Abstract
Background
Previous studies reported that repetitive transcranial magnetic stimulation (rTMS) can reduce cue-elicited craving and decrease cigarette consumption in smokers. The mechanism of this effect however, remains unclear. We used resting-state functional magnetic resonance imaging (rsfMRI) to test the effect of rTMS in non-treatment seeking smokers.
Methods
We used a single blinded, sham-controlled, randomized counterbalanced crossover design where participants underwent two visits separated by at least 1 week. Participants received active rTMS over the left dorsolateral prefrontal cortex (DLPFC) during one of their visits, and sham rTMS during their other visit. They had two rsFMRI scans before and after each rTMS session. We used the same rTMS stimulation parameters as in a previous study (10 Hz, 5 seconds-on, 10 seconds-off, 100% resting motor threshold, 3000 pulses).
Results
Ten non-treatment-seeking, nicotine-dependent, cigarette smokers (6 women, an average age of 39.72 and an average cigarette per day of 17.30) finished the study. rsFMRI results demonstrate that as compared to a single session of sham rTMS, a single session of active rTMS inhibits brain activity in the right insula and thalamus in fractional amplitude of low frequency fluctuation (fALFF). For intrinsic brain connectivity comparisons, active TMS resulted in significantly decreased connectivity from the site of rTMS to the left orbitomedial prefrontal cortex.
Conclusions
This data suggests that one session of rTMS can reduce activity in the right insula and right thalamus as measured by fALFF. The data also demonstrates that rTMS can reduce rsFC between the left DLPFC and the medial orbitofrontal cortex.
Keywords: DLPFC, resting-state fMRI, nicotine dependence, reward circuit, transcranial magnetic stimulation
1. Introduction
Nicotine dependence remains a great global public health concern (Rep., 2011). A previous research reported that the overall worldwide economic cost of smoking (from health expenditures and productivity losses together) reached $1,852 billion in 2012 (Goodchild et al., 2017). Despite the availability of therapeutic options for smoking cessation, relapse rates persist at a high level (Piasecki, 2006; Pollak et al., 2007). Therefore, there is need of new and effective strategies to help cigarette smokers achieve abstinence.
Transcranial magnetic stimulation (TMS) is a non-invasive brain stimulation technique (Barker et al., 1985) which has getting used not only as a research tool (Li et al., 2009; Ziemann, 2004) but also as a therapeutic intervention (Chae et al., 2004; George et al., 1997) for neuropsychiatric disorders. Recent studies, including our groups previous work, reveal a therapeutic effect of repetitive TMS (rTMS) on cigarette consumption (Amiaz et al., 2009; Dinur-Klein et al., 2014; Eichhammer et al., 2003) and cue-induced craving in nicotine dependence (Li et al., 2013b; Rose et al., 2011). No matter outstanding development in understanding the mechanisms of TMS for smoking cessation, it is clear that advanced innovative research remains needed.
Resting-state functional MRI (rsfMRI) has become an extremely interesting research field (Biswal et al., 2010; Murphy et al., 2013). Functional MRI was initially used to measure resting-state networks by Biswal and colleagues (Biswal et al., 1995; Fedota and Stein, 2015). rsfMRI is believed in relation to spontaneous, low frequency fluctuations in blood oxygen level-dependent (BOLD) signal (Zou et al., 2008). Two commonly used methods to measure resting-state functional connectivity (rsFC) are linear correlation and independent component analysis (ICA) s(Lee et al., 2013). Signal fluctuation reflects the strength of a functional connection between brain regions and offer a systems-level understanding of neural function (Honey et al., 2009; Rogers et al., 2007; Song et al., 2011). However, for clinical studies, abnormal functional connectivity between two or more related brain regions can not be interpreted as a disordered neural connectivity because the assessment is only the correlation between the two regions (Song et al., 2011). An alternative analytical method for rsfMRI is to measure the amplitude of low frequency fluctuation (ALFF) (Zou et al., 2008). The local spontaneous low frequency oscillations (LFO) are associated with local neuronal activation in brain disorders (Scholvinck et al., 2010) (Jiang et al., 2011; Liu et al., 2006; Zang et al., 2007). Recently, clinical treatment studies demonstrated that the accurate localization of abnormal spontaneous brain activity was crucial for the therapeutic effects of drugs in specific diseases, e.g., depression and schizophrenia (Fryer et al., 2015; Liu et al., 2015). As an advance ALFF method, fractional ALFF (fALFF) has also been utilized in resting-state fMRI research (Zou et al., 2008). Therefore, we used fALFF to measure brain function of rsFMRI. fALFF allows, researchers to obtain stable data across scan sessions (Brewer et al., 2011) and making it an ideal tool for longitudinal studies, including pre-post treatment assessments (Song et al., 2011).
It has been reported that nicotine affects different neuronal circuits which are relative to disparate cognitive and affective processes (e.g., reward, learning, affect, executive control) (Fedota and Stein, 2015; Koob and Volkow, 2010). As shown in task activation studies (Hartwell et al., 2011; Li et al., 2013a), chronic nicotine exposure leads to abnormal functional connectivity in specific networks (Sutherland et al., 2013) and large-scale networks (Weiland et al., 2015). The left dorsolateral prefrontal cortex (DLPFC) is considered to be integral in the exertion of executive control over emotions of craving and limbic reward circuits related with smoking (da Silva et al., 2013; Goldstein and Volkow, 2011). In fMRI studies, researchers reported that smokers had hypo-activation of the DLPFC and hyper-activation of subcortical areas as compared to both controls and former smokers (Nestor et al., 2011). Recently another study demonstrated that decreased left DLPFC and increased PCC of percent BOLD signal change predicted subsequent smoking relapse (Loughead et al., 2015). Therefore, these findings suggest that hypo-activation of the DLPFC can cause the lack of control over smoking cigarette.
