar]
- Yosef N, Ubogu EE. alpha(M)beta(2)-integrin-intercellular adhesion molecule-1 interactions drive the flow-dependent trafficking of Guillain-Barre syndrome patient derived mononuclear leukocytes at the blood-nerve barrier in vitro. J Cell Physiol. 2012;227:3857–3875. doi: 10.1002/jcp.24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosef N, Xia RH, Ubogu EE. Development and characterization of a novel human in vitro blood-nerve barrier model using primary endoneurial endothelial cells. Journal of neuropathology and experimental neurology. 2010;69:82–97. doi: 10.1097/NEN.0b013e3181c84a9a. [DOI] [PubMed] [Google Scholar]
- Yuan F, Yosef N, Lakshmana Reddy C, Huang A, Chiang SC, Tithi HR, Ubogu EE. CCR2 gene deletion and pharmacologic blockade ameliorate a severe murine experimental autoimmune neuritis model of Guillain-Barre syndrome. PloS one. 2014;9:e90463. doi: 10.1371/journal.pone.0090463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


