Abstract
Background
This paper compares adolescents with primary opioid problem use (OPU) to those with primary marijuana or alcohol problem use (MAPU) who received up to six months of Adolescent Community Reinforcement Approach (A-CRA), an empirically supported treatment.
Methods
Intake clinical characteristics, treatment implementation measures, and clinical outcomes of two substance problem groups (OPU and MAPU) were compared using data from 1,712 adolescents receiving A-CRA treatment. Data were collected at intake and 3, 6, and 12 months post-intake.
Results
At intake, adolescents in the OPU group were more likely than those in the MAPU group to be Caucasian, older, female, and not attending school; report greater substance and mental health problems; and engage in social and health risk behaviors. There was statistical equivalence between groups in rates of A-CRA treatment initiation, engagement, retention, and satisfaction. Both groups decreased significantly on most substance use outcomes, with the OPU group showing greater improvement; however, the OPU group had more severe problems at intake and continued to report higher frequency of opioid use and more days of emotional problems and residential treatment over 12 months.
Conclusions
The feasibility and acceptability of A-CRA for OPUs was demonstrated. Despite significantly greater improvement by the OPU group, they did not improve to the level of the MAPU group over 12 months, suggesting that they may benefit from A-CRA continuing care up to 12 months, medication to address opioid withdrawal and craving, and the inclusion of opioid-focused A-CRA procedures.
Keywords: Adolescent Community Reinforcement Approach, opioid, substance use treatment, adolescent, outcomes
1.0 Introduction
During the past decade, use of non-heroin opioids by secondary school students has almost doubled (Johnston et al., 2012), and there has been a corresponding increase in adolescent admissions to publicly-funded substance use treatment programs for opioid use (SAMHSA, 2015). A handful of studies have compared adolescents presenting for treatment with problematic opioid use to adolescents with problematic use of other commonly abused substances, notably alcohol and marijuana. Results indicate that adolescents with opioid use problems are more likely to be Caucasian, older, middle class, and suburban. As a group, they tend to have a higher proportion of females. They also have higher rates of school drop-out, substance use severity, multiple substance use disorders, health risk behaviors, and psychological distress (Clemmey et al., 2004; Gordon et al., 2004; Hopfer et al., 2000, 2002; Marsch et al., 2005; Subramaniam et al., 2009, 2010). In addition, Clemmey et al. (2004) found that adolescent heroin users engaged in more days of criminal behavior than non-heroin users. The above findings suggest that adolescents with opioid use problems tend to have a poor long-term prognosis (Subramaniam et al., 2009). This implication is supported by one study that found adolescent heroin users responded to substance use treatment in the same remitting/relapsing pattern as non-heroin users, but continued to report a higher percentage of days of substance use and greater number of substance abuse and dependence symptoms over 12 months of follow-up (Clemmey et al., 2004).
Opioid agonist treatment, such as methadone, buprenorphine, and buprenorphine plus naloxone, is well-researched and effective for adults with opioid dependence (Mattick et al., 2009; National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction, 1998). Controlled treatment research of medications to enhance opioid abstinence outcomes for adolescents is limited. Emerging literature provides initial support for the safety and efficacy of buprenorphine for this population (Marsch et al., 2005; Woody et al., 2008), and there is one ongoing open label trial currently underway in Clincaltrials.gov studying retention in medication-assisted treatment for youth with substance use disorders. However, to date we are unaware of published studies with adolescents comparing the effectiveness of combined medication and psychosocial treatment to the effectiveness of psychosocial treatment alone. It is, therefore, important to examine the impact of existing effective and manualized psychosocial treatments for substance use on adolescent opioid use and concomitant problems.
The Adolescent Community Reinforcement Approach (A-CRA) is an empirically-supported psychosocial treatment for adolescent substance use that has been widely implemented with standardized clinical training and supervision. Originally developed as the Community Reinforcement Approach (CRA), it was tested successfully with adults (Azrin et al., 1982; Hunt and Azrin, 1973) and then adapted for application with adolescents and clinically validated in several randomized trials with this age group (Dennis et al., 2004a; Godley et al., 2007, 2014; Henderson et al., 2016; Slesnick et al., 2007). A-CRA is predicated on helping clients better engage in their community. The “community” includes family, friends, school, work, and other organizations, and extra-curricular activities. Operant behavior change techniques are used to help adolescents develop a nonsubstance using lifestyle that becomes more rewarding than using (Azrin, 1976; Hunt and Azrin, 1973). Nineteen procedures are part of A-CRA and include problem solving, communication, anger management, and relapse prevention skills, among others. Medication monitoring and adherence is another unique procedure that can help facilitate compliance with prescribed medications. Clinicians choose from a menu of procedures to individualize treatment according to the needs of each adolescent (Godley et al., 2001; Meyers and Smith, 1995). Additionally, there are sessions designed for parents/caregivers and joint family sessions for the adolescent and parent/caregiver. Prior research has shown that A-CRA has relatively high and equivalent rates of treatment initiation, engagement, retention, and participant satisfaction across gender and ethnic groups (Godley et al., 2011b), co-occuring substance and mental health disorders, and juvenile justice problems (Godley et al., 2014; Henderson et al., 2016). Although CRA in combination with methadone or naltrexone has been successfully tested in studies with adults (Abbott, 2009; DeJong et al., 2007), there are no published studies of A-CRA for opioid problems with or without medication assistance and some researchers have called for the need to do so (Clemmey et al., 2004).
The purpose of this paper is to compare adolescents with primary opioid problem use (OPU) to those with primary marijuana or alcohol problem use (MAPU) who have received A-CRA treatment. Based on the literature, we hypothesized that relative to the MAPU group: a) adolescents in the OPU group would have similar rates of A-CRA treatment initiation, engagement, retention, and treatment satisfaction; and b) adolescents in the OPU group would respond to treatment similarly to the MAPU group, but continue to report greater rates of substance use and mental health problems over time. Results from this study will help the field better understand whether an evidence-based treatment such as A-CRA has potential as an outpatient treatment for adolescents with OPU and for further testing in randomized clinical trials with this population.
