Abstract
Currently, it is not well understood how ligands of the Aryl hydrocarbon Receptor (AhR) modify inflammatory responses triggered by Toll like receptor (TLR) agonists in human dendritic cells (DCs). Here, we show that AhR ligands 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the tryptophan derivatives 6-formylindolo[3,2-b]carbazole (FICZ), Kynurenine (kyn), and the natural dietary compound Indole-3-carbinole (I3C) differentially modify cytokine expression in human monocyte-derived DCs (MoDCs). The results show that TLR-activated MoDCs express higher levels of AhR and are more sensitive towards the effects of AhR ligands. Depending on the cytokine, treatment with AhR ligands led to a synergistic or antagonistic effect of the TLR-triggered response in MoDCs. Thus, activation of AhR increased the expression of Interleukin (IL)-1β, but decreased the expression of IL-12A in TLR-activated MoDCs. Furthermore, TCDD and FICZ may have opposite effects on the expression of cytochrome P4501A1 (CYP1A1) in TLR8-activated MoDCs indicating that the effect of the specific AhR ligand may depend on the presence of the specific TLR agonist. Gene silencing showed that synergistic effects of AhR ligands on TLR-induced expression of IL-1β requires a functional AhR and the expression of NF-κB RelB. On the other hand, repression of IL-12A by TCDD and FICZ involved the induction of the caudal type homeobox 2 (CDX2) transcription factor. Additionally, the levels of DC surface markers were decreased in MoDCs by TCDD, FICZ and I3C, but not by kyn. Overall, these data demonstrate that AhR modulates TLR-induced expression of cytokines and DC-specific surface markers in MoDCs involving NFκB RelB and the immune regulatory factor CDX2.
Keywords: AhR, dendritic cells, cytokines, TLR, CDX2, NF-κB
Introduction
There is strong evidence that environmental factors including toxic chemicals and dietary components contribute to the development of autoimmune diseases (Pollard 2015; Selmi et al. 2012). The aryl hydrocarbon receptor (AhR) is a cytosolic receptor protein that responds to environmental and physiological stress. The AhR plays an important role in regulating immune responses and exposure to AhR-activating toxicants contributes to the promotion of immune system disorders (Marshall and Kerkvliet 2010). The AhR affects the expression of immunoregulatory genes and the function of inflammatory DCs (Jin et al. 2010; Bankoti et al. 2010; Vogel et al. 2008). Several studies show that exposure to AhR agonists like the environmental toxicant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) leads to profound immunosuppression and an increase of regulatory T cells (Treg) (Kerkvliet 2002; Mitchell and Lawrence 2003). On the other hand, exposure to AhR ligands like the tryptophan metabolite 6-formylindolo[3,2-b]carbazole (FICZ) may promote differentiation of inflammatory T helper (Th)17 cells (Quintana et al. 2008, Veldhoen et al. 2008). Recent studies show that the AhR is a key regulator for development and function of interleukin (IL)-22-secreting innate lymphoid cells in the lamina propria (Lee et al. 2012; Lawrence and Sherr 2012), and activation of AhR by dietary substances such as indole-3-carbinol (I3C) is required for the development and/or maintenance of intestinal immune function and host defense mechanisms (Li et al. 2011; Kiss et al. 2011). The mechanisms underlying AhR regulation of human DC fate and function, and the effects of AhR signaling in human DCs during inflammatory responses are largely unknown. DC function and differentiation are both highly regulated by nuclear factor-kappa-B (NF-κB, Burkly et al. 1995). Conversely, NF-κB RelB deficiency affects the development of DCs and lymphocytes and is associated with immunodeficiency and autoimmunity in humans (Sharfe et al. 2015). A crosstalk between AhR and NF-κB has been reported from several studies (Tian 2009; Vogel and Matsumura 2009), but the mechanism and biological consequences are not completely understood. Recent reports demonstrate that the AhR provides additional activation (hyperexpression) or inhibition of TLR-/NF-κB-mediated gene transcription when the cell receives further stimuli (TCDD), transforming the TLR/NF-κB signal into an activator or inhibitor of gene transcription in the presence of an activated AhR (Vogel et al. 2013; Lahoti et al. 2015; Ruby et al. 2002).
To advance our understanding of the effects of AhR activation in human DCs, we generated human monocyte-derived DCs (MoDCs) from CD14+ monocytes isolated from healthy individuals. It is well established that the AhR can regulate the generation of various immune responses, particularly those involving the differentiation and function of DCs and T cells (Marshall and Kerkvliet 2010). While the mechanisms by which ligands of the AhR alter the function of DCs and the differentiation of T cells are not fully elucidated, an increasing number of studies suggest that interactions between the AhR and the TLR and NF-κB pathways likely play a highly significant role (Bankoti et al. 2010; Vogel et al. 2013).
In the current study we investigated the effects of various AhR ligands including TCDD, I3C, kyn and FICZ on the expression of cytokines in resting and TLR-activated MoDCs. Family members of TLR as well as IL-1R share a common motif called the Toll/IL-1 receptor (TIR) domain (Kawai and Akira 2007), which is critically involved in TLR signaling. Two major TIR domain–containing adapters, MyD88 (myeloid differentiation primary response gene 88) and TRIF (TIR domain–containing adapter-inducing IFN-β), have been described (Martin and Wesche 2002). For instance, TLR3 agonists mediate their response via TRIF-dependent pathway, whereas TLR4 and TLR8 agonists trigger their response through MyD88. Using human MoDCs we addressed the molecular basis of the altered expression of AhR/NF-κB regulated cytokines and DC surface markers after treatment with various AhR and TLR ligands. The current study specifically tested the role of the NF-κB member RelB, which has been shown to interact with the AhR signaling pathway and the expression of cytokines induced by AHR ligands (Vogel et al. 2007a; Vogel et al. 2007b; Memari et al. 2015). In order to identify key factors, which might be responsible for the repression of TLR-induced cytokines by AhR ligands we analyzed expression of activating transcription factor 3 (ATF3), indoleamine 2,3-dioxygenase (IDO1) and the caudal type homeobox 2 (CDX2) transcription factor. All three genes have been described to potentially modify or negatively regulate TLR- and NF-κB-regulated signaling and cytokine expression (Gilchrist et al. 2006; Shon et al. 2015; Ryu et al. 2008). The current study specifically tested the role of the NF-κB member RelB and CDX2, a caudal-related homeobox gene important in intestinal homeostasis and inflammation (Ryu et al. 2008; Coskun et al. 2011), by gene silencing, an approach that allowed us to examine the important interplay between RelB and CDX2 in AhR signaling in human DCs.
Materials and Methods
Reagents and antibodies
Dimethylsulfoxide (DMSO), phorbol-12-myristate-13-acetate (PMA), and bacterial lipopolysaccharide (LPS) from Escherichia coli O127:B8 were obtained from Sigma (St. Louis, MO). TLR ligands CL075 and polyinosinic:polycytidylic acid (poly(I:C)) were purchased from InvivoGen (San Diego, CA). LPS is the major structural component of the outer wall of all Gram-negative bacteria and recognized by TLR4. CL075 is a thiazoloquinolone derivative that stimulates TLR8 in human peripheral blood mononuclear cells and poly(I:C) is a synthetic analog of double-stranded RNA, a molecular pattern associated with viral infection and TLR3 activation. TCDD (>99% purity) was originally obtained from Dow Chemical Co. (Midland, MI). Other molecular biological reagents were purchased from Qiagen (Valencia, CA) and Roche Clinical Laboratories (Indianapolis, IN). The polyclonal RelB (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), polyclonal AhR (Novus Biologicals, Littleton, CO; Abnova, Walnut, CA), and the monoclonal CDX2 (Developmental Studies Hybridoma Bank, University of Iowa, IA) antibodies were used for Western blot analyses.
Monocyte-derived dendritic cell (MoDC) cultures and transfection experiments
Leukocyte-enriched buffy coat from healthy donors were obtained from the Stanford Blood Center (Mountain View, CA). Isolation of CD14+ cells and generation of MoDC cultures were performed as described previously (Chang et al. 2004) and all human cell work was approved by the IRB at University of California Davis. Cells were maintained in RPMI 1640 medium containing 50 μm 2-mercaptoethanol, 10 mM HEPES, 10% endotoxin-free fetal calf serum, recombinant human GM-CSF, and IL-4 (1000 units/ml each) for 7 days. Cells were treated on day 6 with TCDD (10 nM), FICZ (100 nM), I3C (50 μM) and kynurenine (kyn, 50 μM) for the indicated amount of time in absence or presence of LPS (50 ng/ml), CL075 (5 μg/ml) or poly(I:C) (5 μg/ml). Transfection of siRNA into MoDCs was performed via Nucleofector technology as described (Vogel et al., 2014). Briefly, MoDCs were resuspended in 100 μl Nucleofector Solution for human dendritic cells (Amaxa GmbH, Köln, Germany) and nucleofected with 1.0 μg siRNA or 0.2 μg plasmid DNA using program U-002, which is preprogrammed into the Nucleofector device (Amaxa GmbH). Cdx2 siRNA duplex (sc-43680) and Control siRNA duplex (sc-37007) were from Santa Cruz Biotechnology. The siRNAs to target human AhR (target sequence: TTCGTTTACCTTCAAACTTTA) and RelB (target sequence: GCGGATTTGCCGAATTAACAA) were synthesized by Qiagen.