High frequency rTMS (greater than 5 pulses/second) can increase cortical excitability as demonstrated in motor cortex excitability paradigms (Chen, 2000). Subsequently, rTMS may exert its anti-craving effect by normalizing DLPFC function in smokers (Ni and Chen, 2015). In this study, we hypothesized that high-frequency TMS applied to the DLPFC would modulate rsFC in the frontostriatal pathway including the DLPFC, medial orbitofrontal cortex, and insula. Thus, we used rsfMRI to investigate the effect of a single session of rTMS on brain activity using fALFF and intrinsic functional connectivity of the left DLPFC-related circuits.
2. Methods and Materials
2.1. Study design
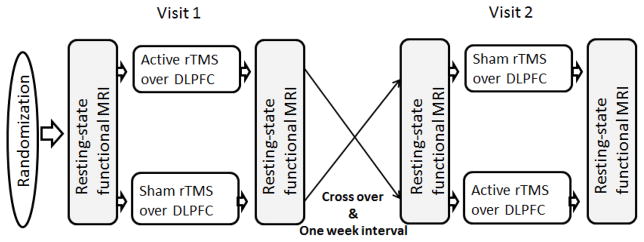
This was a randomized, single-blind, sham-controlled crossover study in which participants received both active and sham rTMS over the left DLPFC, with a 1-week interval between treatments to avoid carryover effects (Figure 1). The order of stimulation was randomized and counterbalanced across participants. The randomization was performed with a web-based randomization generator (www.randomization.com). Participants were blinded to treatment condition. Structural and resting state functional MRIs were performed before and after each rTMS session. At baseline, demographic and smoking habits profile data were collected. Participants were instructed to keep their regular smoking habits with the exception of not smoking for 2 hours prior to each experimental visit. This brief period of abstinence was to ensure that participants had some degree of baseline craving and responsiveness to cigarette cues without the potential confound of a ceiling effect from prolonged abstinence (Hartwell et al., 2013a; Li et al., 2013a; Li et al., 2013b). Craving assessments were performed before and after each cigarette cue presentation in a fashion validated in our previous experiment (Li et al., 2013b).
Figure 1.
Study design
2.2. Participants
We recruited 11 (6 women and 5 men) healthy, non-treatment seeking, nicotine-dependent cigarette smokers (DSM-IV) (≥10 cigarettes/day) between the ages of 18 and 60 through flyers, newspaper and internet advertisements. We excluded potential participants who used tobacco products other than cigarettes, those who currently used nicotine replacement therapy, those currently taking smoking cessation medications (Exe. bupropion or varenicline), or any other psychoactive medications, those with any medical conditions, those with significant current or past DSM-IV Axis I disorders, those who were pregnant, and those with non-nicotine substance dependence or abuse. All study procedures were approved by the Medical University of South Carolina Institutional Review Board and were in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki. Participants provided informed consent and completed an initial assessment. Recent smoking was confirmed by collecting an exhaled carbon monoxide (CO) level (≥ 10ppm) measured with a Micro-Smokelyzer (Bedfont Scientific Ltd., Kent, UK). We also completed a detailed tobacco use history, including the Fagerström Test for Nicotine Dependence (FTND) (Fagerstrom, 1978), and the Questionnaire of Smoking Urges-Brief (QSU-B) (Cox et al., 2001). We assessed current health by performing a physical examination, and we collected a urine sample to screen for illicit drug use.
2.3. Repetitive TMS
2.3.1. Determining resting motor threshold (rMT) and locating cortical targets
We determined the rMT of each participant at the beginning of each experimental visit prior to any exposure or ratings. RMT was determined using a MagPro double blinded rTMS Research System (MagVenture, Denmark) with a Cool-B65 Butterfly Coil (a combined active and sham coil). We positioned the coil over the area of the skull corresponding to the motor cortex, and adjusted the location until each pulse resulted in isolated movements of the right thumb (abductor pollicis brevis – APB). We then adjusted the output of the magnetic pulse until we found the lowest intensity that reliably produced thumb movement 50% of the time (Ziemann and Hallett, 2000). We used the position of the coil used for rMT assessment as the motor cortex target (M1). We then determined the approximate location of the left DLPFC by moving the TMS coil 6 cm anterior to M1 along a parasagittal line (George and Post, 2011; Herbsman et al., 2009). On the second visit, we repeated the rMT procedure and the treatment position was reproduced using MagVenture TMS repositioning caps.
2.3.2. Active rTMS procedures
We utilized the same treatment parameters for this study as we did for a previous investigation demonstrating that rTMS can reduce cue induced cigarette craving (Li et al., 2013b). Treatment was standardized at 100% rMT, at 10 pulses per second (10 Hz) for 5 seconds, with an inter-train interval of 10 seconds. Each treatment session lasted for 15 minutes and we subsequently delivered a total of 3000 pulses.
2.3.3. Sham-TMS procedures
After we determined the rMT and located the left DLPFC as described above, participants were fitted with two electrodes on the scalp just below the hairline. Electrodes were connected to the Cool-B65 Butterfly Coil sham stimulator. The electrical current of the sham system using a sham rTMS coil was titrated to a level matching participants’ sensory ratings of active rTMS in order to make active and sham stimulation indistinguishable (Borckardt et al., 2008). We used 10 Hz, 5 seconds-on, 10 seconds-off for 15 minutes in sham conditions. The sham-TMS scalp discomfort was matched to that of active TMS at 100% (Borckardt et al., 2008). During active TMS there was no current flowing through the scalp electrodes.
