Abstract
Background
Chronic inflammation has been associated with the development and progression of human cancers including prostate cancer. The exact role of the inflammatory Th17-IL-17 pathway in prostate cancer remains unknown. In this study, we aimed to determine the importance of Th17 cells and IL-17 in a Pten-null prostate cancer mouse model.
Methods
The Pten-null mice were treated by Th17 inhibitor SR1001 or anti-mouse IL-17 monoclonal antibody from 6 weeks of age up to 12 weeks of age. For SR1001 treatment, the mice were injected i.p. twice a day with vehicle or SR1001, which was dissolved in a dimethylsulfoxide (DMSO) solution. All mice were euthanized for necropsy at 12 weeks of age. For IL-17 antibody treatment, the mice were injected i.v. once every two weeks with control IgG or rat anti-mouse IL-17 monoclonal antibody, which was dissolved in PBS. The injection time points were at 6, 8, and 10-week-old. All mice were analyzed for the prostate phenotypes at 12 weeks of age.
Results
We found that either SR1001 or anti-IL-17 antibody treatment decreased the formation of micro-invasive prostate cancer in Pten-null mice. The SR1001 or anti-IL-17 antibody treated mouse prostates had reduced proliferation, increased apoptosis, and reduced angiogenesis, as well as reduced inflammatory cell infiltration. By assessing the epithelial-to-mesenchymal transition (EMT) markers, we found that SR1001 or anti-IL-17 antibody treated prostate tissues had weaker EMT phenotype compared to the control treated prostates.
Conclusions
These results demonstrated that Th17-IL-17 pathway plays a key role in prostate cancer progression in Pten-null mice. Targeting Th17-IL-17 pathway could prevent micro-invasive prostate cancer formation in mice.
Keywords: Th17-IL-17 pathway, SR1001, Anti-IL-17 antibody, Prostate cancer, Pten-null mice
Introduction
Chronic inflammation plays an important role in the initiation and/or progression of many human cancers [1]. Up to 20% of all cancer cases worldwide are associated with chronic inflammation, chronic infection, or both [1]. Inflammation affects carcinogenesis at the molecular level by regulating the tumor microenvironment through alteration of the balance of cytokines, chemokines, matrix-degrading enzymes, transcriptional factors and reactive oxygen species. Recent evidence points toward a major role for inflammatory processes in prostate cancer (PCa) pathogenesis [2]. Interleukin (IL)-17 (commonly termed IL-17) is a family of pro-inflammatory cytokines implicated in a variety of immune responses and is composed of six members, IL-17A to IL-17F. The receptor of IL-17 (IL-17R) is a transmembrane protein. Five members of the IL-17R family have been identified so far and designated as IL-17RA to IL-17RE. The binding of IL-17 to its receptors on haematopoietic and nonhaematopoietic cells such as epithelial and endothelial cells triggers intracellular signaling that induces the production of pro-inflammatory cytokines such as IL-6, C-X-C chemokines such as chemokine 8 (CXCL8), CXCL9, CXCL10, and CXCL11, and beta-defensin-2 [3]. IL-17A is secreted not only by Th17 cells but also by other cell types, including natural killer (NK) T cells, γδ T cells, noncytotoxic CD8+ T cells, neutrophils, and master cells [4].
The identification of Th17 as a third subset of T helper cells in 2005 changed the classical Th1/Th2 paradigm of T helper cell differentiation [5]. Distinct from Th1 and Th2 subsets, Th17 cells are characterized in particular by the production of pro-inflammatory cytokines IL-17A, IL-17F, and IL-22 as signature cytokines and expression of retinoic acid-related orphan receptor gamma t (RORγt) as master transcriptional factor [5]. T-cell immunity can promote or inhibit cancer development and growth and it is therefore critical to determine how specific T-cell lineages selectively affect cancer growth. Th17 cells have functions in autoimmune diseases, inflammation and host defense against infectious pathogens [5]. However, the role of Th17 cells and in particular IL-17A in tumor immunity remains controversial with report that Th17 cells and IL-17A could either promote or suppress tumor growth, depending on the type of malignancy or means of therapeutic intervention [6]. Thus, it is important to study further the specific nature of inflammatory response and the tissue context, so that the positive or negative effects of Th17 cells on tumor immunopathology can be determined.
We have taken a genetic approach to assess the role of IL-17A in PCa by interbreeding IL-17 receptor C (IL-17RC) deficient mice with mice that are conditionally null for Pten in the prostatic epithelium [7,8]. We found that IL-17RC-deficient mice displayed smaller prostate than IL-17RC-sufficient mice. In addition, the IL-17RC-deficient mice had decreased expression of matrix metalloproteinase 7 (MMP7) (also known as putative metalloproteinase I or matrilysin), which is why IL-17RC-deficient mice had reduced invasive cancer formation and growth compared to IL-17RC-sufficient mice. To further understand the mechanisms of how IL-17 promotes PCa, we generated an MMP7 and Pten double knockout (KO, or null) mouse model and demonstrated that IL-17 promotes PCa via MMP7-induced epithelial-to-mesenchymal transition (EMT) [9]. IL-17A induces MMP7 expression to disrupt E-cadherin/β-catenin complex and releasesβ-catenin in Pten-null mice prostates, thus enhancing EMT and tumor cell invasion. This indicates the IL-17-MMP7-EMT axis as a potential target for developing new strategies in the prevention and treatment of PCa [9]. By treating the Pten-null mice with Compound III (a selective MMP7 inhibitor) for 5–6 weeks, there was no significant difference in GU-bloc weight between the control and MMP7 inhibitor treatment groups. However, the percentage of invasive PCa was significantly less in the MMP7 inhibitor treatment group than the control group [9].
