Abstract
Background and aims
One challenge to HCV elimination through therapeutic intervention is reinfection. The aim of this analysis was to calculate the incidence of HCV reinfection among both HIV-positive and negative individuals treated for recent HCV infection (estimated infection duration <18 months).
Methods
Individuals with recent HCV infection who achieved an end-of-treatment response in four open-label studies between 2004 and 2015 in Australia and New Zealand were assessed for HCV reinfection, confirmed by sequencing of the Core-E2 and/or NS5B regions. Reinfection incidence was calculated using person-time of observation. Exact Poisson regression analysis was used to assess factors associated with HCV reinfection.
Results
The cohort at-risk for reinfection (n=120; 83% male; median age 36 years) was composed of HIV-positive men-who-have-sex-with-men (53%) and people who inject drugs (current 49%, ever 69%). Total follow-up time at-risk was 135 person-years (median 1.08 years, range 0.17, 2.53). Ten cases of HCV reinfection were identified, for an incidence of 7.4 per 100 py (95% CI 4.0, 13.8). Reinfection incidence was significantly higher amongst participants who reported injection drug use at end of or post-treatment, irrespective of HIV status (15.5 per 100 py, 95% CI 7.8, 31.1). In adjusted analysis, factors associated with reinfection were older age (aIRR 5.3, 95% CI 1.15, 51.5, p=0.042) and injection drug use at end of or post-treatment (aIRR 7.9, 95%CI 1.6, 77.2, p=0.008).
Conclusions
High reinfection incidence following treatment for recent HCV infection in individuals with ongoing risk behaviour emphasises the need for post-treatment surveillance, harm reduction strategies and education in at-risk populations.
Keywords: Hepatitis C infection, HIV, recent, acute, reinfection, treatment
Highly effective, well tolerated interferon-free direct-acting antivirals (DAA) have revolutionised hepatitis C virus (HCV) therapeutics (1), with daily fixed-dose combination DAA regimens providing cure in greater than 95% of individuals with chronic infection (2, 3). The availability of DAA therapy has led to significant therapeutic optimism with the possibility of broad treatment uptake and subsequent HCV elimination (4–7). One challenge to HCV elimination though therapeutic intervention is reinfection.
There is concern that HCV reinfection may compromise the individual and population level benefits of HCV treatment in some populations with the risk of reinfection cited as a reason for not offering treatment to people who inject drugs (PWID) (8, 9). However, in general, the incidence of HCV reinfection in PWID treated for chronic HCV infection ranges between one and five per 100 py (summarised in Supplementary Table 1). Reinfection incidence following treatment in individuals with HIV/HCV co-infection is varied, with high incidence reported in some cohorts of HIV-positive men-who-have-sex-with-men (MSM) (10–12). There is uncertainty around these reinfection estimates due to sample size, retrospective study designs, exclusion of recent PWID from trials, varied definitions for recent injection drug use and time at-risk for reinfection, and the inability to accurately distinguish relapse from reinfection.
Mathematical modelling suggests that substantial reductions in HCV incidence and prevalence could be achieved by targeted DAA treatment scale-up amongst those at highest risk of ongoing transmission, including PWID and HIV-positive MSM with recently diagnosed HCV infection (5, 13–15). Despite the high cost of DAA therapy, treating recent PWID and HIV-positive MSM with early liver disease appears to be cost-effective compared to delaying until cirrhosis, given the reduction in liver-related complications and additional benefit of averting secondary infections (6, 7, 16). However, ongoing risk behaviours associated with HCV transmission may contribute to reinfection and compromise the population-level benefits of Treatment as Prevention (5, 17, 18). Few studies have evaluated the incidence of HCV reinfection following treatment of recent HCV infection (summarised in Supplementary Table 1) (11, 12, 19), a high-risk group for onward transmission and of importance as DAA treatment access expands to traditionally marginalised populations. The aim of this analysis was to calculate the incidence of HCV reinfection among individuals treated for recent HCV infection (estimated infection duration <18 months) and assess clinical and behavioural factors associated with reinfection.
Methods
Study participants
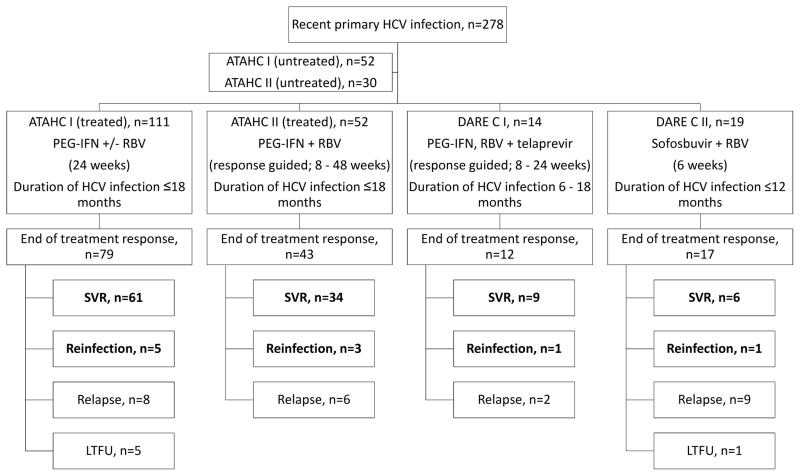
Individuals with recent HCV infection (infection duration <18 months) who received treatment in four prospective open-label studies (ATAHC I, ATAHC II, DARE-C I and DARE-C II) between 2004 and 2015 in Australia and New Zealand were assessed for HCV reinfection (20–22) (Figure 1). The primary endpoints of these studies (20–22) and an analysis of HCV superinfection and reinfection in treated and untreated participants in ATAHC I (19) have been presented or published previously.
Figure 1.
Participant disposition.
Participants highlighted in bold constitute the cohort at-risk for reinfection.
Recent primary HCV infection was defined as initial detection of serum anti-HCV antibody and/or HCV RNA within six months of enrolment and either (i) documented recent HCV seroconversion (anti-HCV antibody negative result in the 18 [DARE-C II] or 24 [ATAHC, ATAHC II, DARE-C I] months prior to enrolment) or (ii) acute clinical hepatitis (jaundice or alanine aminotransferase [ALT] greater than 10 times the upper limit of normal [ULN]) within the previous 12 months with the exclusion of other causes of acute hepatitis, with estimated duration of HCV infection less than 12 [DARE-C II] or 18 [ATAHC, ATAHC II, DARE-C I] months at screening.
HCV RNA testing and sequencing
The presence of HCV RNA was assessed at all scheduled study visits (see Supplementary Material). In ATAHC I, HCV RNA was assessed using a qualitative HCV RNA assay (Versant transcription-mediated amplification [TMA]; Bayer, Australia; LLoD 10 IU/ml) and if positive, a quantitative HCV RNA assay (Versant HCV RNA 3.0; Bayer, Australia; LLoD 615 IU/ml). In ATAHC II, DARE-C I and DARE-C II, HCV RNA was assessed using a quantitative HCV RNA assay (COBAS Taqman 2.0; Roche Diagnostics, USA; LLoD 15 IU/mL). Population-based HCV RNA sequencing was performed on the first available pre-treatment quantifiable HCV RNA sample and the first available quantifiable HCV RNA sample following HCV RNA recurrence using an in-house assay with methods described previously (23, 24). See Supplementary Material for more details.
