Abstract
Background
Ethanol (EtOH) intake correlates with increased risk-taking, and sex differences exist in both EtOH use and risk-taking in humans and rats. However, the interaction of sex and gonadal hormones to affect risk-taking under the influence of EtOH has not been determined. This was the focus of the current study.
Methods
Adult Long-Evans rats (n=18 males and females) were gonadectomized and received hormone replacement at physiologic levels or blank implants (n=7–9/group). Risk-taking was assessed with probability discounting, requiring rats to choose between a small/certain reward and a large/uncertain reward delivered with decreasing probability throughout each daily session. Before testing, rats received saline or EtOH (0.5 or 1.0 g/kg) ip.
Results
In males, EtOH increased preference for the large/uncertain reward lever (F2,28=10.462, p<0.05). However, there was no effect of EtOH on lever preference in females (F1,30=0.914, p>0.05). At baseline, ORCHX+T males showed a greater preference for the large/uncertain reward lever then ORCHX males (F1,14=13.805, p<0.05). In females only, EtOH decreased choice latency relative to baseline (F1,10=7.25, p<0.05). EtOH decreased loss sensitivity in both sexes, with all rats exhibiting decreased lose-shift ratios (males: F2,18=5.10, p<0.05; females F2,10=4.37, p<0.05).
Conclusions
These results show that EtOH, sex, and hormones interact to influence decision making. EtOH increases risk taking in males, but not in females. However, EtOH selectively decreases choice latency in females, and decreases loss sensitivity in both sexes. These findings are relevant to understanding human behavior, particularly in adolescents who experience increased hormone levels and often drink EtOH and engage in risky behavior.
Keywords: sex characteristics, gonadal hormones, decision making, choice behavior, operant behavior, reward
1. INTRODUCTION
Ethanol (EtOH) is the most-commonly used drug worldwide (Winstock, 2014), with 87.6% of American adults reporting EtOH consumption during their lifetime (NIAAA, 2016). According to The Independent Scientific Committee on Drugs, EtOH causes more harm than any other illicit substance, due to its prevalence and detriments to both the user and society (Nutt et al., 2010). National surveys show that drinking, impulsive behavior and injury are positively correlated (NIAAA, 2016; Cherpitel, 1993; Borges et al., 2004). Additionally, drinking is associated with gambling behavior, as research suggests that alcohol use positively correlates with both gambling frequency and gambling losses (Martens et al., 2009). However, in these large-scale human studies, it is difficult to establish cause and effect, and to examine underlying mechanisms. Furthermore, sex differences exist in both EtOH use and risk-taking, with men consuming more alcohol and engaging in more risky behavior such as criminal activity, drug use, biking or driving without a helmet or seatbelt, and problematic gambling (Johansson et al., 2009; Carroll et al., 2004; MacArthur et al., 2012). However, it is unknown if EtOH has differential effects on risk-taking in men and women, an issue which has important implications for public health and safety.
Gonadal steroids also stimulate risk-taking behavior. Under the influence of gonadal steroids, male and female adolescents engage in increased risk-taking behaviors (such as hazardous drinking, drug use, and unprotected sex) relative to pre-pubertal years (MacArther et al., 2012; Kuntsche and Gmel, 2013). Additionally, endogenous testosterone levels in human men correlate with increased risk taking under uncertainty in both the Iowa Gambling Task (Stanton et al., 2011) and the stock market (Coates and Herbert, 2008). Furthermore, EtOH use in teen girls correlates with circulating estradiol levels (Martin et al., 1999) and higher androgen levels in pubertal boys correlate with greater probability of lifetime EtOH use (de Water et al., 2013). Therefore, the presence of circulating gonadal steroids may enhance EtOH effects on behavior, potentially increasing risk-taking in one or both sexes. To address this possibility, the present study compared gonadectomized male and female rats with and without hormone replacement to investigate the influence of EtOH and gonadal steroids on the response to uncertainty as a measure of risk-taking behavior.
Sex differences exist in animal models of both risk-taking and EtOH use. Female rats, like female humans, are more risk-averse than males in an experimental model of decision-making (Orsini et al., 2016). Unlike humans, female rats exhibit greater preference for and higher voluntary intake of EtOH (McGlacken et al., 1995). However, this difference may be due to cultural constraints on women in regard to EtOH use. Animal models allow the study of sex and hormone effects on behavior and eliminate the potential confound of gender expectations. To test decision making in rats, discounting paradigms require subjects to choose between two rewards: a small “safe” reward, and a large reward that is “discounted” or made less desirable by pairing with a cost. Probability discounting tests risk taking in the context of reward uncertainty, a paradigm related to human risk taking in the form of gambling. Rats choose between a small/certain reward of 1 pellet, and a large/uncertain reward of 3–4 pellets, delivered with decreasing probability in successive blocks of trials. Probability discounting has been used to show effects of drugs such as amphetamine on risk taking (St. Onge and Floresco, 2009). However, EtOH effects on risky behavior in probability discounting have not been determined.
Because drinking, risk taking, and gambling are correlated in human studies, we hypothesized that EtOH would increase tolerance to uncertainty in rats performing probability discounting. Like EtOH, gonadal steroids influence decision-making, with increased hormone levels facilitating risk taking in human males (Stanton et al., 2011; Coates et al., 2008). Therefore, we expected that rats receiving gonadal steroid replacement would exhibit greater risk taking than their counterparts without steroids. Finally, EtOH may interact with sex and/or hormonal milieu. Because drinking and risk taking increase during human adolescence, EtOH may have a stronger effect on risky decision making in steroid-treated rats.