Flow cytometry
Multiparameter flow cytometry was performed using the LSRFortessa X-20 cell analyzer operated by FACSDiva software (BD Biosciences). MoDCs were immunostained using directly conjugated monoclonal antibodies against CD83, CD86, CD195 (CCR5), CD196 (CCR6), CD197 (CCR7), and HLA-DR (BD Biosciences and Tonbo Biosciences). Data were analyzed and illustrated using FlowJo software (Tree Star).
RNA isolation and quantitative real-time RT-PCR
The preparation of total RNA was performed with a Quick RNA isolation kit (Zymo Research) and synthesis of cDNA were conducted as described previously (Vogel et al. 2014). qPCR was then performed with the LightCycler (Roche, Indianapolis, IN) or StepOnePlus Real-Time PCR System using the Fast SYBR Green Master Mix (Applied Biosystems Inc.) according to the manufacturer’s protocol. The data were normalized to the housekeeping gene ribosomal protein S13 (rps13) with the primer sequences CTCCCTTTCGTTGCCTGAT (forward primer) and CCCTTCTTGGCCAGTTTGTA (reverse primer).
Western blotting analysis
To analyze the level of AhR, RelB and CDX2 protein in MoDCs, whole cell protein (25 μg) was separated on a 10% SDS-polyacrylamide gel and blotted onto a polyvinylidine difluoride membrane (Immuno-Blot; Bio-Rad) as described (Vogel et al. 2014). The antigen-antibody complexes were visualized using the chemoluminescence substrate SuperSignal West Pico (Pierce) as recommended by the manufacturer.
Statistics
The results were analyzed by GraphPad Prism software. All experiments were repeated a minimum of three times, and data are expressed as mean ± S.D. Differences were considered significant at p < 0.05. A comparison of two groups was made with an unpaired, two-tailed Student’s t test. A comparison of multiple groups was made with analysis of variance (ANOVA) followed by a Dunnett’s or Tukey’s test. A two-way ANOVA was used when data with more than one factor were analyzed.
Results
Time-dependent effects of TCDD on the LPS-induced expression of cytokines in MoDCs
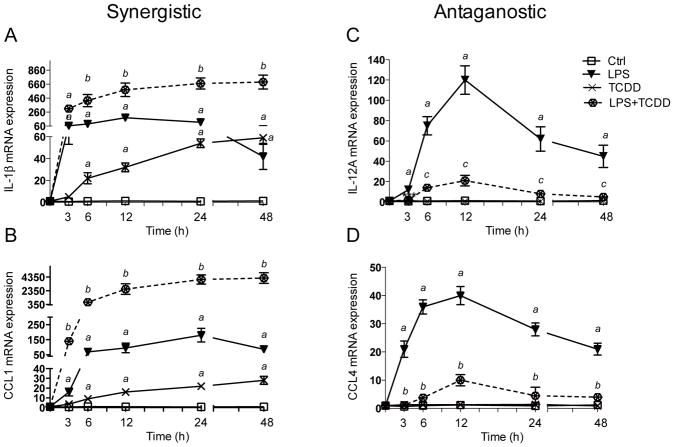
Initial experiments tested the time-dependent effects of AhR activation on LPS-induced cytokine expression in MoDCs. TCDD led to a 23-fold increase of IL-1β mRNA expression after 6 h treatment and the level of IL-1β increased over time to a maximum induction level of 59-fold at 48 h after treatment with TCDD compared to control cells (Fig. 1A). LPS rapidly increased IL-1β 65-fold after 3 h treatment with a maximum level of 180-fold at 12 h, which declined to 42-fold increase above control at 48 h after treatment with LPS. TCDD significantly increased the LPS-induced expression of IL-1β and led to a sustained elevated level of 690-fold at 48 h after co-treatment with TCDD and LPS. The expression of CCL1 was also significantly increased by TCDD after 6 h treatment and further increased to a maximum level of 28-fold above control after 48 h (Fig. 1B). LPS led to an early increase of CCL1 after 3 h and maximally increased CCL1 expression at 24 h after treatment with LPS. As in the case of IL-1β, CCL1 was synergistically induced by co-treatment with TCDD and LPS. The level of CCL1 mRNA was more than 4000-fold increased compared to control after 24 h and 48 h treatment with TCDD in presence of LPS. In contrast, TCDD reduced the LPS-induced expression of IL-12A as well as CCL4 (Fig. 1C and D). LPS induced the expression of IL-12A and CCL4 mRNA by 120-fold and 40-fold, respectively, after 12 h treatment. The LPS-induced mRNA level of IL-12A and CCL4 was significantly suppressed by 84% and 75%, respectively, in presence of TCDD at 12 h after treatment. TCDD by itself did not change the expression of IL-12A or CCL4 (Fig. 1C and D). Human myeloid cell lines like THP-1 or U937 are commonly used as an alternative cell model to replace human primary DCs to test the potential immunotoxic effects of various chemicals including allergens or skin sensitizers (Ashikaga et al. 2002; Python et al. 2007). THP-1 and U937 are monocytic cell lines, and after differentiation with GM-CSF and IL-4 display the morphologic, phenotypic, molecular, and functional characteristics of DCs generated from human donor-derived monocytes (Berges et al. 2005; Chanput et al. 2015). Differentiated U937-derived DCs exhibit de novo cell-surface expression of CD80, CD86, CD40, and CD1a, expression of DC specific cytokines and acquire characteristic stellate morphology, which is further enhanced by AhR activation (Vogel et al. 2008). In order to test if TCDD similarly affects MoDCs and U937-derived DCs, we next analyzed the effects of TCDD in resting and LPS-activated DCs derived from the U937 human cell line. The expression of IL-1β and CCL1 was significantly induced as early as three hours after treatment with 50 ng/ml LPS with a maximal increase of IL-1β by 750-fold at 24 h and 78-fold increase of CCL1 at 24 h (Fig. S1A and B). TCDD induced IL-1β at 6 h and continued to increase the expression of IL-1β by about 8-fold at 48 h after treatment with TCDD. CCL1 expression was induced by TCDD about 3.5-fold at 24 h and 48 h compared to control cells. Concomitant treatment of U937-derived DCs with LPS and TCDD led to a further increase of IL-1β up to 1600-fold at 24 h. TCDD also stimulated the LPS-induced expression of CCL1 to a maximal increase of 780-fold at 48 h. In contrast, the expression of CCL4 was significantly inhibited by TCDD in LPS-treated U937-derived DCs. LPS induced CCL4 very early at 3 h by about 800-fold, which was suppressed by about 70% when cells were co-treated with TCDD (Fig. S1D). In contrast to MoDCs, mRNA expression of IL-12A did not change significantly after treatment with TCDD and/or LPS (Fig. S1C).
Figure 1.
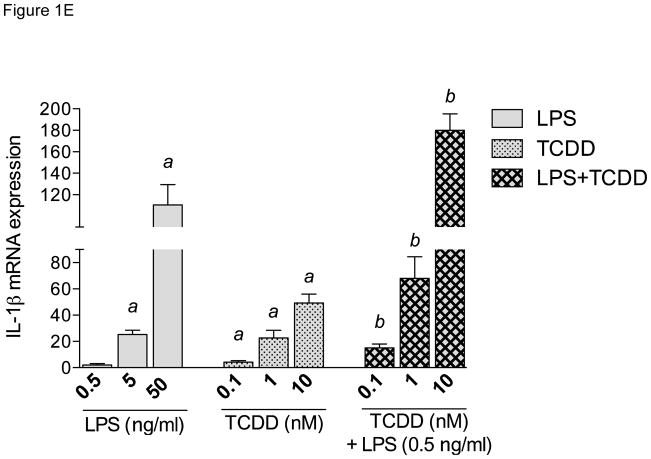
Synergistic and antagonistic effects of TCDD on time-dependent expression of cytokines in LPS-stimulated MoDCs. Human primary DCs were treated with LPS (50 ng/ml), TCDD (10 nM), or co-treated with TCDD plus LPS (dotted line) for 3 to 48h. The expression of A) IL-1β, B) CCL1, C) IL-12A, and D) CCL4 was analyzed using real-time PCR. E) Dose-dependent effects of LPS and TCDD on IL-1β mRNA expression in MoDC. Cells were treated with various concentrations of LPS (0.5–50 ng/ml), TCDD (0.1–10 nM) or co-treated with 0.5 ng/ml LPS and TCDD for 24 h. Results are presented as mean ± SEM and the y-axis represents mRNA expression level as fold increase above control. a significantly higher than control, p < 0.05; b significantly higher than TCDD- or LPS-treated cells, p < 0.05; c significantly lower than LPS-treated cells, p < 0.05.