Study definitions and outcomes
An end-of-treatment response (ETR) was defined as HCV RNA below the lower limit of detection, target not detected (LLoD, TND). HCV RNA recurrence was defined as quantifiable HCV RNA following ETR. Post-treatment relapse was defined as the presence of quantifiable HCV RNA after an ETR, confirmed as homologous virus on sequencing of Core-E2 and/or NS5B regions as described (23, 24). Confirmed reinfection was defined by the presence of quantifiable HCV RNA after an ETR with detection of an HCV strain that was distinct from the primary infecting strain (heterologous virus on sequencing). Possible reinfection was defined by the presence of quantifiable HCV RNA after an ETR without sequence data, but occurring after post treatment week 24 and with documentation of HCV RNA TND at post treatment week 12 or 24 to exclude post-treatment relapse. Persistent reinfection was defined by the presence of quantifiable HCV RNA in a repeated sample taken at least 12 weeks after HCV RNA recurrence.
The time at risk for reinfection was calculated from the date of end of treatment in individuals with an ETR to date of reinfection or last undetectable HCV RNA. The estimated date of reinfection was calculated as the midpoint between the dates of the last undetectable HCV RNA test and the first quantifiable HCV RNA test during follow-up. The primary study outcome was HCV reinfection incidence.
Study oversight
All study participants provided written informed consent before study procedures. The study protocols were approved by St Vincent’s Hospital, Sydney Human Research Ethics Committee (primary study committee), as well as local ethics committees at all study sites. The studies were conducted according to the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice (ICH/GCP) guidelines. The studies were registered with clinicaltrials.gov registry (ATAHC I: NCT00192569; ATAHC II: NCT01336010; DARE-C I: NCT01743521; DARE-C II: NCT02156570).
Statistical analysis
In the cohort at-risk for reinfection (ETR without post-treatment relapse), categorical parameters were summarised as number and proportion and continuous variables were summarised by either mean and standard deviation (SD) or median and interquartile range (IQR), as appropriate. Reinfection incidence was calculated using person-time of observation. Confidence intervals (CI) for rates were calculated using Poisson distribution.
Exact Poisson regression analysis was used to assess factors associated with HCV reinfection, with time at risk (years) as the exposure variable. In unadjusted analyses, potential predictors were determined a priori and included sex, age at study enrolment, income, education level, social functioning score at enrolment (median), mode of HCV acquisition (injection drug use, sexual, other), HIV infection, injection drug use (ever, previous 6 months at enrolment, previous 30 days at enrolment), and injection drug use at end of and/or post-treatment. Social functioning was calculated using a validated scale from the Opiate Treatment Index (25) and addressed employment, residential stability, interpersonal conflict and social support (higher score reflects poorer social functioning, range score 0–48). All variables with p<0.2 in univariate analysis were considered in multivariate regression models using a backwards stepwise approach. Statistically significant differences were assessed at p<0.05; p-values were two-sided. Additional models assessed factors associated with reinfection among lifetime PWID (participants who reported injection drug use at least once) and HIV-positive MSM. See Supplementary Material for more details.
Analysis was performed using STATA version 14.0 (StataCorp, College Station, TX).
Results
Participant disposition
Between 2004 and 2015, 278 participants with recent HCV infection were enrolled in Australia and New Zealand with 196 participants included in the intention-to-treat population; 82 participants were enrolled into the untreated arms of ATAHC I and II (Figure 1). An end of treatment response (ETR) was documented in 77% (n=151). Six participants (4%) were lost to follow up (LTFU) after ETR. Viral recurrence following ETR was seen in 35 (23%), confirmed by sequencing as relapse in 25 (17%) and reinfection in 10 (6%). Participants with relapse and LTFU after ETR were excluded from subsequent analysis.
The enrolment characteristics of the cohort at-risk for reinfection (n=120; male 83%; median age 36 years, IQR 29–46) are shown in Table 1. HIV co-infection was documented in 53%; all of whom identified as MSM. Injection drug use ever prior to enrolment, within six months and within 30 days of enrolment was reported by 69%, 49% and 43%, respectively. Of those participants who reported injection drug use within 30 days of enrolment (n=52), the drugs most commonly injected were amphetamines (61%) and heroin or other opiates (29%) (Supplementary Table 2). Injection drug use at end of or post treatment was reported by 38% (n=45). Among those reporting injection drug use during follow-up, 71% (n=32) reported predominantly injecting amphetamines and 22% (n=10) reported use of unsterile needles and/or syringes. Different drug use behaviours were observed among participants with HIV/HCV co-infection as compared with HCV mono-infection; while participants with HIV/HCV co-infection were less likely to have ever injected drugs (61% vs 80%; p=0.021), those who did were older at commencement of injection drug use (30 years [IQR 25, 41] vs 23 years [IQR 18, 30]; p<0.001) (Supplementary Table 3).
Table 1.
Enrolment demographic and clinical characteristics of participants at-risk for reinfection
| Variables | Overall N=120 |
HCV Reinfection N=10 |
No reinfection a N=110 |
|---|---|---|---|
| Age at enrolment, median (IQR) | 36 (29–46) | 44 (36–49) | 35 (24–46) |
| Gender | |||
| Male | 100 (83) | 10 (100) | 90 (82) |
| Female | 19 (16) | 0 | 19 (17) |
| Transgender | 1 (1) | 0 | 1 (1) |
| Full or part-time employment | 68 (56) | 5 (50) | 63 (57) |
| Tertiary education or greater, n (%) | 70 (58) | 8 (80) | 62 (56) |
| Social functioning score, median (IQR) | 11 (6–16) | 16 (12–17) | 11 (6–15) |
| HIV infection, n (%) | 64 (53) | 7 (70) | 57 (52) |
| On cART, n (%) | 52 (81) | 5 (71) | 47 (82) |
| IDU, n (%) | |||
| Ever prior to enrolment | 83 (69) | 7 (70) | 77 (69) |
| Previous 6 months prior to enrolment | 59 (49) | 6 (60) | 53 (48) |
| Previous 30 days prior to enrolment | 52 (43) | 6 (60) | 46 (42) |
| Age at first IDU, median (IQR) | 25 (20–34) | 35 (30–46) | 25 (20–32) |
| OST, n (%) | |||
| Ever prior to enrolment | 14 (12) | 1 (10) | 13 (13) |
| At enrolment | 6 (5) | 1 (10) | 5 (5) |
| Mode of primary HCV acquisition, n (%) | |||
| IDU | 66 (55) | 6 (60) | 60 (55) |
| Sexual exposure | 51 (43) | 4 (40) | 47 (43) |
| Other | 3 (3) | 0 | 3 (3) |
| Weeks between estimated date of HCV infection and treatment commencement, median (IQR) | 36 (30–46) | 34 (27–52) | 37 (30–46) |
| HCV treatment | |||
| PEG ± RBV | 103 (86) | 8 (80) | 95 (86) |
| PEG-IFN + RBV + telaprevir | 10 (8) | 1 (10) | 9 (8) |
| Sofosbuvir + RBV | 7 (6) | 1 (10) | 6 (5) |
| Treatment weeks, median (IQR) | 24 (16–24) | 20 (8–24) | 24 (16–24) |
| Time at risk of reinfection (years), median (IQR) | 1.08 (0.59, 1.50) | 0.67 (0.35, 1.23) | 1.19 (0.60, 1.52) |
Includes participants with ETR and no reinfection or relapse
Abbreviations: Injecting drug use (IDU); opiate substitution therapy (OST); pegylated interferon (PEG-IFN); ribavirin (RBV)
Total follow-up time post treatment was 141 person-years (py; median 1.22 py, range 0.19, 2.53). Total follow-up time at-risk for reinfection (censured at estimated date of reinfection) was 135 py (median 1.08 years, range 0.17, 2.53).
