Abstract
Objective
To determine time trends in the incidence and survival of polymyalgia rheumatica (PMR) over a 15 year period in Olmsted County, Minnesota, USA and to examine trends in incidence of PMR in the population by comparing this time period to a previous incidence cohort from the same population base.
Methods
All cases of incident PMR among Olmsted County, Minnesota residents in 2000-2014 were identified to extend the previous 1970-1999 cohort. Detailed review of all individual medical records was performed. Incidence rates were age and sex adjusted to the US white 2010 population. Survival rates were compared with the expected rates in the population of Minnesota.
Results
There were 377 incident cases of PMR during the 15 year study period. Of these 64% were female and the mean age at incidence was 74.1 years. The overall age and sex adjusted annual incidence of PMR was 63.9 (95% confidence interval [CI] 57.4, 70.4) per 100,000 population aged ≥50 years. Incidence rates increased with age in both sexes, but incidence fell after age 80 years. There was a slight increase in incidence of PMR in the recent time period compared to 1970-1999 (p=0.063). Mortality among individuals with PMR was not significantly worse than that expected in the general population (standardized mortality ratio: 0.70; 95% CI: 0.57, 0.85).
Conclusion
The incidence of PMR has increased slightly in the past 15 years compared to previous decades. Survivorship in patients with PMR is not worse than in the general population.
Keywords: Polymyalgia rheumatica, epidemiology, incidence, survival
Polymyalgia rheumatica (PMR) is a rheumatic disorder associated with moderate-to-severe musculoskeletal pain and stiffness in the neck, shoulder and hip area. It affects individuals aged >50 years. The etiology is not clearly known but is associated with immune activation and likely environmental and genetic factors.
The incidence of PMR increases with age and appears to vary in different parts of the world. The majority of studies of PMR incidence date from the 1980s, and generally report incidence rates of 50-68/100,000 in Northern Europe and North America, with much lower rates in the Mediterranean basin [1,4,5,6,8,9]. It is unknown whether there has been any change in the incidence or survivorship of patients with PMR in recent years.
The population of Olmsted County, Minnesota is predominately white and of Northern European origin. The incidence of PMR in Olmsted County from 1970 to 1999 was 59/100,000, with survivorship similar to the general population [1, 2]. The purpose of this study was to examine incidence and survival in PMR in recent years, and describe these trends over the past 45 years in this population-based cohort.
MATERIALS AND METHODS
The population of Olmsted County, Minnesota is well suited for investigation of the epidemiology of PMR because comprehensive unit medical records for all residents seeking medical care are available. The US Census estimated the population of Olmsted County aged ≥50 years at 31,098 persons in 2000 with a mean age of 64.5 years and 55% females. A record linkage system allows ready access to the medical records from all health care providers for the local population, including the Mayo Clinic and Olmsted Medical Center and their affiliated hospitals, local nursing homes, and the few private practitioners. The potential of this data system for population based studies has been described [3]. This system assures virtually complete ascertainment of all clinically recognized cases of PMR among Olmsted County residents.
Using this data resource, an inception cohort of all cases of PMR first diagnosed between January 1, 1970 and December 31, 1999, among Olmsted Country residents was previously identified as described [1,2]. The same method of case ascertainment was used to expand this cohort to include all incident cases of PMR among residents between January 1, 2000, and December 31, 2014. Patients were followed up until death or January 1, 2015. Detailed review of all individual medical records was performed. Individuals were included as PMR cases if they fulfilled European League Against Rheumatism/American College of Rheumatology classification criteria for PMR [4]. All cases were physician diagnosed, and all of these cases fulfilled these criteria. Medical records were reviewed to document anorexia, weight loss, fatigue, maximum temperature, and morning stiffness (bilateral aching and morning stiffness involving the following areas: neck or torso, shoulders or proximal regions of arms, and hips or proximal aspects of the thighs). Physician diagnosis of giant cell arteritis (GCA) before, at or after diagnosis of PMR was also collected. Exclusion criteria were the presence of other diseases that could explain the symptoms, such as active rheumatoid arthritis, systemic lupus erythematosus, polymyositis, chronic infection or multiple myeloma.
