Abstract
Various preclinical studies have demonstrated that the success of immunotherapeutic strategies in inhibiting tumor progression in animal models of Glioblastoma multiforme (GBM). It is also evident that tumor-induced immune suppression drastically impacts the efficacy of immune based therapies. Among the mechanisms employed by GBM to induce immunosuppression is the accumulation of regulatory T cells (Tregs) and Myeloid derived suppressor cells (MDSCs). Advancing our understanding about the pathways regulating the expansion, accumulation and activity of MDSCs will allow for the development of therapies aimed at abolishing the inhibitory effect of these cells on immunotherapeutic approaches. In this review, we have focused on the origin, expansion and immunosuppressive mechanisms of MDSCs in animal models and human cancer, in particular GBM.
Keywords: Glioma, myeloid derived suppressor cells, immunotherapy, tumor microenvironment, T cells
1. Glioblastoma multiforme (GBM) and immunesuppression in GBM
Glioblastoma multiforme is the most common and aggressive form of glioma, with a 5-year relative survival rate of about 5% [1]. These primary brain tumors most often occur in the cerebral hemispheres and include astrocytomas, oligodendriogliomas, and oligoastrocytomas [2]. Current WHO classification of gliomas into prognostically useful grades is based on histological classification of morphological features, separating diffuse lower grade gliomas, which are classified as WHO grades II and III, from GBM, a WHO grade IV glioma. However, the utility of histopathological classification is limited by assessor variability and morphological ambiguity, resulting in a diagnostic and therapeutic challenge [3, 4]. Recent advances in molecular characterization of gliomas reveal a wide spectrum of genetic diversity which may inform prognosis and treatment for a particular glioma with greater specificity. Histologically indistinguishable categories of primary glioblastomas, which arise de novo, and secondary glioblastoma can now be molecularly distinguished by the presence of IDH1 mutation in secondary glioblastoma, indicating a more favorable clinical prognosis [2, 3, 5]. Similarly, molecular markers have identified up-regulation of PDGFRA as a hallmark of pediatric gliomas, distinguishing these tumors from adult primary glioblastomas which often show EGFR amplification and PTEN mutation [6]. The emerging picture of diverse biomarker expression between tumors in addition to heterogeneity of biomarker expression within a single tumor suggests a need for high resolution classification schemes and targeted treatments [7]. While current standard of care includes resection followed by radiotherapy and chemotherapy with temozolomide, greater specificity in molecular characterization and targeted, precision-based immunotherapies may vastly improve upon the current treatment strategies [8].
Various preclinical studies have demonstrated that immunotherapeutic strategies can be successful in animal models of GBM, including: gene therapy [9], passive immunotherapy with antibodies against tumor antigens [10], adoptive T-cell transfer with T cells activated against tumor antigens or engineered T cells to express chimeric antigen receptors (CARs) [11-13]. In addition, immune modulatory strategies can be aimed at inhibiting the immune checkpoints used by tumors to escape from immune surveillance [14, 15] as well as active immunotherapy, employing peptide or dendritic cell (DC) vaccines [16]. It is also evident that successful immunotherapy for glioma needs to address the mechanisms of tumor-induced immune suppression.
It was previously accepted that the brain exhibits a dampened immune response or “immune privilege” because of the presence of the blood brain barrier, lack of traditional lymphatic structures and lack of antigen presenting cells (APCs) within the brain parenchyma [17, 18]. Gliomas have been shown to employ a variety of mechanisms to suppress the immune system including secreted cytokines such as TGFβ, IL-10 and VEGF, markers expressed on tumor cells such as programmed death ligand 1 (PDL1) and Fas-L, and immunosuppressive supporting cells [19-24]. Targeting specific mechanisms of immune suppression may not only be useful in increasing the effectiveness of immunotherapies but may also bolster the immune system against severe lymphopenia caused by standard treatment with radiation and temozolomide [25].
T-cell defects have also been recognized in GBM patients. It has been shown that glioblastoma causes significant CD4+ lymphopenia, leaving immune modulatory Tregs as an increased fraction of the CD4+ compartment [26]. Tregs are a subset of CD4+ cells which physiologically inhibit T cell activation to induce tolerance toward self-antigens and prevent autoimmunity. Surprisingly, removal of Tregs from patients with glioblastoma resulted in normal CD4+ T cell function, indicating that normal immune function may be restored by abrogating effects of immune cells which have been skewed towards an immunosuppressive phenotype. Gliomas may further suppress the immune system by stimulating a subset of Natural Killer T cells called NKT type II cells, which secrete immunosuppressive cytokines IL-13 and TGF-β, and M2 polarized macrophages, which secrete immunosuppressive cytokines IL-10 and TGF-β and inhibit T cell proliferation [27, 28]. Gliomas are also infiltrated by a unique population of immune cells termed myeloid-derived suppressor cells, or MDSCs [29, 30]. As reviewed below, MDSCs support glioma progression and invasion, mediate immune suppression and inhibit the efficacy of anti-tumor immunity.
2. Origin and subtypes of MDSCs
MDSCs are a heterogeneous population of immature myeloid cells and myeloid progenitor cells [31-34]. Under physiological situations, these immature cells would normally give rise to myeloid cells such as granulocytes, macrophages and dendritic cells, however under conditions of stress, such as in infection, trauma, autoimmune diseases and cancer, a block in their usual differentiation pathway leads to the expansion of immature myeloid cells [31, 33, 34]. While MDSCs are a heterogeneous population of cells, three characteristics define these cells: their myeloid origin, immature status and ability to suppress T cell responses [31, 33, 34]. Although the term MDSCs was formally adapted in 2007, these cells have been described 35 years ago and referred to natural suppressor (NS) cells, immature myeloid cells (IMC) or myeloid suppressor cells (MSC) [35, 36]. In mice MDSCs are identified as CD11b+, Gr-1+ cells that lack the expression of markers typically expressed on mature differentiated monocytes, macrophages or DCs [31-33, 37]. Normal mouse bone contains 20-40% of these CD11b, Gr-1 expressing cells while the spleen contains about 2-4% [31]. Based on the expression of Gr-1, MDSCs in mice have been divided into two main subtypes, Gr-1high (polymorphonuclear; PMN MDSCs) and Gr-1low (monocytic; M-MDSCs) MDSCs [31-33, 38, 39]. Use of epitope specific antibodies has refined the classification as CD11b+, Ly6G+, Ly6Clow Granulocytic MDSCs and CD11b+, Ly6G−, Ly6Chigh monocytic MDSCs [39, 40]. The Gr-1high MDSCs are phenotypically similar to neutrophils while the Gr-1low MDSCs are morphologically and phenotypically similar to monocytes. Because of the broad nature of the markers used for MDSC classification, it can be technically challenging to distinguish these cells from tumor associated macrophages (TAMs) and granulocytes. MDSCs have typically been associated with lower F4/80 and higher Gr-1expression than TAMs [31]. What further complicates the identity of these cells is that within the tumor microenvironment, MDSCs can differentiate into TAMs [38]. In humans, MDSCs are defined as CD14−, CD11b+ cells or cells that express CD33 but lack the expression of myeloid or lymphoid markers and of MHC II molecule HLA-DR [30, 33, 41-49]. In humans, PMN-MDSCs are CD14−, CD11b+, CD33+, CD15+ while the M-MDSCs are CD14+, HLA-DR−/low cells [33]. Additionally a third population identified as Lin−, HLA-DR−, CD33+ represents a mixed group of enriched myeloid progenitors and all three populations are required to be analyzed in cancer patients for accurate analysis [33, 41, 42, 45, 48, 50]. As discussed later the potency and mechanism of suppression exerted by the various subsets are distinct from each other.