HCV reinfection among participants treated for recent HCV infection
Ten cases of HCV reinfection were identified (eight confirmed, two possible), with persistent reinfection in five and spontaneous clearance in three cases (Figure 2). Reinfection outcome was indeterminate in two cases due to lack of on-study follow-up testing. Of the ten participants (seven HIV-positive MSM) with reinfection, eight reported injection drug use during follow up; one HIV-positive MSM who reported never injecting drugs prior to study enrolment subsequently injected anabolic steroids during follow-up. The remaining two cases occurred in HIV-positive MSM who denied ever injecting drugs. Detailed demographic and clinical characteristics of the ten participants with reinfection are displayed in Table 2.
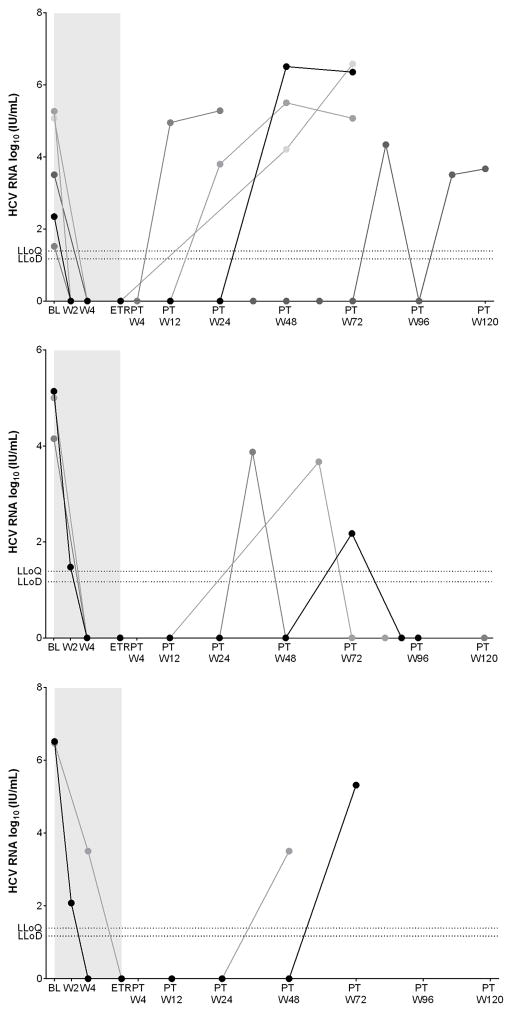
Figure 2.
Viral kinetics on and post-treatment in participants with HCV reinfection.
Panel A: Persistent HCV reinfection. Panel B: Spontaneous clearance of HCV reinfection.
Panel C: Indeterminate HCV reinfection outcome.
Shaded area indicates period on treatment.
LLoQ 25 IU/mL and LLoD 15 IU/mL for COBAS Taqman 2.0, Roche Diagnostics, USA.
Abbreviations: Baseline (BL), end-of-treatment response (ETR), lower limit of detection (LLoD), lower limit of quantitation (LLoQ), week (W), post treatment (PT)
Table 2.
Detailed demographic, behavioural and virological characteristics of participants with HCV reinfection (n=10)
| Gender, age1 | HIV | Mode of primary HCV | IDU | HCV genotype | Region sequenced | Time between EOT and reinfection (weeks) | ALT | Reinfection outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| At screening2 | During follow-up | Primary | Reinfection | EOT | Reinfection | ||||||
|
Confirmed reinfection
| |||||||||||
| Male 29 |
No | IDU | Yes | Yes | 3a | 1a | E1/HVR1 | 18 | 21 | 53 | Spontaneous clearance |
| Male 49 |
Yes | IDU | Yes | Yes | 3a/1a | 3a/1a | Core-E2 | 81 | 83 | 1135 | Persistence |
| Male 36 |
No | IDU | Yes | Yes | 1a | 3a | E1/HVR1 Core-E2 |
30 | 26 | 319 | Persistence |
| Male 44 |
Yes | Sexual | No | No | 2a | 1a/3a | Core-E2 | 40 | 58 | 565 | Indeterminate |
| Male 50 |
Yes | Sexual | No | Yes | 1a | 1a | NS5B | 65 | 18 | 15 | Indeterminate |
| Male 62 |
Yes | IDU | Yes | Yes | 3a | 1a | Core-E2 NS5B |
39 | 15 | 65 | Persistence |
| Male 45 |
Yes | Sexual | No | No | 1a | 1a | Core-E2 NS5B |
18 | 29 | 54 | Persistence |
| Male 41 |
Yes | IDU | Yes | Yes | 3a | 1a | Core-E2 NS5B |
9 | 23 | 910 | Persistence |
|
| |||||||||||
|
Possible reinfection
| |||||||||||
| Male 35 |
No | IDU | Yes | Yes | 3a | NA3 | InnoLipa | 31 | 15 | 14 | Spontaneous clearance |
| Male 46 |
Yes | Sexual | No | Yes4 | 1a | NA3 | InnoLipa | 64 | 71 | 43 | Spontaneous clearance |
Age at study enrolment
IDU within 6 months of study enrolment
Unable to sequence due to low HCV RNA at time of viral recurrence
Anabolic steroids
Abbreviations: ALT, alanine aminotransferase; EOT, end of treatment; IDU, injecting drug use1 ALT, normal range 0–30 U/L
Among eight cases of confirmed reinfection, median estimated time to HCV reinfection was 35 weeks (range 9 – 81 weeks) from end of treatment. The shortest time to reinfection was noted in an HIV-positive MSM following short duration interferon-free DAA therapy who reported high-risk drug and sexual behaviour. Despite confirmation of sustained virological response at week 4 post treatment (SVR4, HCV RNA TND), HCV RNA was quantifiable at post treatment week 12 with an HCV genotype switch from 3a (screening) to 1a (post treatment week 12) in association with an acute clinical illness and transaminitis (ALT 910 U/L). Amongst participants with confirmed reinfection, median ALT at end of treatment and following diagnosis of reinfection were 25 U/L (range 15 – 83 U/L) and 192 U/L (range 15 – 1135 U/L), respectively.
Low level quantifiable HCV RNA was detected in the two cases of possible reinfection and as such, reinfection could not be confirmed by sequencing. Estimated time to possible reinfection was 31 and 64 weeks from end of treatment, respectively. In the first case, quantifiable HCV RNA (3.87 log10 IU/mL) was detected at a single time point at post treatment week 36 (TND at post treatment week 24) and was repeatedly negative between post treatment weeks 48 and 120. In the second case, quantifiable HCV RNA (2.18 log10 IU/mL) was detected at post treatment week 72 (TND at post treatment week 48) and was repeatedly negative subsequent to this.
Among all participants at-risk for reinfection, reinfection incidence was 7.4 per 100 py (95% CI 4.0, 13.8) (Table 3 and Supplementary Table 4), for a projected cumulative reinfection incidence of 7.2% at one year and 14.5% at two years from end of treatment (Supplementary Figure 1). The incidence of HCV reinfection was 4.5 per 100 py (95% CI 1.4, 13.9) amongst those with HCV mono-infection, compared to 10.3 per 100 py (95% CI 4.9, 21.7) in those with HIV/HCV co-infection (p=0.232). The incidence of HCV reinfection was 8.5 per 100 py (95% CI 4.2, 16.9) in those who had ever injected drugs, compared to 4.9 per 100 py (95% CI 1.2, 19.8) in those who had never injected drugs (p=0.532). HCV reinfection incidence was significantly higher amongst participants who reported injection drug use at end of and/or post treatment (15.5 per 100 py, 95% CI 7.8, 31.1) as compared with those who did not inject drugs during follow up (2.6 per 100 py, 95% CI 0.6, 10.3) (p=0.023).
Table 3.