2.0 Material and methods
This study uses data from a large dissemination project funded by the Substance Abuse and Mental Health Services Administration’s (SAMHSA) Center for Substance Abuse Treatment (CSAT) from 2007 to 2012. Seventy-eight substance use disorder treatment organizations received grants to implement A-CRA treatment (Godley et al., 2011a). These organizations represented 26 states across the United States, including urban (42), rural (8), and mixed (28) communities. Most were community-based, not-for-profit agencies operating outpatient clinics. Across sites, each clinician delivering A-CRA received standardized training, cross-site supervision, ongoing fidelity assessment, and coaching (Godley et al., 2011a). Each site followed their respective IRB-approved consent procedures with adolescents.
2.1 Participants and design
The initial pool of data were collected from 4,027 adolescents. Data were included in these analyses for all participants who were in treatment long enough to allow calculation of study variables and who completed both the intake and 12-month post-intake interviews. Data were excluded from sites completing less than 50% of expected follow-up interviews and for participants not yet due for their 12-month follow-up at the close of the project. A final sample size of 1,712 participants from 71 sites was determined, and using these data, follow-up rates of 89% at 3 months post-intake, 84% at 6 months, and 75% at 12 months were achieved.
Participants were classified into two mutually exclusive substance problem groups at A-CRA treatment intake based on adolescents’ responses to questions administered as part of the Global Appraisal of Individual Needs (GAIN) upon treatment entry (Dennis et al., 2003). The “Marijuana or Alcohol Problem Use (MAPU)” group consisted of adolescents who either reported symptoms in the DSM-IV-TR indicative of marijuana and/or alcohol abuse (30%; 18%) or dependency (33%; 8%) in the past year or reported at least weekly use of marijuana (65%) and/or alcohol (15%) while in the community. They did not report opioid use or related problems sufficient to meet criteria for the opioid problem use group as described below.
The “Opioid Problem Use (OPU)” group consisted of adolescents who either reported symptoms in the DSM-IV-TR indicative of opioid abuse (22%) or dependence (31%) in the past year or reported at least weekly use of opioids while in the community (49%) with or without MAPU. While the MAPU group averaged less than one day of opioid use in the 90 days prior to intake, the OPU group averaged 25 days.
Adolescents reporting “at least weekly use” of the indicated substance were included in the MAPU and OPU groups because 43 sites were not required to, and opted out from, asking DSM-IV-TR abuse and dependence symptoms by specific drug. Instead, abuse and dependence symptoms were asked for any substance. As a result, 1,512 MAPU participants and 113 OPU participants were categorized as reporting “at least weekly use.” A more detailed inspection of intake data from these adolescents suggested that those with “at least weekly use” actually used marijuana or opioids more frequently than those with documented marijuana or opioid dependence. Specifically, in the MAPU group, “at least weekly” users of marijuana stated that they used marijuana 49% of the 90 days prior to treatment, as compared to 42% for those with dependence and 30% for those with abuse. In the OPU group, “at least weekly” users of opioids stated that they used opioids 52% of the 90 days prior to treatment, as compared to 23.6% for those with dependence and 11.5% for those with abuse. See the supplemental materials1 for detailed information on the OPU subgroups (“at least weekly use,” “ abuse,” and “dependence”).
2.2 Measures
2.2.1 Treatment initiation, engagement, retention, and satisfaction measures
Treatment initiation, engagement, and retention data were recorded in a secure online database by clinicians during the scheduled 12–14 week A-CRA program. Continuing care A-CRA sessions were also documented and could be provided for an additional 12 weeks. Measures of initiation and engagement are dichotomous yes/no variables. In accordance with definitions developed and tested by the Washington Circle Group (Garnick et al., 2009), if participants received a second treatment session within 14 days of the first treatment session, they met criteria for initiation. If participants received two additional treatment sessions within 30 days of the initiation date, they met criteria for engagement. Treatment retention was measured through the total number of client, caregiver or significant other, and family sessions documented for each adolescent. Treatment Satisfaction was assessed through the Treatment Satisfaction Scale (TxSS; Cronbach’s alpha = .96) of the GAIN administered to youth 3 months after treatment admission and is a count of 14 yes/no items measuring general satisfaction with treatment services and staff. The scale ranges from 0 to 14, with higher values indicating greater general satisfaction with treatment services and staff.
2.2.2 Treatment outcome measures
GAINs administered at treatment intake and at 3, 6, and 12 months post-intake were included in outcome analyses. Outcome measures included frequency of substance use (percent of days); number of abuse and dependence symptoms for any substance; average days of emotional problems; days in the past 90 the adolescent was incarcerated; days in the past 90 spent in residential substance use treatment; and the percent of days in the past 90 that participants reported using any alcohol, opioids, marijuana, and other illicit psychoactive drugs while living in the community.
2.3 Analytic plan
2.3.1 Intake analyses
First, intake values were compared between the MAPU and OPU groups. To control for any nesting effects due to similarities between participants in the same treatment facility, a binomial HLM analysis using HLM version 7.0 (Raudenbush et al., 2011) was conducted. HLM ensures that the assumption of independence of observations was not violated by partitioning variance into within and between treatment facility variance. The following binary measurements were analyzed: gender, current criminal justice involvement, single parent family status, symptoms of externalizing mental health disorders, symptoms of internalizing mental health disorders, and symptoms of any co-occurring mental health disorders. The intake analyses also examined the percent of days using AOD, alcohol, marijuana, and opioids, average number of substance use disorder symptoms, average days of emotional problems, days in a controlled environment, and race. Missing data were dropped from the analyses because of the large sample size and small rates of missing data.