2.2 Expansion of MDSCs
Initial studies described the marked systemic expansion of MDSCs in tumor bearing animals and cancer patients. Since then expansion and accumulation of these cells have been shown to occur during bacterial and parasitic infections such as Trypanosoma cruzi infection [51], acute toxoplasmosis [52], polymicrobial sepsis [53], helminth infections [54], Candida albicans [55] and Porphrymonas gingivalis [56]. Autoimmune diseases and inflammatory conditions such as experimental autoimmune encephalomyelitis, experimental autoimmune uveoretinitis and inflammatory bowel disease have also been associated with MDSC expansion [57-59]. As mentioned above, immature myeloid cells are part of myelopoiesis, a process that is tightly regulated by cytokines such as granulocyte/macrophage colony stimulating factor (GMCSF) [60, 61], granulocyte colony stimulating factor (GCSF) [62, 63], macrophage colony stimulating factor (MCSF) [64], stem cell factor (SCF) [65], IL-3 and FMS-related tyrosine kinase 3 (Flt3L) among others [31]. Aberrant production of these and other cytokines by tumor cells can directly skew myelopoiesis and the generation of MDSCs. Exposure of bone marrow stem cells to tumor-cell cultures results in expansion of MDSCs [66]. When CD14+ monocytes from healthy donor plasma was cultured with glioma cells, increase in the expression of immunosuppressive factors such as IL-10, TGFβ and PDL1 was observed along with an increase in their ability to induce apoptosis in activated T cells [67]. Other factors shown to cause MDSC expansion include cyclooxygenase 2 (COX2) [68], prostaglandins [69], IL-6 [70, 71] and vascular endothelial growth factor [72]). A number of studies have highlighted the importance of STAT3 signaling in regulating MDSC expansion [31, 73]. STAT3 in myeloid cells drives Bcl-xL, c-myc and cyclin D1 expression which prevents cell apoptosis and promotes cell proliferation [73]. STAT3 and JAK2 were activated in hematopoietic cells exposed to tumor cell cultures and the expansion of MDSCs was abrogated when STAT3 was inhibited [74, 75]. MDSCs from tumor bearing animals also have increased levels of phosphorylated STAT3 compared to those from naïve mice [75]. Ablation of STAT3 also reduced the expansion of MDSCs and increased T cell responses in tumor bearing mice [76]. STAT3 dependent induction of S100A8 and S100A9 expression by myeloid progenitor cells inhibited their differentiation and induced expansion of MDSCs in the spleens of tumor bearing mice [77]. In addition to factors that cause MDSC expansion, several other factors have been identified that activate MDSCs to exert their immunosuppressive potential. Blockade of IFNγ for example abrogates MDSC mediated T cell suppression [38]. IFNγ results in the induction of arginase 1 and iNOS expression through STAT1; thus, MDSCs from STAT1−/− mice did not suppress T cell responses [78]. A second pathway implicated in the activation of MDSCs occurs through the activation of IL-4Rα-STAT6 signaling leading to the induction of arginase 1 expression [79, 80]. MyD88 signaling downstream of Toll-like receptor activation is potentially involved in the expansion and activation of MDSCs in polymicrobial sepsis [53]. HIF-1α has emerged as another important factor regulating MDSC expansion and function. In response to hypoxia in the TME, HIF-1α was shown to induce arginase 1 and iNOS in tumor infiltrating MDSCs and further promoted their differentiation into TAMs [81]. Additionally HIF-1α also regulates the expression of PDL1 (binds to PD-1 on T cells to activate checkpoint blockade) on MDSCs [82]. HMGB1 has also been shown to drive the generation of MDSCs from bone marrow cells, contributed to the T cell suppressive ability of MDSCs and promoted IL-10 release from MDSCs [83]. TNFα prevents MDSC differentiation in a S100A8/A9 dependent manner and enhances the T cell suppressive potential of the immature cells [84].
2.3 Mechanisms of immunosuppressive activity
One of the salient properties of these cells is their ability to inhibit T cell responses [31, 33, 37]. It is important to mention that CD11b+, Gr-1+ myeloid cells from tumor free animals do not inhibit antigen-specific T cell responses as shown by Kusmartsev et al. and our unpublished observations [85]. PMN-MDSCs and M-MDSCs also use distinct mechanisms to exhibit immunosuppression; while PMN-MDSCs express high levels of ROS and low NO, M-MDSCs primarily use NO production and cytokine release [38, 39]. M-MDSCs also consistently show higher suppressive activity than PMN-MDSCs on a per cell basis.
2.3.1 Arginase 1 and iNOS
The most common mechanism through which MDSCs work is associated with L-arginine metabolism. Both arginase 1 and iNOS metabolize L-arginine to produce urea and L-ornithine, and nitric oxide (NO) and L-citrulline respectively [86]. The enhanced activity of arginase 1 and iNOS in MDSCs leads to increased catabolism of L-arginine from the extracellular environment thus depleting this non-essential amino acid from the microenvironment [86]. Depletion of L-arginine from the extracellular environment inhibits T cell proliferation by interfering with the expression of the CD3ζ chain, by preventing the upregulation of cell cycle regulators cyclin D3 and cdk4 [87, 88]. NO at the same time inhibits JAK3 and STAT5 function in T cells, inhibits expression of MHC II and induction of T cell apoptosis [31]. Studies of human T cells have also shown that NO affects the stability of mRNA encoding IL-2 and the release of IL-2 by activated lymphocytes [86]. Additionally by depleting L-arginine from the extracellular environment, the activity of NOS is switched from mainly producing NO to mostly O2− [86]. Reactive oxygen species (ROS) such as these as explained in the next section also suppress T cell responses.
2.3.2 ROS
Several tumor derived factors such as IL-3, IL-6, IL-10 and GMCSF induce ROS production in MDSCs [31]. Oxidative stress caused by macrophages derived from tumor bearing mice inhibited ζ chain expression in T cells and antigen-induced T cell proliferation [89]. Treatment of these macrophages with N-acetylcysteine abrogated the effect on CD3ζ chain expression on T cells [89]. Addition of oxidants such as hydrogen peroxide (H2O2) and diamide to T cells also reduced the expression of CD3 ζ chain [89]. Kusmartsev et al. showed that arginase activity in MDSCs led to significant increase in ROS levels that subsequently inhibited antigen-specific T cell proliferation [85]. The inhibition of T cell proliferation was abolished in the presence of catalase or an arginase inhibitor (nor-NOHA) [85]. Peroxynitrite (ONOO−) is one of the most powerful oxidants produced in the body as a result of the reaction between NO and superoxide anion (O2−) [86]. It induces the nitration and nitrosylation of amino acids cysteine, methionine, tryptophan and tyrosine. Direct contact of MDSCs with T cells resulted in the nitration of the T cell receptor and the CD8 molecule, which rendered T cells unresponsive to non-specific stimuli [90]. Use of peroxynitrite scavenger resulted in a block in MDSC immunosuppression [85]. Both peroxynitrites and H2O2 are also thought to have a direct role in inducing apoptosis of antigen-activated T cells [86].
