Abstract
Purpose
18F-fluorodeoxyglucose positron emission tomopraphy/computed tomography (FDGPET/CT) has been proven to be useful for imaging many types of cancer; however, its role is not well defined in hepatocellular carcinoma (HCC). We assessed the prognostic value of metabolic imaging biomarkers as established by baseline pretreatment FDG PET/CT in patients with HCC.
Methods
We retrospectively analyzed the records of patients with HCC who underwent FDG PET/CT before initial treatment from May 2013 through May 2014. Four PET/CT parameters were measured: maximum standardized uptake value (SUVmax), total lesion glycolysis (TLG), metabolic tumor volume (MTV), and tumor-to-normal-liver SUV ratio (TNR). Optimal cut-off values for the PET/CT parameters to stratify patients in terms of overall survival (OS) were determined. Multivariate analysis was performed to determine whether the PET/CT parameters could add to the prognostic value of the Cancer of the Liver Italian Program (CLIP) scoring system and the Barcelona-Clinic Liver Cancer (BCLC) staging system.
Results
The analysis included 56 patients. Univariate analysis of the association between OS and continuous variables, including the PET/CT parameters SUVmax, TLG, tumor size, total bilirubin level, and alkaline phosphatase level were significant predictors of OS. SUVmax ≥ 11.7, TLG ≥ 1,341, MTV ≥ 230 mL, and TNR ≥ 4.8 were identified as cut-off values. Multivariate analysis revealed that SUVmax ≥ 11.7 and TNR ≥ 4.8 were independent factors predicting a poor prognosis in both the CLIP scoring system and the BCLC staging system, as was TLG in the BCLC staging system.
Conclusion
Pretreatment FDG PET/CT in patients with HCC can add to the prognostic value of standard clinical measures. Incorporation of imaging biomarkers derived from FDG PET/CT into HCC staging systems should be considered.
Keywords: FDG PET/CT, Hepatocellular carcinoma, CLIP scoring system, BCLC staging system
Introduction
Liver cancer is the second leading cause of cancer-related death in the world [1]. The most common form of liver cancer is hepatocellular carcinoma (HCC). The occurrence of liver cancer is closely associated with chronic liver damage, such as that caused by chronic hepatitis due to hepatitis virus infection, liver cirrhosis, or fatty liver disease [2]. Other metabolic diseases (such as obesity and diabetes mellitus) and alcohol intake are also well known risk factors for liver cancer [3, 4].
In patients with cancer, prognostic modeling can facilitate decision making regarding treatment; however, in patients with HCC, evaluating prognosis is difficult and complicated because cirrhosis is often present [5]. Both tumor features and functional hepatic reserve must be taken into account. At present, several different staging systems for HCC are available, and there is no universally accepted staging system [6]. The Cancer of the Liver Italian Program (CLIP) scoring system for HCC takes into account both liver function and tumor characteristics relevant to prognosis [7]. The Barcelona Clinic Liver Cancer (BCLC) staging system was constructed on the basis of the results of several cohort studies and randomized controlled trials conducted by the Barcelona group [8]. Other staging systems are also available for HCC, including the American Joint Committee on Cancer staging system, the Japanese Integrated Staging system, the Okuda staging system, Groupe d’Etude et de Traitement du Carcinoma Hépatocellulaire (GRETCH), and the Chinese University Prognostic Index (CUPI) [5, 9–11]. Some of these staging systems have been shown to be applicable for all stages of HCC and are widely accepted; however, none is predictive in every situation, so there is room for improvement. Although both the CLIP scoring system and the BCLC staging system have been validated in different cohorts of patients, a more reliable prognostic classification for HCC is still needed.
In patients with cancer, 18F-fluorodeoxyglucose positron emission tomography (FDG PET) has been used for surveillance after treatment and for evaluation of response to therapy. During the past decade, combined FDG PET and computed tomography (FDG PET/CT) has also been used for imaging various malignancies [12]. However, the use of FDG PET/CT in HCC is limited because, whereas FDG uptake in cholangiocarcinoma, hepatocholangiocarcinoma, and liver metastases is higher than in normal liver tissue [13–15], FDG uptake in well-differentiated HCC is similar to uptake in normal liver tissue because of a high rate of gluconeogenesis in well-differentiated HCC [16]. In patients with many types of cancer, not only the highest metabolic activity within the tumor (maximum standard uptake value; SUVmax) in a two-dimensional region of interest, but also total lesion glycolysis (TLG) and metabolic tumor volume (MTV) in a three-dimensional region of interest are considered to provide valuable prognostic information. However, the prognostic value of findings on baseline pretreatment FDG PET/CT in patients with HCC remains to be elucidated.
The aim of this study was to determine the associations between overall survival (OS) and established prognostic factors and FDG PET/CT parameters and to determine whether baseline pretreatment FDG PET/CT parameters add to the prognostic value of the CLIP score or BCLC staging system in patients with HCC.
Materials and methods
Patients
This study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center, which waived the requirement for informed consent, and was performed in compliance with the Health Insurance Portability and Accountability Act. We retrospectively reviewed the database of MD Anderson’s Tumor Registry and identified 98 consecutive patients with liver tumors who were referred to our institution for treatment during the period from May 2013 through May 2014. Of these patients, 30 were excluded because they did not undergo FDG PET/CT before initial treatment. An additional 12 patients were excluded because OS data were unavailable (five patients), the patient had another type of liver cancer (cholangiocarcinoma, gallbladder carcinoma, hepatic adenoma, or adenomatosis; four patients), the patient had already had surgery at the time of referral to MD Anderson Cancer Center (two patients), or FDG PET/CT was false-negative for HCC (one patient). The remaining 56 patients were included in the analysis. In all patients, the diagnosis of HCC was confirmed at MD Anderson as part of the institution’s standard practice based on pathological or radiological HCC characteristics.
