Abstract
We describe the case of a 27-year-old woman with a history of sickle cell trait (SCT) who presented with several months of hematuria and was found to have nutcracker syndrome (NCS). While SCT is a common cause of hematuria resulting from renal papillary necrosis, our patient had concomitant abdominal pain and anemia, prompting further evaluation and the subsequent diagnosis of NCS. Interestingly, the anoxia in the left renal vein from NCS predisposes patients with SCT to sickling. Our case highlights key clinical features of both NCS and SCT and the relationship between the two disease processes.
KEY WORDS: nutcracker syndrome, sickle cell trait, hematuria
INTRODUCTION
Nutcracker syndrome (NCS) is characterized by complex and nonspecific symptoms secondary to the impingement of the left renal vein (LRV) against the aorta by the superior mesenteric artery (SMA). There is a paucity of data regarding the syndrome, and currently no validated diagnostic or therapeutic guidelines exist to identify the syndrome, resulting in an unknown prevalence and incidence.1 However, a higher rate of symptomatic NCS has been proposed in patients with sickle cell trait (SCT).2 , 3 Hematuria is a common complaint in patients with SCT, and many times does not result in further investigation; however, hematuria is often the only presenting sign of NCS and is thought to be more significant in patients with SCT secondary to increased sickling from venous compression.1 , 2 Here, we present a case of a young woman with SCT who presented with symptomatic anemia and hematuria and was subsequently diagnosed with NCS.
CASE PRESENTATION
A previously healthy 27-year-old African-American woman with SCT presented to the emergency department with a 6-month history of persistent, grossly bloody urine, diffuse abdominal pain following urination which worsened with movement, fatigue, and dyspnea on exertion. She noted a dark urine stream at the initiation of urination, which did not clear. She had also developed shortness of breath with daily activities. She had been seen multiple times at urgent care clinics and repeatedly diagnosed and empirically treated for urinary tract infections without improvement. She denied any cough, chest pain, fever, joint pains, changes in weight, swelling, recent illnesses, or history of easy bleeding/bruising.
Her family history was significant only for sickle cell disease in her father. She denied taking any prescribed or over-the-counter medications. On physical examination, her pulse was 115 beats/min with a blood pressure of 97/50 mmHg and body mass index of 18.8 kg/m2. She had conjunctival pallor but no abdominal or flank tenderness.
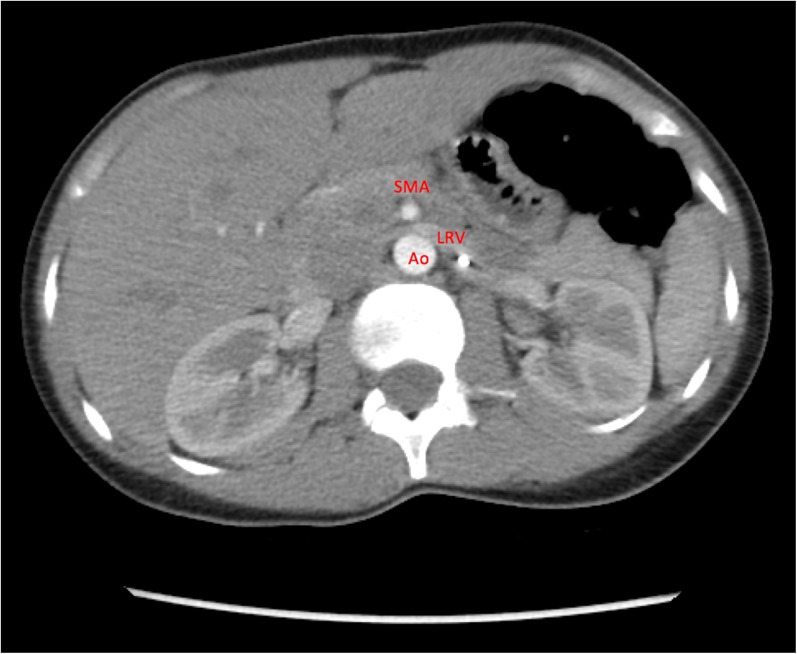
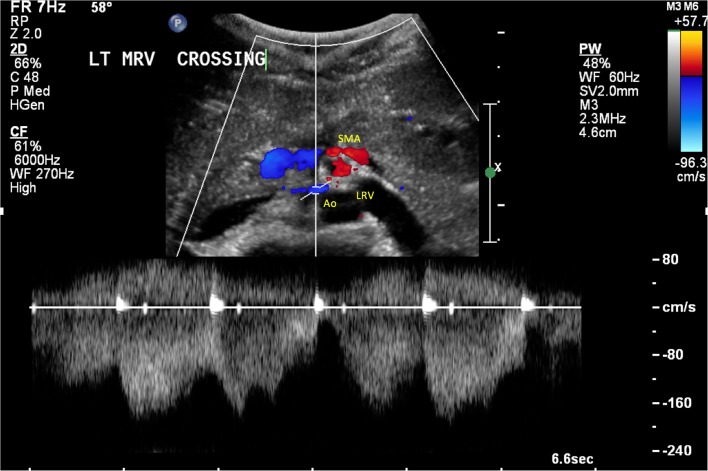
A basic metabolic profile did not reveal any abnormalities in her renal function or electrolytes, but a urinalysis showed >25 red blood cells/high-power field and 3+ protein. Her blood counts were hemoglobin 5.3 g/dL, hematocrit 16%, and mean corpuscular volume 68 fL. Iron studies were significant for ferritin 82 ng/mL, iron 25 mcg/dL, and transferrin 265 mg/dL. Her SCT was confirmed by hemoglobin electrophoresis, and no other hemoglobinopathy was identified. Transfusion of one unit of packed red blood cells was administered, with improvement in her shortness of breath and fatigue. A non-contrast CT scan and ultrasound of her abdomen showed no acute abnormalities, but a cystoscopy revealed bloody efflux at the left ureteral orifice. Subsequently, a CT scan of the abdomen with and without contrast showed pinching of the left renal vein between the SMA and aorta (Fig. 1). A follow-up renal sonogram with abdominal Doppler showed a narrowing of the left renal vein at the level of the SMA, with a peak velocity gradient greater than 10:1 (Fig. 2), consistent with left renal vein stenosis and NCS.