Dose-dependent effect of TCDD on LPS-activated IL-1β expression
Dose-response studies showed that 5 and 50 ng/ml LPS and 0.1, 1.0 and 10 nM TCDD induced IL-1β expression in a dose-dependent manner in MoDC (Fig. 1E). No significant increase of IL-1β was observed at the low concentration of 0.5 ng/ml LPS. However, co-treatment of DCs with a low concentration of LPS (0.5 ng/ml) and TCDD (0.1 nM) caused a significant 10-fold increase of IL-1β expression (Fig. 1E).
Effect of different AhR ligands on cytokine and chemokine expression in TLR-activated MoDC
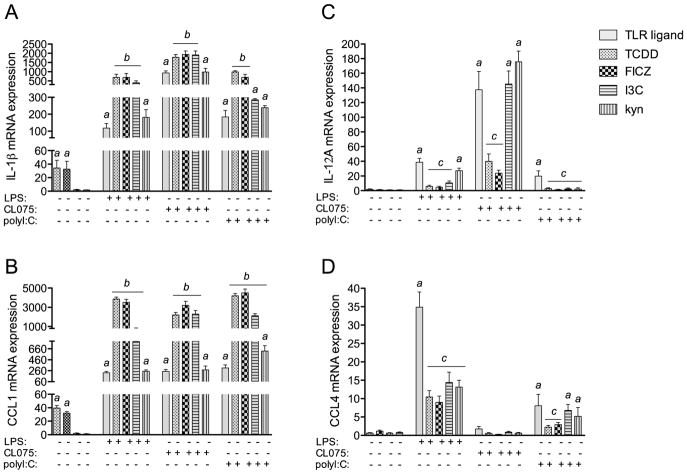
To test the hypothesis that activation of human DCs is selectively affected by AhR ligands in a ligand-specific manner, the effects of four defined AhR ligands were evaluated on cytokine expression in resting and TLR-activated MoDCs. The effects of the AhR ligands (TCDD, FICZ, I3C, and kyn) were assessed on the expression of IL-1β, IL-12A, CCL1, and CCL4 in MoDCs activated by three different TLR ligands (LPS, CL075, and polyI:C). The cytokines and chemokines above were selected since they are important for the innate function of DCs and their expression levels were found to be affected by AhR ligands in LPS-activated MoDCs using a PCR screening assay (data not shown). Furthermore, AhR activation has previously been reported to change the expression of CCL and CXCL chemokines in vitro and in vivo (Vogel et al. 2013; Lahoti et al. 2015; Vogel et al. 2016), but has not yet been evaluated in primary human MoDCs. MoDCs were treated for 24 h and the mRNA expression of cytokines was analyzed after treatment with TLR ligands in presence or absence of AhR ligands. The expression of IL-1β was induced by TCDD and FICZ about 30-fold above control (Fig. 2A). LPS treatment led to 100-fold increase of IL-1β, which was further enhanced by TCDD, FICZ and I3C, but not by kyn. The TLR8 ligand CL075 induced IL-1β expression by 1000-fold above control. TCDD, FICZ and I3C stimulated the CL075 induced expression of IL-1β up to 2000-fold. We observed a 200-fold increase of IL-1β after challenge with polyI:C which was further increased by TCDD and FICZ to a 1000-fold increase above control level. Treatment with I3C or kyn did not significantly increase the polyI:C-induced expression of IL-1β. CCL1 was induced by TCDD and FICZ by 40-fold and 35-fold above control, respectively (Fig. 2B). The TLR ligands LPS, CL075, and polyI:C induced CCL1 about 200-fold. TCDD, FICZ, and I3C synergistically induced TLR-activated CCL1 expression by more than 2000-fold. On the other hand, the expression of the cytokine IL-12A was induced 40-fold by LPS and significantly repressed by TCDD, FICZ and I3C (Fig. 2C). The TLR8 ligand CL075 increased the expression of IL-12A by 130-fold, which was repressed by TCDD and FICZ by about 75%. The TLR3 ligand polyI:C led to a 17-fold increase of IL-12A, which was repressed significantly by all AhR ligands tested. The chemokine CCL4 was 35-fold induced by LPS and was more than 50% repressed by simultaneous treatment with TCDD, FICZ, I3C, and kyn (Fig. 2D). CL075 did not induce the expression of CCL4 significantly. PolyI:C led to relatively small increase of CCL4 by 7-fold above control. TCDD and FICZ, but not I3C or kyn repressed the TLR3-mediated expression of CCL4.
Figure 2.
Effect of AhR ligands on cytokine and CC chemokine expression in MoDCs. The mRNA expression of A) IL-1β, B) CCL1, C) IL-12A and D) CCL4 by AhR ligands in TLR-activated MoDCs is shown. Cells were treated with 10 nM TCDD, 100 nM FICZ, 50 μM I3C, or 50 μM kyn in absence or presence of LPS (50 ng/ml), CL075 (5 μg/ml), or poly(I:C) (5 μg/ml) for 24h. Results from real-time PCR are presented as mean ± SEM and the y-axis represents mRNA expression level as fold increase above control. a significantly higher than non-activated DC control, p < 0.05; b significantly higher than TLR-activated MoDCs, p < 0.05; c significantly lower than TLR-activated MoDCs, p < 0.05
Expression of negative regulators of TLR and NF-κB responses in MoDCs
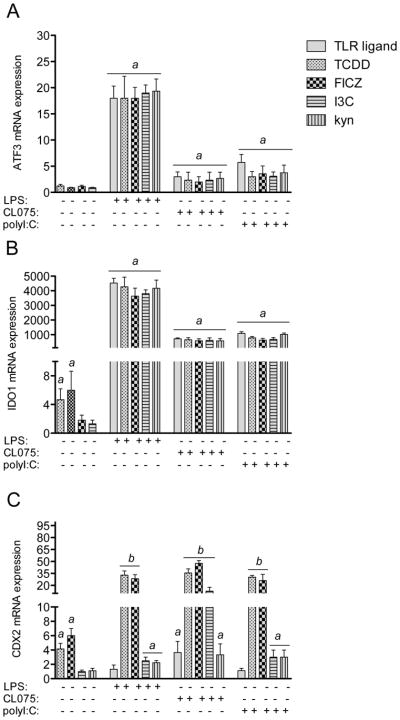
In order to identify factors which would be responsible for the repression of TLR responses by AhR ligands on IL-12A and CCL4 we focused on the expression of ATF3, IDO1 and CDX2. All three factors ATF3, IDO1 and CDX2 were described to modify and negatively regulate TLR- and NF-κB-regulated signaling and cytokine expression (Gilchrist et al. 2006; Shon et al. 2015; Ryu et al. 2008). We found a significant increase of ATF3 by LPS and a moderate induction of ATF3 by the TLR ligands CL075 and polyI:C (Fig. 3A). Activation of AhR by the various ligands tested did not change the expression of ATF3. The expression of IDO1 was significantly induced by TCDD and FICZ as initially reported in U937-derived DCs (Vogel et al. 2008), but not by I3C or kyn (Fig. 3B). The TLR-mediated increase of IDO1 was not significantly changed by AhR ligands. On the other hand, IDO2 expression was not induced by AhR ligands with the exception of TCDD (Fig. S2). As for IDO1 the TLR-induced expression of IDO2 was not significantly affected by the AhR ligands. On the other hand, expression of CDX2 was 4-fold and 5-fold upregulated by TCDD and FICZ, respectively (Fig. 3C). With the exception of a 4-fold increase by CL075, the TLR ligands LPS or polyI:C did not significantly increase the expression of CDX2. However, TCDD and FICZ significantly induced the expression of CDX2 by more than 30-fold above control in presence of the TLR ligands LPS, CL075 and polyI:C.
Figure 3.