Patient information was obtained by direct chart review. Information extracted from charts included demographics, HCC risk factors, clinical characteristics, Eastern Cooperative Oncology Group (ECOG) performance status, and pathological differentiation. Furthermore, patients’ radiological images were reviewed to retrieve different tumor parameters mandatory for stage calculation including number of tumor nodules, tumor size, endovascular invasion, lymph node, and distant metastasis. Accordingly, TNM stage (seventh edition), CLIP score and BCLC staging system were calculated using information in the patients’ charts. The CLIP score is calculated by assigning a score (0, 1, or 2) to each of four clinical factors: (1) Child-Turcotte-Pugh score (CTP), (2) number of tumor nodules and whether the tumor occupies ≤50 % or >50 % of the liver on radiological images obtained on first presentation, (3) alfa-fetoprotein level, and (4) portal vein thrombosis. These scores are summed to calculate the CLIP score that ranges from 0 to 6 [7]. The BCLC staging system classifies disease into four categories based on several independent prognostic factors identified in several studies: (1) early stage, (2) intermediate stage, (3) advanced stage, and (4) end stage. These factors are variables related to tumor stage including number of tumor nodules, tumor size, distant metastasis, lymph node involvement, and vascular invasion. The BCLC staging system also includes parameters to assess underlying liver functional status in terms of CTP score, the patient’s ECOG performance status, and cancer-related symptoms [17].
FDG PET/CT imaging
FDG PET/CT scans were performed using a standard clinical protocol. Briefly, patients fasted for 6 h before 18F-FDG administration. All patients were confirmed to have a serum blood glucose level of ≤200 mg/dL before injection of the radiopharmaceutical. 18F-FDG (typically 259 – 444 MBq/7 – 12 mCi) was administered intravenously. Approximately 60 min later, imaging data were acquired using an integrated PET/CT system (Discovery ST, STe, or RX; GE Healthcare). CT was performed concomitantly with each PET acquisition for anatomical localization and attenuation correction with the following parameters: axial slice thickness 3.75 mm, 140 kV, and 120 mA, table speed 13.5 mm. PET was performed in three-dimensional mode at 3 to 5 min per bed position based on body mass index. PET, CT, and fusion images were displayed in slices of 3.75 mm. Datasets with and without attenuation correction were reconstructed. All FDG PET/CT scans were obtained during the 30 days before initiation of treatment.
Image review and tumor analysis
Imaging data were reviewed by experienced nuclear medicine physicians and radiologists at MD Anderson. Four PET/CT parameters, SUVmax, TLG, MTV, and tumor-to-normal-liver SUV ratio (TNR), were measured using a MIM workstation (MIM Software, Cleveland, OH). Volumetric parameters were defined using MIM contouring software as described previously [18]. SUV was defined as measured activity concentration (becquerels per gram) multiplied by body weight (grams) divided by injected activity (becquerels). TLG was defined as average metabolic activity within the tumor multiplied by tumor volume. MTV (mL) was defined using an automated contouring program based on the SUV.
Statistical analysis
Statistical analyses were performed with S-Plus software, version 8.2 (TIBCO, Palo Alto, CA), and SAS software, version 9.3 (SAS Institute Inc., Cary, NC). OS was defined as the time from the date of initial treatment to death from any cause or last follow-up. A univariate Cox model was used to determine the association between OS and continuous variables, including PET/CT parameters. The log-rank test was used to compare OS stratified by various potential prognostic factors. Martingale residual plots and recursive partitioning and regression trees analysis were used to determine cut-off values for PET/CT parameters to stratify patients in terms of OS. A Cox proportional hazards regression model was used for multivariate analysis to evaluate significant PET/CT parameters for both the CLIP score and the BCLC staging system. Fisher’s exact test was used to assess the association between variables. P < 0.05 was considered statistically significant.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. Of the 56 patients, 39 (70 %) had stage III or IV disease. The median time from initial treatment to last follow-up was 5.3 months (range 0.1 – 26.9 months), and 24 patients (43 %) died during follow-up. The estimated median OS was 17.0 months (95 % confidence interval 5.1 months – not assessable). In 26 patients (46 %) the HCC was well or moderately differentiated. Hepatitis B or C virus infection was seen in 27 patients (48 %). Multifocality and capsular nodularity were seen in 29 patients (52 %) and 37 patients (66 %), respectively, and 31 patients (55 %) had a history of alcohol consumption. Moderate or slight ascites was seen in 23 patients (41 %), and portal vein thrombosis was seen in 23 patients (41 %). The median alfafetoprotein value was 60.7 ng/mL (range 1.3 – 76,717.2 ng/mL). The median tumor size was 8.2 cm (range 1.1 – 18.2 cm), and 20 patients (36 %) had a tumor volume more than 50 % of the liver volume.
Table 1.