Since blocking IL-17 signaling by genetic knockout of IL-17RC significantly inhibits PCa formation and growth [7], we hypothesized that blocking IL-17 signaling by using pharmacologic agents can also inhibit PCa formation and growth. Although IL-17A is the signature cytokine of Th17 cells, the production of IL-17A is not the sole function of Th17 cells. Thus, the biological activities of IL-17A should not be equated with the biological activities of Th17 cells. In the current study, we aimed to assess the efficacy of both the small molecule inhibitor of Th17 cells (namely, SR1001) and the anti-mouse IL-17 monoclonal antibody in preventing micro-invasive PCa formation and growth in Pten-null mice. Our results showed that the SR1001 or anti-IL-17 antibody treated mouse prostates had reduced formation of micro-invasive PCa compared to the corresponding control treated mouse prostates. Further, there was reduced EMT in SR1001 or anti-IL-17 antibody treated mouse prostates compared to their corresponding control treated mouse prostates. EMT has been associated with cellular invasiveness [10] and cancer metastasis [11-13]. Our results suggest that targeting either Th17 axis or IL-17A pathway can prevent the formation of micro-invasive PCa in this mouse model.
Materials and Methods
Mice
Animal study was approved by the Animal Care and Use Committee of Tulane University. The breeding strategy for generating prostate-specific Pten-null mice has been described previously [7].
SR1001 and IL-17 antibody treatments
For SR1001 (formula: C15H13F6N3O4S2) [14] treatment, thirteen 6-week-old male Pten-null mice were randomly distributed to SR1001 treatment group (n=8) and vehicle control group (n=5). The Pten-null mice were injected intraperitoneally (i.p.) twice a day with 25 mg/kg (mouse body weight) SR1001 that was dissolved in a 10% dimethylsulfoxide (DMSO) and 90% phosphate-buffered saline (PBS) solution. The control group was injected with equal amount of vehicle (10% DMSO/90% PBS). At 12 weeks of age all mice were euthanized for necropsy. For IL-17 antibody treatment, thirteen 6-week-old male Pten-null mice were randomly distributed into treatment group (n=8) and control group (n=5). The Pten-null mice were injected intravenously (i.v.) once every two weeks with 3 mg/kg (mouse body weight) with control IgG (the control group) or rat anti-mouse IL-17A monoclonal antibody (the treatment group, antibody catalog # MAB421, R&D systems, Inc. Minneapolis, MN, USA), which was dissolved in PBS. The injection time points were at 6, 8, and 10-week-old. All animals were euthanized for necropsy at 12 weeks of age.
Histopathology
Mice were euthanized and weighed at 12 weeks of age. The GU-blocs were photographed, weighed with an empty bladder, and fixed as described [7]. Thirty-two consecutive 4-μm sections of each prostate were cut and eight sections (from every 8th section) per sample were H&E stained for histopathologic assessment in a group-blinded fashion per the Bar Harbor Classification [15]. The prostatic glands were assessed under low- and high-power magnifications, and 78 - 235 prostatic glands in each prostate were counted with a total of over 700 prostatic glands in eight or five mouse prostates per treatment or control group. The number of inflammatory cells in the stromal space between the prostatic glands was counted in five high-power fields (×200 magnification) of each prostate lobe including dorsal, lateral, and ventral prostatic lobes; the average numbers of inflammatory cells per high-power field in eight or five mouse prostates per treatment or control group were compared.
Immunohistochemistry (IHC) and Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Staining
IHC staining was performed per previously established protocols [7,8], using VECTSTAIN ABC kits and DAB Substrate Kits (Vector Laboratories, Burlingame, CA, USA) per the manufacturer's instructions. The primary antibodies used were: rabbit anti-laminin (1:100 dilution, Sigma-Aldrich, St. Louis, MO, USA); rabbit anti-E-cadherin (#3195, 1:200 dilution), anti-β-catenin (#8480, 1:200 dilution), anti-ZO-1 (#8193, 1:100 dilution), anti-Snail (#3879, 1:500 dilution), anti-Slug (#9585, 1:50 dilution), and anti-TCF8/ZEB1 (#3396, 1:100 dilution) (Cell Signaling Technology, Beverly, MA, USA); goat anti-HIF-1α (Y-15, sc-12542, 1:50 dilution), rabbit anti-VEGFA (A-20, sc-152, 1:200 dilution) (Santa Cruz Biotechnology, Dallas, TX, USA); rabbit anti-CD31 (Ab28364, 1:50 dilution) (Abcam, Cambridge, MA, USA); anti-Ki-67 (1:200 dilution) (Millipore, Billerica, MA, USA). IHC results were analyzed by semi-quantitative analysis as previously described [16,17]. TUNEL staining was performed using TACS.XL® Blue Label in Situ Apoptosis Detection Kit (Trevigen, Gaithersburg, MD, USA) per the manufacturer's instructions. To quantify Ki-67-positive and TUNEL-positive cells, three animals from each group were randomly selected; three representative prostate sections from each animal were stained; approximately 300 cells per field of 6 high-power fields (×200 magnification) of each prostate were counted; and the percentages of positive cells were calculated as the number of positive cells divided by the total number of cells. The density of microvessels was evaluated by counting the number of CD31-positive microvessels in 6 high-power fields (×200 magnification) per prostate section; the average numbers of per high-power field of three random mouse prostates per group were compared. The number of inflammatory cells in the stromal space between the prostatic glands was counted in six high-power fields (×200 magnification) per prostate section; the average numbers of inflammatory cells per high-power field in eight or five mouse prostates in the treatment or control groups were compared.
Statistical Analysis
Quantitative data are presented as mean ± standard error of the mean (SEM, error bar). Comparisons of the GU-bloc weight and other quantitative data were analyzed using Student's t test. The χ2 test was used to compare the incidences of micro-invasive adenocarcinoma. Statistical significance was defined as P < 0.05.