2.3.4. Presentation of Smoking-Related Cues
Smoking cues were presented during rTMS sessions. The smoking related cues consisted of a house made smoking cue video, and a series of smoking related images. The video consisted of a person lighting up and smoking a cigarette. The smoking-related images included the heads and mouths of people smoking, hands holding a cigarette and cigarettes in ashtrays or in a pack. Cue effectiveness was measured before and after each rTMS session using a visual analog scale (VAS) (0–10) ranking the relative position of the participants’ response to the question “Right now, I would rate the amount of my craving to smoke as 0–10”.
2.4. Resting-state functional MRI
2.4.1. Image data acquisition and protocol
Imaging data were acquired using a Siemens 3.0 Tesla Total Imaging Matrix (TIM) system in the imaging center at the Medical University of South Carolina. During the resting-state scan, subjects had been instructed to relax with their eyes closed and hold their heads still without falling asleep. After the experiment, they were asked if they had fallen asleep during the MRI scan. The data were excluded if the subject fell asleep. Resting-state fMRI images were collected using an EPI sequence (TR/TE = 2000 ms/27 ms, flip angle = 90°, matrix = 64×64, FOV = 240×240 mm2, slice thickness/gap =5 mm/0 mm, and 36 axial slices covered the whole brain) to yield 178 brain volumes lasting for 356 seconds. 3D T1-weighted anatomical images were also acquired (TR/TE = 8.35 ms/3.27 ms, flip angle = 12°, FOV = 240 × 240 mm2; matrix = 256 × 256, slice thickness = 1 mm, and 156 sagittal slices).
2.4.2. Image pre-processing
Pre-processing was carried out using Data Processing Assistant for Resting-State FMRI (DPARSF) (Chao-Gan and Yu-Feng, 2010) (http://www.restfmri.net) which is based on Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm) and Resting-State fMRI Data Analysis Toolkit (REST) (Song et al., 2011). Functional images, underwent slice-timing correction and realignment. Data from the subjects whose head motion exceeded 3 mm or rotation exceeded 1° during scanning were excluded. Individual 3D T1-weighted anatomical images were co-registered to functional images. Normalized data were re-sliced at a resolution of 3 × 3 × 3 mm3 and spatially smoothed with a 6-mm full width at half-maximum Gaussian kernel. Finally, functional images with linear trend were removed. Several sources of spurious variance (24 head motion parameters, averaged signal from white matter, cerebrospinal fluid (CSF) and global signal) were regressed out using multiple linear regression analysis.
2.4.3. fALFF analysis
We computed the fALFF of each voxel. We defined fALFF as the ratio of power spectrum of low frequency (0.01–0.08 Hz) range to that of the entire frequency range computed (Zou et al., 2008). Finally, the fALFF value of each voxel became normalized through dividing the global mean fALFF value.
2.4.4. Functional connectivity
We used a temporal correlation approach to measure resting-state functional connectivity. (Fox et al., 2005). Left DLPFC was defined as the seed (5 mm radius sphere). We chose the MNI coordinates (x=−42, y=34, z=30) as the location of the left DLPFC (Mylius et al., 2013). A correlation analysis was carried out between the seed and remaining voxels in the brain. The resulting r values were converted using Fisher’s r-to-z transformation to improve the Gaussianity of their distribution. We then performed within-group comparisons (within the gray matter mask) for pre- and post- rTMS in each session (one-sample t-test) with AlphaSim corrected p <0.05 (combined p<0.05 with clusters ≥22). A union mask of each one-sample T image was calculated with significance using REST software, which was prepared to make use of the subsequent two-sample T-test.
2.4.5. Imaging data statistics
We first calculated the D-value image of fALFF/functional connectivity (post-TMS minus pre-TMS) of active and sham TMS conditions, separately. We then compared the D-value images between active and sham TMS conditions using a paired t-test The significance threshold was set at p<0.05 (AlphaSim-corrected; combined height threshold p<0.05 and a minimum cluster size of 22 voxels). AlphaSim can provide a means of estimating the overall significance level (the probability of a false detection) for an entire 3 D functional image. This is accomplished by way of Monte Carlo stimulation of the process of image generation, spatial correlation of voxels, voxel intensity thresholding, masking, and cluster identification (Ward, 2000). Subsequently, we did a post-hoc analysis of region of interest (ROI) with significant differences in the first step. We extracted the signal of fALFF/functional connectivity of regions in every time point, and compared the pre to post-TMS in active and sham-TMS conditions (p<0.05). Last, we did a ROI wise correlation analysis of changed behavioral scores with changed imaging data in active and sham TMS conditions. We computed the Pearson’s correlation coefficients with GraphPAD Prism software between D-value image of fALFF/functional connectivity and VAS scores (p<0.05).
2.5. General Data Analyses
Analyses were completed with SPSS statistical software, version 20.0 (IBM, Endicott, New York). The primary behavioral measure of cue craving (VAS) was taken to be the contrast (difference) between active rTMS and sham rTMS in the pre- to post experiment change in subjective craving. Two-way repeated measure analysis of variances (sham vs. active rTMS x pre- vs. post treatment) were performed on cigarette-craving ratings (VAS). A partial correlation analysis was applied to the change of pre post VAS craving rating and the change of pre post fALFF measures in ROIs.