Patient demographics and clinical characteristics
| No. of patient (%) | |
|---|---|
| Characteristic | (n=56) |
| Age, median (range) | 65 years (21-91 years) |
| Sex | |
| Male | 49 (88) |
| Female | 7 (12) |
| Race/ethnicity | |
| Caucasian | 36 (64) |
| Latino | 10 (18) |
| African American | 7 (13) |
| Asian | 3 (5) |
| ECOG performance status | |
| 0-1 | 45 (80) |
| 2-3 | 9 (16) |
| Unknown | 2 (4) |
| Tumor differentiation | |
| Well | 8 (14) |
| Moderately | 18 (32) |
| Poorly | 6 (11) |
| Unknown | 24 (43) |
| Hepatitis infection | |
| HBV | 6 (11) |
| HCV | 20 (36) |
| HBV and HCV | 1 (1) |
| None | 29 (52) |
| Cirrhosis | |
| Yes | 33 (59) |
| No | 23 (41) |
| Varices | |
| Yes | 15 (27) |
| No | 41 (73) |
| Endovascular invasion | |
| Yes | 30 (54) |
| No | 26 (46) |
| Child-Pugh score | |
| A | 37 (66) |
| B | 17 (30) |
| C | 1 (2) |
| Unknown | 1 (2) |
| TNM stage | |
| I | 6 (11) |
| II | 8 (14) |
| III | 18 (32) |
| IV | 24 (43) |
| CLIP score | |
| 0 | 8 (14) |
| 1 | 8 (14) |
| 2 | 12 (21) |
| 3 | 14 (25) |
| 4 | 4 (8) |
| 5 | 3 (5) |
| Unknown | 7 (13) |
| BCLC stage | |
| 0 | 1 (2) |
| A | 4 (7) |
| B | 6 (11) |
| C | 42 (75) |
| D | 3 (5) |
BCLC, Barcelona-Clinic Liver Cancer; CLIP, Cancer of the Liver Italian Program; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus.
Associations between OS, established prognostic factors, and FDG PET/CT parameters
The median values of FDG PET/CT parameters for the primary lesion were as follows: SUVmax 6.0 (range 2.2 – 20.0), TLG 541.0 (range 4.6 – 5,631.7), MTV 151.9 mL (range 8.3 – 2,003.8), and TNR 2.0 (range 0.8 – 12.9). Table 2 shows the results of the univariate analysis of the associations between OS and continuous variables, including PET/CT parameters. SUVmax, TLG, tumor size, total bilirubin level, and alkaline phosphatase level were significant predictors of OS. MTV and TNR were not significant predictors of OS. Table 3 shows the results of the log-rank test to compare OS between patient subgroups. If there are no data for the 2-year OS rate, the patient died or was censored within 1 year so that no follow-up data were available beyond 1 year. ECOG performance status 0 or 1, no endovascular invasion, CTP score A, tumor volume ≤50 % of liver volume, TNM stage I/II, and CLIP score 0 were significant good predictors of OS.
Table 2.
Univariate analysis of the association between OS and continuous variables
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| SUVmax | 1.11 | 1.0026-1.23 | 0.045* |
| TLG | 1.00020 | 1.0000-1.00050 | 0.024* |
| MTV | 1.00050 | 0.99-1.0010 | 0.099 |
| TNR | 1.17 | 0.97-1.42 | 0.11 |
| Age | 1.00030 | 0.97-1.032 | 0.98 |
| Creatinine level | 1.74 | 0.74-4.11 | 0.20 |
| Albumin level | 0.72 | 0.38-1.36 | 0.31 |
| Tumor size | 1.11 | 1.015-1.21 | 0.022* |
| PT | 1.084 | 0.81-1.46 | 0.59 |
| PT-INR | 8.58 | 0.50-147.25 | 0.14 |
| Total bilirubin level | 1.20 | 1.011-1.41 | 0.037* |
| ALK | 1.0033 | 1.00050-1.0061 | 0.020* |
P < 0.05
OS, overall survival; SUVmax, maximum standardized uptake value; TLG, total lesion glycolysis; MTV, maximum tumor volume; TNR, tumor-to-normal liver ratios of SUV; PT, prothrombin time; PT-INR, prothrombin time-international normalized ratio; ALK, alkaline phosphatase; HR, hazard ratio; CI, confidence interval.glycolysis; TNR, tumor-to-normal liver ratios of SUV.
Table 3.