Figure 1.
CT of the abdomen/pelvis with and without contrast showing impingement of the left renal vein (LRV) between the superior mesenteric artery (SMA) and aorta (Ao). Although suggestive of nutcracker syndrome, further diagnostic studies were needed.
Figure 2.
Doppler ultrasound showing impingement of the left renal vein (LRV) between the superior mesenteric artery (SMA) and aorta (Ao).
She was referred to a vascular surgeon, who performed a left venogram which revealed left renal vein compression, further supporting the diagnosis of NCS. The patient initially declined any surgical intervention; however, after her abdominal pain persisted and she required further blood transfusions for symptomatic anemia, she elected to have a left renal vein bypass to decompress her renal vein. Following the procedure, her hematuria and abdominal pain resolved.
Outpatient follow-up at 1 and 2 years showed sustained resolution of hematuria and anemia. She reported no further episodes of abdominal pain.
DISCUSSION
Hematuria may be either grossly visible (macroscopic) or seen only with examination of the urine (microscopic). Microscopic hematuria is defined as three or more red blood cells per high-power field, although there is no acceptable lower limit where significant disease is excluded.4 Microscopic and gross hematuria not explained by an obvious cause occurs in up to 40% of young adults and is typically transient without any long-term significance.5 , 6 However, when hematuria is persistent, further evaluation is warranted. A more detailed history focusing on the temporal description of hematuria may aid in the localization of the lesion. Initial hematuria, with bleeding at the onset of urination, suggests a lesion in the urethra or prostate. Terminal hematuria, with bleeding at the end of urination, is typically due to a lesion in the bladder or prostate. Total hematuria, with bleeding throughout urination, is concerning for a lesion in the bladder, ureter, or kidneys.7 While the etiology of hematuria is not identified in many cases; the most common causes of hematuria are urinary tract infection, benign prostatic hyperplasia, and urinary calculi.8 Less frequently, the cause is urologic malignancy or renal disease.
SCT is a carrier state of sickle cell disease, as patients have one normal beta globulin gene and one sickle variant gene, producing hemoglobin AS. The prevalence in the United States is 8–10% in the African-American population.9 Microscopic and macroscopic hematuria is the most frequent complication associated with SCT, with half of these cases thought to be due to renal papillary necrosis resulting from micro-infarctions in the renal medulla. SCT renal papillary necrosis produces a painless hematuria which is not associated with anemia.10 Despite its prevalence, clinicians should not assume that hematuria in a SCT patient is due to renal papillary necrosis. Clinicians should perform an appropriate evaluation for the cause of the hematuria, especially if signs and symptoms of alternate etiologies are present. In our patient (who had abdominal pain and anemia in addition to the hematuria), further investigation revealed clinically significant NCS, which was surgically corrected.