2.3.3 Induction of Tregs
MDSCs have also been shown to support the development of FoxP3+ regulatory T cells (Tregs). Several mechanisms have been shown to be involved in this process. In a mouse colon carcinoma model, IFNγ activated Gr-1+ CD115+ M-MDSC were shown to produce IL-10 and TGFβ to mediate the development of CD4+ CD25+ Treg cells [91]. In a mouse model of ID8 ovarian tumors, expression of CD80 on MDSCs was essential for the induction of Tregs [92]. Another group showed that MDSCs promoted Treg development through arginase 1 and independent of TGFβ [93]. A new population of MDSCs in hepatocellular carcinoma patients induced CD4+CD25+ Foxp3 T Cells [43]. In contrast some other studies have ruled out the contribution of MDSCs in inducing Tregs suggesting that immunosuppression by MDSCs probably occurs through multiple mechanisms, all of which may not be active in the same tumor. Our unpublished observations suggest that the phenotype and functionality of the tumor infiltrating MDSCs is influenced by the genetic makeup of the tumor. Differential cytokine release by the tumor cells can influence the activation of these cells.
2.3.4 Decreased expression of L-selectin by T cells
L-selectin also known as CD62L plays an essential role in the homing of lymphocytes to the lymph nodes and the TME. MDSCs, potentially though the expression of ADAM17, an enzyme that cleaves the CD62L ectodomain, downregulate L-selectin levels on naïve T cells, thus interfering with their trafficking to sites where they would be activated [94].
2.3.5 Checkpoint blockade on T cells
Another mechanism by which MDSCs can potentially block T cell responses is by expressing ligands that bind to checkpoint receptors on T cells and cause inhibition of T cell proliferation [95]. CD80 was observed to be upregulated on MDSCs from an ovarian tumor model compared to naïve mice and the CD80-CTLA-4 interaction was crucial for T cell suppression by these MDSCs [92]. In glioma patients, circulating monocytes showed elevated expression of PDL1 as compared to monocytes from normal individuals. When normal monocytes were exposed to GBM culture media, PDL1 expression was further enhanced and led to T cell apoptosis [96].
Independent of their immunosuppressive activity, MDSCs also support angiogenesis, tissue invasion, establishment of a pre-metastatic niche and metastasis through the release of VEGF, bFGF, Bv8 and MMP9. Increased tumor angiogenesis, vascular maturation and decreased tumor cell apoptosis was observed when tumor derived CD11b+, Gr-1+ cells were co-injected with MC26 cells [97]. The effect was dependent on MMP9 release by CD11b+, Gr-1+ cells. Ortiz et al showed that immature myeloid cells lacking immunosuppressive potential could recruit IL-17 producing CD4 T cells and promote tumor growth in a model of epidermal carcinogenesis [98]. Of note, accumulation of these immature lineages apart from directly affecting T cell responses and tumor growth, also interferes with the generation of functional mature APCs that are so crucially required for the activation of anti-tumor T cell response [99].
2.4 Effect on tumor progression
Aberrant myelopoiesis, affecting one or more cells of the myeloid lineage has been described in cancer patients [41]. The use of aberrant myelopoiesis as a biomarker and prognostic indicator for tumor progression has been however hampered by the wide range of markers that have been used to define these cells as mentioned by Messmer et al. [44]. Despite this, several studies have convincingly shown the relation between the accumulation of immunosuppressive myeloid cells and tumor progression in a range of tumor types [41-43, 45-47, 50, 100, 101]. Like MDSCs from murine tumor models, MDSCs from cancer patients show elevated expression of PDL1, iNOS, arginase, ROS and IDO, molecules implicated in T cell suppression [48, 102]. MDSCs isolated from melanoma, glioma, squamous cell carcinoma, non-small cell lung cancer, breast cancer, hepatocellular carcinoma, gastric cancer, colorectal and chronic myeloid leukemia cancer patients have been shown to suppress antigen-specific and CD3-ligation induced T cell proliferation [29, 30, 42, 45, 103]. MDSC expansion has been negatively associated with tumor stage, metastatic burden, response to therapy and progression-free or overall survival [44, 46, 47, 104, 105] .
2.5 Therapeutic targeting of MDSCs
Defective T cell function is one of the major mechanisms of tumor escape. T cell immunosuppression by MDSCs creates a huge barrier to the efficacy of immunotherapeutic approaches and therefore it is imperative that strategies that can efficiently block the activity of these cells be developed. As indicated by Messmer et al, certain tumor derived factors such as HMGB1 and S100A8/A9 may act within the tumor microenvironment, while factors like GMCSF, IL-6 and VEGF may act at the systemic level [44]. This is an important consideration for developing therapies that would either be working at the systemic level or locally within the tumor. A number of methods have recently been shown to be effective in either depleting these cells or inhibiting their activity.
2.5.1 Promoting myeloid cell differentiation
Since the hallmark feature of these cells is their immature status, efforts have been made to promote their differentiation into mature lineages. In this regard, Vitamin A and its metabolites such as retinoic acid (all-trans retinoic acid; ATRA) have been used to trigger the differentiation of immature myeloid cells into DCs and macrophages [106]. ATRA has also been shown to reduce ROS levels in MDSCs. Administration of all-trans retinoic acid resulted in a decrease in MDSCs in tumor bearing mice, improved tumor-specific T cell responses and enhanced the efficacy of vaccination [101]. Administration of ATRA with dendritic cell vaccination in extensive stage SCLC patients decreased MDSC numbers by 2-fold and enhanced cytotoxic CD8 T cell response [100]. Likewise in patients with metastatic renal cell carcinoma, ATRA administration led to a decrease in MDSCs in the blood and improved antigen-specific T cell responses [101].
2.5.2 Inhibition of MDSC expansion
Several studies have focused on trying to block tumor derived factors that can cause MDSC expansion. Blocking of SCF-KIT-induced signaling decreased MDSC expansion and tumor angiogenesis [65]. Likewise administration of avastin, a VEGF blocking antibody resulted in a decrease in the population of CD11b+, VEGFR1+ MDSCs in the peripheral blood of patients with metastatic renal cell carcinoma [107]. Use of neutralizing antibodies against GCSF and GMCSF, have shown promise in mouse models. Shojaei et al showed that blocking GCSF drastically reduced CD11b+, Gr-1+ cells in the tumor, inhibited angiogenesis and delayed the growth of EL4 and LLC tumors that were refractory to anti-VEGF treatment [108]. GCSF neutralization with antibody or with short hairpin mediated knockdown also reduced MDSC burden and tumor growth in 4T1 and AT-3 tumor bearing mice [62]. Blocking the release of GMCSF in pancreatic cancer cells inhibited MDSC generation and tumor growth in CD8 T cell dependent manner [109]. Use of anti-IL-6 receptor monoclonal antibody decreased MDSC accumulation in the tumor and spleen and enhanced anti-tumor T cell response [70]. Use of certain chemotherapy drugs such as gemcitabine [110] and sunitinib [111, 112], 5-fluorouracil [113] and docetaxel [114] have been shown to eliminate MDSCs from the tumor and spleen and a significant increase in the immunotherapy induced anti-tumor T cell responses. Sunitinib treatment in GBM mouse models also resulted in decreased circulating and TME infiltrating MDSCs and increase in CD3 and CD4 T cell counts. Total T cell proliferation and IFNγ release was also increased in the spleens of sunitinib treated mice [29].