2.3.2 Treatment initiation, engagement, retention, and satisfaction analyses
Since a finding of statistical nonsignificance between groups does not necessarily indicate equivalence, equivalence testing was conducted using methods described in Rogers et al. (1993) to determine if the two conditions were similar on the dichotomous variables of initiation and engagement, as well as the following continuous variables: number of client-only sessions, number of caregiver- or significant other-only sessions, number of family sessions, total number of sessions, and score on the Treatment Satisfaction Scale. For dichotomous variables, δ1 and δ2 (i.e., the minimum acceptable difference in a positive and negative direction for equivalence between the MAPU and OPU groups) were established as 20% of the MAPU group proportion. For continuous variables, δ1 and δ2 were established as .2 SD of the MAPU condition. The significance of the equivalence was tested using two one-tailed z-tests. All measures were completed by 100% of the eligible sample except for the Treatment Satisfaction Scale measured at the 3-month interview, which was completed by 81.6% of the eligible sample.
2.3.3 Treatment outcome analyses
A two-level hierarchical multivariate linear model (HMLM2) with maximum likelihood estimation was used to analyze the following outcomes: percent of days using AOD, alcohol, marijuana, opioids, and other illicit substances while living in the community, average number of substance use disorder symptoms, and average days of emotional problems. In this analysis, observation waves across time were nested within participants and participants within treatment sites. For longitudinal analyses, missing data was replaced using the RMV function in SPSS for 3- and 6-month follow-up interviews. For the RMV function, cases were sorted by observation wave, treatment agency, gender, race, and age. The median of the two values above and two values below missing data points were used to replace the missing value. As an additional analysis, a two-level HLM was conducted to test for differences by substance problem group in the outcome variables at 12 months.
Finally, an analysis of intake characteristics was conducted to compare adolescents in the analytic sample to those adolescents not included due to less than a 50% follow-up rate or because their 12-month interview was not yet due.
3.0 Results
3.1 Demographic and clinical characteristics at intake
Participants in the OPU group were significantly more likely to be Caucasian and female, report symptoms of any co-occurring mental health disorder, report unprotected sex, report violent activity, endorse high treatment readiness, have received prior substance use and mental health treatment, report higher recovery environment and social risk, and report more illegal activity (see Table 1). They were also older by an average of two months. Participants who were in school and had higher self-efficacy to resist substance use were significantly less likely to be in the OPU group. A higher percent of all AOD use variables, except marijuana use, significantly increased the likelihood of being in the OPU group, as did higher average days of emotional problems, days incarcerated, and days in residential treatment.
Table 1.
Results of HLM Binomial Logistic regression analyses of treatment intake characteristics (N = 4,027).
| Intake characteristic | Marijuana or Alcohol Problem Use (n = 3,721)
|
Opioid Problem Use (n=306)
|
||
|---|---|---|---|---|
| M (SD)/N (%) | M (SD)/N (%) | OR | p | |
| Age | 15.65 (1.19) | 15.85 (1.13) | 1.14 | 0.046 |
| Female | 851 (23%) | 128 (42%) | 2.35 | <.001 |
| Race | ||||
| White | 1227 (33%) | 191 (63%) | 3.46 | <.001 |
| African American | 522 (14%) | 1 (1%) | 0.05 | <.001 |
| Hispanic | 1,256 (34%) | 62 (20%) | 0.28 | <.001 |
| Other | 714 (19%) | 49 (16%) | 0.46 | <.001 |
| Single parent family | 1,850 (50%) | 138 (45%) | 0.86 | 0.251 |
| Criminal justice involvement | 2,442 (66%) | 190 (62%) | 0.76 | 0.066 |
| Ever Homeless or Runaway | 1,356 (37%) | 176 (58%) | 2.29 | <.001 |
| In School in past 90 days | 3,450 (93%) | 255 (83%) | 0.39 | <.001 |
| Weekly Family Problems | 998 (27%) | 112 (37%) | 1.41 | .008 |
| Social peers using drugs | 2,709 (74%) | 235 (78%) | 1.14 | .351 |
| Internalizing disorders | 1,622 (44%) | 235 (77%) | 3.87 | <.001 |
| Major Depressive Disorder | 1,326 (36%) | 214 (70%) | 3.74 | <.001 |
| Generalized Anxiety Disorder | 410 (11%) | 93 (30%) | 3.27 | <.001 |
| Suicidal Thoughts | 434 (12%) | 87 (29%) | 2.53 | <.001 |
| High Traumatic Stress | 925 (25%) | 157 (51%) | 2.95 | <.001 |
| Externalizing disorders | 2,265 (61%) | 254 (83%) | 2.69 | <.001 |
| ADHD | 1,482 (40%) | 190 (63%) | 2.06 | <.001 |
| Conduct Disorder | 1,926 (52%) | 218 (71%) | 2.08 | <.001 |
| Any Unprotected Sex | 1,095 (31%) | 136 (46%) | 1.69 | <.001 |
| Any Physical Violence | 2,572 (69%) | 251 (82%) | 1.87 | <.001 |
| High Treatment Readiness | 1,466 (41%) | 169 (59%) | 1.91 | <.001 |
| Any Prior Substance Use Treatment | 1,274 (34%) | 196 (64%) | 2.98 | <.001 |
| Any Prior Mental Health Treatment | 1,559 (42%) | 217 (71%) | 2.92 | <.001 |
| Self-efficacy Scale | 4.14 (1.25) | 3.59 (1.47) | 0.70 | <.001 |
| Recovery Environment Risk Index | 0.25 (0.08) | 0.28 (0.10) | 1.35 | <.001 |
| Social Risk Index | 13.77 (4.29) | 14.52 (5.06) | 1.15 | .018 |
| Illegal Activity Scale | 0.11 (0.11) | 0.16 (0.17) | 1.26 | <.001 |
| Percent of days using alcohol | 9% (.18) | 15% (.23) | 1.27 | <.001 |
| Percent of days using opioids | 0.8% (.04) | 28% (.33) | 17.24 | <.001 |
| Percent of days using marijuana | 41% (.36) | 37% (.39) | 0.87 | 0.101 |
| Percent of days using Other Drugs | 4% (.15) | 26% (.35) | 1.75 | <.001 |
| Substance Use Disorder Symptoms | 1.80 (2.47) | 2.38 (3.14) | 1.08 | 0.023 |
| Days of Emotional Problems | 13.13 (25.42) | 25.45 (31.94) | 1.33 | <.001 |
| Days Incarcerated | 3.58 (12.14) | 6.86 (17.88) | 1.21 | 0.001 |
| Days in Residential Treatment | 6.19 (17.88) | 19.31 (29.57) | 1.60 | <.001 |
Note: percent of days used variables, indices and scales are transformed to z-scores so the odds ratio is based on change of 1 standard deviation.