2. METHODS
2.1 Animals
Young adult male and female Long-Evans rats (8–10 weeks of age at the start, Charles River Laboratories, MA) were pair-housed under a reversed 14L:10D photoperiod. As in our previous studies (Cooper et al., 2014; Wallin and Wood, 2015), rats were food-restricted to maintain a slow rate of growth (Figure 1B; (Charles River, 2016) and facilitate operant responding. Experimental procedures were approved by USC’s Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th Ed (National Research Council, National Academies Press, Washington DC; 2011).
Figure 1.
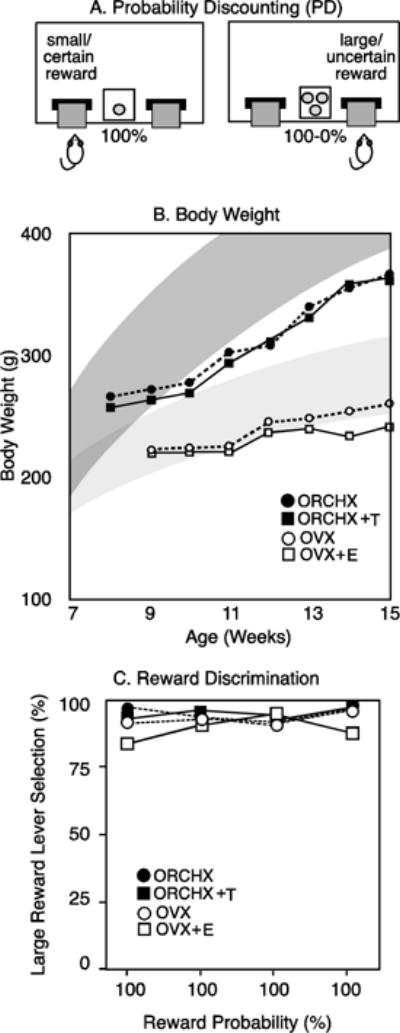
A) Probability Discounting to measure risk taking with operant behavior. Rats choose between a small/certain reward (1 pellet delivered with 100% probability) and a large/uncertain reward (3 pellets delivered with decreasing probability). B) Average body weights of each group throughout the experiment, relative to the range of normal body weights for male and female Long-Evans rats fed ad libitum (Charles River, 2016) C) Large reward lever selection in free-choice trials by each of the 4 groups during reward discrimination training.
2.2 Gonadectomy
All rats (16 male, 18 female) were gonadectomized under ketamine/xylazine anesthesia, and half received hormone replacement (n=7–9/group) as in our previous studies (Antzoulatos et al., 2011; Kent et al., 2013). Hormone implants were made from Silastic tubing (id: 1.57 mm, od: 3.18 mm, Dow Corning, Midland, MI) filled with crystalline steroid to provide chronic replacement of testosterone or estradiol at physiologic levels (Moger, 1976; Bridges, 1984). Males were castrated via a mid-line scrotal incision, and received a 10mm Silastic implant s.c. either filled with testosterone (ORCHX+T) or blank (ORCHX). These testosterone implants maintain serum testosterone at the level of intact adult male rats (~2.5 ng/ml) (Moger, 1976; Damassa et al., 1977). Females were ovariectomized via bilateral dorsal flank incisions and received a 5mm implant s.c. filled with 17β-estradiol (OVX+E) or blank (OVX). These estradiol implants maintain serum 17β-estradiol at 50–100 pg/ml, corresponding to periovulatory estrogen levels during proestrus in rats (Becker et al., 2005; Mosquera et al., 2015). Rats were allowed to recover from surgery for at least 2 weeks before behavioral testing. This time-course is sufficient to demonstrate decreases in hormone-driven behaviors (e.g., mating) after gonadectomy (Hull et al., 2006).
2.3 Training
2.3.1 Operant Chambers
Training and testing took place during the first 4 hours of the dark phase in daily sessions, 5 days/week. Testing was conducted in operant chambers (Med Associates, VT) with 2 retractable levers flanking a pellet dispenser and food cup (Figure 1A). Chambers were illuminated by a house light and were enclosed in sound-attenuating boxes with fans for ventilation.
2.3.2 Lever Training
Initially, rats were trained to respond on each lever (one lever per session, continuously available) to receive 45mg sucrose pellets (Bio-Serv Inc., Frenchtown, NJ). Next, rats were habituated to lever insertion. Each 20-second trial began in darkness with both levers retracted in the inter-trial interval (ITI) state. Three seconds later, the house-light was illuminated and 1 lever was inserted into the chamber. Left and right levers were each inserted once per pair of trials in random order. If the rat responded within 10 seconds, 1 pellet was delivered and the house-light stayed on for 4 seconds before returning to ITI. If the rat failed to respond within 10 seconds, the chamber reverted to ITI and the trial counted as an omission. Training continued until rats omitted <5 of 90 trials.
2.3.3 Reward discrimination
Each daily session consisted of 48 trials divided into 4 blocks. Each block consisted of 6 forced-choice trials and 6 free-choice trials. In the forced-choice trials, 1 lever was inserted into the chamber on each trial (3 trials/lever). In free-choice trials, both levers were inserted, and the rat could select either the small or large reward lever. No probability cost was imposed during reward discrimination. A response on the small reward lever delivered 1 pellet, and a response on the large reward lever delivered 3 pellets (Figure 1A). Location of the small and large reward levers (left vs. right) was counterbalanced among rats. Rats were required to complete 48 trials with >80% selection of the large reward lever.
2.4 Testing
2.4.1 Probability Discounting
Procedures were modified from St. Onge and Floresco. Probability discounting was identical to reward discrimination, except that delivery of the large reward became uncertain. Selection of the small/certain reward lever always resulted in delivery of 1 pellet. Selection of the large/uncertain reward lever delivered 3 pellets with decreasing probability in each block (100, 66.6, 33.3, and 0%). On rewarded trials, the house light remained illuminated for 2 seconds after lever selection while pellets were delivered. On unrewarded trials, the chamber reverted immediately to ITI after the rat responded on the large/uncertain reward lever. The number of trials and the reward schedule were adapted from previous studies (St. Onge and Floresco, 2009; Wallin et al., 2015) so that females would not become satiated during a daily test session.