3. Results
3.1. General Subject Characteristics
Eleven non-treatment seeking participants (6 women) signed informed consent and 10 participants completed the study. One participant was terminated from the study for missing his second appointment. The average age of participants was 39.72 ± 13.27 SD years old and participants smoked an average of 17.30 ± 5.92 SD cigarettes per day. The FTND scores were categorically moderate on average, with an average score of 5.20 ± 1.75 SD. The average QSU-Brief score was 43.10 ± 15.48 SD. Random exhaled CO levels were 12.70 ± 4.61 SD ppm prior to the sham rTMS visit and 13.15 ± 8.32 SD prior to the active rTMS visit.
3.2. Effects of rTMS on cue craving measures
Regarding behavioral data, repeated measure analysis did not show a statistically significant difference between active TMS (Pre 6.75 ± 2.32 SD vs. post 6.35 ± 2.40 SD) and sham TMS (Pre 5.35 ± 2.43 SDvs. post TMS 6.45 ± 2.08 SD) (F1, 9 = 0.69, p = 0.43), although there was a tendency interaction of treatment condition (sham vs. TMS) and measure time (pre vs. post) (F1, 9 = 4.36, p = 0.067). When percent change was used to calculate pre- to post-craving, active rTMS produced a trend towards a significant reduction of cue craving as compared to sham TMS (3.89 ± 6.98 vs. −37.54 ± 15.84; Related-Samples Wilcoxon Signed Rank Test, p = 0.074). These results were consistent with our repeated measure analysis findings.
3.3. Effects of rTMS on fALFF
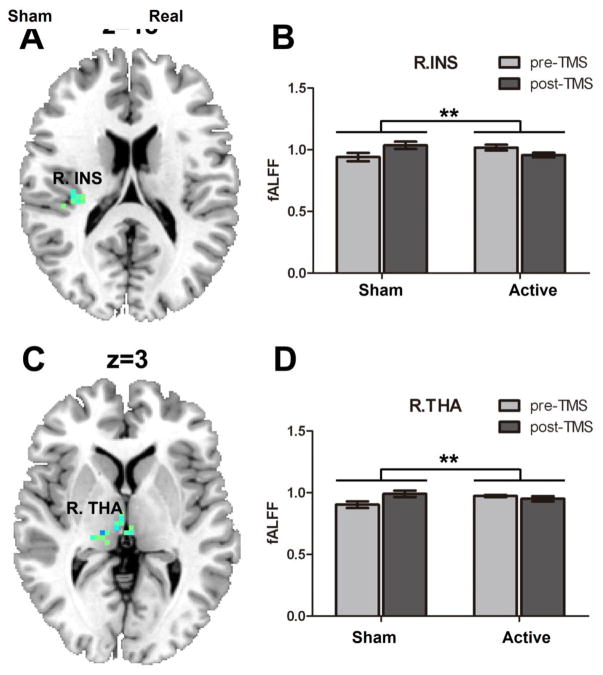
For local intrinsic brain activity comparisons, compared to the sham-TMS condition, active TMS showed a significantly decreased D-value of fALFF in the right thalamus (15, −24, 3), the cluster size of 34 voxels and insula (33, −24, 18), the cluster size of 22 voxels (AlphaSim-corrected; combined height threshold p<0.05). Post-hoc analysis showed that following sham rTMS, fALFF in the insula and thalamus were significantly increased, while there were no significant effects following active TMS (See Figure 2).
Figure 2.
One session of rTMS of 3000 pulse over the left DLPFC reduced brain activity in (A) right insula (p<0.05, AlphaSim corrected); (B) The bar graph shows the mean fALFF of insula (33, −24, 18); (C) right thalamus (p<0.05, AlphaSim corrected); (D) The bar graph shows the mean fALFF of right thalamus (15, −24, 3). * p<.05, **p<.01
3.4. Effects of rTMS on resting state functional connectivity (rsFC)
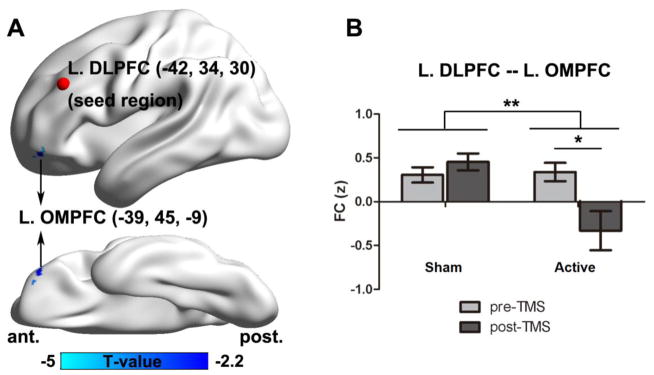
For intrinsic brain connectivity comparisons, compared to sham-TMS, active TMS showed significantly decreased connectivity (D-value images of post-TMS minus pre-TMS) between the left DLPFC (−42, 34, 30) (Mylius et al., 2013) and the left orbital middle prefrontal cortex (OMPFC) (−39, 45, −9) (AlphaSim-corrected; combined height threshold p<0.05 and minimum cluster size 27 voxels). Post-hoc analysis showed that following active TMS the connectivity between the left DLPFC and OMPFC (change of correlation polarity from positive to negative) was significantly decreased, while there were no significant effects following sham-TMS (See Figure 3).
Figure 3.
The comparison of the connectivity of the DLPFC and the orbital medial prefrontal cortex (OMPFC) between active and sham-TMS (A). The connectivity of DLPFC (−42, 34, 30) and OMPFC (−39, 45, −9) (B). The bar graph shows the mean functional connectivity (FC) in sham and active TMS treatment. * p<.05, **p<.01
3.5. Correlation analysis of rTMS-induced fALFF and craving ratings percent changes
There were no statistically significant correlations between the rTMS induced changes of fALFF measures and rTMS induced changes of subjective craving ratings (measured by VAS scores) in either active (insula: r = 0.385, p = 0.271; thalamus: r = 0.024, p = 0.9947) or sham (insula: r = −0.46, p =0.181; thalamus: r = −0.025, p = 0.946) conditions.