OR= odds ratio
3.2 Treatment initiation, engagement, retention, and satisfaction
Both substance problem groups received significantly equivalent A-CRA treatment across all treatment measures (see Table 2).
Table 2.
Results of equivalence testing for treatment engagement, retention, and satisfaction measures.
| Marijuana or Alcohol Problem Use
|
Opioid Problem Use
|
SE | z1 | z2 | Cohen’s d | |||
|---|---|---|---|---|---|---|---|---|
| Mean (%) | SD | Mean (%) | SD | |||||
| Initiation | 79% | 0.4 | 82% | 0.4 | 0.02 | 8.08 | −5.77 | 0.07 |
| Engagement | 63% | 0.5 | 67% | 0.5 | 0.03 | 5.91 | −3.10 | 0.08 |
| Count of client only sessions a | 7.1 | 4.5 | 7.0 | 4.0 | 0.32 | 2.50 | −3.10 | −0.02 |
| Count caregiver or significant other only session a | 0.9 | 1.3 | 0.9 | 1.1 | 0.09 | 2.62 | −2.99 | −0.01 |
| Count family session a | 0.8 | 1.2 | 0.9 | 1.2 | 0.09 | 4.33 | −1.23 | 0.11 |
| Total number of sessions a | 8.9 | 5.7 | 8.9 | 5.0 | 0.41 | 2.63 | −2.99 | −0.01 |
| Total number of sessions in Months 4 – 6 | 1.7 | 3.4 | 2.1 | 4.0 | 0.21 | 5.15 | −1.28 | 0.12 |
| Treatment Satisfaction Scale | 11.7 | 6.0 | 12.5 | 4.5 | 0.38 | 5.45 | −0.84 | 0.15 |
Notes.
Bold type indicates the two groups were significantly equivalent.
Effect sizes can be interpreted as Cohen’s d, where a small effect ≥ .20; medium effect ≥ .5; and large effect ≥ .80.
Intake through 3 months.
3.3 Treatment outcomes
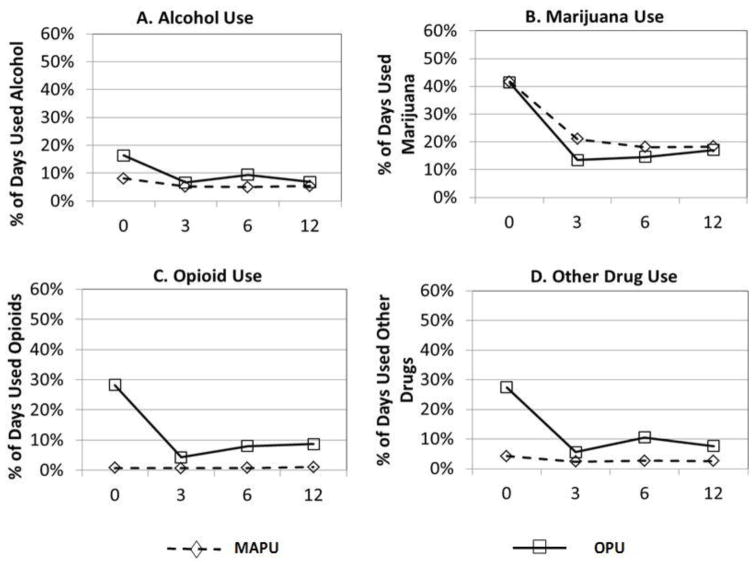
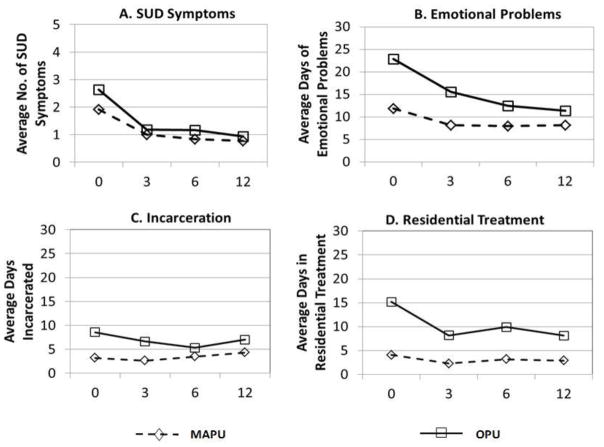
Figure 1 displays the growth curves for percent of days using alcohol, marijuana, opioids, and other drugs in the community at intake by substance problem group. The OPU group had significantly more use at intake than the MAPU group on all substance use variables except percent of days used marijuana (alcohol: b = 0.055; t = 4.28; p < 0.001; opioids: b = 0.14; t = 21.17; p < 0.001; marijuana: b = −0.035; t = −1.25; p = 0.21; other drugs: b = 0.11; t = 9.74; p<0.001). The OPU group also scored significantly higher on average number of substance use disorder symptoms in the 30 days prior to treatment intake (b =0. 485; t = 2.57, p < .05; see Figure 2).