Results
SR1001 or IL-17 antibody treatment prevents formation of invasive prostate adenocarcinoma
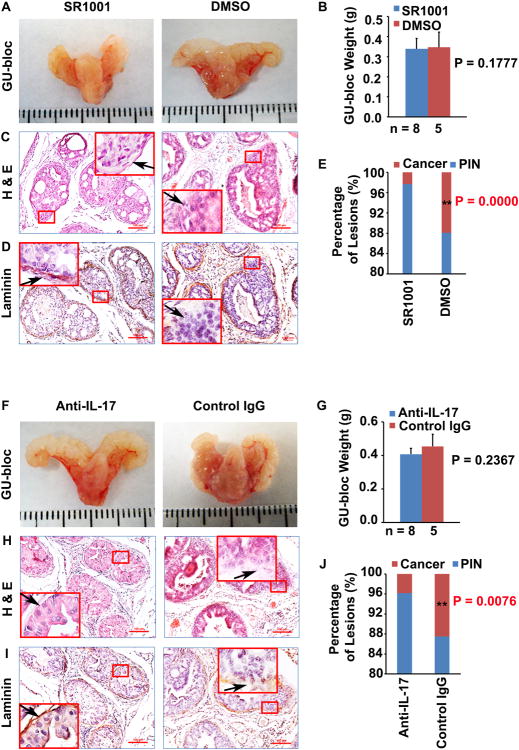
The GU-bloc weight has often been used to represent the prostate tumor burden [7]. Although prostate tumors in SR1001 or IL-17 antibody treatment groups were smaller than those in the corresponding control groups (Fig. 1A and 1F), the GU-bloc weight showed no significant difference between treatment and control animals (Fig. 1B and 1G). However, we found that the micro-invasive prostate adenocarcinoma formation rates were significantly different between SR1001 or IL-17 antibody treatment group and the corresponding control group. In the SR1001 treatment group, only 2.3% of prostatic glands presented with micro-invasive prostate adenocarcinoma. In contrast, 11% of prostatic glands showed micro-invasive prostate adenocarcinoma in the control mice. In the IL-17 antibody treatment group, only 4% of prostatic glands presented with micro-invasive prostate adenocarcinoma. In contrast, 12.5% of prostatic glands showed micro-invasive prostate adenocarcinoma in control IgG treatment mice. The differences in the percentages of lesions were statistically significant between the SR1001 treated and control treated mice (P < 0.01, Fig. 1E) or between IL-17 antibody and control IgG treated mice (P < 0.01, Fig. 1J). These results suggested that SR1001 or IL-17 antibody treatment prevents formation of micro-invasive prostate adenocarcinoma.
Fig. 1. SR1001 or IL-17 antibody decreases the formation of invasive prostate adenocarcinoma in mice.
A: Representative photographs of the GU-blocs in SR1001 and DMSO (control)-treated mice. B: GU-bloc weight; the number of animals in each group is shown under the abscissa. C and D: Representative sections of dorsolateral prostatic lobes stained with H&E and anti-laminin; original magnification, ×100 for photomicrographs and ×400 for inserts; arrow indicates a micro-invasive site where continuity of laminin staining is broken in control mice and a non-invasive site where the continuity of staining is not broken in SR1001-treated mice; the tissue sections in D were consecutive sections to C. E: Percentages of PIN and cancer (i.e., micro-invasive prostate adenocarcinoma) in ventral, dorsal and lateral prostatic lobes of SR1001-treated and control mice; n = 8 and 5 animals in SR1001 and DMSO treatment groups respectively, **P < 0.01 compared with control mice. F: Representative photographs of the GU-blocs in IL-17 antibody and control IgG-treated mice. G: GU-bloc weight; the number of animals in each group is shown under the abscissa. H and I: Representative sections of dorsolateral prostatic lobes stained with H&E and anti-laminin; original magnification, ×100 for photomicrographs and ×400 for inserts; arrow indicates a micro-invasive site where continuity of laminin staining is broken in control IgG-treated mice and a non-invasive site where the continuity of staining is not broken in IL-17 antibody-treated mice; the tissue sections in I were consecutive sections to H. J: Percentages of PIN and micro-invasive cancer in ventral, dorsal and lateral prostatic lobes of IL-17 antibody and control IgG-treated mice; n = 8 and 5 animals in IL-17 antibody and control IgG treatment groups respectively, **P < 0.01 compared with control mice.
SR1001 or IL-17 antibody treatment decreases cellular proliferation and increases apoptosis in prostate lesions
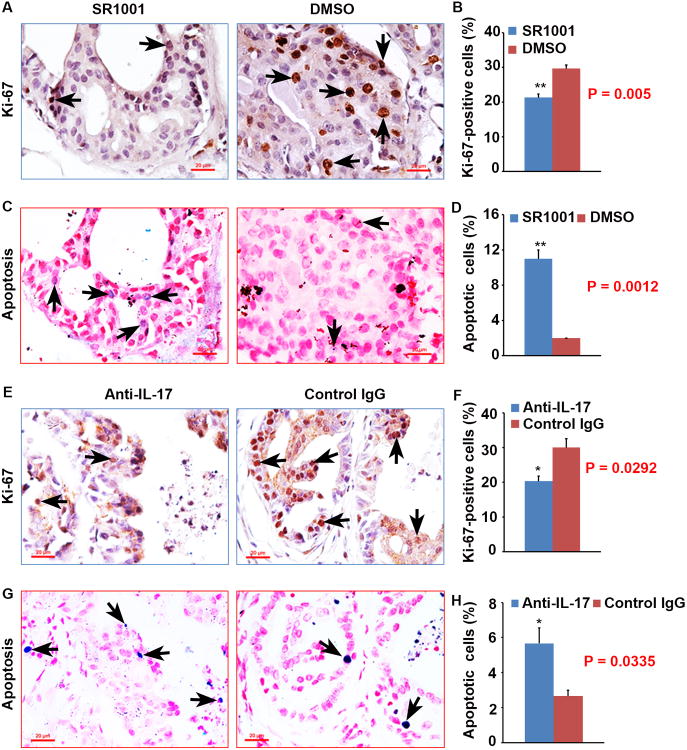
To reveal the underlying cause of the differences in prostate tumor burden between the animals at 12 weeks of age, we assessed cellular proliferation and apoptosis by conducting Ki-67 staining and TUNEL assay on each prostate. We found that there were significantly more Ki-67-positive epithelial cells in vehicle treatment prostates than in SR1001 treatment prostates (P < 0.01, Fig. 2A and B). In addition, there were significantly fewer apoptotic cells in vehicle treatment prostates than in SR1001 treatment prostates (P < 0.01, Fig. 2C and D). There were significantly more Ki-67-positive epithelial cells in control IgG treatment prostates than in anti-IL-17 antibody treatment prostates (P < 0.05, Fig. 2E and F). In addition, there were significantly fewer apoptotic cells in control IgG treatment prostates than in anti-IL-17 antibody treatment prostates (P < 0.05, Fig. 2G and H). These results suggested that the decreased prostate tumor burden in SR1001 or IL-17 antibody treatment mice was due to decreased cellular proliferation and increased apoptosis in mouse prostates.