Expression of A) ATF3, B) IDO1, and C) CDX2 in AhR- and TLR-activated MoDCs. Cells were treated with 10 nM TCDD, 100 nM FICZ, 50 μM I3C, or 50 μM kyn in absence or presence of LPS (50 ng/ml), CL075 (5 μg/ml), or poly(I:C) (5 μg/ml) for 24h. Results from real-time PCR are presented as mean ± SEM and the y-axis represents mRNA expression level as fold increase above control. a significantly higher than non-activated DC control, p < 0.05; b significantly higher than TLR- and AhR-activated MoDCs, p < 0.05
Small interfering RNA transfection of AhR, RelB and CDX2
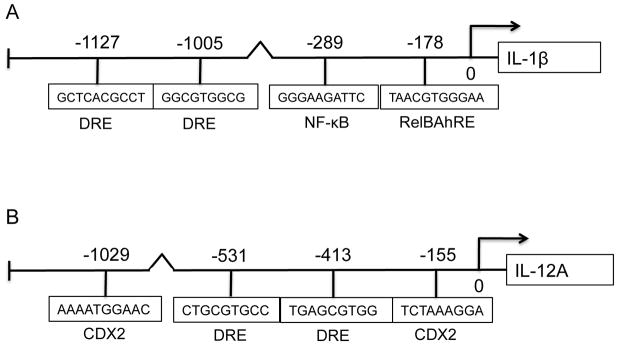
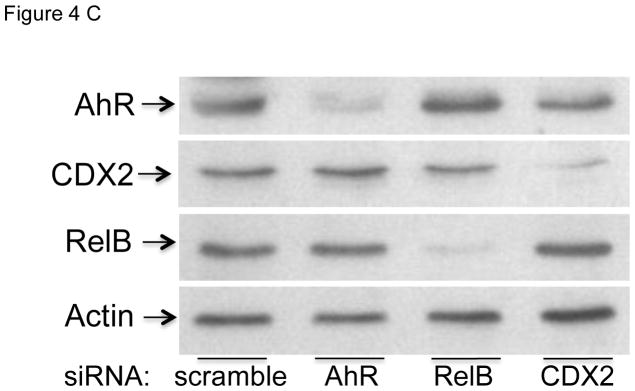
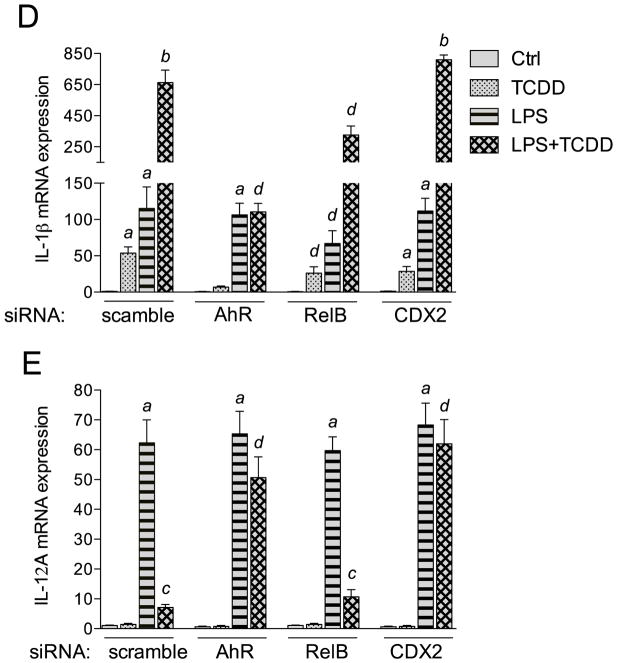
In the present study we found that activation of the AhR by TCDD and FICZ leads to a sustained induction of the pro-inflammatory cytokine IL-1β, but on the other hand TCDD and FICZ repressed the expression of IL-12A in MoDCs in a time-dependent manner (Fig. 1). Previously we showed that the AhR-mediated induction of IL-8 and CCL1 involved the interaction of AhR with RelB (Vogel et al. 2007a; Vogel et al. 2007b). Therefore we used the TFSEARCH program (Heinemeyer et al. 1998) to identify potential RelBAhRE binding sites on the IL-1β promoter. Besides two DRE core sequences and one NF-κB site we identified a potential RelBAhRE binding element at −178 bp upstream the start site of IL-1β (Fig. 4A) as described for the human IL-8 gene (Vogel et al. 2007a). Promoter analysis of the human IL-12A gene revealed two potential DRE core elements and two CDX2 binding sites within the first 1100 bp upstream of the start site of IL-12A (Fig. 4B). Transient transfection of MoDCs for 24 h with short interfering RNA (siRNA) targeting AhR, RelB, and CDX2 significantly reduced the protein levels of AhR, RelB, and CDX2 respectively (Fig. 4C). The level of AhR protein was also significantly reduced by siAhR knockdown in LPS-activated MoDCs (Fig. S3). Results from transfection studies with siRNA into MoDC to target AhR shows that TCDD activates IL-1β and represses IL-12A through an AhR-dependent mechanism (Fig. 4D and E). The AhR-dependent effect was confirmed via the inhibition of the TCDD-induced expression of IL-1β, CCL1 as well as CYP1A1 in presence of the AhR antagonist 3′-methoxy-4′nitroflavone (MNF) (Fig. S4). Transient transfection of MoDCs with siRNA targeting RelB reduced the TCDD-mediated induction of IL-1β mRNA expression significantly. Furthermore, the induction of IL-1β by LPS was suppressed in cells transfected with siRelB by about 40%. On the other hand, the suppressive effect of TCDD on LPS-induced mRNA expression of IL-12A required the expression of AhR, but was independent from RelB (Fig. 4E). However, gene silencing of the homeobox gene CDX2, which has been described as a critical regulator of intestinal homeostasis and inflammation (Coskun et al. 2011), eliminated the suppressive effect of TCDD on LPS-induced expression of IL-12A (Fig. 4E).
Figure 4.
Effect of gene silencing of AhR, RelB, and CDX2 on the expression of IL-1β and IL-12A. Schematic illustration of the promoter of the human A) IL-1β gene. Positions of the two DRE consensus elements, one putative NF-κB recognition site, and one RelBAhRE binding site are presented. B) The promoter of the human IL-12A gene containing two DRE consensus elements and two CDX2 binding sites. C) Western blot analysis of AhR, RelB, and CDX2 protein levels 24h post-transfection of cells with the indicated siRNAs. D) IL-1β and E) IL-12A mRNA expression analyses after treatment with TCDD and LPS or co-treatment with LPS plus TCDD for 24 h as analyzed by real-time PCR. Total RNA was prepared 48 h post-transfection with either a scrambled siRNA or a specific siRNA targeted against AhR, RelB, or CDX2. After 24 h transfected cells were treated with 10 nM TCDD or 50 ng/ml LPS for 24 h. Results are presented as mean ± SEM and the y-axis represents mRNA expression level as fold increase above control. a significantly higher compared to control, p < 0.05; b significantly higher than cells treated with TCDD or LPS alone, p < 0.05; c significantly lower than cells treated with LPS alone, p < 0.05; d significantly different compared to cells transfected with scrambled siRNA, p < 0.05
Modulation of surface markers in MoDCs after treatment with various AhR ligands
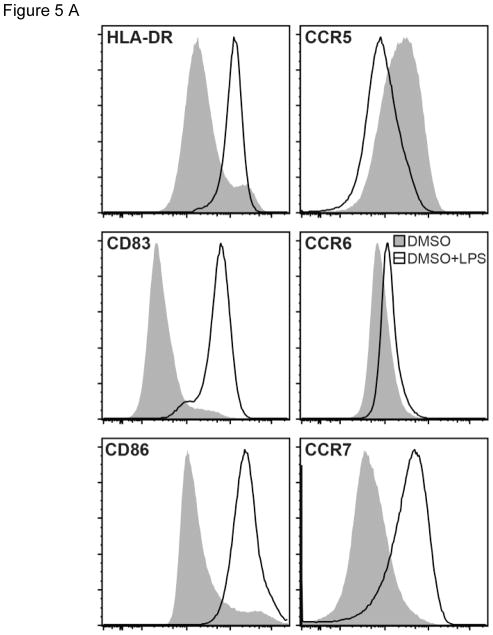
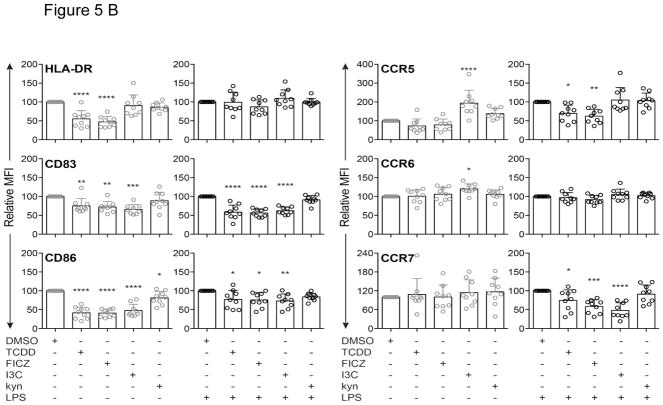
In order to examine the role of AhR activation on surface markers integral for DC-mediated T cell activation, the effects of TCDD, FICZ, I3C and kyn were investigated via flow cytometry. The AhR ligands TCDD and FICZ, but not I3C or kyn decreased expression of HLA-DR in unstimulated MoDC derived from 9 different healthy donors (Fig. 5A and B). TCDD, FICZ, and I3C suppressed CD83 expression in resting and LPS-activated MoDC. CD86 expression was decreased by TCDD, FICZ, and I3C, but not by kyn in non-activated DC. In contrast, I3C increased the level of CCR5 in non-activated MoDC, whereas the AhR ligands TCDD, FICZ or kyn did not affect expression of CCR5 in MoDC. CCR6 expression was not affected by AhR ligands, however FICZ, I3C and for some donors TCDD lowered the expression of CCR7 in LPS-activated MoDC (Fig. 5A and B).
Figure 5.
Modulation of MoDC surface markers by AhR ligands in the presence or absence of LPS. A) Representative overlaid histograms of surface marker expression by MoDCs treated with LPS (50 ng/ml) or DMSO control. B) Comparison of surface marker expression by MoDCs treated with TCDD (10 nM), FICZ (100 nM), I3C (50 μM), or kyn (50 μM) in absence or presence of LPS for 24 h. The surface markers HLA-DR, CD83, CD86, CCR5, CCR6, and CCR7 were analyzed by flow cytometry and data are presented as relative mean fluorescence intensity (MFI) with DMSO control set as 100. Data are presented as mean ± SD with each data point representing an individual donor (n = 9). The significance of difference between DMSO control and each treatment was determined by one-way ANOVA, with Dunnett’s multiple comparison test used to perform pairwise analysis. Symbol: * p < 0.05; ** p<0.01;*** p<0.001; **** p<0.0001.