New incidence cases were added to those already identified from the previous incidence cohort. Age - and sex-specific incidence rates were then calculated using the number of incidence cases as the numerator and population estimates based on decennial census counts as the denominator, with linear interpolation to estimate population size for intercensal years. Annual incidence rates were illustrated using a 3 year centered moving average. Overall rates were age and sex adjusted to the 2010 United States white population. Ninety-five percent confidence intervals were computed for incidence rates assuming that the observed number of cases follows a Poisson distribution. Poisson regression models with smoothing splines to allow for non-linear effects were used to evaluate time trends in incidence rates.
Survival following the diagnosis of PMR was estimated using the Kaplan-Meier product-limit method. Observed and expected survival was compared using the log-rank test, where expected survival was based on the sex and age of the study population and on death rates from the Minnesota Caucasian life tables. The standardized mortality ratio (SMR), the ratio of observed number of deaths to the expected number, was estimated and a 95% CI obtained assuming that the observed number of deaths follows a Poisson distribution. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
There were 377 Olmsted County residents who first fulfilled criteria for PMR between 2000 and 2014, amounting to a total of 760 incident cases of PMR between 1970 and 2014. Two hundred forty-three (64%) of these individuals were female, 134 (36%) were male, and the mean age at incidence of PMR was 74.1 years (Table 1). Prior or concurrent diagnosis of giant cell arteritis was found in 18 (5%) patients. An additional 5 cases of GCA developed during follow-up (10 year rate of GCA development: 1.6%; 95% confidence interval [CI]: 0.1%, 3.1%).
Table 1.
Characteristics of 377 Olmsted County, Minnesota residents with incident polymyalgia rheumatic in 2000-2014
| Characteristic | Value* |
|---|---|
| Age at diagnosis, years, mean (±SD) | 74.1 (±9.50) |
| Sex, female | 243 (64%) |
| Ethnicity | |
| White | 363 (96%) |
| Black | 5 (1%) |
| Asian | 5 (1%) |
| Am. Indian | 1 (0%) |
| Other/Mixed | 3 (1%) |
| Length of follow-up, years, median (interquartile range) | 6.6 (3.7, 10.4) |
| Morning Stiffness | |
| Missing | 1 |
| Yes (>30 minutes) | 13 (3%) |
| Yes (>45 minutes) | 103 (27%) |
| Yes (unspecified) | 183 (49%) |
| No | 77 (20%) |
| Aching/ Stiffness Neck | 150 (40%) |
| Aching/ Stiffness Shoulder/Arm | 357 (95%) |
| Aching/ Stiffness Hip/Thigh | 321 (85%) |
| Anorexia | 41 (11%) |
| Malaise/ Fatigue | 287 (76%) |
| Weight loss (>5 kg) | 164 (44%) |
| Highest Fever Celsius, mean (±SD) | 37.9 (±3.4) |
| ESR, mm/hr, median (interquartile range) | 44 (31, 63) |
| CRP, mg/L, median (interquartile range)(n=275 tested) | 39.7 (16, 74) |
| Rheumatoid Factor Positive (n=219 tested) | 15 (7%) |
| Prior or concurrent giant cell arteritis | 18 (5%) |
ESR, erythrocyte sedimentation rate; CRP, C- reactive protein.
The age and sex adjusted annual incidence rate of PMR in 2000-2014 was 63.9 (95% CI 57.4, 70.4) per 100,000 population aged 50 years and older. The total annual incidence rates increased with advancing age to a maximum in the 70-79 age group, after which the incidence rate fell. Furthermore, the age-specific rates were higher in women compared to men (Table 2).
Table 2.
Incidence of polymyalgia rheumatic among residents of Olmsted County, Minnesota in 2000-2014, by sex and age group, per 100,000 population age ≥ 50 years.