Nutcracker phenomenon was first described in 1937 as an anatomical variation seen in cadavers, at which time the compression of the LRV against the aorta by the SMA was likened to a nut within the jaws of a nutcracker.11 This type of anatomical variation resulting in compression has been seen in other clinical entities, including May–Thurner syndrome (compression of the left common iliac vein between the right common iliac artery and underlying vertebral body), SMA syndrome (compression of the third portion of the duodenum between the SMA and aorta), and median arcuate ligament syndrome (compression of the celiac artery by the median arcuate ligament). The first documented case of clinically significant NCS was described in 1950.12 The prevalence of the condition remains unknown. Most cases are seen in patients in their second and third decade of life, and a second peak in occurrence is seen in the sixth decade of life in women.13 Women, especially those with low body mass index in which there is little retroperitoneal adipose tissue causing reduction of the mesoaortic angle, are at increased risk of NCS.14
The clinical features of NCS are variable, ranging from asymptomatic hematuria to severe pelvic congestion with significant pain. Hematuria, the most common presenting symptom in NCS, results from rupturing of thin-walled peri- and pararenal varicosities and collaterals that form from increased pressure in the LRV.14 The hematuria can be macroscopic or microscopic depending on the severity of the renal venous hypertension.1 Abdominal pain in NCS can be caused by left ureteral colic from blood clots passing down the left ureter, while mesoaortic compression of the LRV results in left gonadal vein obstruction and reflux resulting in symptoms of pelvic congestion (pelvic pain, dyspareunia, dysuria, and dysmenorrhea in women and varicocele in men).1 , 15 Both hematuria and abdominal pain can be aggravated by physical activity.1 Orthostatic proteinuria is also commonly seen and is thought to be secondary to elevated levels of norepinephrine and angiotensin II.16
No validated diagnostic criteria exist to diagnose NCS. CT scans are able to determine the LRV diameter, collaterals, and mesoaortic angle, but cannot measure velocity changes. Venography is often considered the “gold standard” for diagnosis, as it measures the reno-caval pressure gradient; however, Doppler ultrasound can measure the LRV/inferior vena cava gradient and is often preferred due to its non-invasive nature.1 , 17
Conservative treatment is recommended for mild hematuria, especially in patients younger than 18 years of age, where 75% have complete resolution without specific interventions, while surgical decompression of the LRV is reserved for patients with refractory symptoms.1 , 14 Angiotensin inhibitors have been shown to decrease the proteinuria in patients with NCS.18 Overall, most surgical interventions attempt to reduce LRV hypertension, with poorer outcomes associated with patients who had lower pressure gradients preoperatively.19
In our case, the diagnosis of NCS was further supported by a concurrent diagnosis of SCT, as each clinical entity likely exacerbated the other. Patients with SCT are four times as likely to have left kidney renal medulla involvement resulting in hematuria. This occurs since the longer left renal vein is normally slightly compressed between the aorta and superior mesenteric artery, causing increased blood pressure in the vein, increasing relative anoxia in the renal medulla, and promoting sickling.2 This phenomenon is worsened and perpetuated by clinically significant NCS.
KEY POINTS
• Transient hematuria is often benign in young patients, but persistent hematuria deserves an evaluation for underlying pathology. The most common causes of hematuria are urinary tract infection, benign prostatic hyperplasia, and urinary calculi.
• Hematuria is a common complication of sickle cell trait (SCT), with half the cases due to renal papillary necrosis. Patients with SCT who develop hematuria should be evaluated for less common etiologies, such as nutcracker syndrome (NCS), based on their presentation and physical exam.
• NCS is a cause of hematuria secondary to the impingement of the left renal vein against the aorta by the superior mesenteric artery. In SCT patients, NCS can lead to anemia from red blood cell sickling due to anoxia from the compressed left renal vein
• No validated diagnostic criteria exist to diagnose NCS, although venography is often considered the “gold standard” for diagnosis. Conservative treatment is recommended for mild hematuria, while surgical decompression of the left renal vein is reserved for patients with refractory symptoms.
Compliance with Ethical Standards
Conflict of Interest
To the best of our knowledge, no conflict of interest, financial or other, exists.
Disclosures
The opinions expressed in this article are those of the authors alone and do not reflect the views of the Department of Veterans Affairs.