Effects of TLR agonists on the expression of AhR in MoDC
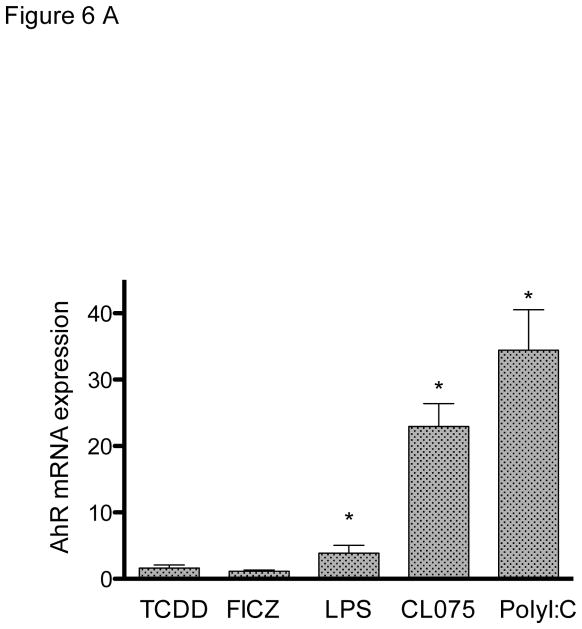
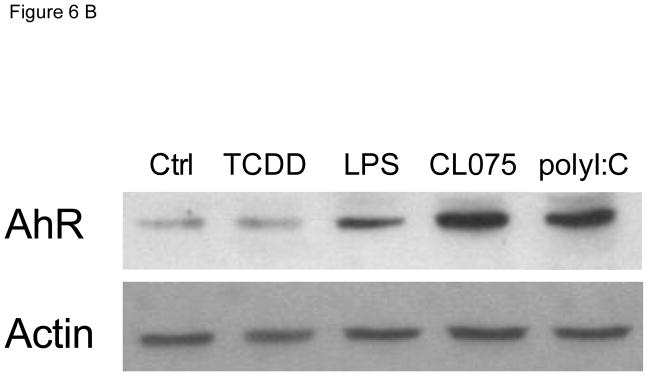
In order to test the effect of TLR activation on the expression of AhR, MoDCs were treated with three different TLR ligands. As shown previously (Vogel et al. 2014), LPS induced the mRNA expression of AhR in MoDC by about 4-fold above control level (Fig. 6A). However, activation of MoDCs with TLR ligands CL075 and poly(I:C) led to an even greater induction of AhR by 22-fold and 34-fold, respectively. The TLR-mediated induction of AhR mRNA was confirmed at the protein level via Western blotting using an AhR-specific antibody (Fig. 6B).
Figure 6.
Effect of TLR ligands on the expression of AhR in MoDCs. A) MoDCs were treated with LPS (50 ng/ml), CL075 (5 μg/ml), or poly(I:C) (5 μg/ml) for 24h. The mRNA expression of AhR was determined using real-time PCR and presented as mean ± SD and the y-axis represents mRNA expression level as fold increase above control. *significantly higher than control, p < 0.05. B) Western blot analysis of AhR protein levels 24h after treatment with TLR ligands. Equivalent amounts of whole cell protein (25 μg of protein) were loaded in each lane on 10% SDS-polyacrylamide gels and analyzed by immunoblotting using an AhR-specific antibody. A representative gel image is shown for Western blot.
Effect of AHR ligands on prototypical AhR-responsive genes in TLR-activated MoDCs
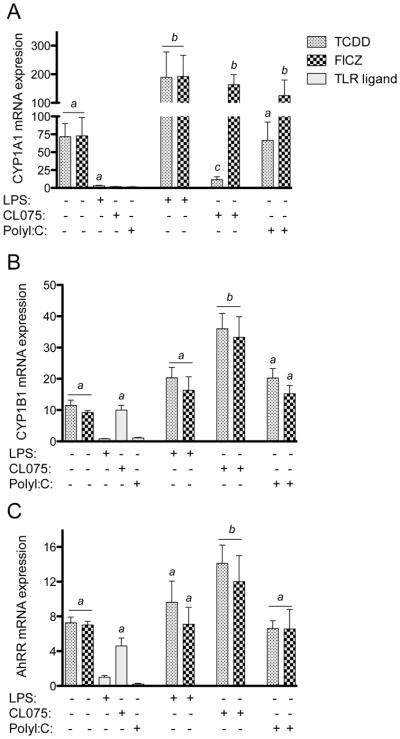
Here we analyzed the effect of structurally different AhR ligands on the expression of AhR-DRE regulated genes, such as CYP1A1, CYP1B1 and AhRR in MoDCs. Cells were treated with 10 nM TCDD, 100 nM FICZ, 50 μM I3C and 50 μM kyn for 24h. The effect of the AhR ligands was tested in MoDCs, activated with 5 μg/ml poly(I:C) as a TLR3 agonist (TRIF-dependent pathway), 50 ng/ml LPS as a TLR4 agonist or with 5 μg/ml of the synthetic thiazoloquinoline immunostimulatory compound CL075 as a TLR8 agonist (MyD88-dependent response). Cells were co-treated with AhR ligands and TLR agonists for 24h. Both, TCDD and FICZ significantly increased CYP1A1 expression by about 60-fold compared to control (Fig. 7A). Besides LPS, the TLR agonists poly(I:C) and CL075 had no significant effect on CYP1A1 expression in the absence of AhR activation. TCDD- and FICZ-induced CYP1A1 expression was further enhanced in LPS-activated MoDCs. In MoDCs stimulated with either CL075 or poly(I:C), only FICZ increased CYP1A1 expression levels. In contrast, the TCDD-mediated induction of CYP1A1 was suppressed by over 80% in CL075-activated MoDCs (Fig. 7A). The expression of CYP1B1 and AhRR was induced by TCDD and FICZ 12- and 5-fold, respectively (Figs. 7B and C). Interestingly, the expression of CYP1B1 and AhRR was increased 10-fold and 5-fold, respectively, by the TLR ligand CL075 in the absence of AhR activation. LPS or poly(I:C) treatment did not significantly affect CYP1B1 or AhRR mRNA expression. Only CL075 enhanced the TCDD- and FICZ-induced expression of CYP1B1 (Fig. 7B) and AhRR (Fig. 7C), whereas LPS and poly(I:C) did not change the TCDD- or FICZ-induced expression of CYP1B1 or AhRR. In contrast to TCDD and FICZ, Kyn and I3C had no significant effect on CYP1A1, CYP1B1 or AhRR expression in MoDCs in absence or presence of LPS (Fig. S5).
Figure 7.
Effect of AhR ligands on the expression of A) CYP1A1, B) CYP1B1, and C) AhRR in MoDCs activated with TLR ligands. MoDCs were treated with TCDD (10 nM) or FICZ (100 nM) in absence or presence of LPS (50 ng/ml), CL075 (5 μg/ml), or poly(I:C) (5 μg/ml). Results from real-time PCR are presented as mean ± SEM and the y-axis represents mRNA expression level as fold increase above control. a significantly higher than control, p < 0.05; b significantly higher than cells treated with TCDD or FICZ alone, p < 0.05; c significantly lower than cells treated with TCDD or FICZ alone, p < 0.05.