| Male | Female | Total | ||||
|---|---|---|---|---|---|---|
| Age group | N | Rate | N | Rate | N | Rate |
| 50-59 | 11 | 8.5 | 21 | 15.3 | 32 | 12.0 |
| 60-69 | 36 | 46.4 | 45 | 52.5 | 81 | 49.6 |
| 70-79 | 58 | 124.3 | 102 | 181.7 | 160 | 155.7 |
| 80+ | 29 | 112.6 | 75 | 157.8 | 104 | 141.9 |
| Overall | 134 | 55.6* (43.6, 61.6) | 243 | 72.7* (63.5, 81.9) | 377 | 63.9** (57.4, 70.4) |
Age adjusted to US white 2010 population
Age and sex adjusted to US white 2010 population
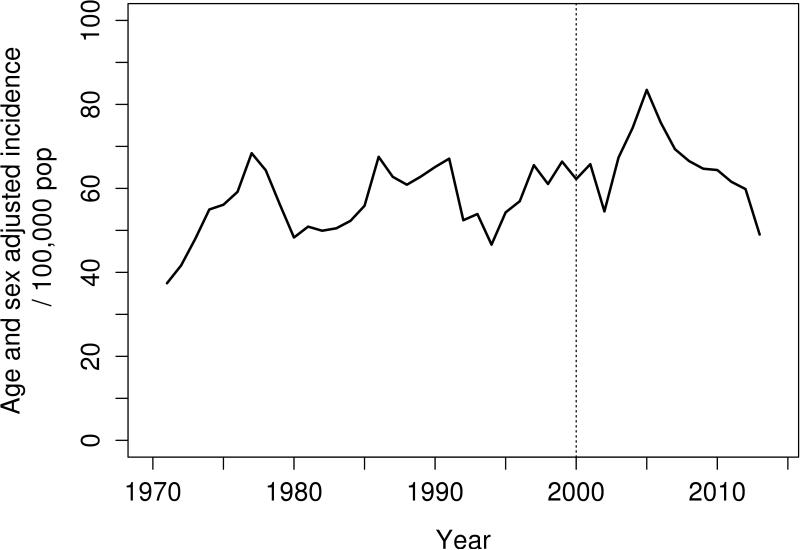
The incidence of PMR in this long-term study demonstrates variability at specific time points with a slight overall increase in incidence over the past 45 years. There was no evidence of seasonality in incidence (data not shown). The 3 year moving average annual incidence rates per 100,000 population are shown in Figure 1 to illustrate trends over the entire study period. The peaks were evenly spaced in 1975, 1990 and 2005. There was a significant increase in incidence of PMR in the recent time period (2000-2014) compared to 1970-1999 (age and sex adjusted rate: 55.8 per 100,000; p=0.063).
Figure 1.
Overall incidence of polymyalgia rheumatica incidence over 45 years
During median follow-up of 6.6 years, 107 patients died. Mortality among individuals with PMR was not significantly worse than that expected in the general population (standardized mortality ratio: 0.70; 95% CI: 0.57, 0.85).
DISCUSSION
The annual incidence of PMR in Olmsted County is 63.9/100,000 population for the years 2000-2014. This compares to an average incidence rate of 58.7/100,000 in this population for 1975-1999 [2]. The 45 year incidence observation period in this study is the longest period a population has been assessed for PMR. Using the same case ascertainment procedures for PMR from 1970 to 2014, the incidence of PMR has increased slightly in the last 15 years.
In this study, there was no signal of seasonal variation with regard to the incidence of PMR, but there was a decline in incidence in the 80+ age group. Such a decline in incidence in older age groups has been noted in other studies [2]. The reasons for this are uncertain, but may relate to reduced suspicion of disease in older patients, perhaps resulting in under diagnosis, changes in immune response in very high age, or other as yet poorly understood factors.
The reported incidence rates from different populations are quite variable, with a clear tendency for higher rates in studies performed in Northern European populations. The incidence of PMR has been found to be higher in individuals of Scandinavian background [5-7]. The annual incidence rate of PMR in Ribe County, Denmark over the period 1982-1985 was 68.3/100,000 for the population aged ≥ 50 years, which is similar to the estimate in the current study from Olmsted County [5]. Similarly, the incidence of PMR in Göteborg, Sweden between 1985 and 1987 for the population age ≥ 50 years was 50/100,000 [7].
The annual incidence of PMR is lower in Southern European countries. The annual incidence rate of PMR in Reggio Emilia, Italy, over the period 1980-1988 for the population age ≥ 50 years was 12.7/100,000 [8]. Analogously, the annual incidence rate in Lugo, Spain, for the population age ≥50 years over the period 1987-1996 was 18.7/100,000 for overall PMR (with or without associated with GCA) and 13.5/100,00 for subjects with isolated pure PMR [9]. A rate of 3.15/100,000 has been reported from Turkey, markedly lower than that of European populations [10]. Results from the current study are closer to those from Northern Europe, which probably relates to the fact that the population of Minnesota is composed primarily of people of Northern European descent.
The mortality rates among both men and women with PMR in this study were not higher than the general Minnesota population.