Fig. 2. SR1001 or IL-17 antibody decreases proliferation and increases apoptosis in mouse prostates.
A: Representative prostate sections stained for Ki-67 in SR1001 and DMSO-treated mice; arrows indicate positive cells. B: Percentages of Ki-67-positive cells in SR1001 and DMSO-treated mouse prostates; data are represented as mean ± s.e.m., n = 3 animals per group, **P < 0.01. C: Representative prostate sections stained for apoptosis (TUNEL assay) in SR1001 and DMSO-treated mice; arrows indicate positive cells. D: Percentages of apoptotic cells in SR1001 and DMSO-treated mouse prostates; data are represented as mean ± s.e.m., n = 3 animals per group, **P < 0.01. E: Representative prostate sections stained for Ki-67 in IL-17 antibody and control IgG-treated mice; arrows indicate positive cells. F: Percentages of Ki-67-positive cells in IL-17 antibody and control IgG-treated mouse prostates; data are represented as mean ± s.e.m., n = 3 animals per group, *P < 0.05. G: Representative prostate sections stained for apoptosis in IL-17 antibody and control IgG-treated mice; arrows indicate positive cells. H: Percentages of apoptotic cells in IL-17 antibody and control IgG- treated mice; data are represented as mean ± s.e.m., n = 3 animals per group, *P < 0.05.
SR1001 or IL-17 antibody treatment decreases inflammatory cell infiltration in prostate lesions
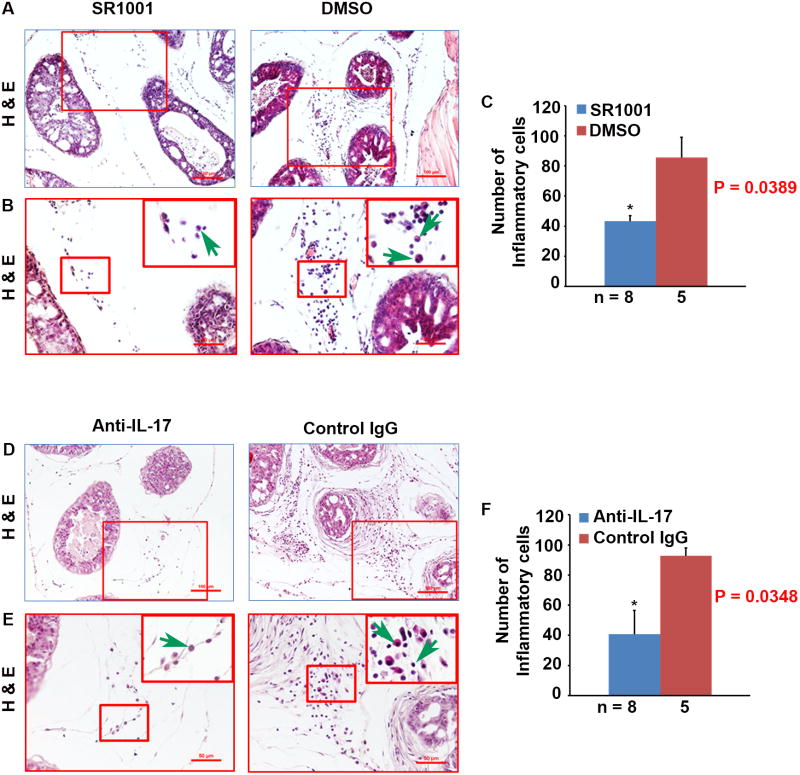
Th17 and IL-17 have been shown to provide protective immunity to infections by fungi and extracellular bacteria but are involved in chronic inflammation and autoimmunity. We found that the DMSO and IgG control treated mouse prostates had many inflammatory cells in the stromal space between the prostatic glands; however, the number of inflammatory cells was significantly reduced in either SR1001 (P < 0.05, Fig. 3A - C) or anti-IL-17 antibody (P < 0.05, Fig. 3D -F) treated mouse prostates. The inflammatory cell population was mainly composed of macrophages and lymphocytes, and few neutrophils (Fig. 3A, B and D, E). This phenomenon is consistent with our previous report in the intact Pten-null mouse prostate and the number of inflammatory cells was significantly reduced in Il-17rc-deficient prostate compared to Il-17rc-suficient prostate [7].
Fig. 3. SR1001 or IL-17 antibody treatment decreases inflammatory cell infiltration in mouse prostate tumors.
A: Representatives of H&E-stained sections from SR1001 and DMSO-treated mouse anterior prostatic lobes; original magnification, ×100. B: ×200 magnification of the selected regions in A; ×400 for inserts; arrows indicate different inflammatory cells. C: Number of inflammatory cells counted on H&E-stained sections from SR1001 and DMSO-treated mouse ventral, dorsal, and lateral prostatic lobes; the number of animals in each group is shown under the abscissa; data are represented as mean ± s.e.m., *P < 0.05. D: Representatives of H&E-stained sections from IL-17 antibody and control IgG-treated mouse anterior prostatic lobes; original magnification, ×100. E: ×200 magnification of the selected regions in D; ×400 for inserts; arrows indicate different inflammatory cells. F: Number of inflammatory cells counted on H&E-stained sections from IL-17 antibody and control IgG-treated mouse ventral, dorsal, and lateral prostatic lobes; the number of animals in each group is shown under the abscissa; data are represented as mean ± s.e.m., *P < 0.05.