Discussion
The critical role of TLR responses in activation of the NF-κB signaling pathway for transcriptional regulation of cytokines in DCs is well established (Vallabhapurapu and Karin 2009). Members of the TLR family function as sensors for a wide variety of microbial components to elicit immune responses (Kawai and Akira 2007). On the other hand, the cytosolic ligand-activated receptor and transcription factor AhR acts as a sensor for environmental pollutants such as dioxins and dioxin-like compounds (Denison et al. 2011). More recently endogenous and natural compounds have been identified as potential AhR agonists (Barouki et al. 2012). Moreover, the AhR has been shown to recognize bacterial pigmented virulence factors suggesting a role as an intracellular pattern recognition receptor (Moura-Alves et al. 2014). The results of the current study provide insight into the critical role of the AhR as a modifier of TLR-triggered activation of the NF-κB pathway and regulation of cytokines in MoDCs. The current study shows that depending on the specific cytokine, concomitant treatment with AhR ligands may lead to a synergistic or antagonistic effect of the TLR-triggered response in MoDCs. As shown for IL-1β and IL-12A, activation of AhR may cause hyperexpression or inhibition, respectively, of TLR-mediated induction of the cytokines. The synergistic increase of TLR-triggered induction of IL-1β involves AhR as well as NF-κB member RelB, whereas the AhR-mediated inhibition of IL-12A in TLR activated MoDCs was not affected by gene silencing of RelB. The data confirm previous findings indicating the requirement of AhR and RelB for the induction of chemokines such as IL-8 and CCL1 by TCDD (Vogel et al. 2007a and 2007b). Furthermore, RelB seems to play a critical role also in LPS- and TLR4-mediated induction of IL-1β in MoDCs. The results confirm data from a recent study showing the positive regulation of inflammatory marker genes including IL-1β by RelB in TLR4-as well as AhR-activated DCs (Shih et al. 2012; Memari et al. 2015). The results received from AhR-mediated induction of IL-1β support the interaction of the AhR and NF-κB signaling pathways which has been described previously for other genes in different cell models (DiNatale et al. 2010; Luecke et al. 2010; Vogel and Matsumura 2009; Tian 2009). It is interesting to note, that the production of IL-1β in DCs seems to be important to retain the expression of IL-22 and AhR in human natural killer cells (NK) in secondary lymphoid tissues (Hughes et al. 2010). With regards to the repression of TLR-mediated responses by AhR activation we investigated the effect of AhR on the expression of negative regulators of TLR responses and NF-κB signaling including ATF3, IDO1 and CDX2. Importantly, we identified the intestinal differentiation factor and immune regulatory gene CDX2 as a new target gene of AhR. DNA binding of CDX2 to a specific proximal site of the Pregnane X Receptor (PXR) has been shown to be required for the reduction of gastrointestinal inflammation (Dou et al. 2012). Treatment of MoDCs with TCDD and FICZ led to an increased expression of CDX2. Interestingly, the TLR4 and TLR3 ligands did not elevate the expression of CDX2, however the expression of CDX2 was hyperactivated when cells were co-treated with the AhR ligands TCDD and FICZ. The reduced expression of CDX2 in MoDCs by gene silencing significantly eliminated the repressive effect of TCDD on LPS-induced expression of IL-12A. The results show that the repression of TLR-induced expression of the IL-12A gene, which contains CDX2 binding elements on its promoter region, involves the induction of the transcription factor CDX2. Therefore, the role of CDX2 in immune regulatory processes mediated through AhR activation should be further explored in future studies, especially in DCs due to their critical importance in regulating both innate and adaptive immune responses. Results from the dose-response study on the expression of IL-1β indicate the high sensitivity of the AhR and NF-κB cross-talk leading to significant effects of very low concentrations of LPS and TCDD only under co-treatment conditions in MoDCs. Similar results were obtained from a dose-response study analyzing the expression of IL-8 after treatment with TCDD and LPS in MoDCs (Fig. S6). The importance of the interaction of AhR and NF-κB mediating the induction of IL-8 has been described previously in U937-derived DCs (Vogel et al. 2007a). This finding could suggest that low LPS concentrations are not sufficient to induce the expression of the above cytokines, but might prime DCs for an enhanced sensitivity to the activation by AhR ligands. For instance, a low-dose of AhR ligand (TCDD) can significantly alter the classical inflammatory response or allergic responses elicited by a low dose of pathogen-associated molecules such as LPS. A critical role of the ligand activated AhR has been demonstrated for the expression of IL-22 (Veldhoen et al. 2008; Rohlman et al. 2012) as well as the development and function of innate lymphoid cells in the lamina propria (Li et al. 2011; Lowe et al. 2014). The expression of IL-22 has been shown to be induced by LPS (Dumoutier et al. 2011) and recently we demonstrated that the expression of IL-22 was markedly upregulated when LPS-stimulated cells were treated with TCDD (Vogel et al. 2013). Therefore, it is possible that the crosstalk between AhR and NF-κB is not only leading to a dysregulation of cytokine expression, but is also essential for the effective and adequate expression of cytokines such as IL-22 for instance, which is important to maintain the function of intestinal immune cells and gut immunity (Lawrence and Sherr 2012).
Members of the AhR gene battery such as CYP1A1, CYP1B1, and AhRR, were significantly induced after treatment of non-activated MoDCs with AhR ligands TCDD and FICZ, but not I3C or kyn, in the absence of TLR agonists. TCDD and FICZ were also more effective than I3C or kyn in changing the expression of the cytokines or CYP1A1 in TLR-activated cells. On the other hand, all AhR ligands tested including I3C and kyn repressed the expression of TLR3-activated IL-12A and TLR4-activated CCL4. However, only TCDD and FICZ, but not I3C or kyn suppressed the TLR8-mediated induction of IL-12A and TLR3-induced expression of CCL4. These results show clearly that TLR-activated DCs are more sensitive to the effects of AhR ligands than non-activated DCs. One contributing factor to the increased sensitivity could be the significantly elevated levels of AhR expression in MoDCs induced by TLR activation. This is in line with our recent finding, showing the increased expression of AhR associated with induction of CYP1A1 through activation of TLR4 and NF-κB by LPS (Vogel et al. 2014). Furthermore, the specific effect of an AhR ligand may depend on the particular TLR agonist activating the DC. In this regard, it is interesting to note that TCDD and FICZ led to opposite effects on the expression of CYP1A1 in TLR8-, but not in TLR4-activated MoDCs suggesting a notable AhR ligand-specific effect. Treatment with the TLR ligand CL075 induced the expression of CYP1B1 and AhRR. However, the TLR-mediated increased expression of AhR does not fully explain this effect, since polyI:C or LPS did not affect the expression of CYP1B1 or AhRR, but increased the expression of AhR. It is possible that TLR8-triggered responses by CL075 interact with critical factors of the AhR pathway, which is not the case with agonists of other TLR signaling pathways. Interestingly, the expression of CYP1A1 is silenced in TLR2-deficient mice, even when under exposure to AhR ligands (Do et al. 2012). Therefore, it is possible that the interaction of TLRs with AhR signaling depends on the particular TLR ligand or TLR signaling pathway. So far the reason for the TLR-specific effects on AhR signaling are unknown and future studies are warranted to identify critical factors involved in TLR-specific effects on AhR-mediated gene expression. In addition, we have to consider that certain AhR ligands may suppress TLR-mediated responses through inhibition of NF-κB activation as shown for I3C (Takada et al. 2005). Significant differences also exist between TCDD and FICZ in MoDCs treated with the AhR antagonist MNF. MNF efficiently inhibited the effect of TCDD, but MNF could not block FICZ-induced gene expression of CYP1A1, IL-1β and CCL1 indicating the selectivity of ligand binding of FICZ and TCDD, which is supported by findings using AhR mutants within the ligand-binding domain of the AhR (Soshilov and Denison 2014). The inability of MNF to antagonize FICZ-induced CYP1A1 expression is not limited to MoDCs and has been observed in other cell lines including U937-derived DCs and MCF-7 breast tumor cells (unpublished results).
The expression of critical DC surface markers was monitored via flow cytometry in MoDCs. The results show that the level of the surface marker HLA-DR was suppressed in MoDCs by AhR ligands TCDD and FICZ, whereas CD83 and CD86 expression was reduced in the presence of TCDD, FICZ, and I3C, but not by kyn in both unstimulated and LPS-activated MoDCs. In contrast, expression of CCR5 was increased in MoDC following treatment with I3C and kyn, but not after exposure to TCDD or FICZ, whereas the level of CCR7 was decreased only by FICZ and I3C in LPS-activated MoDCs. The data confirm that AhR ligands may exert ligand-specific effects on certain markers of DCs, which also depends on the maturation and activation status of DCs. The repressed surface marker expression (CD83, CD86, and CCR7 in particular) by TCDD, FICZ, and I3C in LPS-treated MoDCs is consistent with decreased levels of CCL4 and IL-12A mRNA, but opposite to the increase of IL-1β and CCL1 mRNA levels. These data suggest, that DCs exposed to these AhR ligands might (1) stay in the peripheral and are less likely to migrate to draining lymph nodes (lower CCR7); (2) interact poorly with naive T cells (lower CD83, CD86, CCL4, and IL-12A); (3) attract and activate more cells expressing CCR8, including NK cells, monocytes, macrophages, and immature B cells (higher CCL1 and IL-1β). Overall, with the exception of CCR5, the suppressive effects of AhR ligands on the surface markers tested in MoDCs would predict a decreased antigen-presenting capacity in these cells. In summary, AhR ligands synergize or antagonize the action of TLR agonists depending on the specific cytokine and agonist of TLR. The current data illustrate that the AhR provides additional activation (hyperexpression) or inhibition of TLR-/NF-κB-mediated gene transcription when the cells receive further stimuli (e.g. TCDD or FICZ), transforming the TLR/NF-κB signal into an activator or inhibitor of gene transcription in the presence of an activated AhR. Therefore, the NF-κB/AhR crosstalk presents a critical component modulating the fine-tuning of cytokines, which is likely critical not only for the proper function of DCs in response to microbial infections, but also for appropriate control of inflammatory and immune responses to prevent immune dysregulation and/or immune-mediated disease.
Supplementary Material
Figure S1. Time-dependent expression of cytokines in U937 derived DCs after treatment with TCDD and LPS. U937 DCs were treated with 10 nM TCDD, 100 ng/ml LPS or co-treated with LPS and TCDD. Cells were treated for various time points from 3 h to 48 h and the expression of IL-1β, CCL1, IL-12A, and CCL4 was analyzed. Results from real-time PCR are presented as mean ± SD and the y-axis represents mRNA expression level as fold increase above control. a Significantly higher than control p < 0.05, b significantly higher than LPS-treated U937 derived DC p < 0.05, c significantly lower than LPS-treated U937 derived DC p < 0.05.