2.4.2 Ethanol treatment
Rats were tested on probability discounting for 12 days until performance stabilized. Stability was determined as in previous studies (Cooper et al., 2014; Wallin et al., 2015) by Repeated Measures (RM)-ANOVA with test day as the repeated measure. Decision-making behavior was considered stable when there was no effect of test day on selection of the large reward for 3 consecutive days. Next, rats were tested for probability discounting after injection of saline and increasing doses of EtOH (0.5 and 1.0 g/kg) in a within-subjects design. 15 minutes prior to behavioral testing, rats were injected ip with 20% EtOH prepared from a commercially-available neutral grain spirit (Everclear, Luxco, St. Louis MO; Crabbe et al., 2008) or an equivalent volume of 0.9% saline (Winchester Laboratories, Riviera Beach, FL). Rats were first treated with saline for 5 days. Choice behavior from the last 3 days of saline treatment was averaged for each rat. Subsequently, rats were treated with EtOH at 0.5 and 1.0 g/kg (3 days/dose). After each EtOH dose, rats were retested on probability discounting for 2 days with saline treatment to control for any drift in baseline choice behavior.
2.5 Loss of Righting Reflex (LORR)
After all operant behavior testing was completed, all rats were tested for loss of righting reflex according to (Silveri and Spear, 1999). Rats were given a hypnotic dose of EtOH (3.5g/kg) ip and placed on their backs until the righting reflex was regained, defined as rats successfully turning over and righting themselves three times. Sleep time was measured as the duration between loss and regaining of righting reflex.
2.6 Data Analysis
2.6.1 Choice behavior
Data were analyzed as in St. Onge and Floresco (2009). For each free-choice trial, rats could respond on the large reward lever, the small reward lever, or omit a response. Large reward preference was calculated as the number of free-choice trials where the rat responded on the large reward lever divided by the number of completed trials (trials with large or small reward lever responses). Choice behavior in each block was averaged for each rat over the 3 days of testing under each dose of saline or EtOH. Behavior was then averaged for each of the 4 hormone groups (ORCHX, ORCHX+T, OVX, and OVX+E) in each block.
Data from males and females were analyzed separately. Within each sex, large reward preference was analyzed by Repeated Measures-Analysis of Variance (RM-ANOVA) with hormone as the between-subjects factor and dose (saline, 0.5, and 1.0 g/kg EtOH) and probability block as repeated measures. When there was an interaction with or a main effect of dose, data from each EtOH dose were compared to data from saline treatment, as in (St. Onge and Floresco, 2009). To investigate hormone effects on decision making in the absence of alcohol, baseline data from rats with and without hormone replacement after saline were compared. Choice latency (elapsed time before the rat selected a lever) under each EtOH dose was also analyzed for each hormone group and compared by RM-ANOVA. Trial omissions and LORR were analyzed by two-way ANOVA, with sex and hormone as factors.
2.6.2 Win-Stay and Lose-Shift Ratios
To investigate sensitivity to reward delivery and omission in probability discounting, Win-Stay (WS) and Lose-Shift (LS) behavior was analyzed on a trial-by-trial basis as in (Wallin et al., 2015). A WS occurred when the rat received the large reward (win), and responded on the large reward lever again in the following trial (stay). A LS occurred when the rat received no pellets from the large reward lever (loss), and selected the small reward on the following trial (shift). WS and LS ratios were computed as the number of times each behavior occurred divided by the total number of wins or losses, respectively. WS and LS ratios were averaged for each group and compared by RM-ANOVA. Note that WS ratios could not be calculated for the 0% block, as wins were not possible, and LS ratios could not be calculated for the 100% block, since losses were not possible.
3. RESULTS
3.1 Selection of large reward lever
As shown in Fig. 1C, males and females made a strong and consistent responses for the large reward lever in free-choice trials during reward discrimination training. During training, rats completed 48 trails/day (24 each, free-choice and forced-choice) where selection of the large reward delivered 3 pellets with 100% probability across all blocks. Successful completion of reward discrimination training required rats to select the large reward lever on ≥80% of free-choice trials. As a consequence, each rat received at least 100 sugar pellets, more than the maximum number of pellets available during probability discounting (72 pellets). From their responses during reward discrimination training, neither male nor female rats show evidence of satiety in 48 trials/day.
Figure 2 presents preference for the large/uncertain reward lever in males and females with and without hormone replacement in response to saline, 0.5, and 1.0 g/kg EtOH during 6 free-choice trials in each probability block. Preference for the large/uncertain reward lever in saline-treated ORCHX+T males was similar to that of vehicle-treated intact males in previous studies (Wallin et al., 2015). This group selected the large/uncertain reward on 84.0% and 23.9% of completed trials in the 100% and 0% probability blocks, respectively. For every behavioral measure (large reward lever preference, choice latency, WS and LS ratios), there was a significant effect of probability block by RM-ANOVA. In male rats, there was a significant main effect of dose on large reward lever preference, with EtOH treatment increasing preference for the large/uncertain reward lever (F2,28=10.462, p<0.05). In males, there was also a main effect of hormone at baseline, such that ORCHX+T males showed a greater preference for the large/uncertain reward lever then ORCHX males after saline (F1,14=13.805, p<0.05). By contrast, there was no main effect of EtOH dose preference for the large, uncertain reward lever in females (F1,30=0.914, p>0.05), although there was a dose x probability block interaction (F6,90=3.41, p=0.05). At baseline, there was a trend toward OVX+E females exhibiting a greater preference for the large, uncertain reward lever than OVX females (F1,16=3.83, p=0.06). To further examine the effects of EtOH on response to uncertainty, large reward lever preference at each EtOH dose was compared to baseline behavior after saline, as in (St. Onge and Floresco, 2009). As shown in Figure 2, both 0.5g/kg (F1,14=5.99, p<0.05) and 1.0 g/kg EtOH (F1,14=15.97, p<0.05) significantly increased large reward preference above baseline in males. However, in females, neither EtOH dose was sufficient to significantly increase large reward lever preference above baseline levels (F1,16=0.98, p<0.05 and F1,15=1.4, p>0.05, respectively).