Incidence of HCV reinfection among participants treated for recent HCV infection
| Participant type | Cases of reinfection (n) | Participants at risk (n) | Person years follow-up | Incidence/100 person-years | 95% CI |
|---|---|---|---|---|---|
| Overall | |||||
| Confirmed/possible reinfection | 10 | 120 | 135 | 7.4 | 4.0, 13.8 |
| Confirmed reinfection | 8 | 120 | 135 | 5.9 | 3.0, 11.9 |
| Confirmed persistent reinfection | 5 | 120 | 135 | 3.7 | 1.5, 8.9 |
| HCV mono-infection | |||||
| Confirmed/possible reinfection | 3 | 56 | 67 | 4.5 | 1.4, 13.9 |
| Confirmed reinfection | 2 | 56 | 67 | 3.0 | 0.7, 11.9 |
| Confirmed persistent reinfection | 1 | 56 | 67 | 1.5 | 0.2, 10.6 |
| HIV/HCV co-infection# | |||||
| Confirmed/possible reinfection | 7 | 64 | 68 | 10.3 | 4.9, 21.7 |
| Confirmed reinfection | 6 | 64 | 68 | 8.9 | 4.0, 19.7 |
| Confirmed persistent reinfection | 4 | 64 | 68 | 5.9 | 2.2, 15.7 |
| IDU ever | |||||
| Confirmed/possible reinfection | 8 | 84 | 94 | 8.5 | 4.2, 16.9 |
| Confirmed reinfection | 6 | 84 | 94 | 6.4 | 2.9, 14.1 |
| Confirmed persistent reinfection | 4 | 84 | 94 | 4.2 | 1.6, 11.3 |
| IDU never | |||||
| Confirmed/possible reinfection | 2 | 36 | 41 | 4.9 | 1.2, 19.8 |
| Confirmed reinfection | 2 | 36 | 41 | 4.9 | 1.2, 19.8 |
| Confirmed persistent reinfection | 1 | 36 | 41 | 2.5 | 0.3, 17.5 |
| IDU at end of and/or post-treatment | |||||
| Confirmed/possible reinfection | 8 | 45* | 52 | 15.3 | 7.7, 30.6 |
| Confirmed reinfection | 6 | 45* | 52 | 11.5 | 5.2, 25.5 |
| Confirmed persistent reinfection | 4 | 45* | 52 | 7.7 | 2.9, 20.4 |
| No IDU at end of and/or post-treatment | |||||
| Confirmed/possible reinfection | 2 | 72* | 77 | 2.6 | 0.7, 10.4 |
| Confirmed reinfection | 2 | 72* | 77 | 2.6 | 0.7, 10.4 |
| Confirmed persistent reinfection | 1 | 72* | 77 | 1.3 | 0.2, 9.2 |
All participants with HIV/HCV are HIV-positive MSM
Numbers do not equal 120 due to missing data on injecting during follow-up in 3 participants
Abbreviations: Confidence interval (CI)
Risk factors for HCV reinfection following treatment for recent infection
Factors associated with HCV reinfection were assessed using exact Poisson regression analysis (Table 4). In adjusted analysis, factors independently associated with reinfection included older age (aIRR 5.42, 95% CI 1.06, 52.93, p=0.040) and injection drug use at end of and/or post-treatment (aIRR 7.86, 95%CI 1.54, 76.79, p=0.008) (Table 4). Factors associated with reinfection were unchanged when the analysis was limited to those with confirmed reinfection (Supplementary Table 5).
Table 4.
Factors associated with reinfection following treatment for recent HCV infection – Exact Poisson regression analysis
| HCV reinfection N=10 |
No reinfection N=110 |
IRR | 95% CI | P | aIRR | 95% CI | P | |
|---|---|---|---|---|---|---|---|---|
| Sex, n (%) | * | |||||||
| Male | 10 (100) | 90 (82) | 1.00 | - | ||||
| Female | 0 | 19 (17) | 0.37 | 0.00, 2.28 | 0.336 | |||
| Transgender | 0 | 1 (1) | 3.35 | 0.00, 20.82 | 1.000 | |||
| Age category (divided at median), n (%) | ||||||||
| ≤36 | 2 (20) | 54 (49) | 1.00 | - | 1.00 | - | ||
| >36 | 8 (80) | 56 (51) | 3.72 | 0.74, 35.99 | 0.137 | 5.42 | 1.06, 52.93 | 0.040 |
| Tertiary education, n (%) | ||||||||
| No | 2 (20) | 48 (44) | 1.00 | - | - | |||
| Yes | 8 (80) | 62 (56) | 2.79 | 0.56, 26.97 | 1.000 | |||
| Full or part-time employment, n (%) | ||||||||
| No | 5 (50) | 47 (43) | 1.00 | - | - | |||
| Yes | 5 (50) | 63 (57) | 0.84 | 0.19, 3.64 | 1.000 | |||
| Social functioning score at enrolment, n (%) | * | |||||||
| ≤11 | 2 (20) | 58 (53) | 1.00 | - | ||||
| >11 | 7 (70) | 45 (41) | 4.60 | 0.88, 45.34 | 0.079 | |||
| Missing | 1 (10) | 7 (6) | 3.06 | 0.05, 58.77 | 0.730 | |||
| Mode of primary HCV acquisition, n (%) | ||||||||
| IDU | 6 (60) | 60 (55) | 1.00 | - | ||||
| Sexual | 4 (40) | 47 (43) | 0.98 | 0.20, 4.14 | 1.000 | |||
| Other | 0 | 3 (3) | 3.66 | 0.00, 25.36 | 1.000 | |||
| HIV infection, n (%) | ||||||||
| No | 3 (30) | 53 (48) | 1.00 | - | ||||
| Yes | 7 (70) | 57 (52) | 2.31 | 0.53, 13.86 | 0.351 | |||
| Injecting drug use ever at enrolment, n (%) | ||||||||
| No | 3 (30) | 33 (30) | 1.00 | - | ||||
| Yes | 7 (70) | 77 (70) | 1.04 | 0.24, 6.26 | 1.000 | |||
| Injection drug use in previous 6 months at enrolment, n (%) | * | |||||||
| No | 4(40) | 55 (50) | 1.00 | - | ||||
| Yes | 6 (60) | 53 (48) | 1.40 | 0.33, 6.76 | 0.838 | |||
| Missing | 0 | 2 (2) | 5.98 | 0.00, 47.84 | 1.000 | |||
| Injection drug use in previous 30 days at enrolment, n (%) | ||||||||
| No | 4 (40) | 64 (58) | 1.00 | - | ||||
| Yes | 6 (60) | 46 (42) | 2.77 | 0.64, 12.02 | 0.195 | |||
| IDU at end of treatment and/or post treatment, n (%) | ** | ** | ||||||
| No | 2 (20) | 70 (64) | 1.00 | - | 1.00 | - | ||
| Yes | 8 (80) | 37 (34) | 5.88 | 1.17, 56.82 | 0.027 | 7.86 | 1.54, 76.79 | 0.008 |
| Missing | 0 | 3 (3) | 5.51 | 0.00, 71.51 | 1.000 | 5.78 | 0.00, 74.36 | 1.000 |
P overall for categorical variables:
≥0.05,
0.001–0.05,
<0.001
Univariate analysis - P overall: Sex, p=0.281, social functioning score, p=0.079, IDU in previous 6 months, p=0.838, IDU at end of and/or post treatment, p=0.027
Multivariate analysis – P overall: IDU at end of and/or post treatment, p=0.008
Factors associated with HCV reinfection were also assessed amongst lifetime PWID (n=84; 46% HIV-positive MSM) and HIV-positive MSM (n=64). Among lifetime PWID, 54% reported injection drug use at end of and/or post-treatment. Median age at first injection drug use was 25 years (IQR 20–34), significantly older among those with reinfection (35 years [IQR 30–46] vs 25 years [IQR 20–32]; p=0.013). Median duration of injection drug use at enrolment was 5.5 years (IQR 2.2, 11.0), with shorter duration and more recent onset of injection drug use among those with reinfection (2.8 years [IQR 0.5, 5.2] vs 6.2 years [IQR 2.6, 12.5]; p=0.046). In unadjusted analysis, HCV reinfection in PWID was associated with methamphetamine injecting during follow up (p=0.010) and use of unsterile needles and/or syringes during follow up (p=0.002). On multivariate analysis, reinfection was associated with older age (aIRR 23.26, 95% CI 2.49, 319.35, p=0.003), shorter duration of injection drug use (duration >5.5 years: aIRR 0.05, 95% CI 0.00, 0.59; p=0.010) and use of unsterile needles and/or syringes during follow up (aIRR 43.27, 95%CI 5.52, 368.14, p<0.001) (Supplementary Table 6). Among HIV-positive MSM, injection drug use at end of and/or post-treatment was reported by 23% (ever injection drug use 61%); this was the only factor associated with HCV reinfection in this sub-group (aIRR 8.19, 95%CI 1.34, 85.99, p=0.019; adjusted for age) (Supplementary Table 7).