4. Discussion
To our knowledge, this is the first study that systematically evaluates the effects of rTMS over the DLPFC on LFO amplitudes (as indexed fALFF) or resting state functional connectivity (FC) in cigarette smokers. We found that compared to sham rTMS, active rTMS a decreased the fALFF in the right insula and thalamus. Additionally, following active rTMS of the DLPFC, the temporal connectivity between the left DLPFC and the left OMPFC diminished.
The frontal cortex and its executive functions have been reported to relate with addiction (Goldstein and Volkow, 2002). An intervention targeting the top-down prefrontal-striatal brain networks can reduce smoking cue craving (Volkow et al., 2012). Recently, in comparison with non-smokers, smokers revealed a decreased prefrontal response in the course of cognitive (Froeliger et al., 2013) and cue craving processing (Augustus Diggs et al., 2013). In line with this idea, the prefrontal cortical activity is critical in modulating ventral-striatal mediated cigarette craving (Kober et al., 2010). Given this data in aggregate, one would hypothesize that high-frequency stimulation of the DLPFC might result in increased prefrontal cortex function and decreased mesolimbic function via descending lateral pathways (Volkow et al., 2012). Supporting this hypothesis, one recent study of combined TMS and fMRI reported that the craving-induced signal in the medial orbitofrontal cortex was attenuated by deactivation of the DLPFC in smokers (Hayashi et al., 2013). Another interleaved TMS/fMRI study completed by our group, found that TMS over the DLPFC resulted in decreased activity of the anterior cingulate cortex (ACC) in depressed patients (Li et al., 2004b). In this study, we used a cross-over design to examine how rTMS over the left DLPFC affected the connectivity of brain regions, and the regional cerebral function based on whole brain measured with fALFF during resting-state fMRI. In addition to the above findings, we also found that rTMS over the left DLPFC reduced the connectivity linking the DLPFC and mOPFC. Current results are consistent with previous studies (Hayashi et al., 2013; Li et al., 2004b). The current findings suggest that the DLPFC may have a modulating effect on reward pathways which includes the DLPFC-OFC top-down connectivity. This functional connectivity is also consistent with the recognized existence of anatomical connections between the DLPFC and mOPFC based on work in primates (Cavada and Schultz, 2000; Hayashi et al., 2013).
As a newly-developed method for analyzing resting-state fMRI data, fALFF can be used to locate specific brain pathways. Biswal et al first reported that spontaneous blood oxygen level-dependent low-frequency (0.01–0.08Hz) fluctuations were closely involved in spontaneous neural activities and were important for physiological function (Biswal et al., 1995). fALFF has been used for addiction research. Recently Denier and colleagues reported that heroin treatment increased fALFF in thalamus (Denier et al., 2015). Previous studies of neurobiology in addictive disorders demonstrate that insula is involved in the subjective, interoceptive awareness of drug craving (Garavan, 2010; Naqvi et al., 2014; Naqvi et al., 2007). Among cigarette smokers, insula activity has been proven to correlate with self-reported smoking cue-induced craving after smoking treatment (Brody et al., 2002; Franklin et al., 2011). A recent study of rsfMRI revealed that the vulnerability of relapse is related to decreased connectivity between the posterior insula and primary sensorimotor cortices in smokers (Addicott et al., 2015). Another fMRI study completed by our group reported that cue-elicited craving may be relative with the connectivity between the right anterior insula and the precunneus (Moran-Santa Maria et al., 2015). The current results demonstrate that compared to sham TMS, active rTMS decreases fALFF measure in the right insula and thalamus. Therefore, our results are consistent with the previous findings that the insula is involved in the cue craving process (Addicott et al., 2015; Naqvi and Bechara, 2010; Naqvi et al., 2014). Furthermore, the current study also supported that fALFF can be a useful measure for rsfMRI study.
Our findings using fALFF demonstrate predominantly remote region effects, rather than local effects (contralateral insula and thalamus). This is consistent with the findings of numerous studies completed by our laboratory which have reported TMS produces brain effects in remote brain areas through trans-synaptic connections (Li et al., 2004a; Li et al., 2004b; Li et al., 2010). Importantly, we found that excitation of the left DLPFC resulted in decreased connectivity of the mOPFC, which suggests that there is connectivity between left DLPFC and left mOPFC, and the high-frequency rTMS of the DLPFC may cause functional changes in the left mOPFC. Several previous imaging studies completed by our team demonstrate that mOPFC is associated with cue-induced smoking cue craving in nicotine dependence (Hanlon et al., 2013; Hartwell et al., 2016; Hartwell et al., 2011). In healthy volunteers Cho and Strafella found that rTMS of the left DLPFC induced dopamine (DA) release in mOPFC (Cho and Strafella, 2009). Another recent study found that the craving-related signal in the medial orbitofrontal cortex was reduced by the deactivation of the DLPFC, and support a model wherein aberrant circuitry connecting dorsolateral prefrontal to orbitofrontal cortices may underlie addiction (Hayashi et al., 2013). Hence, our findings are subsequently consistent with and further support previous studies.
Although our study did not find an effect of rTMS on cue-elicited craving, a trend towards a significant (p=0.07) behavioral effect of active rTMS on craving in comparison with sham was demonstrated. This appears inconsistent with earlier studies demonstrating a transient effect of rTMS on nicotine craving (Dinur-Klein et al., 2014; Li et al., 2013b; Pripfl et al., 2014), however this study was prospectively designed and powered as an imaging study rather than a behavioral study. The small craving reduction effect size may be due to low stimulation dose (100%rMT, one session).