Figure 2.
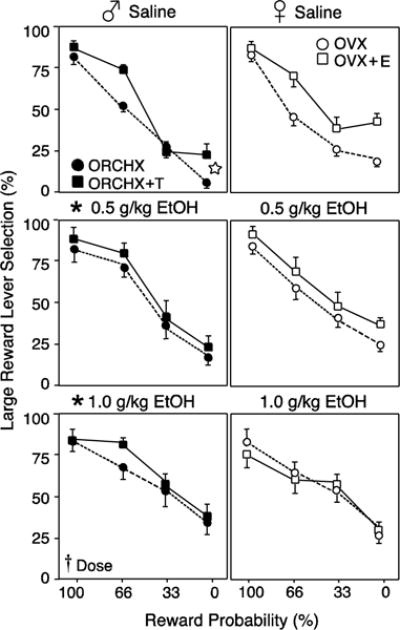
Large reward lever selection (mean±SEM) during 6 free-choice trials in each probability block after saline, 0.5 g/kg EtOH, or 1.0 g/kg EtOH. Rats were gonadectomized males (♂, left, closed symbols) and females (♀, right, open symbols), with (squares) and without (circles) hormone replacement. Cross indicates main effect of EtOH dose (p<0.05) by RM-ANOVA. Asterisks indicate significant effect of each dose compared to baseline. Star indicates significant effect of hormone at baseline.
3.2 Choice Latency
Figure 3 compares choice latency among all groups under saline, 0.5g/kg, and 1.0g/kg EtOH in each probability block. In males, there was no effect of EtOH or hormone treatment on choice latency, and no significant interactions (all p>0.05). In contrast, EtOH treatment affected choice latency in females, indicated by a dose × probability block interaction (F6,36=3.64, p<0.05). Further analysis comparing choice latencies at each EtOH dose to baseline behavior revealed that pretreatment with 1.0g/kg EtOH significantly decreased choice latency in females rats only (F1,10=7.25, p<0.05). This suggests that EtOH increases impulsivity selectively in females by decreasing the time spent deliberating choices.
Figure 3.
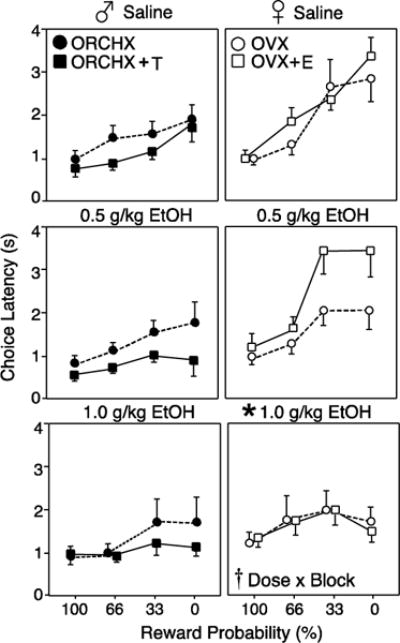
Latency to choose a lever (seconds; mean±SEM) with probability discounting. Behavior was measured during 6 free-choice trials in each probability block after saline, 0.5 g/kg EtOH, or 1.0 g/kg EtOH. Rats were gonadectomized males (♂, left, closed symbols) and females (♀, right, open symbols), with (squares) and without (circles) hormone replacement. Cross indicates significant interaction of EtOH dose × probability block (p<0.05) by RM-ANOVA. Asterisk indicates significant effect of dose compared to baseline.
3.3 Lose-Shift and Win-Stay behavior
The LS ratio indicates the likelihood that a rat will shift to the small/safe lever after a loss on the large/risky lever, and is a measure of sensitivity to reward omission. Figure 4 shows that the LS ratio increased for all groups as probability of receiving the large reward decreased (males: F2,18=30.66, p<0.05; females: F2,10=8.74, p<0.05). EtOH treatment decreased loss sensitivity in both sexes. There was a main effect of EtOH dose on LS ratos in both males (F2,18=5.10, p<0.05) and females (F2,10=4.37, p<0.05). 1.0g/kg EtOH significantly decreased LS ratios in both males and females (F1,10=17.75, p<0.05 and F1,7=6.64, p<0.05; respectively). However, compared to baseline levels, 0.5g/kg EtOH only significantly decreased LS ratios in female rats (F1,7=7.04, p<0.05) and did not significantly affect males. These data show that rats were significantly less likely to shift to the safe lever after a loss on the large lever under the influence of EtOH, and suggests that females are more sensitive to the effect of EtOH to decrease loss sensitivity. In contrast to LS, the WS ratio indicates likelihood of choosing the large/risky lever again after a win, and provides a measure of sensitivity to reward delivery. There was no effect of hormone or EtOH on WS behavior in either sex, nor were there any interactions (all p’s >0.05; data not shown), suggesting that EtOH does not increase reward sensitivity.
Figure 4.