Log-rank test to compare OS among patient subgroups
| Variable | No. of patients |
No. of events |
Median OS (95% CI), mo |
1-year OS rate (95% CI) |
2-year OS rate (95% CI) |
P
value |
|---|---|---|---|---|---|---|
| Age, years | ||||||
| ≤ 60 | 22 | 10 | 4.9 (3.67-NA) | 0.40 (0.22-0.73) | 0.16 | |
| > 60 | 34 | 14 | 17.0 (9.13-NA) | 0.64 (0.49-0.84) | 0.41 (0.23-0.73) | |
| Sex | ||||||
| Female | 7 | 2 | NA (7.1-NA) | 0.64 (0.34-1.00) | 0.66 | |
| Male | 49 | 22 | 16.3 (4.8-NA) | 0.55 (0.41-0.72) | 0.38 (0.23-0.63) | |
| Race/ethnicity | ||||||
| Other | 20 | 8 | 17.0 (3.7-NA) | 0.55 (0.35-0.87) | 0.28 (0.060-1.00) |
0.87 |
| Caucasian | 36 | 16 | 16.3(4.9-NA) | 0.56 (0.41-0.76) | ||
| ECOG PS | ||||||
| 0-1 | 45 | 20 | 17.0 (5.1-NA) | 0.57 (0.43-0.75) | 0.4 (0.24-0.66) | 0.02* |
| 2-3 | 9 | 4 | 2.9 (1.3-NA) | 0.25 (0.05-1.00) | ||
| Hepatitis infection |
||||||
| HBV/HCV | 27 | 13 | 4.9 (3.7-NA) | 0.49 (0.32-0.76) | 0.26 (0.10-0.72) | 0.21 |
| None | 29 | 11 | 17.0 (7.1-NA) | 0.61 (0.44-0.84) | ||
| Cirrhosis | ||||||
| No | 23 | 10 | 17.0 (5.1-NA) | 0.61 (0.43-0.87) | 0.38 (0.18-0.81) | 0.47 |
| Yes | 33 | 14 | 17.6 (4.2-NA) | 0.51 (0.34-0.75) | ||
| Varices | ||||||
| No | 41 | 18 | 17.0 (4.5-NA) | 0.53 (0.39-0.72) | 0.42 (0.25-0.72) | 0.69 |
| Yes | 15 | 6 | 17.6 (7.1-NA) | 0.65 (0.42-1.00) | ||
| Endovascular invasion |
||||||
| No | 26 | 8 | NA (16.3-NA) | 0.74 (0.57-0.94) | 0.55 (0.33-0.89) | 0.0025* |
| Yes | 30 | 16 | 4.5 (3.2-NA) | 0.37 (0.21-0.63) | ||
| Focality | ||||||
| Multifocal | 29 | 14 | 5.1 (3.5-NA) | 0.47 (0.30-0.74) | 0.058 | |
| Unifocal | 27 | 10 | NA (9.1-NA) | 0.64 (0.47-0.86) | 0.56 (0.37-0.83) | |
| AFP level, ng/mL | ||||||
| < 400 | 34 | 12 | NA (9.1-NA) | 0.63 (0.47-0.83) | 0.55 (0.37-0.81) | 0.16 |
| ≥ 400 | 20 | 10 | 7.1 (4.2-NA) | 0.5 (0.3-0.82) | ||
| Child-Pugh score | ||||||
| A | 37 | 14 | 17.6 (7.1-NA) | 0.63 (0.48-0.82) | 0.47 (0.28-0.77) | 0.0016* |
| B | 17 | 8 | 5.1 (2.6-NA) | 0.47 (0.26-0.84) | ||
| C | 1 | 1 | 1.3 (NA-NA) | |||
| Tumor volume as a percentage of liver volume |
||||||
| ≤ 50% | 32 | 10 | NA (16.3-NA) | 0.71 (0.56-0.91) | 0.55 (0.36-0.85) | 0.019 |
| > 50% | 20 | 11 | 4.8 (3.7-NA) | 0.38 (0.21-0.72) | ||
| TNM stage | ||||||
| III/IV | 42 | 22 | 5.1 (4.2-NA) | 0.43 (0.29-0.64) | 0.0059 | |
| I/II | 14 | 2 | NA (NA-NA) | 0.92 (0.77-1.00) | 0.76 (0.51-1.00) | |
| Ascites | ||||||
| Moderate | 8 | 4 | 2.1 (1.1-NA) | 0.34 (0.11-1.00) | 0.059 | |
| None | 33 | 14 | 17.6 (7.1-NA) | 0.61 (0.46-0.81) | 0.43 (0.24-0.77) | |
| Slight | 15 | 6 | 16.97 (3.5 , NA) | 0.51 (0.28 , 0.94) | ||
| CLIP score | ||||||
| 0 | 8 | 1 | NA (16.3-NA) | 1.00 (1.00-1.00) | 0.75 (0.43-1.00) | 0.018* |
| 1 | 8 | 4 | 7.1 (4.9-NA) | 0.47 (0.21-1.00) | ||
| 2 | 12 | 3 | 17.6 (17.6-NA) | 0.82 (0.63-1.00) | ||
| 3 | 14 | 7 | 4.8 (3.7-NA) | 0.37 (0.17-0.80) | ||
| 4 | 4 | 2 | 3.1 (1.7-NA) | |||
| 5 | 3 | 2 | 17.0 (2.6-NA) | 0.67 (0.30-1.00) | ||
| BCLC stage | ||||||
| 0 | 1 | 0 | NA (NA-NA) | 0.18 | ||
| A | 4 | 1 | 17.0 (16.3-NA) | 1.00 (1.00-1.00) | ||
| B | 6 | 2 | NA (17.6-NA) | 0.83 (0.58-1.00) | 0.56 (0.23-1.00) | |
| C | 42 | 20 | 9.1 (4.5-NA) | 0.47 (0.33-0.67) | ||
| D | 3 | 1 | 1.8 (1.3-NA) |
P < 0.05
OS, overall survival; AFP, alfa-fetoprotein; CI, confidence interval; CLIP, Cancer of the Liver Italian Program; BCLC, Barcelona-Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group Performance Status; NA, not available.