2.5.3 Inhibition of MDSC function
Another approach to block MDSC activity is to inhibit the induction of immunosuppressive molecules in these cells. COX2 inhibitor for example downregulated the expression of arginase 1 by MDSCs and improved immunotherapy induced anti-tumor T cell responses [48, 115]. This was thought to occur through the inhibition of prostaglandin E2, which in turn has been shown to induce arginase 1 expression in MDSCs. Similarly administration of sildenafil, a phosphodiesterase inhibitor resulted in a substantial delay in tumor progression in several mouse models by downregulating the expression of arginase 1 and iNOS in MDSCs [116].
2.5.4 Inhibition of MDSC trafficking
Interventions that target the migration of MDSCs to the TME are currently being developed. Since various mechanisms regulating MDSC migration to the TME are in play across the variety of tumor types, therapies in this case will probably have to be tumor specific. Examples include inhibition of CSF-1 receptor signaling blockade, CCL2/CCR2 blockade, CXCL2/CXCR2 blockade, CXCL12/CXCR4 blockade, COX2 and PDE-5 inhibitors [31, 33, 34]. Pharmacologic inhibition of CSF1R signaling using GW2580 blocked the trafficking of M-MDSCs and in combination with anti-VEGFR2 antibody suppressed tumor angiogenesis and tumor growth [64].
2.5.5 Use of myeloid depleting antibodies
Gr-1 or Ly6G specific antibodies have been used in a variety of mouse models to deplete cells expressing Gr-1 or more specifically Ly6G [68]. The success of these antibodies in mouse models reinforces the notion that MDSC depletion can potently enhance anti-tumor immune responses. Both of these antibodies would however also deplete cells other than MDSCs, such as granulocytes, neutrophils and myeloid progenitors that in turn can negatively impact the generation of functional APCs. Our data with immune-stimulatory gene therapy in mouse GBM models has highlighted that the timing of depletion is a crucial factor and maximum benefit is seen when MDSCs are depleted during the proliferative phase of the anti-tumor T cell response. The development of a MDSC depleting antibody in humans will be technically challenging though because of the significant heterogeneity in the population and the lack of a single defining marker.
3. MDSCs in Glioma
Significant infiltration of MDSCs has been observed in both de novo and transplantable rodent GBM models [29, 68, 80, 102, 117, 118]. MDSCs identified as CD33+/Lin−/HLA-DR− cells were expanded in the blood of GBM patients as compared to healthy donors [67]. High levels of PDL1 were also seen on the MDSCs obtained from these patients compared to peripheral blood monocytes from the same samples. Raychaudhuri et al analyzed the MDSC frequency in 28 patients with newly diagnosed glioblastoma and compared it to 11 normal donors [30]. Increased circulating MDSCs were seen in the blood of GBM patients and interestingly the frequency of circulating MDSCs were the highest as compared to patients with RCC, melanoma and bladder carcinoma [30]. Not only that, but Sippel et al showed that the CD11b+ cells in the blood were most expanded in GBM patients as compared to patients with meningioma, pituitary tumors and anaplastic glioma [103]. Similar to the other tumor types, greater expansion of the granulocytic MDSCs was seen as compared to the monocytic subset. 15% of the identified MDSCs were composed of the Lin− cell type (CD33+, CD15−, CD14−, HLA-DR−). T cells from GBM patients also showed suppressed IFNγ release upon stimulation compared to T cells from healthy controls, that was restored by the depletion of MDSCs using anti-CD15 and anti-CD33 antibody coated magnetic beads [30]. They also showed that GBM tumor tissues were infiltrated by MDSCs, the majority of which were Lin−, followed by granulocytic and monocytic subtypes. Since GBM patients are often treated with glucocorticoids, the authors also evaluated the effect of steroids on MDSCs. No correlation was observed between MDSC counts and steroid dosing. Similar results were observed by Dubinski et al [102].
Systemic administration of a CCL2 neutralizing antibody by itself and in combination with temozolomide significantly reduced MDSC and macrophage infiltration in GL261-induced GBM and enhanced the survival of tumor bearing mice [118]. COX2 blockade was shown to suppress GBM growth in a CCL2 dependent manner. MDSC numbers were reduced in the bone marrow along with a reduction in MDSC infiltration in the GBM TME. Inhibition of MDSC accumulation was associated with elevated levels of CXCL10 and cytotoxic T lymphocytes in the TME [68]. Studies have looked at the efficacy of combining COX2 inhibitor celecoxib in combination with immunotherapy [119], dendritic cell vaccination [120], CD40 monoclonal antibody [121] with moderate to good success. GMCSF was upregulated in both human and mouse glioma TME and Kohanbash et al. showed that GMCSF induced IL-4Rα expression on MDSCs in the glioma TME, that in turn induced arginase expression in response to IL-13 [80]. IL-4Rα−/− mice showed slower glioma growth and showed lower levels of arginase in the TME. Incidentally glioma patients have been shown to have elevated plasma arginase activity compared to healthy donors [30]. L-arginine supplementation to T cells reversed the T cell suppression induced by CD11b+ from glioma patients indicating that exogenous L-arginine supplementation may help to reverse some of the MDSC induced T cell defects [103]. While much attention has focused on the effect of MDSCs on CD8 T cells, a recent study has found a strong link between MDSCs and CD4 effector memory T cells (CD4 TEM) in glioma [102]. A positive correlation was observed between the frequencies of granulocytic MDSCs and CD4 TEM at the tumor site. The frequency and expression of PD-1 was significantly higher on tumor infiltrating TEM as compared to circulating TEM or those from healthy donors. Since PD-1 is expressed on activated T cells and is associated with T cell exhaustion, the authors also analysed the expression of PD-1 on the CD4 TEM cells. GBM infiltrating CD4 TEM showed PD-1 upregulation that correlated with reduced IFNγ release by these cells. Tumor derived MDSCs showed PDL1 expression and when tumor derived MDSCs were cultured with CD4 T cells, PD-1 expression on CD4 T cells was induced.
4. Conclusion and future directions
So far studies have conclusively shown the accumulation of MDSCs in the TME and in the circulation of GBM patients. Efforts are underway to understand the mechanism of expansion and activation of MDSCs in GBM and it seems likely that mechanisms and pathways uncovered in GBM will also be encountered in other solid tumors. Glioma cells express several factors such as IL-6, VEGF, IL-10, PGE2 and GMCSF that previously have been associated with MDSC expansion, activation or trafficking in other tumor types. It also appears that several distinct molecules may be involved across the various GBM subtypes and a single approach to block MDSC activity or expansion may not be applicable for all GBMs. A variety of targets including STAT3, COX2, CCL2, arginase and CSF1R have been identified as potential therapeutic strategies to block MDSC-mediated immunosuppression and more research is needed to take these therapies to the bedside.
