Abstract
Background
The microvascular reperfusion injury after retransfusion has not been completely characterized. Specifically, the question of heterogeneity among different microvascular beds needs to be addressed. In addition, the identification of anaerobic metabolism is elusive. The venoarterial PCO2 to arteriovenous oxygen content difference ratio (Pv-aCO2/Ca-vO2) might be a surrogate for respiratory quotient, but this has not been validated. Therefore, our goal was to characterize sublingual and intestinal (mucosal and serosal) microvascular injury after blood resuscitation in hemorrhagic shock and its relation with O2 and CO2 metabolism.
Methods
Anesthetized and mechanically ventilated sheep were assigned to stepwise bleeding and blood retransfusion (n = 10) and sham (n = 7) groups. We performed analysis of expired gases, arterial and mixed venous blood gases, and intestinal and sublingual videomicroscopy.
Results
In the bleeding group during the last step of hemorrhage, and compared to the sham group, there were decreases in oxygen consumption (3.7 [2.8–4.6] vs. 6.8 [5.8–8.0] mL min−1 kg−1, P < 0.001) and increases in respiratory quotient (0.96 [0.91–1.06] vs. 0.72 [0.69–0.77], P < 0.001). Retransfusion normalized these variables. The Pv-aCO2/Ca-vO2 increased in the last step of bleeding (2.4 [2.0–2.8] vs. 1.1 [1.0–1.3], P < 0.001) and remained elevated after retransfusion, compared to the sham group (1.8 [1.5–2.0] vs. 1.1 [0.9–1.3], P < 0.001). Pv-aCO2/Ca-vO2 had a weak correlation with respiratory quotient (Spearman R = 0.42, P < 0.001). All the intestinal and sublingual microcirculatory variables were affected during hemorrhage and improved after retransfusion. The recovery was only complete for intestinal red blood cell velocity and sublingual total and perfused vascular densities.
Conclusions
Although there were some minor differences, intestinal and sublingual microcirculation behaved similarly. Therefore, sublingual mucosa might be an adequate window to track intestinal microvascular reperfusion injury. Additionally, Pv-aCO2/Ca-vO2 was poorly correlated with respiratory quotient, and its physiologic behavior was different. Thus, it might be a misleading surrogate for anaerobic metabolism.
Electronic supplementary material
The online version of this article (doi:10.1186/s40635-017-0136-3) contains supplementary material, which is available to authorized users.
Keywords: Hemorrhage, Shock, Transfusion, Microcirculation, Hypoxia
Background
Hemorrhagic shock is a major cause of morbidity and mortality after trauma and other conditions. The restoration of systemic oxygen transport may not prevent the development of multiple organ failure. Possible explanations for this phenomenon are the oxygen debt accumulated during shock [1], the reperfusion injury mainly related to oxygen species production [2], and the persistent depression of microvascular perfusion [3]. Although the microcirculation in experimental hemorrhagic shock has been extensively described [4, 5], the microcirculatory alterations after blood resuscitation have not been completely studied. Specifically, the issue of perfusion heterogeneity among different microvascular beds needs to be adequately addressed.
The identification of anaerobic metabolism after the normalization of systemic hemodynamics in shock states is elusive. Even though several systemic variables may track the presence of tissue hypoxia, none of them is specific or sensitive. The acute increase in respiratory quotient (RQ) is an excellent marker of ongoing anaerobic metabolism in both exercise and oxygen supply dependency [6–8]. In both circumstances, there is an excess of CO2 production (VCO2) compared to oxygen consumption (VO2), which results from anaerobic VCO2. This arises from bicarbonate buffering of anaerobically generated protons (i.e., lactic acid dissociation, ATP hydrolysis). The measurement of RQ, however, requires analysis of expired gases by means of a metabolic cart. Such monitoring is usually not available in the ICU. Recently, observational studies found that venoarterial PCO2 difference (Pv-aCO2) to arteriovenous oxygen content difference (Ca-vO2) ratio might be a surrogate for RQ [9, 10]. This assumption relies on Fick’s principle, which states that VCO2 and VO2 can be calculated as the product of cardiac output by the respective venoarterial content difference. This also assumes a linear relationship between CO2 content and pressure. Nevertheless, those studies have not compared Pv-aCO2/Ca-vO2 with RQ.
Our goal was to characterize the intestinal mucosal and serosal microvascular alterations during hemorrhagic shock and retransfusion (H/R) and its relation with O2 and CO2 metabolism. A secondary objective was to correlate gut abnormalities with those of sublingual mucosa, a more accessible window in critically ill patients. Our hypotheses were (1) intestinal mucosal microcirculation is more susceptible than the other territories and (2) Pv-aCO2/Ca-vO2 fails to reflect the changes in RQ.
Methods
Anesthesia and ventilation
Seventeen sheep (23 ± 7 kg, mean ± SD) were anesthetized with 30 mg kg−1 of sodium pentobarbital and intubated and mechanically ventilated with a Servo Ventilator 900C (Siemens-Elema AB, Solna, Sweden) with a tidal volume of 15 mL kg−1, a FiO2 of 0.21, and a positive end-expiratory pressure of 6 cmH2O. The initial respiratory rate was set to keep the arterial PCO2 between 35 and 40 mmHg. This respiratory setting was maintained during the rest of the experiment. Neuromuscular blockade was performed with pancuronium bromide (0.06 mg kg−1). Additional pentobarbital boluses (1 mg kg−1) were administered hourly and when clinical signs of inadequate depth of anesthesia were evident. Analgesia was provided by fentanyl as a bolus of 2 μg kg−1, followed by 1 μg h−1 kg−1. These drugs were administered intravenously.