Additional prognostic value of PET/CT parameters
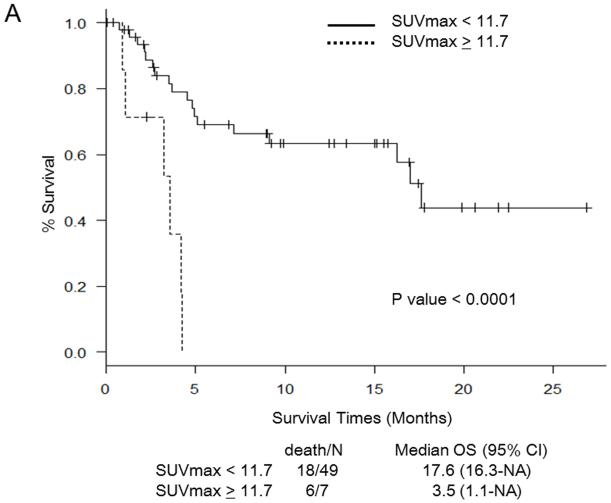
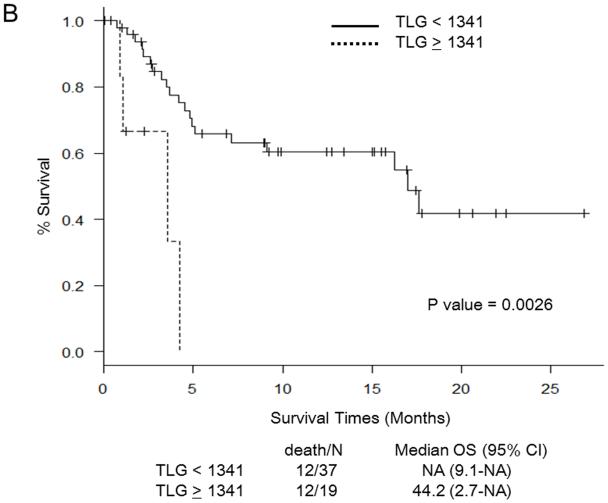
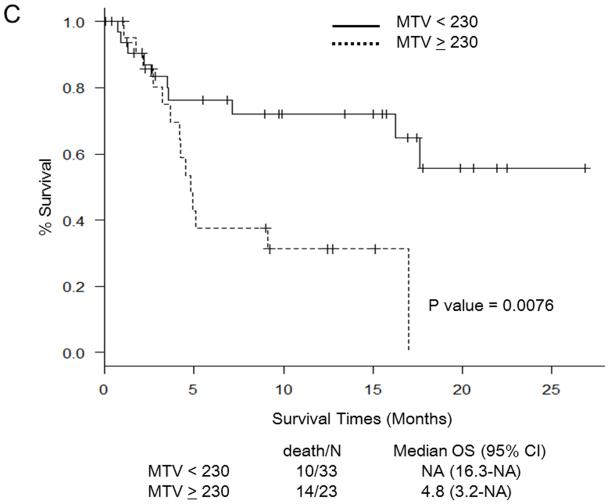
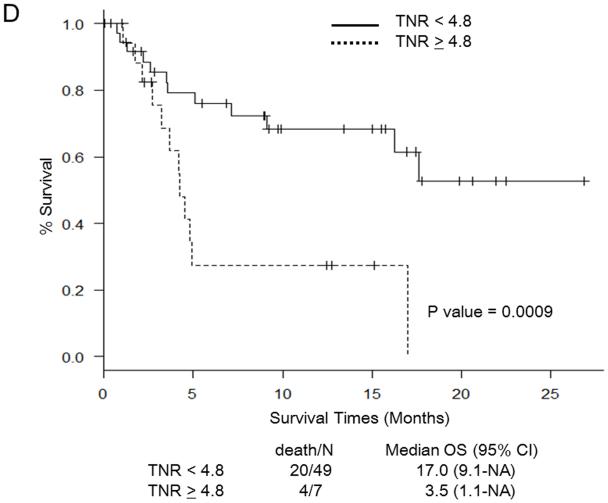
Martingale residual plots and recursive partitioning and regression trees analysis revealed the following optimal cut-off points for PET/CT parameters to stratify patients in terms of OS: SUVmax 11.7, TLG 1,341, MTV 230 mL, and TNR 4.8. Figure 1 shows Kaplan-Meier curves for OS in relation to the determined cut-off values of PET/CT parameters. OS was significantly worse in patients with SUV ≥ 11.7 (Fig. 1a), TLG ≥ 1,341 (Fig. 1b), MTV ≥ 230 (Fig. 1c), and TNR ≥ 4.8 (Fig. 1d). The results of the multivariate analysis to determine whether PET/CT parameters provide additional prognostic value beyond that provided by the CLIP score and the BCLC staging system in predicting OS are shown in Tables 4 and 5, respectively. Each table includes four different sets of data. These correspond to four different models, one for each of the PET/CT parameters. For the CLIP scoring system, both SUVmax ≥ 11.7 and TNR ≥ 4.8 were independent poor prognostic factors (Table 4). For the BCLC staging system, both SUV ≥ 11.7 and TNR ≥ 4.8 were independent poor prognostic factors, as was TLG ≥ 1,341 (Table 5).
Fig. 1.
Survival curves for patients in relation to cut-off values for PET/CT parameters. a Maximum standardized uptake value (SUVmax). b Total lesion glycolysis (TLG). c Metabolic tumor volume (MTV). d Tumor-to-normal-liver SUV ratio (TNR). NA not assessable, OS overall survival, CI confidence interval
Table 4.