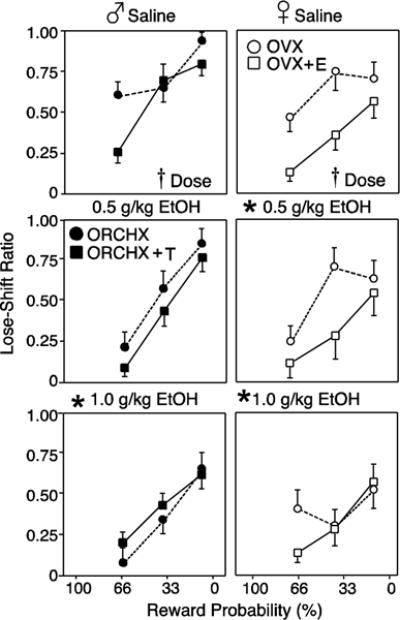
Lose-Shift ratios (mean±SEM) with probability discounting. Behavior was measured during 6 free-choice trials in each probability block after saline 0.5 g/kg EtOH or 1.0 g/kg EtOH. Rats were gonadectomized males (♂, left, closed symbols) and females (♀, right, open symbols), with (squares) and without (circles) hormone replacement. Crosses indicate main effect of EtOH dose (p<0.05) by RM-ANOVA. Asterisks indicates significant effect of each dose compared to baseline.
3.4 Omissions
Figure 5 compares average number of omitted free-choice trials per session by each of the four groups. By two-factor ANOVA, there was a main effect of sex, with females omitting more trials than males during probability discounting (F1,30=19.31, p<0.05). For this reason, large reward preference is presented as a percent of completed trials to correct for omissions. There was no effect of hormone (F1,30=.001, p>0.05) or EtOH dose (F2,29=2.26, p>0.05) on trial omissions.
Figure 5.
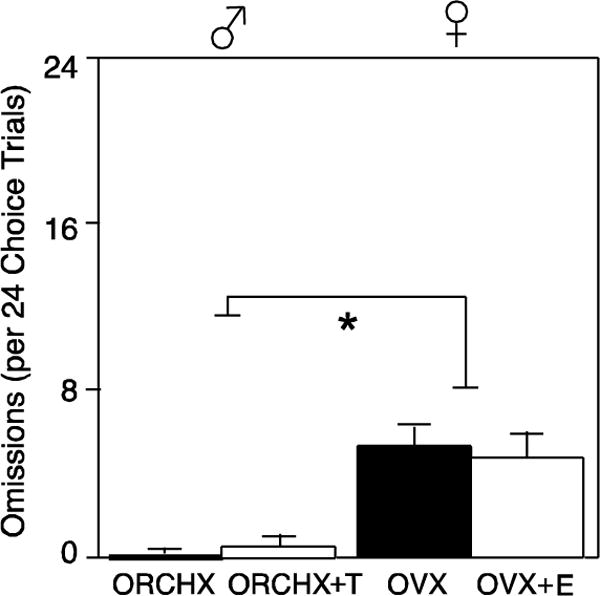
Trial omissions (mean±SEM) per 24 free-choice trials per session by gonadectomized males (♂, left, closed symbols) and females (♀, right, open symbols), with (squares) and without (circles) hormone replacement. Asterisk indicates p<0.05 by ANOVA.
3.5 Loss of Righting Reflex
Figure 6 compares sleep time in the LORR test. By two-factor ANOVA, there was a significant effect of sex, with females waking up significantly faster than males (F1,28=9.82, p<0.05). While there was no main effect of hormone (F1.28=0.70, p>0.05), there was a significant interaction between sex and hormone, with testosterone-treated males taking longer to wake up than males without testosterone (270.6 ± 30.1 vs. 192.3 ± 28.3 minutes, respectively) and estrogen-treated females waking up more quickly than their untreated counterparts (126.9 ± 25.7 vs. 162.4 ± 23.3 minutes, respectively; F1,28=4.34, p<0.05). However, post hoc t-tests with the Bonferonni correction for multiple comparisons revealed no effect of hormone on LORR within each sex, such that ORCHX males did not significantly differ from ORCH+T (p>0.05) and OVX females did not significantly differ from OVX+E (p>0.05).
Figure 6.

Sleep time (minutes, mean±SEM) in loss of righting reflex test after 3.5 g/kg EtOH. Rats were gonadectomized males (♂, left) and females (♀, right), with (closed bars) and without (open bars) hormone replacement. Asterisk indicates p<0.05 by ANOVA.
4. DISCUSSION
The present study investigated effects of EtOH, sex, and gonadal hormones on decision-making in the context of reward uncertainty. As hypothesized, EtOH increased risk taking by increasing preference for the large/uncertain reward lever, but did so only in male rats. Also as predicted, the presence of gonadal hormones increased baseline levels of risk taking. Testosterone significantly increased risk taking in males and estradiol caused an increase in risk taking in females that just missed significance. EtOH also increased impulsivity (indicated by a decrease in choice latency), but had this effect on females only. Finally, EtOH decreased sensitivity to loss (measured by LS behavior) in both sexes. These results show that sex, hormones, and EtOH interact to influence decision making under uncertainty.
There is a long history of testing gonadectomized males and females with and without chronic steroid replacement as a model to investigate sex differences and activational (post-pubertal) effects of gonadal steroid hormones (Miller et al., 2016). Compared with ORCHX males and OVX females, the ORCHX+T and OVX+E groups provide a surgical control, and ensure that any effects of castration or ovariectomy can be attributed to gonadal steroids. They further eliminate differences in steroid levels within and between individuals. In this regard, testosterone levels in male rats vary considerably between individuals (Callies et al., 2003) and follow a circadian pattern of secretion (Waite et al., 2009). Variability in steroid levels is an even greater concern in females, for whom estrogen and progesterone fluctuate across the estrous cycle and could potentially modify cognitive function. However, recent research suggests that behavioral variability in normally-cycling females is not greater than in males (Becker et al., 2016). Furthermore, a recent study in female rats found no effect of estrous cyclicity on decision-making in an effort-discounting task (Uban et al., 2012). While ovariectomy increased selection of a large/high effort reward, estradiol rescued behavior back to the level of ovary-intact females. For these reasons, ORCHX+T and OVX+E rats present a reasonable comparison vs gonadectomized males and females.