This study is among the first to report the incidence of PMR over a long period of time, spanning more than 4 decades. A particular strength of this study is use of the Rochester Epidemiology Project record linkage system, through which all cases of PMR in Olmsted County, Minnesota were identified and verified by individual medical record review, which avoids referral bias within the study cohort. The ability to review the entire medical record of each study subject to collect information pertinent to the study avoids potential recall biases that might arise if data were collected in other ways, such as interviews or questionnaires.
A potential limitation of the retrospective study design may be incomplete documentation in the medical record regarding symptoms suggestive of PMR. Because the population of Olmsted County is predominantly Caucasian, results may not be generalizable to non-Caucasian individuals, however this population represents the cohort at highest risk of developing this disease.
In conclusion, the incidence of PMR has increased somewhat over the past 45 years. Survival among patients with PMR is not worse than the general population. Further research is needed to understand the determinants of the increase in incidence of PMR.
SIGNIFICANCE AND INNOVATIONS.
This study is among the first to report on the incidence of polymyalgia rheumatica (PMR) over a long period including recent decades.
The incidence of PMR in Olmsted County, Minnesota, has increased slightly in the past 15 years compared to previous decades.
Survival among patients with PMR is not worse than the general population.
Acknowledgments
Funding: This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement for all authors: The authors have no financial or non-financial potential conflicts of interest to declare.
REFERENCES
- 1.Salvarani C, Gabriel SE, O'Fallon WM, Hunder GG. Epidemiology of polymyalgia rheumatica in Olmsted County, Minnesota, 1970-1991. Arthritis Rheum. 1995;38:369–73. doi: 10.1002/art.1780380311. [DOI] [PubMed] [Google Scholar]
- 2.Doran MF, Crowson CS, O'Fallon WM, Hunder GG, Gabriel SE. Trends in the Incidence of Polymyalgia Rheumatica over a 30 Year Period in Olmsted County, Minnesota, USA. J Rheumatol. 2002;29:1694–1697. [PubMed] [Google Scholar]
- 3.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Pankratz JJ, Brue SM, Rocca WA. Data resource profile: The Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–24. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasgupta B, Cimmino MA, Maradit Kremers H, Schmidt WA, Schirmer M, Salvarani C, et al. European League Against Rheumatism – American College of Rheumatology provisional classification criteria for polymyalgia rheumatica. Arthritis Rheum. 2012;64(4):943–954. doi: 10.1002/art.34356. [DOI] [PubMed] [Google Scholar]
- 5.Boesen P, Sorensen SF. Giant cell arteritis, temporal arteritis, and polymyalgia rheumatica in a Danish county: a prospective investigation, 1982-1985. Arthritis Rheum. 1987;0:294–9. doi: 10.1002/art.1780300308. [DOI] [PubMed] [Google Scholar]
- 6.Elling P, Olsson AT, Elling H. Synchronous variations of the incidence of temporal arteritis and polymyalgia rheumatica in different regions of Denmark: association with epidemics of Mycoplasma pneumoniae infection. J Rheumatol. 1996;23:112–9. [PubMed] [Google Scholar]
- 7.Schaufelberger C, Bengstsson BA, Andersson R. Epidemiology and mortality in 220 patients with polymyalgia rheumatica. Br J Rheumatol. 1995;34:261–4. doi: 10.1093/rheumatology/34.3.261. [DOI] [PubMed] [Google Scholar]
- 8.Salvarani C, Macchioni P, Zizzi F, Mantovani W, Rossi F, Castri C, et al. Epidemiologic and immunogenetic aspects of polymyalgia rheumatica and giant cell arteritis in northern Italy. Arthritis Rheum. 1991;34:351–6. doi: 10.1002/art.1780340313. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Gay MA, Garcia-Porrua C, Vazquez-Caruncho M, Dababneh A, Hajeer A, Ollier WE. The spectrum of polymyalgia rheumatica in northwestern Spain: incidence and analysis of variables associated with relapse in a 10 year study. J Rheumatol. 1999;26:1326–32. [PubMed] [Google Scholar]
- 10.Pamuk ON, Donmez S, Karahan B, Pamuk GE, Cakir N. Giant cell arteritis and polymyalgia rheumatica in northwestern Turkey: Clinical features and epidemiological data. Clin Exp Rheumatol. 2009;27(5):830–3. [PubMed] [Google Scholar]