Our primary reason for using provocation with smoking cues during rTMS treatment was that cue provocation activates the neurocircuitry involved in craving (Hartwell et al., 2013a; Hartwell et al., 2013b; Li et al., 2013a) which may have counteracted the acute anti-craving effect of rTMS alone (Dinur-Klein et al., 2014). Interestingly, in the absence of active rTMS (sham condition) there was an increase in brain function (fALFF) in both the insula and thalamus, which may be consistent with increased cue craving (Figure 2). Previous studies have reported that smoking cues (Naqvi and Bechara, 2010; Naqvi et al., 2007) and abstinence (McClernon et al., 2005) induces activity in the insula and thalamus in smokers. Lastly, nonsignificant effects of rTMS on craving may have resulted from the lower validity of the VAS scale as a measure of craving. Higher validity measures (e.g. questionnaire of smoking urges-brief – QSU-B) should be used in the future study to measure the efficiency of rTMS.
There are several notable limitations to this study. First, the small sample size may cause Type II errors. However, the within subject design partially mitigates this possibility. Second, the study lacks non-smoking control participants, and thus we are unable to detect abnormal connectivity in nicotine addicts as compared to healthy participants. However, the specific aim of this study was to test the effect of rTMS on resting state brain function in cigarette smokers, and subsequently healthy controls were not necessary for us to achieve this aim. Furthermore, we only carried out a connectivity analysis of the left DLPFC, underneath TMS stimulation site, and subsequently may have overlooked other vital connectivity changes. However, our data illustrated the connectivity of OFC and DLPFC, which supports the left DLPFC could be an ideal region as a target in nicotine addiction.
In conclusion, our results offer preliminary proof that rTMS applied over the DLPFC exerts its anti-craving and smoking cessation by modulating functional connectivity with cortical networks (DLPFC-OFC), and through lowering fALFF within the right insula and thalamus. Prior studies have shown that both the insula (Naqvi et al., 2014) and thalamus (Franklin et al., 2007) are involved in cue craving and drug abuse. Future studies are needed to determine whether and how the immediate activity induced by rTMS relates to its longer-term smoking cessation action. As such, if treated by multi-session rTMS, it is possible that inactivation of the insula and thalamus, as well as the negative connectivity of left DLPFC and OMPFC could underlie subsequent behavioral changes such as smoking cessation.
Highlights.
The mechanism of repetitive transcranial magnetic stimulation (rTMS) over the left dorsolateral prefrontal cortex (DLPFC) to decrease cue-elicited craving in smokers remains unclear
We used resting-state fMRI with fractional amplitude of low frequency fluctuation (fALFF) to further elucidate rTMS’s anti-craving mechanism
rTMS delivered during a smoking cue reduced insular and thalamic resting-state activity
rTMS also modulated functional connectivity of the orbitofrontal cortex
Acknowledgments
Role of funding source
This work was supported by the Psychiatry Chair Fund (XL), R21DA036752 (XL), and MUSC Center for Biomedical Imaging Startup Award (XL).
Footnotes
Clinicaltrials.gov ID: NCT02665338
ClinicalTrials.gov Brief Title: TMS-fMRI for Neural Pathway in Smokers
Financial Disclosures
The authors declare no conflicts of interest.
Author contributions
XL and MSG were responsible for the study design. XL and SH collected the data; XL and LD conducted data analysis; XL, LD, GLS, and BB contributed to the interpretation of the data and wrote the manuscript. All authors approved of the final manuscript before submission.
This data was presented in poster format as an abstract at the Society of Biological Psychiatry 71th Annual Meeting 2016. Atlanta, Georgia.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addicott MA, Sweitzer MM, Froeliger B, Rose JE, McClernon FJ. Increased Functional Connectivity in an Insula-Based Network is Associated with Improved Smoking Cessation Outcomes. Neuropsychopharmacology. 2015;40:2648–2656. doi: 10.1038/npp.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction. 2009;104:653–660. doi: 10.1111/j.1360-0443.2008.02448.x. [DOI] [PubMed] [Google Scholar]
- Augustus Diggs H, Froeliger B, Carlson JM, Gilbert DG. Smoker-nonsmoker differences in neural response to smoking-related and affective cues: an fMRI investigation. Psychiatry Res. 2013;211:85–87. doi: 10.1016/j.pscychresns.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borckardt JJ, Walker J, Branham RK, Rydin-Gray S, Hunter C, Beeson H, Reeves ST, Madan A, Sackeim H, George MS. Development and evaluation of a portable sham transcranial magnetic stimulation system. Brain Stimul. 2008;1:52–59. doi: 10.1016/j.brs.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci U S A. 2011;108:20254–20259. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Cavada C, Schultz W. The mysterious orbitofrontal cortex foreword. Cereb Cortex. 2000;10:205. doi: 10.1093/cercor/10.3.205. [DOI] [PubMed] [Google Scholar]
- Chae JH, Nahas Z, Wassermann E, Li X, Sethuraman G, Gilbert D, Sallee FR, George MS. A pilot safety study of repetitive transcranial magnetic stimulation (rTMS) in Tourette’s syndrome. Cogn Behav Neurol. 2004;17:109–117. doi: 10.1097/01.wnn.0000116253.78804.3a. [DOI] [PubMed] [Google Scholar]
- Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve Suppl. 2000;9:S26–S32. doi: 10.1002/1097-4598(2000)999:9<::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- da Silva MC, Conti CL, Klauss J, Alves LG, do Nascimento Cavalcante HM, Fregni F, Nitsche MA, Nakamura-Palacios EM. Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J Physiol Paris. 2013;107:493–502. doi: 10.1016/j.jphysparis.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Denier N, Schmidt A, Gerber H, Vogel M, Huber CG, Lang UE, Riecher-Rossler A, Wiesbeck GA, Radue EW, Walter M, Borgwardt S. Abnormal functional integration of thalamic low frequency oscillation in the BOLD signal after acute heroin treatment. Hum Brain Mapp. 2015;36:5287–5300. doi: 10.1002/hbm.23011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinur-Klein L, Dannon P, Hadar A, Rosenberg O, Roth Y, Kotler M, Zangen A. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry. 2014;76:742–749. doi: 10.1016/j.biopsych.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Johann M, Kharraz A, Binder H, Pittrow D, Wodarz N, Hajak G. High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J Clin Psychiatry. 2003;64:951–953. doi: 10.4088/jcp.v64n0815. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Fedota JR, Stein EA. Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Ann N Y Acad Sci. 2015;1349:64–82. doi: 10.1111/nyas.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O’Brien CP, Childress AR. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry. 2011;68:516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic Activation to Cigarette Smoking Cues Independent of Nicotine Withdrawal: A Perfusion fMRI Study. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Froeliger B, Modlin LA, Kozink RV, Wang L, Garland EL, Addicott MA, McClernon FJ. Frontoparietal attentional network activation differs between smokers and nonsmokers during affective cognition. Psychiatry Res. 2013;211:57–63. doi: 10.1016/j.pscychresns.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Roach BJ, Ford JM, Turner JA, van Erp TG, Voyvodic J, Preda A, Belger A, Bustillo J, O’Leary D, Mueller BA, Lim KO, McEwen SC, Calhoun VD, Diaz M, Glover G, Greve D, Wible CG, Vaidya J, Potkin SG, Mathalon DH. Relating Intrinsic Low-Frequency BOLD Cortical Oscillations to Cognition in Schizophrenia. Neuropsychopharmacology. 2015;40:2705–2714. doi: 10.1038/npp.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H. Insula and drug cravings. Brain Struct Funct. 2010;214:593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- George MS, Post RM. Daily left prefrontal repetitive transcranial magnetic stimulation for acute treatment of medication-resistant depression. Am J Psychiatry. 2011;168:356–364. doi: 10.1176/appi.ajp.2010.10060864. [DOI] [PubMed] [Google Scholar]
- George MS, Wassermann EM, Kimbrell TA, Little JT, Williams WE, Danielson AL, Greenberg BD, Hallett M, Post RM. Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry. 1997;154:1752–1756. doi: 10.1176/ajp.154.12.1752. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild M, Nargis N, Tursan d’Espaignet E. Global economic cost of smoking-attributable diseases. Tob Control. 2017 doi: 10.1136/tobaccocontrol-2016-053305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Hartwell KJ, Canterberry M, Li X, Owens M, Lematty T, Prisciandaro JJ, Borckardt J, Brady KT, George MS. Reduction of cue-induced craving through realtime neurofeedback in nicotine users: the role of region of interest selection and multiple visits. Psychiatry Res. 2013;213:79–81. doi: 10.1016/j.pscychresns.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell KJ, Hanlon CA, Li X, Borckardt JJ, Canterberry M, Prisciandaro JJ, Moran-Santa Maria MM, LeMatty T, George MS, Brady KT. Individualized real-time fMRI neurofeedback to attenuate craving in nicotine-dependent smokers. J Psychiatry Neurosci. 2016;41:48–55. doi: 10.1503/jpn.140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell KJ, Johnson KA, Li X, Myrick H, LeMatty T, George MS, Brady KT. Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addict Biol. 2011;16:654–666. doi: 10.1111/j.1369-1600.2011.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell KJ, Lematty T, McRae-Clark AL, Gray KM, George MS, Brady KT. Resisting the urge to smoke and craving during a smoking quit attempt on varenicline: results from a pilot fMRI study. Am J Drug Alcohol Abuse. 2013a;39:92–98. doi: 10.3109/00952990.2012.750665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell KJ, Prisciandaro JJ, Borckardt J, Li X, George MS, Brady KT. Real-time fMRI in the treatment of nicotine dependence: a conceptual review and pilot studies. Psychol Addict Behav. 2013b;27:501–509. doi: 10.1037/a0028215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Ko JH, Strafella AP, Dagher A. Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proc Natl Acad Sci U S A. 2013;110:4422–4427. doi: 10.1073/pnas.1212185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbsman T, Avery D, Ramsey D, Holtzheimer P, Wadjik C, Hardaway F, Haynor D, George MS, Nahas Z. More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol Psychiatry. 2009;66:509–515. doi: 10.1016/j.biopsych.2009.04.034. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang GH, Qiu YW, Zhang XL, Han LJ, Lv XF, Li LM, Lin CL, Zhuo FZ, Hu SY, Tian JZ. Amplitude low-frequency oscillation abnormalities in the heroin users: a resting state fMRI study. Neuroimage. 2011;57:149–154. doi: 10.1016/j.neuroimage.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Smyser CD, Shimony JS. Resting-state fMRI: a review of methods and clinical applications. AJNR Am J Neuroradiol. 2013;34:1866–1872. doi: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Borckardt J, Prisciandaro JJ, Saladin ME, Morgan PS, Johnson KA, Lematty T, Brady KT, George MS. Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: a preliminary real-time fMRI study. Addict Biol. 2013a;18:739–748. doi: 10.1111/j.1369-1600.2012.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA, Brady KT, George MS. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry. 2013b;73:714–720. doi: 10.1016/j.biopsych.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Nahas Z, Anderson B, Kozel FA, George MS. Can left prefrontal rTMS be used as a maintenance treatment for bipolar depression? Depress Anxiety. 2004a;20:98–100. doi: 10.1002/da.20027. [DOI] [PubMed] [Google Scholar]