There was a sex difference in risk taking under the influence of EtOH, such that EtOH increased risk taking only in male rats. In this way, EtOH effects on probability discounting resemble amphetamine effects on delay discounting. In delay discounting rats choose between a small/immediate reward and a large/delayed reward. Eubig et al. (2014) investigated sex differences in delay discounting under the influence of amphetamine and found no baseline sex difference in delay discounting. However, drug treatment revealed a sex difference, with males selecting the large/delayed reward more than females when under the influence of amphetamine. Thus, in both studies only males preferred the large/discounted reward more when under the influence of drug. These results show that males and females do not necessarily have the same response to drugs of abuse, and highlight the importance of including sex differences in the study of drug effects on decision-making.
Decision-making behavior is particularly sensitive to DA function in the nucleus accumbens (Acb; Orsini et al., 2015; Mitchell et al., 2014; Stopper et al., 2012). Thus, observed effects of hormones and EtOH in probability discounting may be due to differences in the mesolimbic DA system. Risk taking in probability discounting correlates with DA release in Acb, with greater increases in Acb DA corresponding to stronger preference for the large/uncertain reward (St. Onge et al., 2012). Because EtOH increases DA release in Acb (Blanchard et al., 1993), it is not surprising that EtOH treatment also increased risk taking (relative to saline treatment) in male rats. Modulation of Acb DA by sex and gonadal hormones may also underlie observed differences in risk taking. For instance, females rats have lower baseline levels of Acb DA than males (Xiao and Becker, 1994). This sex difference in Acb DA could explain why EtOH failed to increase risk taking in females. Furthermore, gonadectomy decreases Acb DA levels in both males and females (Xiao and Becker, 1994; Mitchell et al., 1989). Therefore, it is not surprising that gonadectomized rats in the current study exhibited decreased risk taking compared to their hormone-treated counterparts at baseline. Sex and hormone effects on decision may also be attributed to differences in dopamine receptor density and/or function. D1-type dopamine receptors (D1R) are a crucial determinant of risk-taking behavior in probability discounting (Orsini et al., 2015; Stopper et al., 2012). Treatment with D1R antagonists, either systemically (St. Onge and Floresco, 2009) or locally in Acb or mPFC (Stopper et al., 2012; St. Onge et al., 2011), decreases risk taking in probability discounting. In contrast, treatment with a D1R agonist increases risk taking in this task. Adult male rats have a higher density of D1R in the striatum than females, but this difference does not emerge until puberty (Yoest et al., 2014). Therefore, the dependence of striatal D1R density on hormonal milieu may explain the increased risk taking in testosterone-treated males at baseline. Additionally, the sex difference in D1R density may underlie the propensity for increased risk taking under the influence of EtOH exhibited only by males.
Male rats, in addition to taking more risk than females when under the influence of EtOH, took longer to recover in the LORR test. This longer sleep time in males indicates increased sensitivity to the hypnotic effects of EtOH and corresponds with previous findings (Silveri and Spear, 1999). While we did not measure blood ethanol levels, it has previously been shown blood alcohol content does not differ between male and female rats at any time point up to 5 hours after a similar dose of 3.0 g/kg of EtOH (Webb et al., 2002). Interestingly, when Silveri and colleagues(1999) tested LORR in gonadally-intact males and females throughout development, the sex difference in sleep times did not emerge until young adulthood, after puberty and adolescence. This suggests that the sex difference in sensitivity to EtOH’s hypnotic effects is dependent on gonadal hormones. The current study supports these earlier findings, with OVX+E females exhibiting the shortest sleep times and ORCHX+T males sleeping the longest. Gonadectomy reduced this sex difference, with no difference in sleep time between OVX females and ORCHX males. Thus, males are more sensitive to both the hypnotic and risk-enhancing effects of EtOH.
Results from this study and others investigating decision making have both biological and clinical significance. In many social mammal species, individuals (of at least one sex) disperse from the natal group during adolescence. This behavior is adaptive, as it decreases inbreeding and promotes genetic diversity. Dispersal is inherently risky, entailing both uncertainty and physical risk. Therefore, it makes sense that adolescents should be less risk-averse, and that hormone-treated rats in our study took more risks than rats without hormone replacement. In modern human society, the increase in risk taking at adolescence is maladaptive, as accidental injury is the leading cause of death among adolescents, and drug use and problematic gambling are major public health concerns (Centers for Disease Control and Prevention, 2016; Substance Abuse and Mental Health Services Administration, 2014; Kagan et al., 2014). Importantly, behavioral differences in rats correspond to those observed in humans, with males taking more risk than females, and gonadal hormones increasing risk taking in both sexes. Our work furthers understanding of sex differences in decision-making, particularly in response to drugs of abuse. The current study shows that males are especially susceptible to the maladaptive behavioral effects of EtOH. Thus, this model can be used to investigate neural mechanisms underlying sex differences in innate behavioral tendencies, and in sex differences in drug-induced behavioral changes.
5. CONCLUSION
The current study shows that gonadal hormones increase risk taking in the context of reward uncertainty. Furthermore, sex and hormones interact with EtOH with to influence decision-making. At baseline, hormonal milieu is important for determining levels of risk taking. However, with EtOH treatment a sex difference emerges, with males taking more risk under EtOH influence, and female risk taking unaffected by EtOH. These findings have important implications for public health and safety, particularly for adolescents who experience increased hormone levels and often drink EtOH and engage in risky behavior.