Discussion
This analysis assessed HCV reinfection incidence amongst individuals treated for recent HCV infection (duration of infection <18 months) who achieved an end-of-treatment response. High levels of risk behaviour associated with HCV transmission were reported, including 38% reporting injection drug use at end of and/or post treatment, predominantly methamphetamine. Ten cases of reinfection were identified for an overall reinfection incidence of 7.4 per 100 py. All cases occurred in men, the majority of whom were HIV-positive MSM (n=7) and reported injection drug use at end of and/or post treatment (n=8). Two cases occurred in HIV-positive MSM who denied ever injecting drugs.
The incidence of reinfection following treatment for recent HCV infection reported in this analysis is consistent with previous studies amongst HIV-positive MSM and PWID (reinfection incidence: 9.6 – 15.2 per 100 py) (10–12), and expands upon the previous analysis limited to the predominantly HCV mono-infected ATAHC I cohort (19). The higher incidence of HCV reinfection in this and other acute cohorts contrasts with the majority of published studies in individuals treated for chronic HCV infection (summarised in Supplementary Table 1). In a recent meta-analysis, Simmons et al (26) examined the risk of HCV recurrence following interferon-based treatment-induced SVR in three different populations, defined by their perceived risk of reinfection – HCV mono-infected “low risk” (no recognised risk factors for reinfection), HCV mono-infected “high risk” (former or recent injection drug use, incarceration, MSM) and HIV/HCV co-infection. Reinfection incidence was 0.0 per 100 py (95% 0.0, 0.0) in those deemed “low risk”, 1.9 per 100 py (95% CI 1.1, 2.8) in those deemed “high risk” and 3.2 per 100 py (95% CI 0.0, 12.3) in those with HIV/HCV co-infection. However, the proportion of “high risk” or HIV/HCV co-infected individuals continuing to engage in behaviours which facilitated HCV transmission and placed them at risk of reinfection was unclear.
When assessing suitability for HCV therapy, certain populations, including PWID and people with HIV/HCV co-infection, have been considered “high-risk”, primarily based on the apparent potential for reinfection (26). However, these populations are heterogeneous with different levels of risk attributable to specific subgroups. Sub-populations of PWID include those who report injecting an illicit drug at least once (lifetime PWID), those who have ceased injecting drug use (former PWID) and those who continue to inject drugs (recent PWID, with definitions of “recent” varying between one to 12 months) (27). Understanding the definitions for different PWID populations is crucial to accurately define reinfection risk following therapy. Similarly, not all people with HIV/HCV co-infection demonstrate contemporary behaviours placing them at risk of reinfection. While the internationally observed increase in HCV incidence in HIV-positive MSM has been associated with sexual risk behaviour and recreational drug use (10), as with primary HCV infection (28, 29), HIV-positive MSM who inject drugs are at significantly higher risk of HCV reinfection than HIV-positive MSM who do not inject drugs. As exemplified in this cohort, populations at high risk of reinfection, such as PWID and HIV-positive MSM, are not mutually exclusive. While often discussed as separate cohorts, it is important to remember that there is significant overlap.
The risk of HCV reinfection following treatment is significantly higher in those who report ongoing behaviour facilitating HCV transmission, with reinfection incidence ranging between 0.0 – 33.0 per 100 py in PWID treated for chronic HCV infection who reported ongoing injection drug use (19, 30–36). Similarly, in this cohort, HCV reinfection incidence was significantly higher amongst participants who reported injection drug use during follow up as compared with those who did not. However, reinfection was not associated with injection drug use prior to or at commencement of therapy. Particularly in the setting of interferon-based therapy, there may have been considerable selection bias in those PWID deemed suitable, or willing, for treatment. While injecting risk behaviour among PWID appeared to decline during and after interferon-based treatment (37), it is possible that expanded HCV treatment access and DAA therapeutic optimism may be associated with increased risk behaviour, as seen among MSM following the introduction of HIV combination antiretroviral therapy (38).
Reinfection was associated with injection drug use following treatment and older age, the latter appearing to be related to older age and shorter time since injecting onset among PWID with reinfection. Recent onset of injection drug use has been associated with HCV acquisition, though typically among young PWID (39–45). The increased risk of reinfection seen with injection drug use post treatment in association with use of unsterile needles and syringes highlights the need for education and broad access to harm reduction and prevention strategies in concert with HCV treatment. (37)(38)For PWID, access to interventions known to prevent HCV infection, including OST and high coverage needle and syringe access programs (42, 46–49), will be crucial. However, differences in drug use and sexual behaviours among cohorts of HIV-positive MSM as compared with HCV mono-infected populations may necessitate different public health strategies. Much of the literature surrounding HCV acquisition and prevention among PWID focusses on individuals who inject opiates (42, 46–49). Older age at injecting onset, increasing use of stimulant drugs (largely amphetamines) and the phenomena of ‘chemsex’ (illicit drug use before or during sex, by both injecting and non-injecting routes of administration) may necessitate a different approach in MSM (50–54). Evidence supporting sexual behavioural interventions for HCV prevention among MSM is lacking. With serosorting of sexual partners by HIV-status and increasing use of pre-exposure prophylaxis to prevent HIV transmission in HIV-negative MSM, there is the potential for increased sexual risk behaviour and transmission of HCV among MSM populations (52, 55, 56). With DAA treatment scale-up among traditionally marginalised or “high-risk” populations, implementation and evaluation of novel prevention strategies should be a priority.
This study has a number of strengths, including the prospective design, inclusion of active PWID and HIV/HCV co-infected MSM, robust definition of follow up time at-risk for reinfection and use of viral sequencing to accurately delineate relapse and reinfection. The inclusion of a relatively large at-risk population, and documentation of ten cases of reinfection, provided sufficient power to evaluate associations. The prospective design allowed for serial HCV RNA measurements, improving the accuracy of the date of HCV reinfection estimation. Time at-risk for reinfection was calculated from date of end of treatment, where previous analyses have calculated time at-risk from date of SVR. In the era of DAA therapy, reinfection incidence rates will need to be calculated from end of treatment and sequencing used to accurately determine the aetiology of post-treatment HCV RNA recurrence to avoid misclassification, with reinfection occurring prior to the primary endpoint (SVR12) also seen in DAA registration trials (57, 58). However, the sequencing methodology used in this analysis could be considered suboptimal, given the inherent limitations of population-based (Sanger) sequencing, including poor sensitivity to detect minor variants and inability to detect mixed infection (9). Use of next-generation sequencing (NGS) could provide additional clarity in classification of post-treatment viral recurrence.