SR1001 or IL-17 antibody treatment decreases angiogenesis in prostate lesions
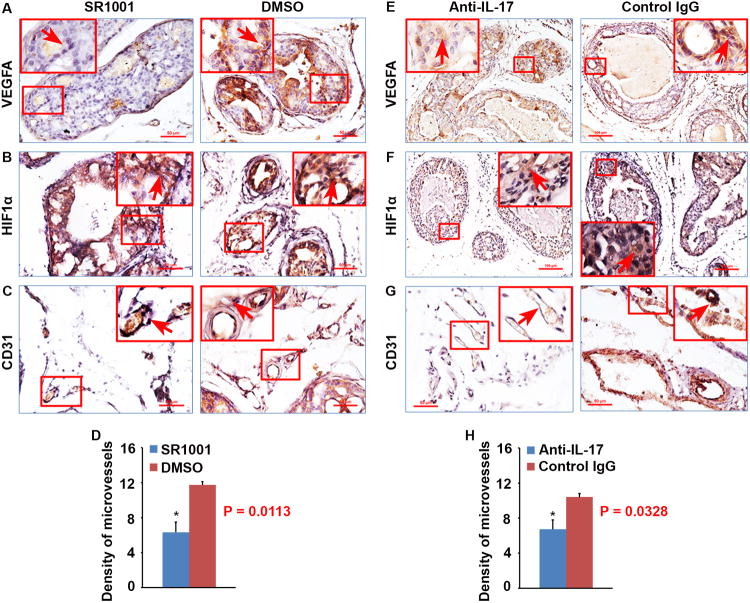
IL-17A produced by Th17 cells is an angiogenic factor that stimulates the migration and cord formation of vascular endothelial cells in vitro and elicits vessel formation in vivo, thus promoting tumor growth and metastasis through de novo carcinogenesis and neovascularization via Stat3 signaling [18]. Therefore, we assessed angiogenesis in mouse prostate tumors using IHC staining of vascular endothelial growth factor A (VEGFA), hypoxia-inducible factor 1-alpha (HIF1α) and CD31. We found that there were significantly more blood vessels in DMSO or IgG control groups of mouse prostates than that in SR1001 (P < 0.05, Fig. 4C and D) or anti-IL-17 antibody (P < 0.05, Fig. 4G and H) treatment groups, which were accompanied with higher levels of HIF1α (Fig. 4B or F) and VEGFA (Fig. 4A or E) in DMSO or IgG control treatment prostates than in SR1001 or anti-IL-17 treatment prostates. These results indicated that reduced HIF1α and VEGFA expression as well as angiogenesis in SR1001 or anti-IL-17 treatment prostates contributed to the decreased prostate tumor burden in SR1001 or anti-IL-17 treatment mice.
Fig. 4. SR1001 or IL-17 antibody treatment inhibits angiogenesis in mouse prostates.
A-C: Representative prostate sections stained for VEGFA, HIF1α, and CD31 in SR1001 and DMSO-treated mice. Arrows indicate positive staining cells or microvessels. D: Density of microvessels in SR1001 and DMSO-treated mouse prostates; data are represented as mean ± s.e.m., n = 3 animals per group, *P < 0.05. E-G: Representative prostate sections stained for VEGFA, HIF1α, and CD31 in IL-17 antibody and control IgG-treated mice. Arrows indicate positive staining cells or microvessels. H: Density of microvessels in IL-17 antibody and control IgG-treated mouse prostates; data are represented as mean ± s.e.m., n = 3 animals per group, *P < 0.05.
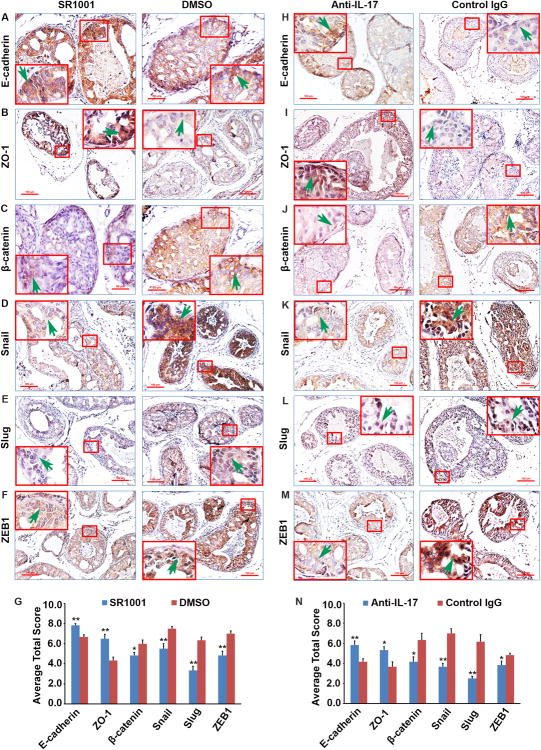
SR1001 or IL-17 antibody treatment inhibits EMT in prostate lesions
To further understand the molecular mechanisms underlying the reduced prostate tumor formation in SR1001 and anti-IL-17 treatment mice, we examined expression of several epithelial and mesenchymal marker proteins in the prostate lesions. We found that SR1001 or anti-IL-17 treatment mice had obviously increased expression of epithelial markers such as E-cadherin and ZO-1, compared with control mice (Fig. 5A, B and G or H, I and N). In contrast, SR1001 or anti-IL-17 treatment mice had reduced expression of mesenchymal markers such as β-catenin, compared with control mice (Fig. 5C and G or J and N). Since EMT is induced by certain transcription factors such as Snail, Slug, and ZEB1, we also assessed their expression levels. We found that the expression levels of Snail, Slug, and ZEB1 were reduced in either SR1001 or anti-IL-17 treatment prostates, compared with their corresponding control prostates (Fig. 5D, E, F, and G or J, K, L, and N). Taken together, these results suggested that SR1001 or anti-IL-17 antibody treated prostates showed higher expression levels of epithelial markers, whereas their corresponding control prostates showed higher expression levels of mesenchymal markers and transcription factors, implying that either SR1001 or anti-IL-17 antibody weakened EMT characteristics in the mouse prostate tumors.