Highlights.
Probability discounting measures decision-making under conditions of uncertainty
Steroid hormones promote risky decision-making in rats
Under the influence of ethanol, males take more risks than females
Acknowledgments
We thank Ms. Grace Li for data collection. This work was supported by the National Institutes of Health (NIH R21-AA020575 to RIW).
Role of Funding Source:
Nothing declared
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
KWM and RIW conceived and designed the study and wrote the manuscript. KWM performed data acquisition and analysis. JRC and JC participated in data acquisition and analysis. All authors read and approved the final manuscript.
Conflict of Interest:
No conflict declared
References
- Alcohol Facts and Statistics. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics. Accessed July 26, 2016.
- Antzoulatos E, Jakowec MW, Petzinger GM, Wood RI. MPTP neurotoxicity and testosterone induce dendritic remodeling of striatal medium spiny neurons in the C57Bl/6 mouse. Parkinsons Dis. 2011 doi: 10.4061/2011/138471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ. 2016;7:34. doi: 10.1186/s13293-016-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, Glick SD. Sex Differences in Ethanol-induced Dopamine Release in Nucleus Accumbens and in Ethanol Consumption in Rats. Alcohol Clin Exp Res. 1993;17:968–973. doi: 10.1111/j.1530-0277.1993.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Borges G, Cherpitel CJ, Medina-Mora ME, Mondragon L. Violence related injuries in the emergency room: alcohol, depression, and conduct problems. Subst Use Misuse. 2004;39:911–930. doi: 10.1081/ja-120030893. [DOI] [PubMed] [Google Scholar]
- Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinol. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- Callies F, Kollenkirchen U, Von Zur Mühlen C, Tomaszewski M, Beer S, Allolio B. Testosterone undecanoate: a useful tool for testosterone administration in rats. Exp Clin Endocrinol Diabetes. 2003;111:203–208. doi: 10.1055/s-2003-40464. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Adolescent Health. 2016 http://www.cdc.gov/nchs/fastats/adolescent-health.htm. Accessed Aug 10, 2016.
- River Charles. Long-Evans Rats 2016 Pricing Catalogue. 2016 http://www.criver.com/files/pdfs/rms/us-model-pricing/rm_rm_c_long_evans_rats.aspx. Accessed November 1, 2016.
- Cherpitel CJ. Alcohol, injury, and risk-taking behavior: Data from a National Sample. Alcohol Clin Exp Res. 1993;17:762–766. doi: 10.1111/j.1530-0277.1993.tb00837.x. [DOI] [PubMed] [Google Scholar]
- Coates JM, Herbert J. Endogenous steroids and financial risk taking on a London trading floor. Proc Natl Acad Sci. 2008;105:6167–6172. doi: 10.1073/pnas.0704025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SE, Goings SP, Kim JY, Wood RI. Testosterone enhances risk tolerance without altering motor impulsivity in male rats. Psychoneuroendocrinol. 2014;40:201–212. doi: 10.1016/j.psyneuen.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Cameron AJ, Munn E, Bunning M, Wahlsten D. Overview of mouse assays of ethanol intoxication. Curr Protoc Neurosci. 2008:9–26. doi: 10.1002/0471142301.ns0926s42. [DOI] [PubMed] [Google Scholar]
- Damassa DA, Smith ER, Tennent B, Davidson JM. The relationship between circulating testosterone levels and male sexual behavior in rats. Horm Behav. 1977;8:275–286. doi: 10.1016/0018-506x(77)90002-2. [DOI] [PubMed] [Google Scholar]
- de Water E, Braams BR, Crone EA, Peper JS. Pubertal maturation and sex steroids are related to alcohol use in adolescents. Horm Behav. 2013;63:392–397. doi: 10.1016/j.yhbeh.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Eubig PA, Noe TE, Floresco SB, Sable JJ, Schantz SL. Sex differences in response to amphetamine in adult Long–Evans rats performing a delay-discounting task. Pharmacol Biochem Behav. 2014;118:1–9. doi: 10.1016/j.pbb.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Wood RI, McKenna KE. Male sexual behavior. In: Neill JD, editor. The Physiology of Reproduction. 3rd. Raven Press; New York: 2006. pp. 1729–1824. [Google Scholar]
- Johansson A, Grant JE, Kim SW, Odlaug BL, Götestam KG. Risk factors for problematic gambling: A critical literature review. J Gambl Stud. 2009;25:67–92. doi: 10.1007/s10899-008-9088-6. [DOI] [PubMed] [Google Scholar]
- Kagan R, Whyte K, Esrick J, Carnevale J. Problem Gambling in the 21st century healthcare system. National Council on Problem Gambling. 2014 http://www.ncpgambling.org/wp-content/uploads/2014/07/ACA-brief-web-layout-publication.pdf. Accessed November 28, 2016.