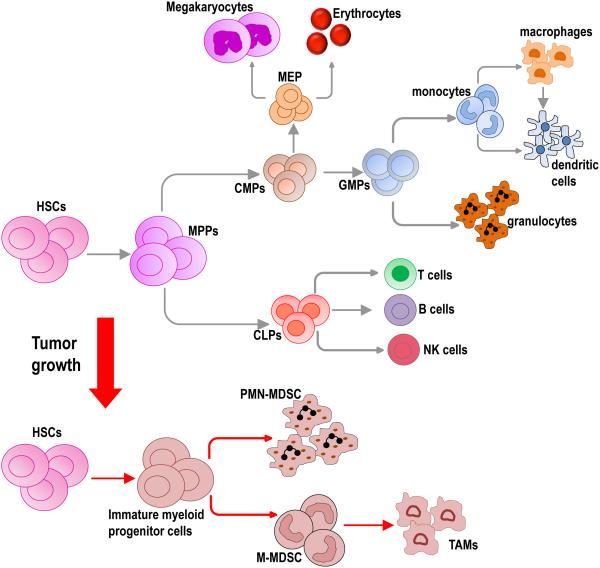
Figure 1. Hematopoiesis and MDSC generation.
Under physiological conditions, hematopoietic stem cells (HSCs) give rise to the various cells that form the hematopoietic system in a tightly regulated process through the generation of multipotent progenitors (MPPs), common myeloid progenitor (CMPs) and common lymphoid progenitors (CLPs). CLPs eventually give rise to cells of the lymphocyte lineage, such as T cells, B cells and NK cells. CMPs give rise to myeloid and erythroid lineages such as granulocytes, monocytes, platelets and red blood cells (RBCs). Tumor growth results in aberrant myelopoiesis leading to the accumulation of myeloid progenitor cells and a mixture of immature myeloid cells. Several tumor derived factors such as GMCSF, MCSF and IL-3, normally involved in myelopoiesis have been implicated in MDSC generation. Two main types of MDSCs have been identified, polymorhonuclear MDSCs (PMN-MDSCs) and monocytic MDSCs (M-MDSCs). M-MDSCs within the TME can differentiate to tumor associated macrophages (TAMs).
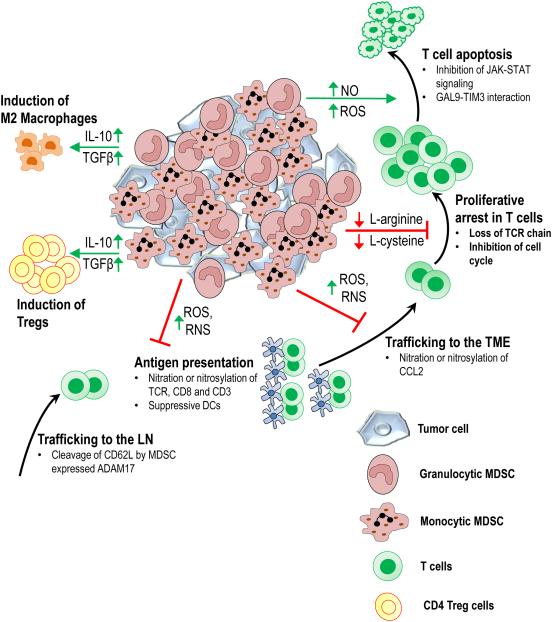
Figure 2. Mechanisms of MDSC-mediated immunosuppression.
MDSCs within and outside the TME can suppress anti-tumor immunity in a variety of ways. Cleavage of CD62L from the surface of T cells by MDSC expressed ADAM17 blocks naïve T cell migration to lymph nodes, where they would be activated by antigen presenting cells. Through production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), MDSCs interfere with T cell activation and recruitment to the TME. By depleting arginine and cysteine from the extracellular environment of the T cells, MDSCs induce proliferative arrest in T cells. Additionally through the release of cytokines such as IL-10 and TGFβ, MDSCs promote induction of Tregs and M2 macrophage polarization.
Acknowledgments
Financial Support
This work was supported by National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grants R01-NS074387, R01-NS057711, R21-NS091555, and R01-NS094804 to M.G.C.; NIH/NINDS Grants R01-NS061107, R01-NS082311, and R21-NS084275 to P.R.L.; Leah’s Happy Hearts, University of Michigan Comprehensive Cancer Center, Chad Tough Foundation, and The Phase One Foundation to M.G.C. and P.R.L.; the Department of Neurosurgery, University of Michigan School of Medicine; the Michigan Institute for Clinical and Health Research, NIH 2UL1-TR000433; University of Michigan Cancer Biology Training Grant, NIH/NCI (National Cancer Institute) T32-CA009676; University of Michigan Training in Clinical and Basic Neuroscience, NIH/NINDS T32-NS007222; and the University of Michigan Medical Scientist Training Program, NIH/NIGMS (National Institute of General Medicine Sciences) T32-GM007863.
List of abbreviations
- GBM
Glioblastoma multiforme
- CARs
Chimeric antigen receptors
- PDL1
Programmed death ligand 1
- IDH1
Isocitrate dehydrogenase 1
- PMN
Polymorphonuclear
- TAMs
Tumor associated macrophages
- HLA
Human leucocyte antigen
- FLT3L
Fms-like tyrosine kinase 3 ligand
- COX2
Cyclooxygenase 2
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- ATRA
All-trans retinoic acid
Footnotes
The authors have declared that no conflict of interest exists.