Figure S2. Expression of IDO2 in TLR- and AhR-activated MoDCs. Cells were treated with 10 nM TCDD, 100 nM FICZ, 50 μM I3C, or 50 μM Kyn in absence or presence of LPS (50 ng/ml), CL075 (5 μg/ml), or poly(I:C) (5 μg/ml) for 24h. Results from real-time PCR are presented as mean ± SEM and the y-axis represents mRNA expression level as fold increase above control. * significantly different from MoDC control, p < 0.05
Figure S3. Effect of gene silencing of AhR in LPS-treated MoDCs. Western blot analysis of AhR protein levels 24h post-transfection of cells with either a scrambled siRNA or a specific siRNA targeted against AhR. MoDCs were treated with 50 ng/ml LPS for 24 h. Equivalent amounts of whole cell protein (25 μg of protein) were loaded in each lane on 10% SDS-polyacrylamide gels and analyzed by immunoblotting using an AhR- or Actin-specific antibody.
Figure S4. Selective effect of AhR antagonist MNF on TCDD- and FICZ-induced expression of CYP1A1 and cytokines IL-1β and CCL1 in MoDCs. MoDCs were treated with AhR ligands TCDD (10 nM) or FICZ (100 nM) in absence or presence of the AhR antagonist MNF (5 μM) for 24h. Expression of CYP1A1 and cytokines IL-1β and CCL1 was analyzed by real-time PCR. Results are presented as mean ± SEM and the y-axis represents mRNA expression level as fold increase above control. a significantly higher than control, p < 0.005; c significantly lower than TCDD-treated cells in absence of MNF, p < 0.005
Figure S5. Effect of AhR ligands on the expression of CYP1A1, CYP1B1, and AhRR in MoDCs. MoDCs were treated with TCDD (10 nM), FICZ (100 nM), I3C (50 μM), or kyn (50 μM) in absence or presence of LPS (50 ng/ml) for 24 h. Results from real-time PCR are presented as mean ± SEM and the y-axis represents mRNA expression level as fold increase above control. a significantly higher than control, p < 0.05; b significantly higher than cells treated with TCDD or FICZ alone, p < 0.05
Fig. S6. Dose-dependent effects of LPS and TCDD on IL-8 mRNA expression in MoDCs. Cells were treated with various concentrations of LPS (0.5–50 ng/ml), TCDD (0.1–10 nM) or co-treated with 0.5 ng/ml LPS and TCDD for 24 h. Results are presented as mean ± SEM and the y-axis represents mRNA expression level as fold increase above control. a significantly higher than control, p < 0.05; b significantly higher than TCDD- or LPS-treated cells, p < 0.05
Acknowledgments
We thank Suzette Smiley-Jewell for critical reading of the manuscript.
Funding
This publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health by Grant Number R01 ES019898 (to C.V.) and NIEHS/NIGMS Grant Number R01 ES013784 (to D.M.S).
References
- Ashikaga T, Hoya M, Itagaki H, Katsumura Y, Aiba S. Evaluation of CD86 expression and MHC class II molecule internalization in THP-1 human monocyte cells as predictive endpoints for contact sensitizers. Toxicol In Vitro. 2002;6:711–716. doi: 10.1016/s0887-2333(02)00060-7. [DOI] [PubMed] [Google Scholar]
- Bankoti J, Rase B, Simones T, Shepherd DM. Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol Appl Pharmacol. 2010;246:18–28. doi: 10.1016/j.taap.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouki R, Aggerbeck M, Aggerbeck L, Coumoul X. The aryl hydrocarbon receptor system. Drug Metabol Drug Interact. 2012;27:3–8. doi: 10.1515/dmdi-2011-0035. [DOI] [PubMed] [Google Scholar]
- Berges C, Naujokat C, Tinapp S, Wieczorek H, Höh A, Sadeghi M, Opelz G, Daniel V. A cell line model for the differentiation of human dendritic cells. Biochem Biophys Res Commun. 2005;333:896–907. doi: 10.1016/j.bbrc.2005.05.171. [DOI] [PubMed] [Google Scholar]
- Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- Chang WL, Baumgarth N, Yu D, Barry PA. Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality. J Virol. 2004;78:8720–8731. doi: 10.1128/JVI.78.16.8720-8731.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanput W, Peters V, Wichers H. THP-1 and U937 cells. In: Verhoeckx K, et al., editors. The impact of food bioactives on gut health. Springer International; 2015. pp. 147–159. [PubMed] [Google Scholar]
- Coskun M, Troelsen JT, Nielsen OH. The role of CDX2 in intestinal homeostasis and inflammation. Biochim Biophys Acta. 2011;1812:283–289. doi: 10.1016/j.bbadis.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124:1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNatale BC, Schroeder JC, Francey LJ, Kusnadi A, Perdew GH. Mechanistic insights into the events that lead to synergistic induction of interleukin 6 transcription upon activation of the aryl hydrocarbon receptor and inflammatory signaling. J Biol Chem. 2010;32:24388–24397. doi: 10.1074/jbc.M110.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KN, Fink LN, Jensen TE, Gautier L, Parlesak A. TLR2 controls intestinal carcinogen detoxication by CYP1A1. PLoS One. 2012;7:e32309. doi: 10.1371/journal.pone.0032309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou W, Mukherjee S, Li H, Venkatesh M, Wang H, Kortagere S, Peleg A, Chilimuri SS, Wang ZT, Feng Y, Fearon ER, Mani S. Alleviation of gut inflammation by Cdx2/Pxr pathway in a mouse model of chemical colitis. PLoS One. 2012;7:e36075. doi: 10.1371/journal.pone.0036075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoutier L, de Heusch M, Orabona C, Satoh-Takayama N, Eberl G, Sirard JC, Di Santo JP, Renauld JC. IL-22 is produced by γC-independent CD25+ CCR6+ innate murine spleen cells upon inflammatory stimuli and contributes to LPS-induced lethality. Eur J Immunol. 2011;41:1075–1085. doi: 10.1002/eji.201040878. [DOI] [PubMed] [Google Scholar]
- Gilchrist M, Thorsson V, Li B, Rust AG, Korb M, Roach JC, Kennedy K, Hai T, Bolouri H, Aderem A. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature. 2006;441:173–178. doi: 10.1038/nature04768. [DOI] [PubMed] [Google Scholar]
- Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel AE, Kel OV, Ignatieva EV, Ananko EA, Podkolodnaya OA, Kolpakov FA, Podkolodny NL, Kolchanov NA. Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res. 1998;26:362–367. doi: 10.1093/nar/26.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, Mao C, Giovenzana C, Nuovo G, Wei L, Zhang X, Gavrilin MA, Wewers MD, Caligiuri MA. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;36:803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G-B, Moore AJ, Head JL, Neumiller JJ, Lawrence BP. Aryl hydrocarbon receptor activation reduces dendritic cell function during influenza virus infection. Toxicol Sci. 2010;116:514–522. doi: 10.1093/toxsci/kfq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kerkvliet NI. Recent advances in understanding the mechanisms of TCDD immunotoxicity. Int Immunopharmacol. 2002;2:277–291. doi: 10.1016/s1567-5769(01)00179-5. [DOI] [PubMed] [Google Scholar]
- Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- Lahoti TS, Boyer JA, Kusnadi A, Muku GE, Murray IA, Perdew GH. Aryl Hydrocarbon Receptor Activation Synergistically Induces Lipopolysaccharide-Mediated Expression of Proinflammatory Chemokine (c-c motif) Ligand 20. Toxicol Sci. 2015;148:229–240. doi: 10.1093/toxsci/kfv178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence BP, Sherr DH. You AhR what you eat? Nat Immunol. 2012;13:117–119. doi: 10.1038/ni.2213. [DOI] [PubMed] [Google Scholar]
- Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, Colonna M. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Lowe MM, Mold JE, Kanwar B, Huang Y, Louie A, Pollastri MP, Wang C, Patel G, Franks DG, Schlezinger J, Sherr DH, Silverstone AE, Hahn ME, McCune JM. Identification of cinnabarinic acid as a novel endogenous aryl hydrocarbon receptor ligand that drives IL-22 production. PLoS One. 2014;9:e87877. doi: 10.1371/journal.pone.0087877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecke S, Wincent E, Backlund M, Rannug U, Rannug A. Cytochrome P450 1A1 gene regulation by UVB involves crosstalk between the aryl hydrocarbon receptor and nuclear factor kappaB. Chem Biol Interact. 2010;3:466–473. doi: 10.1016/j.cbi.2010.01.038. [DOI] [PubMed] [Google Scholar]
- Marshall NB, Kerkvliet NI. Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann N Y Acad Sci. 2010;1183:25–37. doi: 10.1111/j.1749-6632.2009.05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MU, Wesche H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim Biophys Acta. 2002;1592:265–280. doi: 10.1016/s0167-4889(02)00320-8. [DOI] [PubMed] [Google Scholar]