Multivariate Cox regression analysis using PET/CT parameters and Cancer of the Liver Italian Program (CLIP) score
| Variable | Coefficient | P value | HR (95% CI) |
|---|---|---|---|
| CLIP 1 (vs. 0) | 1.52 | 0.181 | 4.59 (0.49-42.97) |
| CLIP 2 (vs. 0) | 1.16 | 0.318 | 3.18 (0.33-30.78) |
| CLIP 3 (vs. 0) | 1.92 | 0.100 | 6.82 (0.69-67.01) |
| CLIP 4 (vs. 0) | 3.78 | 0.004* | 43.96 (3.36-574.48) |
| CLIP 5 (vs. 0) | 2.15 | 0.084 | 8.60 (0.75-99.12) |
| SUVmax ≥ 11.7 (vs. < 11.7) | 2.08 | 0.004* | 8.00 (1.96-32.76) |
| CLIP 1 (vs. 0) | 1.67 | 0.140 | 5.31 (0.58-48.84) |
| CLIP 2 (vs. 0) | 1.11 | 0.340 | 3.03 (0.31-29.49) |
| CLIP 3 (vs. 0) | 2.25 | 0.054 | 9.44 (0.96-93.04) |
| CLIP 4 (vs. 0) | 3.14 | 0.025* | 23.04 (1.50-354.97) |
| CLIP 5 (vs. 0) | 1.88 | 0.149 | 6.57 (0.51-84.38) |
| TLG ≥ 1341 (vs. < 1341) | 0.38 | 0.509 | 1.46 (0.47-4.55) |
| CLIP 1 (vs. 0) | 1.71 | 0.129 | 5.54 (0.61-50.46) |
| CLIP 2 (vs. 0) | 1.12 | 0.336 | 3.06 (0.31-29.70) |
| CLIP 3 (vs. 0) | 2.28 | 0.050* | 9.76 (1.00-94.79) |
| CLIP 4 (vs. 0) | 3.22 | 0.018* | 25.13 (1.72-367.09) |
| CLIP 5 (vs. 0) | 1.95 | 0.128 | 7.04 (0.57-86.91) |
| MTV ≥ 230 mL (vs. < 230 mL) | 0.33 | 0.571 | 1.39 (0.45-4.30) |
| CLIP 1 (vs. 0) | 1.74 | 0.122 | 5.69 (0.63-51.72) |
| CLIP 2 (vs. 0) | 1.14 | 0.327 | 3.11 (0.32-30.15) |
| CLIP 3 (vs. 0) | 2.09 | 0.070 | 8.11 (0.84-78.33) |
| CLIP 4 (vs. 0) | 3.60 | 0.005* | 36.57 (2.91-459.45) |
| CLIP 5 (vs. 0) | 2.13 | 0.088 | 8.38 (0.73-95.94) |
| TNR ≥ 4.8 (vs. < 4.8) | 1.58 | 0.047* | 4.85 (1.02-22.92) |
P < 0.05
CI, confidence interval; HR, hazard ratio; SUVmax, maximum standardized uptake value; TLG, total lesion glycolysis; MTV, maximum tumor volume; TNR, tumor-to-normal liver ratios of SUV.
Table 5.
Multivariate Cox regression analysis using PET/CT parameters and Barcelona-Clinic Liver Cancer (BCLC) stage
| Variable | Coefficient | P value | HR (95% CI) |
|---|---|---|---|
| BCLC B (vs. A/0) | 0.20 | 0.870 | 1.22 (0.11-13.64) |
| BCLC C (vs. A/0) | 1.07 | 0.300 | 2.92 (0.38-22.23) |
| BCLC D (vs. A/0) | 1.63 | 0.284 | 5.09 (0.26-100.01) |
| SUVmax ≥ 11.7 (vs. < 11.7) | 1.65 | 0.003* | 5.19 (1.73-15.56) |
| BCLC B (vs. A/0) | 0.20 | 0.869 | 1.22 (0.11-13.65) |
| BCLC C (vs. A/0) | 0.87 | 0.411 | 2.38 (0.30-18.84) |
| BCLC D (vs. A/0) | 2.11 | 0.160 | 8.29 (0.44-157.75) |
| TLG ≥ 1341 (vs. < 1341) | 0.97 | 0.033* | 2.64 (1.08-6.45) |
| BCLC B (vs. A/0) | 0.05 | 0.968 | 1.05 (0.09-11.80) |
| BCLC C (vs. A/0) | 0.83 | 0.436 | 2.29 (0.28-18.41) |
| BCLC D (vs. A/0) | 2.17 | 0.150 | 8.73 (0.46-166.13) |
| MTV ≥ 230 mL (vs. < 230 mL) | 0.89 | 0.053 | 2.44 (0.99-6.02) |
| BCLC B (vs. A/0) | 0.22 | 0.861 | 1.24 (0.11-13.82) |
| BCLC C (vs. A/0) | 1.16 | 0.260 | 3.19 (0.42-24.08) |
| BCLC D (vs. A/0) | 1.69 | 0.273 | 5.43 (0.26-112.19) |
| TNR ≥ 4.8 (vs. < 4.8) | 1.48 | 0.021* | 4.37 (1.24-15.38) |
P < 0.05
CI, confidence interval; HR, hazard ratio; SUVmax, maximum standardized uptake value; TLG, total lesion glycolysis; MTV, maximum tumor volume; TNR, tumor-to-normal liver ratios of SUV.