- Kent K, Arientyl V, Khachatryan MM, Wood RI. Oxytocin induces a conditioned social preference in female mice. J Neuroendocrinol. 2013;25:803–810. doi: 10.1111/jne.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Gmel G. Alcohol consumption in late adolescence and early adulthood–where is the problem. Swiss Med Wkly. 2013;143:13826. doi: 10.4414/smw.2013.13826. [DOI] [PubMed] [Google Scholar]
- MacArthur GJ, Smith MC, Melotti R, Heron J, Macleod J, Hickman M, Kipping RR, Campbell R, Lewis G. Patterns of alcohol use and multiple risk behaviour by gender during early and late adolescence: The ALSPAC cohort. Public Health. 2012;3:i20–i30. doi: 10.1093/pubmed/fds006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens MP, Rocha TL, Cimini MD, Diaz-Myers A, Rivero EM, Wulfert E. The co-occurrence of alcohol use and gambling activities in first-year college students. J Am Coll Health. 2009;57:597–602. doi: 10.3200/JACH.57.6.597-602. [DOI] [PubMed] [Google Scholar]
- Martin CA, Arch G, Curry T, Martin D. Alcohol use in adolescent females: Correlates with estradiol and testosterone. Am J Addict. 1999;8:9–14. doi: 10.1080/105504999306036. [DOI] [PubMed] [Google Scholar]
- McGlacken G, Jensen RA, Meliska CJ, Bartke A. Ethanol, nicotine, amphetamine, and aspartame consumption and preferences in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav. 1995;50:619–626. doi: 10.1016/0091-3057(94)00354-8. [DOI] [PubMed] [Google Scholar]
- Miller LR, Marks C, Becker JB, Hurn PD, Chen WJ, Woodruff T, McCarthy MM, Sohrabji F, Schiebinger L, Wetherington CL, Makris S. Considering sex as a biological variable in preclinical research. FASEB J. 2017;31:29–34. doi: 10.1096/fj.201600781R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JB, Stewart J. Effects of castration, steroid replacement, and sexual experience on mesolimbic dopamine and sexual behaviors in the male rat. Brain Res. 1989;491:116–127. doi: 10.1016/0006-8993(89)90093-0. [DOI] [PubMed] [Google Scholar]
- Mitchell MR, Weiss VG, Beas BS, Morgan D, Bizon JL, Setlow B. Adolescent risk taking, cocaine self-administration, and striatal dopamine signaling. Neuropsychopharmacol. 2014;39:955–962. doi: 10.1038/npp.2013.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moger WH. Effect of testosterone implants on serum gonadotropin concentrations in the male rat. Biol Reprod. 1976;14:665–669. doi: 10.1095/biolreprod14.5.665. [DOI] [PubMed] [Google Scholar]
- Mosquera L, Shepherd L, Torrado AI, Torres-Diaz YM, Miranda JD, Segarra AC. Comparison of two methods of estradiol replacement: Their physiological and behavioral outcomes. J Vet Sci Technol. 2015;6:276. doi: 10.4172/2157-7579.1000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) Guide for the Care and Use of Laboratory Animals. 8th. Washington (DC): National Academies Press (US); 2011. Committee for the Update of the Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- Nutt DJ, King LA, Phillips LD. Drug harms in the UK: A multicriteria decision analysis. Lancet. 2010;376:1558–1565. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Moorman DE, Young JW, Setlow B, Floresco SB. Neural mechanisms regulating different forms of risk-related decision-making: Insights from animal models. Neurosci Biobehav Rev. 2015;58:147–167. doi: 10.1016/j.neubiorev.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Willis ML, Gilbert RJ, Bizon JL, Setlow B. Sex differences in a rat model of risky decision making. Behav Neurosci. 2016;130:50. doi: 10.1037/bne0000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of rapid tolerance to the hypnotic effects of ethanol. Alcohol Clin Exp Res. 1999;23:1180–4. [PubMed] [Google Scholar]
- St Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci. 2011;31:8625–8633. doi: 10.1523/JNEUROSCI.1020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JRS, Ahn S, Phillips AG, Floresco SB. Dynamic fluctuations in dopamine efflux in the prefrontal cortex and nucleus accumbens during risk-based decision making. J Neurosci. 2012;32:16880–16891. doi: 10.1523/JNEUROSCI.3807-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacol. 2009;34:681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- Stanton SJ, Liening SH, Schultheiss OC. Testosterone is positively associated with risk taking in the Iowa Gambling Task. Horm Behav. 2011;59:252–256. doi: 10.1016/j.yhbeh.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Stopper CM, Khayambashi S, Floresco SB. Receptor-specific modulation of risk-based decision making by nucleus accumbens dopamine. Neuropsychopharmacol. 2013;38:715–728. doi: 10.1038/npp.2012.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Vol. 2014 Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. (NSDUH Series H-48, HHS Publication No. (SMA) 14-4863). [Google Scholar]
- Uban KA, Rummel J, Floresco SB, Galea LA. Estradiol modulates effort-based decision making in female rats. Neuropsychopharmacol. 2012;37:390–401. doi: 10.1038/npp.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite E, Kershaw Y, Spiga F, Lightman SL. A glucocorticoid sensitive biphasic rhythm of testosterone secretion. J Neuroendocrinol. 2009;21:737–741. doi: 10.1111/j.1365-2826.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- Wallin KG, Alves JM, Wood RI. Anabolic–androgenic steroids and decision making: Probability and effort discounting in male rats. Psychoneuroendocrinol. 2015;57:84–92. doi: 10.1016/j.psyneuen.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin KG, Wood RI. Anabolic-androgenic steroids impair set-shifting and reversal learning in male rats. Eur Neuropsychopharmacol. 2015;25:583–90. doi: 10.1016/j.euroneuro.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B, Burnett PW, Walker DW. Sex Differences in Ethanol-Induced Hypnosis and Hypothermia in Young Long-Evans Rats. Alcohol Clin Exp Res. 2002;26:695–704. [PubMed] [Google Scholar]
- Winstock AR. The global drug survey 2014 findings. Global Drug Survey. 2014 https://www.globaldrugsurvey.com. Accessed June 20, 2016.
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett. 1994;180:155–8. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Yoest KE, Cummings JA, Becker JB. Estradiol, dopamine and motivation. Cent Nerv Syst Agents Med Chem. 2014;14:83–89. doi: 10.2174/1871524914666141226103135. [DOI] [PMC free article] [PubMed] [Google Scholar]