Figure 1.
Results of HLM growth models showing the percent of days using A) alcohol, B) opioids, C) marijuana and D) other drug use by problem use groups.
A) OPU > MAPU at baseline, p < .001; OPU decreasing at a greater rate over time, p < .05.
B) No significant differences between OPU and MAPU groups.
C) OPU > MAPU at baseline, p < .001; OPU decreasing at a greater rate over time, p < .001.
D) OPU > MAPU at baseline, p < .001; OPU decreasing at a greater rate over time, p < .001.
Figure 2.
Results of HLM growth model showing A) SUD symptoms, B) days of emotional problems, C) days incarcerated and D) days in residential treatment by problem use groups.
A) OPU > MAPU at baseline, p < .05.
B) OPU > MAPU at baseline, p < .001; OPU decreasing at a greater rate over time, p < .05.
C) OPU > MAPU at baseline, p < .05.
D) OPU > MAPU at baseline, p < .001; OPU decreasing at a greater rate over time, p < .05.
Figure 1 also shows that the MAPU group decreased significantly in all substance use outcomes over 12 months except percent of days used opioids and days used other drugs (alcohol: b = −0.001; t =− 3.75; p < 0.001; opioids: b = 0.000; t = 1.09; p = 0.28; marijuana: b = −0.01; t = −17.56; p < 0.001; other drugs: b = −0.001; t = −1.802; p = 0.071). The MAPU group also decreased significantly in average number of substance use disorder symptoms over time, with no significant difference compared to the OPU group (b = −0.06; t = −11.63; p < 0.001; see Figure 2).
The OPU group decreased significantly more over time than the MAPU group in alcohol, opioid, and other drug use (alcohol: b = −0.00; t = −2.58; p < .05, with a small effect size (ES) of −.15; opioids: b = −0.01; t = −10.3; p < .001, with a large ES of −1.41; and other drugs: b = −0.01; t = −5.12; p < .001, with a moderate ES of −0.45). The opioid finding (Figure 1, Panel C) is an artifact of group membership, since the MAPU group reported very little opioid use at intake.
Figure 2 shows growth curves for average days of emotional problems, average days incarcerated, and average days in residential treatment by substance problem groups. The OPU group scored significantly higher on average days of emotional problems at intake (b = 8.81; t = 5.01; p < .001) and showed significantly greater improvement, (b = −0.20; t = −3.47; p < .001; ES = −.10). For days incarcerated, the OPU group was significantly higher at intake (b = 4.46; t = 4.935; p < .001). The MAPU increased significantly over time (b = 0.11; t = −1.60; p = .004). At intake, the OPU group reported significantly more days in residential treatment (b = 7.22; t = 6.93; p < .001), and while there is no significant change for the MAPU group, the OPU group decrease was significant (b = −0.33; t = −2.07; p = .039; ES = −.06).
Additional outcome analyses testing for differences at the 12-month follow-up period indicated that the OPU group was significantly higher than the MAPU group on percent of days using opioids in the community (b = 0.08, p < .01), days of emotional problems (b = 0.05, p < .01), and days in residential substance use treatment (b = 5.04, p < .05). There were no other significant differences found at 12 months between the two substance problem groups on any of the other treatment outcomes.
Finally, when comparing participants excluded from outcome analyses (because of low site follow-up rates or the 12-month interview was not yet due) to those included in outcome analyses on all intake characteristics reported in Table 1, there was only one statistically significant difference. Those who were included in the analyses were slightly younger at intake: 15.6 years old to 15.8, odds ratio of 0.94 with 95% C.I. (0.89, 0.99). This difference is not considered clinically meaningful.
4.0 Discussion
Consistent with prior research findings, adolescents in the OPU group were more likely to be Caucasian, older, female, and not attending school, and were more likely to report greater substance use severity and more frequent engagement in health risk behaviors (Clemmey et al., 2004; Gordon et al., 2004; Hopfer et al., 2000, 2002; Marsch et al., 2005; Subramaniam et al., 2009, 2010). With the exception of marijuana, adolescents in the OPU group reported more use of substances, more frequent mental health problems, and more substance-related problems at intake than the MAPU group. Subramaniam et al. (2010) and Clemmey et al. (2004) found that adolescent opioid users engaged in more days of criminal behavior than non-opioid users. This study indicated that adolescents in the OPU group were more likely at intake to report time spent incarcerated and engaged in illegal activity than those in the MAPU group.
Both substance problem groups had similar patterns of A-CRA treatment initiation, engagement, retention, and treatment satisfaction, providing support for the hypothesis that they would not differ on these implementation variables. Although adolescents with OPU present to treatment with significantly greater impairment than those in the MAPU group in most every life-health area (e.g., substance use, mental health, juvenile justice, HIV, school risk), A-CRA appears to be feasible to implement and acceptable to adolescents with OPU.
Confirming the second hypothesis, adolescents in the OPU group responded with similar and sometimes significantly greater improvement than those in the MAPU group, but in general, the OPU group did not improve to the same level. Because intake values for nearly all drug use and other problems were higher for the OPU group, it is likely that their higher initial severity contributed to findings of their greater improvement. Like adolescents who used heroin in Clemmey et al. (2004)’s paper, the OPU group in this study continued to report a higher frequency of substance use (in this case, opioids and drugs other than alcohol and marijuana). The OPU group also continued to report more days of emotional problems and residential treatment than the MAPU group despite the fact that they showed greater improvement in emotional problems.