Discussion
This study confirmed associations between established prognostic factors and OS, and also showed statistically significant associations between OS and metabolic imaging biomarkers. In this study in 56 consecutive patients with HCC who underwent FDG PET/CT before initial treatment, FDG PET/CT-derived parameters were significant predictors of OS, and the optimal cut-off values for these parameters were determined. Patients with values higher than the cut-off values had significantly worse survival. PET/CT parameters, especially SUVmax and TNR, added to the prognostic value of the CLIP score and BCLC staging system, and TLG added to the value of BCLC staging system. To our knowledge, this is the first analysis showing that initial pretreatment PET/CT parameters can add to the prognostic value of prognostic scoring systems in patients with untreated HCC.
Previous studies on the role of FDG PET/CT in the evaluation of patients with HCC have indicated that the value of FDG PET/CT may depend on the degree of differentiation or tumor size. In well-differentiated HCC, FDG PET/CT may not be an appropriate modality because a high rate of gluconeogenesis comparable with that in normal liver tissue results in similar uptake of FDG [16]. Trojan et al. found high FDG uptake in patients with moderately or poorly differentiated HCC, with tumors >5 cm, or with elevated alfa-fetoprotein levels [19]. Hayakawa et al. found that less well differentiated HCC express more hexokinase, resulting in higher FDG uptake [20]. In our study, many patients already had large tumors before initiation of treatment (the median tumor size at the time of pretreatment FDG PET/CT was 8.2 cm). Thus, PET/CT can be considered to have been of value in our patient cohort. Information with regard to tumor differentiation was not available in all our patients. Our findings are partly consistent with those of Ahn et al., who found that preoperative FDG PET/CT can predict early recurrence after curative resection of HCC [21]. They found that both TNR ≥2 and SUVmax ≥4 were significant predictors in a univariate analysis. However, the factors they examined were not significant independent predictors in a multivariate analysis.
To our knowledge, this is the first report indicating that PET/CT parameters may add to the prognostic value of staging systems. In a multivariate analysis that SUVmax and TNR added to the prognostic value of the CLIP score and BCLC staging system, and TLG added to the value of BCLC staging system. Our results suggest that both SUVmax and TNR clearly add to the prognostic value of the CLIP score and BCLC staging system, and TLG might also be prognostic; in contrast, TLG was not an independent poor prognostic factor in a multivariate analysis. Tumor volume is not taken into account in the calculation of either SUVmax or TNR. These results contrast with previous findings indicating that volume-based PET/CT parameters aid in predicting prognosis in many types of malignancy, including non-small-cell lung cancer, head and neck cancer, ovarian cancer, and soft tissue sarcoma [22–25]. However, which PET/CT parameter is the most reliable predictor of outcome in patients with HCC is still unknown. Further prospective study is necessary to clarify this issue.
Although we focused on FDG PET/CT, tracers other than 18F-FDG might also be promising for predicting prognosis in patients with HCC. PET tracers that visualize lipid metabolism may be superior to 18F-FDG for the detection of HCC. The sensitivity of 11C-acetate has been reported to be better for the detection of well-differentiated HCC [26, 27]. In a prospective study, Talbot et al. found that 18F-fluorocholine PET/CT shows significantly greater sensitivity than FDG PET/CT in the detection of well-differentiated HCC, similar sensitivity in the detection of less differentiated HCC, and lower sensitivity in the detection of poorly differentiated HCC [28]. The prognostic value of 11C-acetate and 18F-fluorocholine PET/CT in patients with HCC has not yet been examined.
Our study had several potential limitations. This was a single-center retrospective study, with a relatively small number of patients. Cut-off values found in the present study should be validated in a larger patient group. Additionally, we were not able to categorize patients according to type of systemic therapy, which could be a factor in patient outcome. For example, the number of patients treated with a multikinase inhibitor such as sorafenib was unknown. It has been suggested that molecularly targeted therapy might prolong survival in patients with advanced HCC [29]. Additionally, information about tumor differentiation was not available in all patients. 18 F-FDG uptake varies between well-differentiated and poorly differentiated HCC [26]. Despite these limitations, this study is noteworthy in demonstrating that pretreatment FDG PET/CT not only was important in terms of OS but also added to the prognostic value of the CLIP score and BCLC staging system.
Conclusion
This study demonstrated that metabolic imaging parameters derived from FDG PET/CT are prognostic factors for OS in patients with newly diagnosed untreated HCC. Furthermore, FDG PET/CT imaging parameters add to the prognostic value of and complement the CLIP score and BCLC staging system in patients with untreated HCC. SUVmax and TNR seem to be the most prognostically valuable of the FDG PET/CT parameters, but TLG may also be an important prognostic factor. We believe that incorporation of metabolic imaging parameters into HCC staging systems should be considered, with future studies aimed at further defining the use of these biomarkers.
Acknowledgments
We thank all the patients, their families, and the investigators. We also thank the staff of the University of Texas MD Anderson Cancer Center for their assistance. This report was edited by Stephanie Deming in the Department of Scientific Publications at MD Anderson Cancer Center.
Funding This work was supported in part by the MD Anderson Cancer Center James E. Anderson Distinguished Professorship in Nuclear Medicine (to Dr. Macapinlac), the Society of Nuclear Medicine and Molecular Imaging 2012/2014Wagner-Torizuka Fellowship (to Dr. Takeuchi), NIH grants CA170035-01 (to Dr. Kaseb) and CA106458-01 (to Dr. Hassan), and the NIH/NCI under award number P30CA016672.
Footnotes
This work was presented in part at the European Cancer Congress, Vienna, Austria, 25–29 September 2015.