There are other limitations to this study. Firstly, duration of follow up was limited to that stipulated in the original trial protocol, ranging from 48 to 120 weeks post treatment. Two cases of confirmed reinfection were of indeterminate outcome as reinfection occurred at the last study visit. Given the follow up time, it is also possible that some participants were not followed for a sufficient time to allow for spontaneous clearance of reinfection. Additionally, while the cohort at-risk for reinfection was sizeable, the total follow up time post treatment was impacted by short individual follow up time, which could bias reinfection incidence, by creating a “cohort effect” in which those individuals at very high risk are reinfected early, while overall risk reduces over time. Secondly, due to the intervals between HCV RNA tests (12–24 weeks during follow up), some reinfections with rapid clearance may have been missed and as such, the reported reinfection incidence is an underestimate (59). However, this would not have impacted detection of persistent HCV reinfection. Thirdly, sexual behaviour was not collected, and as such, this could not be included in the model. However, the association between ongoing infecting drug use and reinfection in HIV-positive MSM highlights the overlap in these populations. Lastly, only six percent of the cohort received an interferon-free DAA regimen. Data is being to emerge on reinfection following treatment of chronic HCV infection with DAA therapy. In the C-EDGE COSTAR trial among people receiving OST, six cases of reinfection were identified at or prior to post treatment week 24, with five cases of reinfection detected at post treatment week eight (reinfection incidence 4.6 per 100 py, 95% CI 1.7, 10.0) (57). Urine drug screen was positive both during and following treatment in five of the six cases. The incidence of reinfection following DAA-based treatment needs careful evaluation as access to treatment among populations at-risk of ongoing transmission increases.
The treatment paradigm for individuals with HCV infection is evolving rapidly (2, 3, 58, 60). The potential for broad access to highly effective, well tolerated interferon-free DAA regimens has stimulated discussion around HCV treatment-as-prevention. HCV treatment-as-prevention strategies will be enhanced by early diagnosis and increased treatment uptake in recent HCV infection, in order to reduce transmission amongst at-risk populations (5, 13). The significant risk for HCV reinfection following treatment in individuals with ongoing high risk behaviour emphasises the need for post-treatment surveillance, harm reduction strategies and education (12, 61), but must not be considered an impediment to treatment, if HCV elimination is to be achieved.
Supplementary Material
Acknowledgments
Financial support: The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government. The Burnet Institute receives funding from the Victorian Operational Infrastructure Support Program. Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health (R01DA015999), Janssen-Cilag Pty Ltd and Gilead Sciences Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Jason Grebely and Gail Matthews are supported through NHMRC Career Development Fellowships. Gregory Dore is supported through NHMRC Practitioner Fellowships. None of the authors has commercial relationships that might pose a conflict of interest in connection with this manuscript.
Footnotes
Clinical trial number(s): ATAHC I: NCT00192569; ATAHC II: NCT01336010; DARE-C I: NCT01743521; DARE-C II: NCT02156570
Author’s contributions: GVM, GJD, EG, MH, DS, JS KP, TAA, JG and MM were involved in study concept and design. MM, GVM, GJD, EG, MH, DS, and JS were involved in acquisition of data. MM and KP performed the statistical analysis. MM, GVM, GJD and JG were involved in analysis and interpretation of data. MM drafted the manuscript with critical revision of the manuscript for important intellectual content by all authors. GVM and GJD provided overall study supervision. All authors have seen and approved the final version of the manuscript.
Disclosure of Interest Statement: The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government. The Burnet Institute receives funding from the Victorian Operational Infrastructure Support Program. Research reported in this publication was supported by Gilead Sciences Inc. The content is solely the responsibility of the authors. None of the authors has commercial relationships that might pose a conflict of interest in connection with this manuscript. Jason Grebely and Gail Matthews are supported through NHMRC Career Development Fellowships. Gregory Dore is supported through NHMRC Practitioner Fellowships. Margaret Hellard is supported through an NHMRC Principal Research Fellowship.
MM has received speaker payments from Abbvie.
JG is a consultant/advisor and has received research grants from Abbvie, Bristol-Myers Squibb, Gilead Sciences and Merck.
KP has received unrestricted grant funding grants from Boehringer-Ingelheim Pty Ltd, Bristol-Myers Squibb Australia Pty Ltd, Gilead Sciences Pty Limited, Janssen-Cilag Pty Ltd, Merck Sharp & Dohme (Australia) Pty Ltd, Viiv HealthCare.
EG has participated in the advisory boards and also in speakers’ bureau for Gilead Sciences, Janssen and Abbvie.
MH has received funding for investigator initiated research from Gilead, Bristol-Myers Squibb and Abbvie. The Burnet Institute has received un-tied educational grant funding from Gilead Sciences.
DS: No conflict
JS: No conflict
TLA: No conflict
GJD is an advisory board member and has received honoraria from Roche, Merck, Janssen, Gilead, Bristol-Myers Squibb and Abbvie, has received research grant funding from Roche, Merck, Janssen, Gilead, Bristol-Myers Squibb, Vertex, Boeringher Ingelheim and Abbvie, and travel sponsorship from Roche, Merck, Janssen, Gilead, and Bristol-Myers Squibb.
GVM has received advisory board payments from Gilead, speaker fees and honoraria from Gilead, Bristol-Myers Squibb and Abbvie and research grant funding from Janssen, Gilead and Abbvie.