References
- [1].Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh KM, Wrensch MR, Barnholtz-Sloan JS. The epidemiology of glioma in adults: a "state of the science" review. Neuro-oncology. 2014;16:896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].N. Cancer Genome Atlas Research. Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay E, Miller CR, Vitucci M, Morozova O, Robertson AG, Noushmehr H, Laird PW, Cherniack AD, Akbani R, Huse JT, Ciriello G, Poisson LM, Barnholtz-Sloan JS, Berger MS, Brennan C, Colen RR, Colman H, Flanders AE, Giannini C, Grifford M, Iavarone A, Jain R, Joseph I, Kim J, Kasaian K, Mikkelsen T, Murray BA, O'Neill BP, Pachter L, Parsons DW, Sougnez C, Sulman EP, Vandenberg SR, Van Meir EG, von Deimling A, Zhang H, Crain D, Lau K, Mallery D, Morris S, Paulauskis J, Penny R, Shelton T, Sherman M, Yena P, Black A, Bowen J, Dicostanzo K, Gastier-Foster J, Leraas KM, Lichtenberg TM, Pierson CR, Ramirez NC, Taylor C, Weaver S, Wise L, Zmuda E, Davidsen T, Demchok JA, Eley G, Ferguson ML, Hutter CM, Mills Shaw KR, Ozenberger BA, Sheth M, Sofia HJ, Tarnuzzer R, Wang Z, Yang L, Zenklusen JC, Ayala B, Baboud J, Chudamani S, Jensen MA, Liu J, Pihl T, Raman R, Wan Y, Wu Y, Ally A, Auman JT, Balasundaram M, Balu S, Baylin SB, Beroukhim R, Bootwalla MS, Bowlby R, Bristow CA, Brooks D, Butterfield Y, Carlsen R, Carter S, Chin L, Chu A, Chuah E, Cibulskis K, Clarke A, Coetzee SG, Dhalla N, Fennell T, Fisher S, Gabriel S, Getz G, Gibbs R, Guin R, Hadjipanayis A, Hayes DN, Hinoue T, Hoadley K, Holt RA, Hoyle AP, Jefferys SR, Jones S, Jones CD, Kucherlapati R, Lai PH, Lander E, Lee S, Lichtenstein L, Ma Y, Maglinte DT, Mahadeshwar HS, Marra MA, Mayo M, Meng S, Meyerson ML, Mieczkowski PA, Moore RA, Mose LE, Mungall AJ, Pantazi A, Parfenov M, Park PJ, Parker JS, Perou CM, Protopopov A, Ren X, Roach J, Sabedot TS, Schein J, Schumacher SE, Seidman JG, Seth S, Shen H, Simons JV, Sipahimalani P, Soloway MG, Song X, Sun H, Tabak B, Tam A, Tan D, Tang J, Thiessen N, Triche T, Jr., Van Den Berg DJ, Veluvolu U, Waring S, Weisenberger DJ, Wilkerson MD, Wong T, Wu J, Xi L, Xu AW, Yang L, Zack TI, Zhang J, Aksoy BA, Arachchi H, Benz C, Bernard B, Carlin D, Cho J, DiCara D, Frazer S, Fuller GN, Gao J, Gehlenborg N, Haussler D, Heiman DI, Iype L, Jacobsen A, Ju Z, Katzman S, Kim H, Knijnenburg T, Kreisberg RB, Lawrence MS, Lee W, Leinonen K, Lin P, Ling S, Liu W, Liu Y, Liu Y, Lu Y, Mills G, Ng S, Noble MS, Paull E, Rao A, Reynolds S, Saksena G, Sanborn Z, Sander C, Schultz N, Senbabaoglu Y, Shen R, Shmulevich I, Sinha R, Stuart J, Sumer SO, Sun Y, Tasman N, Taylor BS, Voet D, Weinhold N, Weinstein JN, Yang D, Yoshihara K, Zheng S, Zhang W, Zou L, Abel T, Sadeghi S, Cohen ML, Eschbacher J, Hattab EM, Raghunathan A, Schniederjan MJ, Aziz D, Barnett G, Barrett W, Bigner DD, Boice L, Brewer C, Calatozzolo C, Campos B, Carlotti CG, Jr., Chan TA, Cuppini L, Curley E, Cuzzubbo S, Devine K, DiMeco F, Duell R, Elder JB, Fehrenbach A, Finocchiaro G, Friedman W, Fulop J, Gardner J, Hermes B, Herold-Mende C, Jungk C, Kendler A, Lehman NL, Lipp E, Liu O, Mandt R, McGraw M, McLendon R, McPherson C, Neder L, Nguyen P, Noss A, Nunziata R, Ostrom QT, Palmer C, Perin A, Pollo B, Potapov A, Potapova O, Rathmell WK, Rotin D, Scarpace L, Schilero C, Senecal K, Shimmel K, Shurkhay V, Sifri S, Singh R, Sloan AE, Smolenski K, Staugaitis SM, Steele R, Thorne L, Tirapelli DP, Unterberg A, Vallurupalli M, Wang Y, Warnick R, Williams F, Wolinsky Y, Bell S, Rosenberg M, Stewart C, Huang F, Grimsby JL, Radenbaugh AJ, Zhang J. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. The New England journal of medicine. 2015;372:2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Appin CL, Brat DJ. Biomarker-driven diagnosis of diffuse gliomas. Molecular aspects of medicine. 2015;45:87–96. doi: 10.1016/j.mam.2015.05.002. [DOI] [PubMed] [Google Scholar]
- [4].Lai A, Kharbanda S, Pope WB, Tran A, Solis OE, Peale F, Forrest WF, Pujara K, Carrillo JA, Pandita A, Ellingson BM, Bowers CW, Soriano RH, Schmidt NO, Mohan S, Yong WH, Seshagiri S, Modrusan Z, Jiang Z, Aldape KD, Mischel PS, Liau LM, Escovedo CJ, Chen W, Nghiemphu PL, James CD, Prados MD, Westphal M, Lamszus K, Cloughesy T, Phillips HS. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4482–4490. doi: 10.1200/JCO.2010.33.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:764–772. doi: 10.1158/1078-0432.CCR-12-3002. [DOI] [PubMed] [Google Scholar]
- [6].Paugh BS, Qu C, Jones C, Liu Z, Adamowicz-Brice M, Zhang J, Bax DA, Coyle B, Barrow J, Hargrave D, Lowe J, Gajjar A, Zhao W, Broniscer A, Ellison DW, Grundy RG, Baker SJ. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3061–3068. doi: 10.1200/JCO.2009.26.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, Louis DN, Rozenblatt-Rosen O, Suva ML, Regev A, Bernstein BE. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, R. European Organisation for, T. Treatment of Cancer Brain, G. Radiotherapy, G. National Cancer Institute of Canada Clinical Trials Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- [9].Kane JR, Miska J, Young JS, Kanojia D, Kim JW, Lesniak MS. Sui generis: gene therapy and delivery systems for the treatment of glioblastoma. Neuro-oncology. 2015;17(Suppl 2):ii24–ii36. doi: 10.1093/neuonc/nou355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen KS, Mitchell DA. Monoclonal antibody therapy for malignant glioma. Advances in experimental medicine and biology. 2012;746:121–141. doi: 10.1007/978-1-4614-3146-6_10. [DOI] [PubMed] [Google Scholar]
- [11].Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Morgan RA, Johnson LA, Davis JL, Zheng Z, Woolard KD, Reap EA, Feldman SA, Chinnasamy N, Kuan CT, Song H, Zhang W, Fine HA, Rosenberg SA. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Human gene therapy. 2012;23:1043–1053. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ohno M, Natsume A, Ichiro Iwami K, Iwamizu H, Noritake K, Ito D, Toi Y, Ito M, Motomura K, Yoshida J, Yoshikawa K, Wakabayashi T. Retrovirally engineered T-cell-based immunotherapy targeting type III variant epidermal growth factor receptor, a glioma-associated antigen. Cancer science. 2010;101:2518–2524. doi: 10.1111/j.1349-7006.2010.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Callahan MK, Postow MA, Wolchok JD. CTLA-4 and PD-1 Pathway Blockade: Combinations in the Clinic. Frontiers in oncology. 2014;4:385. doi: 10.3389/fonc.2014.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Calinescu AA, Kamran N, Baker G, Mineharu Y, Lowenstein PR, Castro MG. Overview of current immunotherapeutic strategies for glioma. Immunotherapy. 2015;7:1073–1104. doi: 10.2217/imt.15.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Perng P, Lim M. Immunosuppressive Mechanisms of Malignant Gliomas: Parallels at Non-CNS Sites. Frontiers in oncology. 2015;5:153. doi: 10.3389/fonc.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jackson CM, Lim M, Drake CG. Immunotherapy for brain cancer: recent progress and future promise. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:3651–3659. doi: 10.1158/1078-0432.CCR-13-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Qiu B, Zhang D, Wang C, Tao J, Tie X, Qiao Y, Xu K, Wang Y, Wu A. IL-10 and TGF-beta2 are overexpressed in tumor spheres cultured from human gliomas. Molecular biology reports. 2011;38:3585–3591. doi: 10.1007/s11033-010-0469-4. [DOI] [PubMed] [Google Scholar]
- [20].Fontana A, Constam DB, Frei K, Malipiero U, Pfister HW. Modulation of the immune response by transforming growth factor beta. International archives of allergy and immunology. 1992;99:1–7. doi: 10.1159/000236328. [DOI] [PubMed] [Google Scholar]
- [21].Hishii M, Nitta T, Ishida H, Ebato M, Kurosu A, Yagita H, Sato K, Okumura K. Human glioma-derived interleukin-10 inhibits antitumor immune responses in vitro. Neurosurgery. 1995;37:1160–1166. doi: 10.1227/00006123-199512000-00016. discussion 1166-1167. [DOI] [PubMed] [Google Scholar]
- [22].Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer research. 2003;63:7462–7467. [PubMed] [Google Scholar]