Fig. 5. SR1001 or IL-17 antibody treatment decreases EMT in mouse prostate tumors.
A-F: Representative prostate sections from SR1001 and DMSO-treated mice stained for EMT markers. Arrows indicate positive staining cells. H-M: Representative prostate sections from IL-17 antibody and control IgG-treated mice stained for EMT markers; arrows indicate positive staining cells. G and N: Quantification of EMT markers staining; Total Score (TS) = Proportion Score (PS, range 0 - 5) + Intensity Score (IS, rang 0 - 3); data are represented as mean ± s.e.m., n = 3 animals per group, *P < 0.05, **P < 0.01.
Discussion
Th17 cells and IL-17A have been implicated in many inflammatory and autoimmune diseases, but their role in cancer has not been fully elucidated [6,19]. Th17 cells have been found in many different types of human tumors, including lymphoma [20], myeloma [21], breast cancer [22], colon cancer [23], gastric cancer [24], hepatocellular cancer [25], melanoma [26], ovarian cancer [27], pancreatic cancer [28], and PCa [29]. Most studies focus on the Th17 effector cytokine IL-17A. Recent studies using animal models of autochthonous cancer from many independent groups have demonstrated that the Th17 axis and IL-17A promotes development of colon [30], skin [31], breast [32], prostate [7-9,16], lung [33], and pancreas cancer [34]. Th17 cells and IL-17A are believed to have protumorigenic roles, however, in some specific context they may have antitumorigenic roles [35]. In the current study, we aimed to determine the importance of both the Th17 cells and IL-17A in prostate carcinogenesis.
SR1001 is a high-affinity synthetic ligand specific to both retinoic acid-related orphan receptors ɑ (RORɑ) and γt (RORγt), which inhibits Th17 cell differentiation and function [14]. SR1001 binds specifically to the ligand-binding domains (LBD) of RORɑ and RORγ and inhibits the recruitment of coactivators (e.g., steroid receptor coactivators, SRCs) by disrupting helix 12 of the RORɑ and RORγt ligand-binding pockets and effectively inhibit expression of genes that are preferentially expressed in Th17 cells [14]. SR1001 has been shown to suppress IL-17A gene expression and protein production as well as suppressing the clinical severity of autoimmune disease without obvious toxicity in mice [14]. We found that SR1001 reduced IL-17A and IL-17F mRNA expression in mouse blood cells and prostate tissues (data not shown). No obvious toxicity was noticed as evidenced by similar increase of body weight in both control and treatment groups (data not shown). The dose schedule for SR1001 is based on our preliminary study and published report [14]. In this study, the significant phenotypical difference found between SR1001 treatment and control group was that SR1001 significantly decreased the formation of micro-invasive PCa, though the GU-bloc weights had no significant difference.
Anti-IL-17 antibodies have been shown to inhibit colon and skin cancer formation in animal models [36,37]. In human clinical trials, blocking IL-17 signaling by anti-IL-17 or anti-IL-17RA antibodies is effective in treating psoriasis, rheumatoid arthritis, and uveitis, without increasing any adverse events including infections [38-40]. We used the dose schedule for anti-IL-17 antibody based on our preliminary study and published reports using similar antibodies in animal studies [36,37] and in human clinical trials [38-40]. We found that anti-mouse IL-17A antibody reduced the formation of micro-invasive PCa, although the GU-bloc weights had no significant difference between the treatment and control groups in Pten-null mice. These results are consistent with our previous studies [7-9] and other researchers' studies [32,36]. Wu et al. reported that antibody-mediated blockade of IL-17 and the receptor for IL-23, a key cytokine amplifying TH17 responses, inhibits ETBF-induced colitis, colonic hyperplasia and tumor formation [36]. Novitskiy et al. reported that anti-IL-17-blocking antibodies dramatically decrease tumor growth and the number of myeloid derived suppressor cells (MDSCs) in mice [32]. Our recent publication demonstrated that there is an IL-17-MMP7-EMT axis that exists in prostate carcinogenesis [9]. Our current study showed that reduced EMT characteristics in mouse prostates after blocking either Th17 cell differentiation and function by SR1001 or blocking IL-17 signaling by anti-mouse IL-17A antibody compared with their corresponding control mouse prostates. Other investigators have reported that IL-17 induced EMT via Stat3 in lung adenocarcinoma [41] and enhanced lung cancer cell migration through activating NF-κB by upregulating ZEB1 expression [42]. IL-17 promotes nasopharyngeal carcinoma cell migration and invasion by regulation of the expression of MMP-2/-9 and EMT via the p38-NF-κB signaling pathway [43]. Tumor hypoxia with deregulated expression of HIF leads to poor prognosis of patients diagnosed with solid tumors [44]. Emerging evidence also suggests that hypoxia and HIF signaling pathways contribute to the acquisition of EMT [11]. Further, VEGFA has been shown to be a direct downstream target gene of β-catenin signaling [45], thus the observed reduced angiogenesis phenotype may be linked to IL-17-activated β-catenin signaling.
IL-17 signaling plays an important pro-inflammatory role in many diseases. We have previously found that IL-17RC-deficient mouse prostates had significantly less inflammatory cell infiltration than IL-17RC-sufficient mouse prostates, and the main cell types in the mouse prostate were macrophages, neutrophils, and lymphocytes [7,8]. These previous studies indicated that IL-17 signaling may promote tumor growth via enhancing inflammation. In the current study, we found that blocking Th17-IL-17 pathway either by inhibiting the Th17 cells differentiation and function using SR1001 or by blocking Th17 signature cytokine IL-17A significantly decreased the inflammatory cell infiltration in mouse prostates. These findings are consistent with the pro-inflammatory function of Th17 cells and IL-17A. The main cell types in prostate tissues were macrophages, neutrophils, and lymphocytes. IL-17A has been reported to cause recruitment of neutrophils into the rat airway [46], macrophages in lung cancer [33] and inflammatory infiltrates in a colon cancer model [36]. We have reported that 70% of macrophages in human lung tumors are M2 macrophages [47]. M2 macrophages are believed to promote tumor growth and metastasis by secretion of growth factors, VEGF, and immunosuppressive cytokines/chemokines [48]. Thus, the reduction in inflammatory cell infiltration may partially contribute to the observed inhibition of micro-invasive cancer formation after SR1001 or anti-IL-17 antibody treatment.