Reference List
- 1.Dore GJ, Feld JJ. Hepatitis C virus therapeutic development: in pursuit of “perfectovir”. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(12):1829–36. doi: 10.1093/cid/civ197. [DOI] [PubMed] [Google Scholar]
- 2.Feld JJ, Jacobson IM, Hezode C, Asselah T, Ruane PJ, Gruener N, et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. The New England journal of medicine. 2015;373(27):2599–607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 3.Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, et al. Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1. The New England journal of medicine. 2015;373(8):705–13. doi: 10.1056/NEJMoa1501315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cousien A, Tran VC, Deuffic-Burban S, Jauffret-Roustide M, Dhersin JS, Yazdanpanah Y. Hepatitis C treatment as prevention of viral transmission and liver-related morbidity in persons who inject drugs. Hepatology. 2016;63(4):1090–101. doi: 10.1002/hep.28227. [DOI] [PubMed] [Google Scholar]
- 5.Martin NK, Thornton A, Hickman M, Sabin C, Nelson M, Cooke GS, et al. Can Hepatitis C Virus (HCV) Direct-Acting Antiviral Treatment as Prevention Reverse the HCV Epidemic Among Men Who Have Sex With Men in the United Kingdom? Epidemiological and Modeling Insights. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62(9):1072–80. doi: 10.1093/cid/ciw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin NK, Vickerman P, Dore GJ, Grebely J, Miners A, Cairns J, et al. Prioritization of HCV treatment in the direct-acting antiviral era: An economic evaluation. Journal of hepatology. 2016;65(1):17–25. doi: 10.1016/j.jhep.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott N, McBryde ES, Thompson A, Doyle JS, Hellard ME. Treatment scale-up to achieve global HCV incidence and mortality elimination targets: a cost-effectiveness model. Gut. 2016 doi: 10.1136/gutjnl-2016-311504. [DOI] [PubMed] [Google Scholar]
- 8.Asher AK, Portillo CJ, Cooper BA, Dawson-Rose C, Vlahov D, Page KA. Clinicians’ Views of Hepatitis C Virus Treatment Candidacy With Direct-Acting Antiviral Regimens for People Who Inject Drugs. Substance use & misuse. 2016;51(9):1218–23. doi: 10.3109/10826084.2016.1161054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham EB, Applegate TL, Lloyd AR, Dore GJ, Grebely J. Mixed HCV infection and reinfection in people who inject drugs--impact on therapy. Nature reviews Gastroenterology & hepatology. 2015;12(4):218–30. doi: 10.1038/nrgastro.2015.36. [DOI] [PubMed] [Google Scholar]
- 10.Hagan H, Jordan AE, Neurer J, Cleland CM. Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men. AIDS (London, England) 2015;29(17):2335–45. doi: 10.1097/QAD.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambers FA, Prins M, Thomas X, Molenkamp R, Kwa D, Brinkman K, et al. Alarming incidence of hepatitis C virus re-infection after treatment of sexually acquired acute hepatitis C virus infection in HIV-infected MSM. AIDS (London, England) 2011;25(17):F21–7. doi: 10.1097/QAD.0b013e32834bac44. [DOI] [PubMed] [Google Scholar]
- 12.Martin TC, Martin NK, Hickman M, Vickerman P, Page EE, Everett R, et al. Hepatitis C virus reinfection incidence and treatment outcome among HIV-positive MSM. AIDS. 2013;27(16):2551–7. doi: 10.1097/QAD.0b013e32836381cc. [DOI] [PubMed] [Google Scholar]
- 13.Hickman M, De Angelis D, Vickerman P, Hutchinson S, Martin NK. Hepatitis C virus treatment as prevention in people who inject drugs: testing the evidence. Current opinion in infectious diseases. 2015;28(6):576–82. doi: 10.1097/QCO.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57(Suppl 2):S39–45. doi: 10.1093/cid/cit296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin NK, Foster GR, Vilar J, Ryder S, Cramp ME, Gordon F, et al. HCV treatment rates and sustained viral response among people who inject drugs in seven UK sites: real world results and modelling of treatment impact. Journal of viral hepatitis. 2015;22(4):399–408. doi: 10.1111/jvh.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahnd C, Salazar-Vizcaya L, Dufour JF, Mullhaupt B, Wandeler G, Kouyos R, et al. Modelling the impact of deferring HCV treatment on liver-related complications in HIV coinfected men who have sex with men. Journal of hepatology. 2016;65(1):26–32. doi: 10.1016/j.jhep.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 17.Salazar-Vizcaya L, Kouyos RD, Zahnd C, Wandeler G, Battegay M, Darling KE, et al. Hepatitis C virus transmission among HIV-infected men who have sex with men: Modeling the effect of behavioral and treatment interventions. Hepatology. 2016;64:1856–1869. doi: 10.1002/hep.28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Razavi H. Reducing a country’s HCV-disease burden. The 4th International Symposium on Hepatitis in Substance Users (INHSU 2015); 7–9 October, 2015; Sydney, Australia. 2015. [Google Scholar]
- 19.Grebely J, Pham ST, Matthews GV, Petoumenos K, Bull RA, Yeung B, et al. Hepatitis C virus reinfection and superinfection among treated and untreated participants with recent infection. Hepatology. 2012;55(4):1058–69. doi: 10.1002/hep.24754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dore GJ, Hellard M, Matthews GV, Grebely J, Haber PS, Petoumenos K, et al. Effective treatment of injecting drug users with recently acquired hepatitis C virus infection. Gastroenterology. 2010;138(1):123–35. e1–2. doi: 10.1053/j.gastro.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinello M, Hellard M, Shaw D, Petoumenos K, Applegate T, Grebely J, et al. Short duration response-guided treatment is effective for most individuals with recent hepatitis C infection: the ATAHC II and DARE-C I studies. Antiviral therapy. 2016;21(5):425–34. doi: 10.3851/IMP3035. [DOI] [PubMed] [Google Scholar]
- 22.Martinello M, Gane E, Hellard M, Sasadeusz J, Shaw D, Petoumenos K, et al. Sofosbuvir and ribavirin for 6 weeks is not effective among people with recent hepatitis C virus infection: The DARE-C II study. Hepatology. 2016;64:1911–1921. doi: 10.1002/hep.28844. [DOI] [PubMed] [Google Scholar]
- 23.Jacka B, Applegate T, Krajden M, Olmstead A, Harrigan PR, Marshall BD, et al. Phylogenetic clustering of hepatitis C virus among people who inject drugs in Vancouver, Canada. Hepatology. 2014;60(5):1571–80. doi: 10.1002/hep.27310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamoury FM, Jacka B, Bartlett S, Bull RA, Wong A, Amin J, et al. The Influence of Hepatitis C Virus Genetic Region on Phylogenetic Clustering Analysis. PLoS One. 2015;10(7):e0131437. doi: 10.1371/journal.pone.0131437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darke S, Hall W, Wodak A, Heather N, Ward J. Development and validation of a multi-dimensional instrument for assessing outcome of treatment among opiate users: the Opiate Treatment Index. British journal of addiction. 1992;87(5):733–42. doi: 10.1111/j.1360-0443.1992.tb02719.x. [DOI] [PubMed] [Google Scholar]
- 26.Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of Late Relapse or Reinfection With Hepatitis C Virus After Achieving a Sustained Virological Response: A Systematic Review and Meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62(6):683–94. doi: 10.1093/cid/civ948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larney S, Grebely J, Hickman M, De Angelis D, Dore GJ, Degenhardt L. Defining populations and injecting parameters among people who inject drugs: Implications for the assessment of hepatitis C treatment programs. The International journal on drug policy. 2015;26(10):950–7. doi: 10.1016/j.drugpo.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Jordan AE, Perlman DC, Neurer J, Smith DJ, Des Jarlais DC, Hagan H. Prevalence of hepatitis C virus infection among HIV+ men who have sex with men: a systematic review and meta-analysis. International journal of STD & AIDS. 2016 doi: 10.1177/0956462416630910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gamage DG, Read TR, Bradshaw CS, Hocking JS, Howley K, Chen MY, et al. Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC infectious diseases. 2011;11:39. doi: 10.1186/1471-2334-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marco A, Esteban JI, Sole C, da Silva A, Ortiz J, Roget M, et al. Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. Journal of hepatology. 2013;59(1):45–51. doi: 10.1016/j.jhep.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Aspinall EJ, Corson S, Doyle JS, Grebely J, Hutchinson SJ, Dore GJ, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57(Suppl 2):S80–9. doi: 10.1093/cid/cit306. [DOI] [PubMed] [Google Scholar]