- [23].Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wohrer A, Dieckmann K, Filipits M, Brandstetter A, Weller M, Kurscheid S, Hegi ME, Zielinski CC, Marosi C, Hainfellner JA, Preusser M, Wick W. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro-oncology. 2015;17:1064–1075. doi: 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Didenko VV, Ngo HN, Minchew C, Baskin DS. Apoptosis of T lymphocytes invading glioblastomas multiforme: a possible tumor defense mechanism. Journal of neurosurgery. 2002;96:580–584. doi: 10.3171/jns.2002.96.3.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S, N.C. Consortium Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE, 2nd, Bigner DD, Dranoff G, Sampson JH. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer research. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- [27].Robertson FC, Berzofsky JA, Terabe M. NKT cell networks in the regulation of tumor immunity. Frontiers in immunology. 2014;5:543. doi: 10.3389/fimmu.2014.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, Sawaya R, Heimberger AB. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro-oncology. 2010;12:1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Raychaudhuri B, Rayman P, Huang P, Grabowski M, Hambardzumyan D, Finke JH, Vogelbaum MA. Myeloid derived suppressor cell infiltration of murine and human gliomas is associated with reduction of tumor infiltrating lymphocytes. Journal of neuro-oncology. 2015;122:293–301. doi: 10.1007/s11060-015-1720-6. [DOI] [PubMed] [Google Scholar]
- [30].Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, Garcia J, Vogelbaum MA, Finke J. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro-oncology. 2011;13:591–599. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews. Immunology. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Khaled YS, Ammori BJ, Elkord E. Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunology and cell biology. 2013;91:493–502. doi: 10.1038/icb.2013.29. [DOI] [PubMed] [Google Scholar]
- [33].Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. The Journal of clinical investigation. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment. Adv Cancer Res. 2015;128:95–139. doi: 10.1016/bs.acr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer research. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bennett JA, Rao VS, Mitchell MS. Systemic bacillus Calmette-Guerin (BCG) activates natural suppressor cells. Proceedings of the National Academy of Sciences of the United States of America. 1978;75:5142–5144. doi: 10.1073/pnas.75.10.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer research. 2008;68:2561–2563. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- [38].Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- [39].Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. Journal of immunology. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SE, Dubois CM, Kopp WC, Longo DL, Keller JR. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. Journal of immunology. 1991;147:22–28. [PubMed] [Google Scholar]
- [41].Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. Journal of immunology. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- [42].Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- [43].Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- [44].Messmer MN, Netherby CS, Banik D, Abrams SI. Tumor-induced myeloid dysfunction and its implications for cancer immunotherapy. Cancer immunology, immunotherapy : CII. 2015;64:1–13. doi: 10.1007/s00262-014-1639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer research. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- [46].Romano A, Parrinello NL, Vetro C, Forte S, Chiarenza A, Figuera A, Motta G, Palumbo GA, Ippolito M, Consoli U, Di Raimondo F. Circulating myeloid-derived suppressor cells correlate with clinical outcome in Hodgkin Lymphoma patients treated up-front with a risk-adapted strategy. British journal of haematology. 2015;168:689–700. doi: 10.1111/bjh.13198. [DOI] [PubMed] [Google Scholar]
- [47].Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. Journal of immunology. 2013;190:794–804. doi: 10.4049/jimmunol.1202088. [DOI] [PubMed] [Google Scholar]
- [48].Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer research. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- [49].Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer journal. 2010;16:348–353. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- [50].Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer immunology, immunotherapy : CII. 2012;61:1155–1167. doi: 10.1007/s00262-012-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Goni O, Alcaide P, Fresno M. Immunosuppression during acute Trypanosoma cruzi infection: involvement of Ly6G (Gr1(+))CD11b(+ )immature myeloid suppressor cells. Int Immunol. 2002;14:1125–1134. doi: 10.1093/intimm/dxf076. [DOI] [PubMed] [Google Scholar]
- [52].Voisin MB, Buzoni-Gatel D, Bout D, Velge-Roussel F. Both expansion of regulatory GR1+ CD11b+ myeloid cells and anergy of T lymphocytes participate in hyporesponsiveness of the lung-associated immune system during acute toxoplasmosis. Infect Immun. 2004;72:5487–5492. doi: 10.1128/IAI.72.9.5487-5492.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. The Journal of experimental medicine. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Terrazas LI, Walsh KL, Piskorska D, McGuire E, Harn DA., Jr. The schistosome oligosaccharide lacto-N-neotetraose expands Gr1(+) cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4(+) cells: a potential mechanism for immune polarization in helminth infections. Journal of immunology. 2001;167:5294–5303. doi: 10.4049/jimmunol.167.9.5294. [DOI] [PubMed] [Google Scholar]
- [55].Mencacci A, Montagnoli C, Bacci A, Cenci E, Pitzurra L, Spreca A, Kopf M, Sharpe AH, Romani L. CD80+Gr-1+ myeloid cells inhibit development of antifungal Th1 immunity in mice with candidiasis. Journal of immunology. 2002;169:3180–3190. doi: 10.4049/jimmunol.169.6.3180. [DOI] [PubMed] [Google Scholar]
- [56].Ezernitchi AV, Vaknin I, Cohen-Daniel L, Levy O, Manaster E, Halabi A, Pikarsky E, Shapira L, Baniyash M. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. Journal of immunology. 2006;177:4763–4772. doi: 10.4049/jimmunol.177.7.4763. [DOI] [PubMed] [Google Scholar]
- [57].Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. Journal of immunology. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- [58].Kerr EC, Raveney BJ, Copland DA, Dick AD, Nicholson LB. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J Autoimmun. 2008;31:354–361. doi: 10.1016/j.jaut.2008.08.006. [DOI] [PubMed] [Google Scholar]
- [59].Haile LA, von Wasielewski R, Gamrekelashvili J, Kruger C, Bachmann O, Westendorf AM, Buer J, Liblau R, Manns MP, Korangy F, Greten TF. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135:871–881. doi: 10.1053/j.gastro.2008.06.032. 881 e871-875. [DOI] [PubMed] [Google Scholar]
- [60].Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, Restifo NP. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. Journal of immunology. 1999;162:5728–5737. [PMC free article] [PubMed] [Google Scholar]
- [61].Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer research. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- [62].Waight JD, Hu Q, Miller A, Liu S, Abrams SI. Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PloS one. 2011;6:e27690. doi: 10.1371/journal.pone.0027690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Okazaki T, Ebihara S, Asada M, Kanda A, Sasaki H, Yamaya M. Granulocyte colony-stimulating factor promotes tumor angiogenesis via increasing circulating endothelial progenitor cells and Gr1+CD11b+ cells in cancer animal models. Int Immunol. 2006;18:1–9. doi: 10.1093/intimm/dxh334. [DOI] [PubMed] [Google Scholar]
- [64].Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, Johnson M, Lusis AJ, Cohen DA, Iruela-Arispe ML, Wu L. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–1471. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. The Journal of experimental medicine. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, Lin Y, Dietz AB, Forsyth PA, Yong VW, Parney IF. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro-oncology. 2010;12:351–365. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, Ohlfest JR, Okada H. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer research. 2011;71:2664–2674. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer research. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- [70].Sumida K, Wakita D, Narita Y, Masuko K, Terada S, Watanabe K, Satoh T, Kitamura H, Nishimura T. Anti-IL-6 receptor mAb eliminates myeloid-derived suppressor cells and inhibits tumor growth by enhancing T-cell responses. European journal of immunology. 2012;42:2060–2072. doi: 10.1002/eji.201142335. [DOI] [PubMed] [Google Scholar]