- Memari B, Bouttier M, Dimitrov V, Ouellette M, Behr MA, Fritz JH, White JH. Engagement of the Aryl Hydrocarbon Receptor in Mycobacterium tuberculosis-Infected Macrophages Has Pleiotropic Effects on Innate Immune Signaling. J Immunol. 2015;159:4479–4491. doi: 10.4049/jimmunol.1501141. [DOI] [PubMed] [Google Scholar]
- Mitchell KA, Lawrence BP. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) renders influenza virus-specific CD8+ T cells hyporesponsive to antigen. Toxicol Sci. 2003;74:74–84. doi: 10.1093/toxsci/kfg110. [DOI] [PubMed] [Google Scholar]
- Moura-Alves P, Faé K, Houthuys E, Dorhoi A, Kreuchwig A, Furkert J, Barison N, Diehl A, Munder A, Constant P, Skrahina T, Guhlich-Bornhof U, Klemm M, Koehler AB, Bandermann S, Goosmann C, Mollenkopf HJ, Hurwitz R, Brinkmann V, Fillatreau S, Daffe M, Tümmler B, Kolbe M, Oschkinat H, Krause G, Kaufmann SH. AhR sensing of bacterial pigments regulates antibacterial defence. Nature. 2014;512:387–392. doi: 10.1038/nature13684. [DOI] [PubMed] [Google Scholar]
- Pollard KM. Environment, autoantibodies, and autoimmunity. Front Immunol. 2015;6:60. doi: 10.3389/fimmu.2015.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Python F, Goebel C, Aeby P. Assessment of the U937 cell line for the detection of contact allergens. Toxicol Appl Pharmacol. 2007;220:113–124. doi: 10.1016/j.taap.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Rohlman D, Pham D, Yu Z, Steppan LB, Kerkvliet NI. Aryl Hydrocarbon Receptor-Mediated Perturbations in Gene Expression during Early Stages of CD4(+) T-cell Differentiation. Front Immunol. 2012;3:223. doi: 10.3389/fimmu.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CE, Leid M, Kerkvliet NI. 2,3,7,8-Tetrachlorodibenzo-p-dioxin suppresses tumor necrosis factor-alpha and anti-CD40-induced activation of NF-kappaB/Rel in dendritic cells: p50 homodimer activation is not affected. Mol Pharmacol. 2002;62:722–728. doi: 10.1124/mol.62.3.722. [DOI] [PubMed] [Google Scholar]
- Ryu J-H, Kim S-H, Lee H-Y, Bai JY, Nam Y-D, Bae J-W, Lee DG, Shin SC, Ha E-M, Lee W-J. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- Selmi C, Leung PSC, Sherr DH, Diaz M, Nyland JF, Monestier M, Rose NR, Gershwin ME. Mechanisms of environmental influence on human autoimmunity: a National Institute of Environmental Health Sciences expert panel workshop. J Autoimmun. 2012;39:272–284. doi: 10.1016/j.jaut.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Sharfe N, Merico D, Karanxha A, Macdonald C, Dadi H, Ngan B, Herbrick J-A, Roifman CM. The effects of RelB deficiency on lymphocyte development and function. J Autoimmun. 2015;65:90–100. doi: 10.1016/j.jaut.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Shih VF-S, Davis-Turak J, Macal M, Huang JQ, Ponomarenko J, Kearns JD, Yu T, Fagerlund R, Asagiri M, Zuniga EI, Hoffmann A. Control of RelB during dendritic cell activation integrates canonical and noncanonical NF-kappaB pathways. Nat Immunol. 2012;13:1162–1170. doi: 10.1038/ni.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shon W-J, Lee Y-K, Shin JH, Choi EY, Shin D-M. Severity of DSS-induced colitis is reduced in Ido1-deficient mice with down-regulation of TLR-MyD88-NF-kB transcriptional networks. Sci Rep. 2015;5:17305. doi: 10.1038/srep17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soshilov AA, Denison MS. Ligand promiscuity of aryl hydrocarbon receptor agonists and antagonists revealed by site-directed mutagenesis. Mol Cell Biol. 2014;34:1707–1719. doi: 10.1128/MCB.01183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Andreeff M, Aggarwal BB. Indole-3-carbinol suppresses NF-kappaB and IkappaBalpha kinase activation, causing inhibition of expression of NF-kappaB-regulated antiapoptotic and metastatic gene products and enhancement of apoptosis in myeloid and leukemia cells. Blood. 2005;106:641–649. doi: 10.1182/blood-2004-12-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y. Ah receptor and NF-kappaB interplay on the stage of epigenome. Biochem Pharmacol. 2009;77:670–680. doi: 10.1016/j.bcp.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld J-C, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol. 2007a;21:2941–2955. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Matsumura F. Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem Biophys Res Commun. 2007b;36:722–726. doi: 10.1016/j.bbrc.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Matsumura F. A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kappaB family. Biochem Pharmacol. 2009;77:734–745. doi: 10.1016/j.bcp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Wu D, Goth SR, Baek J, Lollies A, Domhardt R, Grindel A, Pessah IN. Aryl hydrocarbon receptor signaling regulates NF-kappaB RelB activation during dendritic-cell differentiation. Immunol Cell Biol. 2013;91:568–575. doi: 10.1038/icb.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Khan EM, Leung PSC, Gershwin ME, Chang WLW, Wu D, Haarmann-Stemmann T, Hoffmann A, Denison MS. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-kappaB. J Biol Chem. 2014;289:1866–1875. doi: 10.1074/jbc.M113.505578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Chang WL, Kado S, McCulloh K, Vogel H, Wu D, Haarmann-Stemmann T, Yang G, Leung PS, Matsumura F, Gershwin ME. Transgenic Overexpression of Aryl Hydrocarbon Receptor Repressor (AhRR) and AhR-Mediated Induction of CYP1A1, Cytokines, and Acute Toxicity. Environ Health Perspect. 2016;124:1071–1083. doi: 10.1289/ehp.1510194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino DR, Wilde SP, Johnson N, Juettemann T, Flicek PR. The ensembl regulatory build. Genome Biol. 2015;16:56. doi: 10.1186/s13059-015-0621-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Time-dependent expression of cytokines in U937 derived DCs after treatment with TCDD and LPS. U937 DCs were treated with 10 nM TCDD, 100 ng/ml LPS or co-treated with LPS and TCDD. Cells were treated for various time points from 3 h to 48 h and the expression of IL-1β, CCL1, IL-12A, and CCL4 was analyzed. Results from real-time PCR are presented as mean ± SD and the y-axis represents mRNA expression level as fold increase above control. a Significantly higher than control p < 0.05, b significantly higher than LPS-treated U937 derived DC p < 0.05, c significantly lower than LPS-treated U937 derived DC p < 0.05.
Figure S2. Expression of IDO2 in TLR- and AhR-activated MoDCs. Cells were treated with 10 nM TCDD, 100 nM FICZ, 50 μM I3C, or 50 μM Kyn in absence or presence of LPS (50 ng/ml), CL075 (5 μg/ml), or poly(I:C) (5 μg/ml) for 24h. Results from real-time PCR are presented as mean ± SEM and the y-axis represents mRNA expression level as fold increase above control. * significantly different from MoDC control, p < 0.05
Figure S3. Effect of gene silencing of AhR in LPS-treated MoDCs. Western blot analysis of AhR protein levels 24h post-transfection of cells with either a scrambled siRNA or a specific siRNA targeted against AhR. MoDCs were treated with 50 ng/ml LPS for 24 h. Equivalent amounts of whole cell protein (25 μg of protein) were loaded in each lane on 10% SDS-polyacrylamide gels and analyzed by immunoblotting using an AhR- or Actin-specific antibody.
Figure S4. Selective effect of AhR antagonist MNF on TCDD- and FICZ-induced expression of CYP1A1 and cytokines IL-1β and CCL1 in MoDCs. MoDCs were treated with AhR ligands TCDD (10 nM) or FICZ (100 nM) in absence or presence of the AhR antagonist MNF (5 μM) for 24h. Expression of CYP1A1 and cytokines IL-1β and CCL1 was analyzed by real-time PCR. Results are presented as mean ± SEM and the y-axis represents mRNA expression level as fold increase above control. a significantly higher than control, p < 0.005; c significantly lower than TCDD-treated cells in absence of MNF, p < 0.005
Figure S5. Effect of AhR ligands on the expression of CYP1A1, CYP1B1, and AhRR in MoDCs. MoDCs were treated with TCDD (10 nM), FICZ (100 nM), I3C (50 μM), or kyn (50 μM) in absence or presence of LPS (50 ng/ml) for 24 h. Results from real-time PCR are presented as mean ± SEM and the y-axis represents mRNA expression level as fold increase above control. a significantly higher than control, p < 0.05; b significantly higher than cells treated with TCDD or FICZ alone, p < 0.05
Fig. S6. Dose-dependent effects of LPS and TCDD on IL-8 mRNA expression in MoDCs. Cells were treated with various concentrations of LPS (0.5–50 ng/ml), TCDD (0.1–10 nM) or co-treated with 0.5 ng/ml LPS and TCDD for 24 h. Results are presented as mean ± SEM and the y-axis represents mRNA expression level as fold increase above control. a significantly higher than control, p < 0.05; b significantly higher than TCDD- or LPS-treated cells, p < 0.05