4.1 Strengths and limitations
Strengths of the current study include its large sample of diverse adolescents from substance abuse treatment organizations across the United States and its use of a standardized assessment tool, addressing issues of small sample size and generalizability of other research (Subramaniam et al., 2009). Additionally, the large sample allowed analysis of a more inclusive group of adolescents with opioid use problems rather than focusing solely on heroin use as in other work (Clemmey et al., 2004; Hopfer et al., 2000). Finally, this study is based on participation in an empirically supported treatment for substance use problems that was part of a large-scale dissemination and implementation effort that included standardized training, fidelity monitoring, and A-CRA session documentation on all treated adolescents (Godley et al., 2011a).
There were also limitations to this research that should be regarded when interpreting the results. First, adolescents reporting at least weekly use within the MAPU and OPU groups also described substance use and other psychosocial problems consistent with or more severe than their counterparts with abuse and dependence. These findings suggest that many, if not most, of the adolescents in OPU’s “at least weekly use” subgroup may meet DSM-IV-TR dependence criteria; however, this cannot be confirmed without responses to DSM-IV-TR symptoms by specific drug for these individuals and indicates that the assessment of abuse and dependence symptoms by drug is essential to appropriate treatment planning. Second, substance use was self-reported rather than corroborated through tests of biological specimens or collateral reports. However, adolescent self-report measures of substance use from the GAIN have been shown to be consistent with collateral reports and urine tests (Godley et al., 2002), as well as timeline interviewing methods (Dennis et al., 2004b). Third, in this research design, there is no control group of OPU adolescents that did not receive A-CRA; therefore, prospective randomized trials of A-CRA are needed.
4.2 Recommendations for future research
While adolescents in the OPU group responded positively to A-CRA, their use of drugs other than alcohol and marijuana and their severity of problems continued to be higher than those in the MAPU group across follow-up data collection periods. Future research on outpatient A-CRA treatment for OPU should focus on increasing monitoring and treatment for at least one year, perhaps using adaptive treatment logic (McKay et al., 2010) to determine session frequency and episode duration. Because co-occurring mental health problems are significant among those with OPU, research on matching specific A-CRA procedures to symptoms of specific mental health disorders consistent with prior recommendations (Godley et al., 2014) should be attempted. Results of the present study suggest the need for more focused research using A-CRA procedures to address opioid withdrawal and craving. For example, adding ratings of withdrawal and cravings to the A-CRA self-assessment (Happiness Scale) and cuing the therapist to probe those areas for specific treatment goals could be helpful. In addition, the A-CRA functional analysis procedure could be used to identify high-risk craving situations. Other procedures, including backward chaining and problem solving, could be used to identify plans to avoid or minimize situations that trigger craving, while the medication adherence procedure could be used to increase compliance with prescribed medications for opioid withdrawal and ongoing treatment or other physical or emotional issues.
Pharmacological treatments have been shown to be effective in the management of opioid use disorders in adults (Marsch et al., 2005; Woody et al., 2008), and the American Academy of Pediatrics (AAP) has recently advocated for pediatricians to provide increased access to effective treatment for adolescents with opioid use disorders, including developmentally appropriate counseling and medication (AAP, 2016). Additional research is needed to assess the utility of combining outpatient A-CRA therapy with pharmacological treatments to improve the management of withdrawal and relapse prevention in this complex group of youth. In addition to increasing A-CRA outpatient services for up to one year, ongoing recovery support to prevent or minimize the effects of relapse should be considered in light of the potential for relapse to opioid use. While mutual aid groups such as Narcotics Annonymous can be helpful, especially when oriented toward adolescents (Kelly et al., 2005; Passetti and White, 2008), such meetings may not be available or utilized. Recent feasibility studies focusing on post-treatment recovery support via telephone outreach (Garner et al., 2014) and on-demand support via smartphone applications (Dennis et al., 2015) for youth are encouraging but need randomized trials to establish their utility as a long-term recovery support.
5.0 Conclusions
This study demonstrates the feasibility and acceptability of providing outpatient A-CRA treatment to adolescents with OPU. While promising, additional research is needed to further improve clinical outcomes by a) extending A-CRA continuing care and monitoring; b) applying more focused use of A-CRA procedures; c) combining pharmacological treatments with A-CRA; and d) testing long-term recovery supports. Subsequent randomized clinical trials are needed to experimentally assess the effects of opioid-focused A-CRA combined with medication for OPU. Finally, research employing long-term recovery support strategies should be tested to determine their potential to prevent or reduce the effects of relapse.
Supplementary Material
Highlights.
Youth with opioid problem use (OPU) were compared to youth with mainly marijuana/alcohol problems.
The OPU group was significantly more impaired on most intake measures.
Both groups had similar A-CRA initiation, engagement, retention, and satisfaction rates.
Over 12 months, both groups greatly improved; OPU showed higher problem severity.
The feasibility and acceptability of A-CRA for opioid problem use was demonstrated.
Acknowledgments
Role of Funding Source
Preparation for this manuscript was financially supported by grant R01 AA021118 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Funding source had no role in design, collection, analysis, or interpretation of data, or in the decision to submit this manuscript for publication.
Geetha A. Subramaniam, MD is affiliated with the National Institute on Drug Abuse (NIDA) at the National Institutes of Health (NIH) and the content of this article does not in any way reflect the official position of NIDA. The manuscript is based on her work conducted prior to her employment at NIH.
The opinions and conclusions expressed are solely of the authors and should not be construed as representing the opinions of NIAAA or any agency of the Federal Government. The authors wish to thank the SAMHSA Center for Substance Abuse Treatment grantees and their patients for participation in this project. The authors also acknowledge Kelli Wright for manuscript preparation.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors
All authors directly contributed to the writing and editing of this manuscript, and have approved the final manuscript.