- [71].Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer research. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- [73].Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nature reviews. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, Gabrilovich D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. Journal of immunology. 2004;172:464–474. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- [75].Nefedova Y, Nagaraj S, Rosenbauer A, Muro-Cacho C, Sebti SM, Gabrilovich DI. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer research. 2005;65:9525–9535. doi: 10.1158/0008-5472.CAN-05-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mule J, Kerr WG, Jove R, Pardoll D, Yu H. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nature medicine. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- [77].Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. Journal of immunology. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kusmartsev S, Gabrilovich DI. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. Journal of immunology. 2005;174:4880–4891. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- [79].Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G, Colombo MP, Zanovello P. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. Journal of immunology. 2003;170:270–278. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- [80].Kohanbash G, McKaveney K, Sakaki M, Ueda R, Mintz AH, Amankulor N, Fujita M, Ohlfest JR, Okada H. GM-CSF promotes the immunosuppressive activity of glioma-infiltrating myeloid cells through interleukin-4 receptor-alpha. Cancer research. 2013;73:6413–6423. doi: 10.1158/0008-5472.CAN-12-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. The Journal of experimental medicine. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. The Journal of experimental medicine. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Parker KH, Sinha P, Horn LA, Clements VK, Yang H, Li J, Tracey KJ, Ostrand-Rosenberg S. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer research. 2014;74:5723–5733. doi: 10.1158/0008-5472.CAN-13-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sade-Feldman M, Kanterman J, Ish-Shalom E, Elnekave M, Horwitz E, Baniyash M. Tumor necrosis factor-alpha blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity. 2013;38:541–554. doi: 10.1016/j.immuni.2013.02.007. [DOI] [PubMed] [Google Scholar]
- [85].Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. Journal of immunology. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- [86].Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nature reviews. Immunology. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- [87].Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer research. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- [88].Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. Journal of immunology. 2010;184:3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Otsuji M, Kimura Y, Aoe T, Okamoto Y, Saito T. Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 zeta chain of T-cell receptor complex and antigen-specific T-cell responses. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13119–13124. doi: 10.1073/pnas.93.23.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nature medicine. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer research. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- [92].Yang R, Cai Z, Zhang Y, Yutzy W.H.t., Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer research. 2006;66:6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- [93].Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer research. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. Journal of immunology. 2009;183:937–944. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. The Journal of clinical investigation. 2015;125:3335–3337. doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:3165–3175. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- [98].Ortiz ML, Kumar V, Martner A, Mony S, Donthireddy L, Condamine T, Seykora J, Knight SC, Malietzis G, Lee GH, Moorghen M, Lenox B, Luetteke N, Celis E, Gabrilovich D. Immature myeloid cells directly contribute to skin tumor development by recruiting IL-17-producing CD4+ T cells. The Journal of experimental medicine. 2015;212:351–367. doi: 10.1084/jem.20140835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nature reviews. Immunology. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- [100].Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer immunology, immunotherapy : CII. 2013;62:909–918. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, Yancey D, Dahm P, Vieweg J. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:8270–8278. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- [102].Dubinski D, Wolfer J, Hasselblatt M, Schneider-Hohendorf T, Bogdahn U, Stummer W, Wiendl H, Grauer OM. CD4+ T effector memory cell dysfunction is associated with the accumulation of granulocytic myeloid-derived suppressor cells in glioblastoma patients. Neuro-oncology. 2016;18:807–818. doi: 10.1093/neuonc/nov280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sippel TR, White J, Nag K, Tsvankin V, Klaassen M, Kleinschmidt-DeMasters BK, Waziri A. Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting arginase I. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:6992–7002. doi: 10.1158/1078-0432.CCR-11-1107. [DOI] [PubMed] [Google Scholar]
- [104].Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, Gillanders WE, Linehan DC, Goedegebuure P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer immunology, immunotherapy : CII. 2012;61:1373–1385. doi: 10.1007/s00262-011-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer immunology, immunotherapy : CII. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer research. 2007;67:11021–11028. doi: 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- [107].Kusmartsev S, Eruslanov E, Kubler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, Siemann D, Vieweg J. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. Journal of immunology. 2008;181:346–353. doi: 10.4049/jimmunol.181.1.346. [DOI] [PubMed] [Google Scholar]
- [108].Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, Meng YG, Ferrara N. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6742–6747. doi: 10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- [111].Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- [112].Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, Rini B, Finke JH, Cohen PA. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer research. 2010;70:3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer research. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- [114].Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:4583–4594. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int Immunopharmacol. 2007;7:140–151. doi: 10.1016/j.intimp.2006.09.021. [DOI] [PubMed] [Google Scholar]
- [116].Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. The Journal of experimental medicine. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Prins RM, Scott GP, Merchant RE, Graf MR. Irradiated tumor cell vaccine for treatment of an established glioma. II. Expansion of myeloid suppressor cells that promote tumor progression. Cancer immunology, immunotherapy : CII. 2002;51:190–199. doi: 10.1007/s00262-002-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zhu X, Fujita M, Snyder LA, Okada H. Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. Journal of neuro-oncology. 2011;104:83–92. doi: 10.1007/s11060-010-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Eberstal S, Badn W, Fritzell S, Esbjornsson M, Darabi A, Visse E, Siesjo P. Inhibition of cyclooxygenase-2 enhances immunotherapy against experimental brain tumors. Cancer immunology, immunotherapy : CII. 2012;61:1191–1199. doi: 10.1007/s00262-011-1196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Zhang H, Tian M, Xiu C, Wang Y, Tang G. Enhancement of antitumor activity by combination of tumor lysate-pulsed dendritic cells and celecoxib in a rat glioma model. Oncology research. 2013;20:447–455. doi: 10.3727/096504013x13685487925176. [DOI] [PubMed] [Google Scholar]
- [121].Kosaka A, Ohkuri T, Okada H. Combination of an agonistic anti-CD40 monoclonal antibody and the COX-2 inhibitor celecoxib induces anti-glioma effects by promotion of type-1 immunity in myeloid cells and T-cells. Cancer immunology, immunotherapy : CII. 2014;63:847–857. doi: 10.1007/s00262-014-1561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]