A recent study compared the relative importance of IL-17A and RORγt in murine spontaneous intestinal tumorigenesis [49]. They demonstrated that deficiency of IL-17A, but not RORγt decreases murine spontaneous intestinal tumorigenesis. Although we did not compare the relative efficacy of SR1001 and anti-IL-17 antibody in preventing PCa in our study, we showed that both of them have the efficacy in preventing micro-invasive PCa formation. This may be because the protumor effects of IL-17A in certain cancer types [50]. The finding that anti-IL-17 antibody treated mouse prostates had reduced formation of micro-invasive prostate adenocarcinoma is consistent with our previous studies [7-9] and other researchers' results [32,36], although in PCa patients, a significant inverse correlation was seen between Th17 cell differentiation and tumor progression [29]. The result from SR1001 treatment in the Pten-null model supports the idea that Th17 cells have a protumorigenic function in PCa.
Conclusions
In summary, our findings suggest that loss of either Th17 cell differentiation and functionor IL-17A function diminishes micro-invasive cancer development in a mouse model of PCa. These data imply that targeting Th17-IL-17 pathway may prevent micro-invasive prostate cancer formation in this mouse model.
Acknowledgments
Grant sponsor: Z.Y. was partially supported by National Institutes of Health (R01CA174714 and P20GM103518), Department of Defense (W81XWH-14-1-0050, W81XWH-14-1-0149, W81XWH-14-1-0458 (PI: Feng Chen; Co-I: Z.Y.), and W81XWH-15-1-0444)); the Developmental Fund of Tulane Cancer Center (TCC), Louisiana Cancer Research Consortium (LCRC) Fund; Q.Z. was partially supported by NIH-National Institute of General Medical Sciences (P20GM103629) (PI: S. Michal Jazwinski; Pilot Project PI: Q.Z.). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
Footnotes
Conflict of Interests: The authors declare no conflict of interest.
References
- 1.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) 2002;16(2):217–26. 29. discussion 30-2. [PubMed] [Google Scholar]
- 2.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60(1):199–215. doi: 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian Y, Kang Z, Liu C, Li X. IL-17 signaling in host defense and inflammatory diseases. Cell Mol Immunol. 2010;7(5):328–33. doi: 10.1038/cmi.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones SA, Sutton CE, Cua D, Mills KH. Therapeutic potential of targeting IL-17. Nat Immunol. 2012;13(11):1022–5. doi: 10.1038/ni.2450. [DOI] [PubMed] [Google Scholar]
- 5.Ye J, Livergood RS, Peng G. The role and regulation of human Th17 cells in tumor immunity. Am J Pathol. 2013;182(1):10–20. doi: 10.1016/j.ajpath.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10(4):248–56. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Liu S, Ge D, Xue Y, Xiong Z, Abdel-Mageed AB, Myers L, Hill SM, Rowan BG, Sartor O, Melamed J, Chen Z, You Z. Interleukin-17 promotes formation and growth of prostate adenocarcinoma in mouse models. Cancer Res. 2012;72(10):2589–99. doi: 10.1158/0008-5472.CAN-11-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Liu S, Xiong Z, Wang AR, Myers L, Melamed J, Tang WW, You Z. Interleukin-17 promotes development of castration-resistant prostate cancer potentially through creating an immunotolerant and pro-angiogenic tumor microenvironment. Prostate. 2014;74(8):869–79. doi: 10.1002/pros.22805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q, Liu S, Parajuli KR, Zhang W, Zhang K, Mo Z, Liu J, Chen Z, Yang S, Wang AR, Myers L, You Z. Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene. 2016 doi: 10.1038/onc.2016.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17(5):548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, Gouvin LM, Sharma VM, Mercurio AM. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 17(4):319–32. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–29. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Smit MA, Peeper DS. Zeb1 is required for TrkB-induced epithelial-mesenchymal transition, anoikis resistance and metastasis. Oncogene. 2011;30(35):3735–44. doi: 10.1038/onc.2011.96. [DOI] [PubMed] [Google Scholar]
- 14.Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidovic D, Schurer SC, Xu J, Wagoner G, Drew PD, Griffin PR, Burris TP. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472(7344):491–4. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64(6):2270–305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Zhang Q, Chen C, Ge D, Qu Y, Chen R, Fan YM, Li N, Tang WW, Zhang W, Zhang K, Wang AR, Rowan BG, Hill SM, Sartor O, Abdel-Mageed AB, Myers L, Lin Q, You Z. Hyperinsulinemia enhances interleukin-17-induced inflammation to promote prostate cancer development in obese mice through inhibiting glycogen synthase kinase 3-mediated phosphorylation and degradation of interleukin-17 receptor. Oncotarget. 2016;7(12):13651–66. doi: 10.18632/oncotarget.7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allred DC, Clark GM, Elledge R, Fuqua SA, Brown RW, Chamness GC, Osborne CK, McGuire WL. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993;85(3):200–6. doi: 10.1093/jnci/85.3.200. [DOI] [PubMed] [Google Scholar]
- 18.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101(7):2620–7. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Orozco N, Dong C. The IL-17/IL-23 axis of inflammation in cancer: friend or foe? Curr Opin Investig Drugs. 2009;10(6):543–9. [PubMed] [Google Scholar]