Conflict of interest
Mark D. Godley, PhD oversees A-CRA training of clinicians and supervisors in the U.S. and other countries for Chestnut Health Systems, a not-for-profit organization.
Robert J. Meyers, PhD has a consulting business in which he conducts training in A-CRA and related protocols. Jane E. Smith, PhD participates in this business as well.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mark D. Godley, 448 Wylie Drive, Normal, IL 61761, USA.
Lora L. Passetti, 448 Wylie Drive, Normal, IL 61761, USA.
Geetha A. Subramaniam, 6001 Executive Boulevard, Room 3122, MSC 9557, Bethesda, MD 20892-9593, USA.
Rodney R. Funk, 448 Wylie Drive, Normal, IL 61761, USA.
Jane Ellen Smith, Logan Hall Room 178, Albuquerque, NM 87131, USA.
Robert J. Meyers, 3216 LaMancha Dr. NW, Albuquerque, NM 87104, USA.
References
- Abbott PJ. A review of the Community Reinforcement Aproach in the treatment of opioid dependence. J Psychoactive Drugs. 2009;41:379–385. doi: 10.1080/02791072.2009.10399776. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics (AAP) Committee on Substance Use and Prevention. Medication-assisted treatment of adolescents with opioid use disorders. Pediatrics. 2016;138:e20161893. doi: 10.1542/peds.2016-1893. [DOI] [PubMed] [Google Scholar]
- Azrin NH. Improvements in the community reinforcement approach to alcoholism. Behav Res Ther. 1976;14:339–348. doi: 10.1016/0005-7967(76)90021-8. [DOI] [PubMed] [Google Scholar]
- Azrin NH, Sisson RW, Meyers RJ, Godley MD. Outpatient alcoholism treatment by community reinforcement and disulfiram therapy. J Behav Ther Exp Psychiatry. 1982;13:105–112. doi: 10.1016/0005-7916(82)90050-7. [DOI] [PubMed] [Google Scholar]
- Clemmey P, Payne L, Fishman M. Clinical characteristics and treatment outcomes of adolescent heroin users. J Psychoactive Drugs. 2004;36:85–94. doi: 10.1080/02791072.2004.10399726. [DOI] [PubMed] [Google Scholar]
- De Jong CAJ, Roozen HG, van Rossum LGM, Krabbe PFM, Kerkhof AJFM. High abstinence rates in heroin addicts by a new comprehensive treatment approach. Am J Addict. 2007;16:124–130. doi: 10.1080/10550490601184472. [DOI] [PubMed] [Google Scholar]
- Dennis ML, Godley SH, Diamond GS, Tims FM, Babor T, Donaldson J, Liddle H, Titus JC, Kaminer Y, Webb C, Hamilton N, Funk RR. The Cannabis Youth Treatment (CYT) study: Main findings from two randomized trials. J Subst Abuse Treat. 2004a;27:197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Dennis ML, Scott CK, Funk RR, Nicholson L. A pilot study to examine the feasibility and potential effectiveneness of using smartphones to provide recovery support for adolescents. Subst Abuse. 2015;36:486–492. doi: 10.1080/08897077.2014.970323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ML, Titus JC, White MK, Unsicker J, Hodgkins D. Global Appraisal of Individual Needs (GAIN): Administration guide for the GAIN and related measures. Chestnut Health Systems; Bloomington, IL: 2003. [Accessed July 14, 2016]. from http://www.chestnut.org/li/gain. [Google Scholar]
- Dennis ML, Funk R, Godley SH, Godley MD, Waldron H. Cross-validation of the alcohol and cannabis use measures in the Global Appraisal of Individual Needs (GAIN) and Timeline Followback (TLFB; Form 90) among adolescents in substance abuse treatment. Addiction. 2004b;99:120–128. doi: 10.1111/j.1360-0443.2004.00859.x. [DOI] [PubMed] [Google Scholar]
- Garner BR, Godley MD, Passetti LL, Funk RR, White WL. Recovery support for adolescents with substance use disorders: The impact of recovery support telephone calls provided by pre-professional volunteers. J Subst Abuse Alcohol. 2014;2:1010. [PMC free article] [PubMed] [Google Scholar]
- Garnick DW, Lee MT, Horgan CM, Acevedo A the Washington Circle Public Sector Workgroup. Adapting Washington Circle performance measures for public sector substance abuse treatment systems. J Subst Abuse Treat. 2009;36:265–277. doi: 10.1016/j.jsat.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godley MD, Godley SH, Dennis ML, Funk RR, Passetti LL. Preliminary outcomes from the assertive continuing care experiment for adolescents discharged from residential treatment. J Subst Abuse Treat. 2002;23:21–32. doi: 10.1016/S0740-5472(02)00230-1. [DOI] [PubMed] [Google Scholar]
- Godley MD, Godley SH, Dennis ML, Funk RR, Passetti LL. The effectiveness of assertive continuing care on continuing care linkage, adherence, and abstinence following residential treatment for substance use disorders in adolescents. Addiction. 2007;102:81–93. doi: 10.1111/j.1360-0443.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- Godley SH, Garner BR, Smith JE, Meyers RJ, Godley MD. A large-scale dissemination and implementation model. Clin Psychol Sci Pract. 2011a;18:67–83. doi: 10.1111/j.1468-2850.2011.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godley SH, Hedges K, Hunter B. Gender and racial differences in treatment process and outcome among participants in the Adolescent Community Reinforcement Approach. Psychol Addict Behav. 2011b;25:143–154. doi: 10.1037/a0022179. [DOI] [PubMed] [Google Scholar]
- Godley SH, Meyers RJ, Smith JE, Godley MD, Titus JC, Karvinen T, Dent G, Passetti L, Kelberg P. Adolescent Community Reinforcement Approach (ACRA) for Adolescent Cannabis users: Cannabis Youth Treatment (CYT) Manual Series. Vol. 4. Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; Rockville, MD: 2001. [Accessed July 1, 2016]. (DHHS Publication No (SMA) 01-3489) from http://ebtx.chestnut.org/Portals/0/Documents/ACRA_CYT_v4.pdf. [Google Scholar]