Conflicts of interest None.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of our institution and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This retrospective study was approved by the local institutional review board and the requirement for written informed consent was waived. This article does not describe any studies with animals performed by any of the authors.
References
- 1.Stewart BW, Wild CW, editors. World Cancer Report 2014. International Agency for Research on Cancer; Lyon: 2014. http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014. Accessed 30 Nov 2016. [Google Scholar]
- 2.Shibata T, Aburatani H. Exploration of liver cancer genomes. Nat Rev Gastroenterol Hepatol. 2014;11:340–349. doi: 10.1038/nrgastro.2014.6. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Shen J, Sun TT, Zhang X, Wong N. Obesity, insulin resistance, NASH and hepatocellular carcinoma. Semin Cancer Biol. 2013;23:483–491. doi: 10.1016/j.semcancer.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology. National Comprehensive Cancer Network; Fort Washington, PA: version 2.2016. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed 30 Nov 2016. [DOI] [PubMed] [Google Scholar]
- 6.Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford) 2005;7:35–41. doi: 10.1080/13651820410024058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Cancer of the Liver Italian Program (CLIP) Investigators A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 9.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 10.Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–1769. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 11.Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire. J Hepatol. 1999;31:133–141. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 12.Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–1379. [PubMed] [Google Scholar]
- 13.Lee SW, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, et al. Clinical usefulness of 18F-FDG PET-CT for patients with gallbladder cancer and cholangiocarcinoma. J Gastroenterol. 2010;45:560–566. doi: 10.1007/s00535-009-0188-6. [DOI] [PubMed] [Google Scholar]
- 14.Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674–684. doi: 10.1148/radiol.10100729. [DOI] [PubMed] [Google Scholar]
- 15.Jiang L, Tan H, Panje CM, Yu H, Xiu Y, Shi H. Role of 18F-FDG PET/CT imaging in intrahepatic cholangiocarcinoma. Clin Nucl Med. 2016;41:1–7. doi: 10.1097/RLU.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 16.Torizuka T, Tamaki N, Inokuma T, Magata Y, Sasayama S, Yonekura Y, et al. In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. J Nucl Med. 1995;36:1811–1817. [PubMed] [Google Scholar]
- 17.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 18.Davison J, Mercier G, Russo G, Subramaniam RM. PET-based primary tumor volumetric parameters and survival of patients with non-small cell lung carcinoma. AJR Am J Roentgenol. 2013;200:635–640. doi: 10.2214/AJR.12.9138. [DOI] [PubMed] [Google Scholar]
- 19.Trojan J, Schroeder O, Raedle J, Baum RP, Herrmann G, Jacobi V, et al. Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol. 1999;94:3314–3319. doi: 10.1111/j.1572-0241.1999.01544.x. [DOI] [PubMed] [Google Scholar]
- 20.Hayakawa N, Nakamoto Y, Nakatani K, Hatano E, Seo S, Higashi T, et al. Clinical utility and limitations of FDG PET in detecting recurrent hepatocellular carcinoma in postoperative patients. Int J Clin Oncol. 2014;19:1020–1028. doi: 10.1007/s10147-013-0653-3. [DOI] [PubMed] [Google Scholar]
- 21.Ahn SG, Kim SH, Jeon TJ, Cho HJ, Choi SB, Yun MJ, et al. The role of preoperative [18F]fluorodeoxyglucose positron emission tomography in predicting early recurrence after curative resection of hepatocellular carcinomas. J Gastrointest Surg. 2011;15:2044–2052. doi: 10.1007/s11605-011-1660-1. [DOI] [PubMed] [Google Scholar]
- 22.Chen HH, Chiu NT, Su WC, Guo HR, Lee BF. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology. 2012;264:559–566. doi: 10.1148/radiol.12111148. [DOI] [PubMed] [Google Scholar]
- 23.Lim R, Eaton A, Lee NY, Setton J, Ohri N, Rao S, et al. 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J Nucl Med. 2012;53:1506–1513. doi: 10.2967/jnumed.111.101402. [DOI] [PubMed] [Google Scholar]
- 24.Chung HH, Kwon HW, Kang KW, Park NH, Song YS, Chung JK, et al. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis in patients with epithelial ovarian cancer. Ann Surg Oncol. 2012;19:1966–1972. doi: 10.1245/s10434-011-2153-x. [DOI] [PubMed] [Google Scholar]
- 25.Costelloe CM, Macapinlac HA, Madewell JE, Fitzgerald NE, Mawlawi OR, Rohren EM, et al. 18F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J Nucl Med. 2009;50:340–347. doi: 10.2967/jnumed.108.058461. [DOI] [PubMed] [Google Scholar]
- 26.Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213–221. [PubMed] [Google Scholar]
- 27.Cheung TT, Ho CL, Lo CM, Chen S, Chan SC, Chok KS, et al. 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of Milan criteria: surgeon’s perspective. J Nucl Med. 2013;54:192–200. doi: 10.2967/jnumed.112.107516. [DOI] [PubMed] [Google Scholar]
- 28.Talbot JN, Fartoux L, Balogova S, Nataf V, Kerrou K, Gutman F, et al. Detection of hepatocellular carcinoma with PET/CT: a prospective comparison of 18F-fluorocholine and 18F-FDG in patients with cirrhosis or chronic liver disease. J Nucl Med. 2010;51:1699–1706. doi: 10.2967/jnumed.110.075507. [DOI] [PubMed] [Google Scholar]
- 29.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]