- 20.Galand C, Donnou S, Crozet L, Brunet S, Touitou V, Ouakrim H, Fridman WH, Sautes-Fridman C, Fisson S. Th17 cells are involved in the local control of tumor progression in primary intraocular lymphoma. PLoS One. 2011;6(9):e24622. doi: 10.1371/journal.pone.0024622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhodapkar KM, Barbuto S, Matthews P, Kukreja A, Mazumder A, Vesole D, Jagannath S, Dhodapkar MV. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17-1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112(7):2878–85. doi: 10.1182/blood-2008-03-143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, Yilmazer A, Paish EC, Ellis IO, Patel PM, Jackson AM. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10(6):R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71(4):1263–71. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 24.Maruyama T, Kono K, Mizukami Y, Kawaguchi Y, Mimura K, Watanabe M, Izawa S, Fujii H. Distribution of Th17 cells and FoxP3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci. 2010;101(9):1947–54. doi: 10.1111/j.1349-7006.2010.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Zheng H, Zhang H, Ma W, Wang F, Liu C, He S. Increased interleukin (IL)-8 and decreased IL-17 production in chronic obstructive pulmonary disease (COPD) provoked by cigarette smoke. Cytokine. 56(3):717–25. doi: 10.1016/j.cyto.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112(2):362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charles KA, Kulbe H, Soper R, Escorcio-Correia M, Lawrence T, Schultheis A, Chakravarty P, Thompson RG, Kollias G, Smyth JF, Balkwill FR, Hagemann T. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119(10):3011–23. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141–9. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14(11):3254–61. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyun YS, Han DS, Lee AR, Eun CS, Youn J, Kim HY. Role of IL-17A in the development of colitis-associated cancer. Carcinogenesis. 2012;33(4):931–6. doi: 10.1093/carcin/bgs106. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Yi T, Zhang W, Pardoll DM, Yu H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res. 2010;70(24):10112–20. doi: 10.1158/0008-5472.CAN-10-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novitskiy SV, Pickup MW, Gorska AE, Owens P, Chytil A, Aakre M, Wu H, Shyr Y, Moses HL. TGF-beta receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov. 2011;1(5):430–41. doi: 10.1158/2159-8290.CD-11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Ge D, Ma L, Mei J, Liu S, Zhang Q, Ren F, Liao H, Pu Q, Wang T, You Z. Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. J Thorac Oncol. 2012;7(7):1091–100. doi: 10.1097/JTO.0b013e3182542752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H, Jaffee EM, Drake CG, Housseau F, Maitra A, Kolls JK, Sears CL, Pardoll DM, Leach SD. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 2014;25(5):621–37. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gnerlich JL, Mitchem JB, Weir JS, Sankpal NV, Kashiwagi H, Belt BA, Porembka MR, Herndon JM, Eberlein TJ, Goedegebuure P, Linehan DC. Induction of Th17 cells in the tumor microenvironment improves survival in a murine model of pancreatic cancer. J Immunol. 2010;185(7):4063–71. doi: 10.4049/jimmunol.0902609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15(9):1016–22. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao M, Wang C, Zhang J, Li Z, Zhao X, Qin Z. IFNgamma promotes papilloma development by up-regulating Th17-associated inflammation. Cancer Res. 2009;69(5):2010–7. doi: 10.1158/0008-5472.CAN-08-3479. [DOI] [PubMed] [Google Scholar]
- 38.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, Durez P, Tak PP, Gomez-Reino JJ, Foster CS, Kim RY, Samson CM, Falk NS, Chu DS, Callanan D, Nguyen QD, Rose K, Haider A, Di Padova F. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2(52):52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 39.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, Banerjee S. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366(13):1190–9. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 40.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, Aras G, Li J, Russell CB, Thompson EH, Baumgartner S. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–9. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 41.Huang Q, Han J, Fan J, Duan L, Guo M, Lv Z, Hu G, Chen L, Wu F, Tao X, Xu J, Jin Y. IL-17 induces EMT via Stat3 in lung adenocarcinoma. Am J Cancer Res. 2016;6(2):440–51. [PMC free article] [PubMed] [Google Scholar]
- 42.Gu K, Li MM, Shen J, Liu F, Cao JY, Jin S, Yu Y. Interleukin-17-induced EMT promotes lung cancer cell migration and invasion via NF-kappaB/ZEB1 signal pathway. Am J Cancer Res. 2015;5(3):1169–79. [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Ma R, Kang Z, Zhang Y, Ding H, Guo W, Gao Q, Xu M. Effect of IL-17A on the migration and invasion of NPC cells and related mechanisms. PLoS One. 2014;9(9):e108060. doi: 10.1371/journal.pone.0108060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bao B, Ahmad A, Kong D, Ali S, Azmi AS, Li Y, Banerjee S, Padhye S, Sarkar FH. Hypoxia Induced Aggressiveness of Prostate Cancer Cells Is Linked with Deregulated Expression of VEGF, IL-6 and miRNAs That Are Attenuated by CDF. PLoS One. 7(8):e43726. doi: 10.1371/journal.pone.0043726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Easwaran V, Lee SH, Inge L, Guo L, Goldbeck C, Garrett E, Wiesmann M, Garcia PD, Fuller JH, Chan V, Randazzo F, Gundel R, Warren RS, Escobedo J, Aukerman SL, Taylor RN, Fantl WJ. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63(12):3145–53. [PubMed] [Google Scholar]
- 46.Hoshino H, Lotvall J, Skoogh BE, Linden A. Neutrophil recruitment by interleukin-17 into rat airways in vivo. Role of tachykinins. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1423–8. doi: 10.1164/ajrccm.159.5.9806008. [DOI] [PubMed] [Google Scholar]
- 47.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23(4):344–6. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Shapiro M, Nandi B, Pai C, Samur MK, Pelluru D, Fulciniti M, Prabhala RH, Munshi NC, Gold JS. Deficiency of IL-17A, but not the prototypical Th17 transcription factor RORgammat, decreases murine spontaneous intestinal tumorigenesis. Cancer Immunol Immunother. 2016;65(1):13–24. doi: 10.1007/s00262-015-1769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fabre J, Giustiniani J, Garbar C, Antonicelli F, Merrouche Y, Bensussan A, Bagot M, Al-Dacak R. Targeting the Tumor Microenvironment: The Protumor Effects of IL-17 Related to Cancer Type. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17091433. [DOI] [PMC free article] [PubMed] [Google Scholar]