- 32.Backmund M, Meyer K, Edlin BR. Infrequent reinfection after successful treatment for hepatitis C virus infection in injection drug users. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39(10):1540–3. doi: 10.1086/425361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weir A, McLeod A, Innes H, Valerio H, Aspinall EJ, Goldberg DJ, et al. Hepatitis C reinfection following treatment induced viral clearance among people who have injected drugs. Drug Alcohol Depend. 2016;165:53–60. doi: 10.1016/j.drugalcdep.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Midgard H, Bjoro B, Maeland A, Konopski Z, Kileng H, Damas JK, et al. Hepatitis C reinfection after sustained virological response. Journal of hepatology. 2016;64(5):1020–6. doi: 10.1016/j.jhep.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Grebely J, Knight E, Ngai T, Genoway KA, Raffa JD, Storms M, et al. Reinfection with hepatitis C virus following sustained virological response in injection drug users. Journal of gastroenterology and hepatology. 2010;25(7):1281–4. doi: 10.1111/j.1440-1746.2010.06238.x. [DOI] [PubMed] [Google Scholar]
- 36.Currie SL, Ryan JC, Tracy D, Wright TL, George S, McQuaid R, et al. A prospective study to examine persistent HCV reinfection in injection drug users who have previously cleared the virus. Drug Alcohol Depend. 2008;93(1–2):148–54. doi: 10.1016/j.drugalcdep.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Alavi M, Spelman T, Matthews GV, Haber PS, Day C, van Beek I, et al. Injecting risk behaviours following treatment for hepatitis C virus infection among people who inject drugs: The Australian Trial in Acute Hepatitis C. The International journal on drug policy. 2015;26(10):976–83. doi: 10.1016/j.drugpo.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dukers NH, Goudsmit J, de Wit JB, Prins M, Weverling GJ, Coutinho RA. Sexual risk behaviour relates to the virological and immunological improvements during highly active antiretroviral therapy in HIV-1 infection. AIDS (London, England) 2001;15(3):369–78. doi: 10.1097/00002030-200102160-00010. [DOI] [PubMed] [Google Scholar]
- 39.Maher L, Jalaludin B, Chant KG, Jayasuriya R, Sladden T, Kaldor JM, et al. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction (Abingdon, England) 2006;101(10):1499–508. doi: 10.1111/j.1360-0443.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 40.Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008;168(10):1099–109. doi: 10.1093/aje/kwn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Berg CH, Smit C, Bakker M, Geskus RB, Berkhout B, Jurriaans S, et al. Major decline of hepatitis C virus incidence rate over two decades in a cohort of drug users. Eur J Epidemiol. 2007;22(3):183–93. doi: 10.1007/s10654-006-9089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White B, Dore GJ, Lloyd AR, Rawlinson WD, Maher L. Opioid substitution therapy protects against hepatitis C virus acquisition in people who inject drugs: the HITS-c study. Med J Aust. 2014;201(6):326–9. doi: 10.5694/mja13.00153. [DOI] [PubMed] [Google Scholar]
- 43.Spittal PM, Pearce ME, Chavoshi N, Christian WM, Moniruzzaman A, Teegee M, et al. The Cedar Project: high incidence of HCV infections in a longitudinal study of young Aboriginal people who use drugs in two Canadian cities. BMC public health. 2012;12:632. doi: 10.1186/1471-2458-12-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen EJ, Palmateer NE, Hutchinson SJ, Cameron S, Goldberg DJ, Taylor A. Association between harm reduction intervention uptake and recent hepatitis C infection among people who inject drugs attending sites that provide sterile injecting equipment in Scotland. The International journal on drug policy. 2012;23(5):346–52. doi: 10.1016/j.drugpo.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Jordan AE, Des Jarlais DC, Arasteh K, McKnight C, Nash D, Perlman DC. Incidence and prevalence of hepatitis c virus infection among persons who inject drugs in New York City: 2006–2013. Drug and alcohol dependence. 2015;152:194–200. doi: 10.1016/j.drugalcdep.2015.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nolan S, Dias Lima V, Fairbairn N, Kerr T, Montaner J, Grebely J, et al. The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction (Abingdon, England) 2014;109(12):2053–9. doi: 10.1111/add.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsui JI, Evans JL, Lum PJ, Hahn JA, Page K. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA internal medicine. 2014;174(12):1974–81. doi: 10.1001/jamainternmed.2014.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner KM, Hutchinson S, Vickerman P, Hope V, Craine N, Palmateer N, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction (Abingdon, England) 2011;106(11):1978–88. doi: 10.1111/j.1360-0443.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 49.Coffin PO, Rowe C, Santos GM. Novel interventions to prevent HIV and HCV among persons who inject drugs. Curr HIV/AIDS Rep. 2015;12(1):145–63. doi: 10.1007/s11904-014-0248-2. [DOI] [PubMed] [Google Scholar]
- 50.Khosropour CM, Dombrowski JC, Swanson F, Kerani RP, Katz DA, Barbee LA, et al. Trends in serosorting and the association with HIV/STI risk over time among men who have sex with men (MSM) Journal of acquired immune deficiency syndromes. 1999:2016. doi: 10.1097/QAI.0000000000000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Velter A, Saboni L, Sommen C, Bernillon P, Bajos N, Semaille C. Sexual and prevention practices in men who have sex with men in the era of combination HIV prevention: results from the Presse Gays et Lesbiennes survey, France, 2011. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2015;20(14) doi: 10.2807/1560-7917.es2015.20.14.21090. [DOI] [PubMed] [Google Scholar]
- 52.Apers L, Vanden Berghe W, De Wit S, Kabeya K, Callens S, Buyze J, et al. Risk factors for HCV acquisition among HIV-positive MSM in Belgium. Journal of acquired immune deficiency syndromes (1999) 2015;68(5):585–93. doi: 10.1097/QAI.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 53.Melendez-Torres GJ, Bourne A. Illicit drug use and its association with sexual risk behaviour among MSM: more questions than answers? Current opinion in infectious diseases. 2016;29(1):58–63. doi: 10.1097/QCO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 54.Gilbart VL, Simms I, Jenkins C, Furegato M, Gobin M, Oliver I, et al. Sex, drugs and smart phone applications: findings from semistructured interviews with men who have sex with men diagnosed with Shigella flexneri 3a in England and Wales. Sex Transm Infect. 2015;91(8):598–602. doi: 10.1136/sextrans-2015-052014. [DOI] [PubMed] [Google Scholar]
- 55.Golub SA, Kowalczyk W, Weinberger CL, Parsons JT. Preexposure prophylaxis and predicted condom use among high-risk men who have sex with men. Journal of acquired immune deficiency syndromes (1999) 2010;54(5):548–55. doi: 10.1097/QAI.0b013e3181e19a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McFaul K, Maghlaoui A, Nzuruba M, Farnworth S, Foxton M, Anderson M, et al. Acute hepatitis C infection in HIV-negative men who have sex with men. Journal of viral hepatitis. 2015;22(6):535–8. doi: 10.1111/jvh.12366. [DOI] [PubMed] [Google Scholar]
- 57.Dore GJ, Altice F, Litwin AH, Dalgard O, Gane EJ, Shibolet O, et al. Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Annals of internal medicine. 2016;165(9):625–34. doi: 10.7326/M16-0816. [DOI] [PubMed] [Google Scholar]
- 58.Rockstroh JK, Nelson M, Katlama C, Lalezari J, Mallolas J, Bloch M, et al. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. The lancet HIV. 2015;2(8):e319–27. doi: 10.1016/S2352-3018(15)00114-9. [DOI] [PubMed] [Google Scholar]
- 59.Vickerman P, Grebely J, Dore GJ, Sacks-Davis R, Page K, Thomas DL, et al. The more you look, the more you find: effects of hepatitis C virus testing interval on reinfection incidence and clearance and implications for future vaccine study design. The Journal of infectious diseases. 2012;205(9):1342–50. doi: 10.1093/infdis/jis213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gane EJ, Schwabe C, Hyland RH, Yang Y, Svarovskaia E, Stamm LM, et al. Efficacy of the Combination of Sofosbuvir, Velpatasvir, and the NS3/4A Protease Inhibitor GS-9857 in Treatment-Naive or Previously Treated Patients with Hepatitis C Virus Genotype 1 or 3 Infections. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 61.Ingiliz P, Krznaric I, Stellbrink HJ, Knecht G, Lutz T, Noah C, et al. Multiple hepatitis C virus (HCV) reinfections in HIV-positive men who have sex with men: no influence of HCV genotype switch or interleukin-28B genotype on spontaneous clearance. HIV medicine. 2014;15(6):355–61. doi: 10.1111/hiv.12127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.