REFERENCES
- 1.Kurklinsky AK, Rooke TW. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc. 2010;85(6):552–9. doi: 10.4065/mcp.2009.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Eps LS, de Jong PE. Diseases of the kidney and urinary tract. 2. Boston, Ma: Little, Brown & Co.; 1997. p. 220. [Google Scholar]
- 3.Nath KA, Hebbel RP. Sickle cell disease: renal manifestations and mechanisms. Nat Rev Nephrol. 2015;11(3):161–171. doi: 10.1038/nrneph.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mariani AJ, Mariani MC, Macchioni C, et al. The significance of adult hematuria: 1000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. J Urol. 1989;141:350. doi: 10.1016/s0022-5347(17)40763-4. [DOI] [PubMed] [Google Scholar]
- 5.Patel JV, Chambers CV, Gomella LG. Hematuria: etiology and evaluation for the primary care physician. Can J Urol. 2008;15:54–61. [PubMed] [Google Scholar]
- 6.Froom P, Ribak J, Benbassat J. Significance of microhaematuria in young adults. Br Med J. 1984;288(6410):20. doi: 10.1136/bmj.288.6410.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collier VU. Chapter 41. Hematuria. In: Henderson MC, Tierney LM Jr, Smetana GW, editors. The patient history: an evidence-based approach to differential diagnosis. New York, NY: McGraw-Hill; 2012. [Google Scholar]
- 8.Sharp VJ, Barnes KT, Erickson BA. Assessment of asymptomatic microscopic hematuria in adults. Am Fam Physician. 2013;88(11):747–54. [PubMed] [Google Scholar]
- 9.Motulsky AG. Frequency of sickling disorders in U.S. blacks. N Engl J Med. 1973;288:31–3. doi: 10.1056/NEJM197301042880108. [DOI] [PubMed] [Google Scholar]
- 10.Eckert DE, Jonitlis AJ, Davidson AJ. The incidence and manifestation of urographic papillary abnormalities in patients with S hemoglobinopathies. Radiology. 1974;113:59–63. doi: 10.1148/113.1.59. [DOI] [PubMed] [Google Scholar]
- 11.Grant JC. Method of anatomy. 1. Baltimore, MD: Williams & Wilkins; 1937. p. 158. [Google Scholar]
- 12.El-Sadr AR, Mina E. Anatomical and surgical aspects in the operative management of varicocele. Urol Cutan Rev. 1950;54(5):257–9. [PubMed] [Google Scholar]
- 13.Rudloff U, Holmes RJ, Prem JT, et al. Mesoaortic compression of the left renal vein (nutcracker syndrome): case reports and review of the literature. Ann Vasc Surg. 2006;20:120–9. doi: 10.1007/s10016-005-5016-8. [DOI] [PubMed] [Google Scholar]
- 14.Menard MT. Nutcracker syndrome: when should it be treated and how? Perspect Vasc Surg Endovasc Ther. 2009;21(2):117–124. doi: 10.1177/1531003509338402. [DOI] [PubMed] [Google Scholar]
- 15.Coolsaet BL. Ureteric pathology in relation to right and left gonadal veins. Urology. 1978;12(1):40–9. doi: 10.1016/0090-4295(78)90365-5. [DOI] [PubMed] [Google Scholar]
- 16.Mazzoni MB, Kottanatu L, Simonetti GD, et al. Renal vein obstruction and orthostatic proteinuria: a review. Nephrol Dial Transplant. 2011;26:562–5. doi: 10.1093/ndt/gfq444. [DOI] [PubMed] [Google Scholar]
- 17.Takebayashi S, Ueki T, Ikeda N, et al. Diagnosis of the nutcracker syndrome with color Doppler sonography: correlation with flow patterns on retrograde left renal venography. AJR Am J Roentgenol. 1999;172(1):39–43. doi: 10.2214/ajr.172.1.9888735. [DOI] [PubMed] [Google Scholar]
- 18.Ha TS, Lee EJ. ACE inhibition can improve orthostatic proteinuria associated with nutcracker syndrome. Pediatr Nephrol. 2006;21(11):1765–8. doi: 10.1007/s00467-006-0206-3. [DOI] [PubMed] [Google Scholar]
- 19.Shaper KR, Jackson JE, Williams G. The nutcracker syndrome: an uncommon cause of haematuria. Br J Urol. 1994;74(2):144–6. doi: 10.1111/j.1464-410X.1994.tb16575.x. [DOI] [PubMed] [Google Scholar]