- Li X, Nahas Z, Kozel FA, Anderson B, Bohning DE, George MS. Acute left prefrontal transcranial magnetic stimulation in depressed patients is associated with immediately increased activity in prefrontal cortical as well as subcortical regions. Biol Psychiatry. 2004b;55:882–890. doi: 10.1016/j.biopsych.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Li X, Ricci R, Large CH, Anderson B, Nahas Z, Bohning DE, George MS. Interleaved transcranial magnetic stimulation and fMRI suggests that lamotrigine and valproic acid have different effects on corticolimbic activity. Psychopharmacology (Berl) 2010;209:233–244. doi: 10.1007/s00213-010-1786-y. [DOI] [PubMed] [Google Scholar]
- Li X, Ricci R, Large CH, Anderson B, Nahas Z, George MS. Lamotrigine and valproic acid have different effects on motorcortical neuronal excitability. J Neural Transm. 2009;116:423–429. doi: 10.1007/s00702-009-0195-z. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu Z, Liang M, Hao Y, Tan L, Kuang F, Yi Y, Xu L, Jiang T. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. Neuroreport. 2006;17:19–22. doi: 10.1097/01.wnr.0000195666.22714.35. [DOI] [PubMed] [Google Scholar]
- Liu Y, Du L, Li Y, Liu H, Zhao W, Liu D, Zeng J, Li X, Fu Y, Qiu H, Li X, Qiu T, Hu H, Meng H, Luo Q. Antidepressant Effects of Electroconvulsive Therapy Correlate With Subgenual Anterior Cingulate Activity and Connectivity in Depression. Medicine (Baltimore) 2015;94:e2033. doi: 10.1097/MD.0000000000002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Ruparel K, Falcone M, Hopson R, Gur R, Lerman C. Working memory-related neural activity predicts future smoking relapse. Neuropsychopharmacology. 2015;40:1311–1320. doi: 10.1038/npp.2014.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, Hartwell KJ, Hanlon CA, Canterberry M, Lematty T, Owens M, Brady KT, George MS. Right anterior insula connectivity is important for cue-induced craving in nicotine-dependent smokers. Addict Biol. 2015;20:407–414. doi: 10.1111/adb.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Bandettini PA. Resting-state fMRI confounds and cleanup. Neuroimage. 2013;80:349–359. doi: 10.1016/j.neuroimage.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylius V, Ayache SS, Ahdab R, Farhat WH, Zouari HG, Belke M, Brugieres P, Wehrmann E, Krakow K, Timmesfeld N, Schmidt S, Oertel WH, Knake S, Lefaucheur JP. Definition of DLPFC and M1 according to anatomical landmarks for navigated brain stimulation: inter-rater reliability, accuracy, and influence of gender and age. Neuroimage. 2013;78:224–232. doi: 10.1016/j.neuroimage.2013.03.061. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor L, McCabe E, Jones J, Clancy L, Garavan H. Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: Evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. Neuroimage. 2011;56:2258–2275. doi: 10.1016/j.neuroimage.2011.03.054. [DOI] [PubMed] [Google Scholar]
- Ni Z, Chen R. Transcranial magnetic stimulation to understand pathophysiology and as potential treatment for neurodegenerative diseases. Transl Neurodegener. 2015;4:22. doi: 10.1186/s40035-015-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM. Relapse to smoking. Clin Psychol Rev. 2006;26:196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Pollak KI, Oncken CA, Lipkus IM, Lyna P, Swamy GK, Pletsch PK, Peterson BL, Heine RP, Brouwer RJ, Fish L, Myers ER. Nicotine replacement and behavioral therapy for smoking cessation in pregnancy. Am J Prev Med. 2007;33:297–305. doi: 10.1016/j.amepre.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pripfl J, Tomova L, Riecansky I, Lamm C. Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimul. 2014;7:226–233. doi: 10.1016/j.brs.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Rep MMMW. Quitting Smoking Among Adults --- United States, 2001–2010. 2011:1513–1519. [PubMed] [Google Scholar]
- Rogers BP, Morgan VL, Newton AT, Gore JC. Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging. 2007;25:1347–1357. doi: 10.1016/j.mri.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, McClernon FJ, Froeliger B, Behm FM, Preud’homme X, Krystal AD. Repetitive transcranial magnetic stimulation of the superior frontal gyrus modulates craving for cigarettes. Biol Psychiatry. 2011;70:794–799. doi: 10.1016/j.biopsych.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A. 2010;107:10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Stein EA. Insula’s functional connectivity with ventromedial prefrontal cortex mediates the impact of trait alexithymia on state tobacco craving. Psychopharmacology (Berl) 2013;228:143–155. doi: 10.1007/s00213-013-3018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for FMRI data 2000 [Google Scholar]
- Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Hutchison KE. Reduced executive and default network functional connectivity in cigarette smokers. Hum Brain Mapp. 2015;36:872–882. doi: 10.1002/hbm.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Hallett M. Basic Neurophysiological Studies With TMS. In: George M, Belmaker R, editors. Transcranial Magnetic Stimulation In Neuropsychiatry. American Psychiatric Press, Inc; Washington, DC, U.S.A: 2000. p. 45. [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]