- Godley SH, Smith JE, Passetti LL, Subramaniam G. The Adolescent Community Reinforcement Approach (A-CRA) as a model paradigm for the management of adolescents with substance use disorders and co-occurring psychiatric disorders. Subst Abuse. 2014;35:352–363. doi: 10.1080/08897077.2014.936993. [DOI] [PubMed] [Google Scholar]
- Gordon SM, Mulvaney F, Rowan A. Characteristics of adolescents in residential treatment for heroin dependence. Am J Drug Alcohol Abuse. 2004;30:593–603. doi: 10.1081/ADA-200032300. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Wevodau AL, Henderson SE, Colbourn SL, Gharagozloo L, North LW, Lotts VA. An independent replication of the Adolescent Community Reinforcement Approach with justice-involved youth. Am J Addict. 2016;25:233–240. doi: 10.1111/ajad.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer CJ, Mikulich SK, Crowley TJ. Heroin use among adolescents in treatment for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2000;39:1316–1323. doi: 10.1097/00004583-200010000-00021. [DOI] [PubMed] [Google Scholar]
- Hopfer CJ, Khuri E, Crowley TJ, Hooks S. Adolescent heroin use: A review of the descriptive and treatment literature. J Subst Abuse Treat. 2002;23:231–237. doi: 10.1016/S0740-5472(02)00250-7. [DOI] [PubMed] [Google Scholar]
- Hunt GM, Azrin NH. A community-reinforcement approach to alcoholism. Behav Res Ther. 1973;11:91–104. doi: 10.1016/0005-7967(73)90072-7. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2011. Institute for Social Research, University of Michigan; Ann Arbor, MI: 2012. [Accessed June 16, 2016]. from http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2011.pdf. [Google Scholar]
- Kelly JF, Myers MG, Brown SA. The effects of age composition of 12-step groups on adolescent 12-step participation and substance use outcome. J Child Adolesc Subst Abuse. 2005;15:63–72. doi: 10.1300/J029v15n01_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Jacobs EA. Buprenorphine treatment for opioid dependence: The relative efficacy of daily, twice and thrice weekly dosing. Drug Alcohol Depend. 2005;77:195–204. doi: 10.1016/j.drugalcdep.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadhone maintenance therapy versus no opioid replacement therapy for opioid dependence (Review) Cochrane Database Syst Rev. 2009;8:CD002209. doi: 10.1002/14651858.CD002209.pub2.. [DOI] [PubMed] [Google Scholar]
- McKay JR, Lynch KG, Coviello D, Morrison R, Cary MS, Skalina L, Plebani J. Randomized trial of continuing care enhancements for cocaine-dependent patients following initial engagement. J Consult Clin Psychol. 2010;78:111–120. doi: 10.1037/a0018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers RJ, Smith JE. Clinical Guide to Alcohol Treatment: The Community Reinforcement Approach. Guilford Press; New York: 1995. [Google Scholar]
- National Consensus Development Panel on Effetice Medical Treatment of Opiate Addiction. Effective medical treatment of opiate addiction. JAMA. 1998;280:1936–1943. doi: 10.1001/jama.280.22.1936. [DOI] [PubMed] [Google Scholar]
- Passetti LL, White WL. Recovery support meetings for youths: Considerations when referring young people to 12-step and alternative groups. J Groups Addict Recover. 2008;2:97–121. doi: 10.1080/15560350802081280. [DOI] [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows [computer software] Scientific Software International, Inc; Skokie, IL: 2011. [Google Scholar]
- Rogers JL, Howard KI, Vessey JT. Using significance tests to evaluate equivalence between two experimental groups. Psychol Bull. 1993;113:553–565. doi: 10.1037/0033-2909.113.3.553. [DOI] [PubMed] [Google Scholar]
- Slesnick N, Prestopnik JL, Meyers RJ, Glassman M. Treatment outcome for street-living, homeless youth. Addict Behav. 2007;32:1237–1251. doi: 10.1016/j.addbeh.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam G, Stitzer MA, Woody G, Fishman MJ, Kolodner K. Clinical characteristics of treatment seeking adolescents with opioid versus cannabis/alcohol use disorders. Drug Alcohol Depend. 2009;99:141–149. doi: 10.1016/j.drugalcdep.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam G, Ives ML, Stitzer ML, Dennis ML. The added risk of opioid problem use among treatment-seeking youth with marijuana and/or alcohol problem use. Addiction. 2010;105:686–698. doi: 10.1111/j.1360-0443.2009.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), Center for Behavioral Health Statistics and Quality. Treatment Episode Data Set (TEDS): 2003–2013. National Admissions to Substance Abuse Treatment Services; Rockville, MD: 2015. [Accessed June 22 2016]. BHSIS Series S-75, HHS Publication No (SMA) 15-4934 SAMHSA. from http://www.samhsa.gov/data/sites/default/files/2013_Treatment_Episode_Data_Set_National/2013_Treatment_Episode_Data_Set_National.pdf. [Google Scholar]
- Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbot P, Patkar A, Publucker M, McCain K, Potter JS, Forman R, Vetter V, McNicholas L, Blaine J, Lynch KG, Fudala P. Extended versus short-term buprenorphine-naloxone for treatment of opioid-addicted youth: A randomized trial. JAMA. 2008;300:2003–2011. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.