Surgical preparation
A 7.5-French Swan-Ganz Standard Thermodilution Pulmonary Artery Catheter (Edwards Life Sciences, Irvine, CA, USA) was inserted through an introducer in the right external jugular vein to obtain mixed venous samples; its side port was used to administer fluids and drugs. Catheters were placed in the descending aorta via the left femoral artery to measure blood pressure, perform the bleeding, and obtain blood samples, and in the inferior vena cava to perform the retransfusion.
A midline laparotomy was performed, followed by a gastrostomy to drain gastric contents, and a splenectomy to avoid spleen contraction during the hemorrhage. An electromagnetic flow probe was placed around the superior mesenteric artery to measure blood flow (SMABF). A catheter was introduced in the mesenteric vein through a small vein proximal to the gut to draw blood samples and to measure pressure. A tonometer was inserted through a small ileotomy to measure intramucosal PCO2. A 10- to 15-cm segment of the ileum was mobilized, placed outside the abdomen, and opened 2 cm on the antimesenteric border to allow an examination of mucosal microcirculation. The exteriorized intestinal segment was covered and moisture and temperature preserved by a device. Finally, after complete hemostasis, the abdominal-wall incision was closed, excepting a short segment for externalization of the ileal loop.
Measurements and derived calculations
Systemic VO2, VCO2, and RQ were measured by analysis of expired gases (MedGraphics CPX Ultima, Medical Graphics Corporation, St. Paul, MN). VO2 and VCO2 were adjusted to body weight.
Arterial, mixed venous, and mesenteric venous PO2, PCO2, pH, Hb, and O2 saturation were measured with a blood gas analyzer and a co-oximeter (ABL 5 and OSM 3, Radiometer, Copenhagen, Denmark). Oxygen-derived variables were calculated by standard formulae. Systemic and intestinal Ca-vO2 were calculated using mixed and mesenteric venous O2 saturation and Pv-aCO2/Ca-vO2 by means of mixed venous blood.
Cardiac index (CI) was calculated as VO2 divided by systemic Ca-vO2. Systemic oxygen transport (DO2) was calculated as CI by arterial O2 content.
SMABF was measured by the electromagnetic method (Spectramed Blood Flowmeter model SP 2202 B, Spectramed Inc., Oxnard, CA, USA), with in vitro calibrated transducers of 5–7 mm diameter (Blood Flowmeter Transducer, Spectramed Inc., Oxnard, CA, USA). Occlusive zero was controlled before and after each experiment. Non-occlusive zero was corrected before each measurement. SMABF was referred to gut weight. Intestinal DO2 was calculated as SMABF by arterial O2 content and intestinal VO2 as SMABF by intestinal Ca-vO2.
Intramucosal PCO2 was measured by air tonometry (Tonometrics Catheter and Tonocap, Datex-Ohmeda, Helsinki, Finland). Then, we calculated intramucosal-arterial PCO2 (ΔPCO2).
Arterial lactate was measured with a point-of-care analyzer (Stat Profile Critical Care Xpress, Nova Biomedical, Waltham, MA, USA).
Microvideoscopic measurements and analysis
The microcirculatory network was evaluated in intestinal mucosa and serosa, and sublingual mucosa by means of a sidestream-dark-field (SDF) imaging device (Microscan, MicroVision Medical, Amsterdam, Netherlands) [11]. Different precautions were taken and steps followed to obtain images of adequate quality and to insure satisfactory reproducibility. After gentle removal of saliva by isotonic-saline-drenched gauze, steady images of at least 20 s were obtained while avoiding pressure artifacts with a portable computer and an analog-to-digital video converter (ADVC110, Canopus Co., San Jose, CA, USA). The videos were recorded from three different areas. Video clips were stored as AVI files to allow computerized frame-by-frame image analysis.
Video-image analysis was performed blindly by well-trained researchers. Adequate focus and contrast adjustment were verified, and images of poor quality were discarded. The entire sequence was used to describe the semiquantitative characteristics of the microvascular flow and, particularly, the presence of stopped or intermittent flow.
We used an image-analysis software (Microscan analysis software®–AVA 3.0–MicroVision Medical, Amsterdam, Netherlands) [12] to determine total vascular density. An analysis based on semiquantitative criteria that distinguished no flow (0), intermittent flow [1], sluggish flow [2], and continuous flow [3] was performed on individual vessels [3]. The overall score, called microvascular flow index (MFI), is the average of the individual values [13]. Quantitative red blood cell (RBC) velocity was determined using space-time diagrams [12]. We also calculated the proportion of perfused vessels, the perfused vascular density (i.e., the total vascular density multiplied by the fraction of perfused vessels), and the heterogeneity flow index as highest-lowest MFI divided mean MFI [14].
In sheep, most of sublingual vascular density (97 ± 1%) and all intestinal vessels consist of small vessels (diameter <25 μm) [5], so analysis was focused on these types of vessels, whereas the vessels of higher diameter were assessed only for ruling out compression artifacts.
Experimental procedure
Basal measurements were taken after a period of no less than 30 min after systemic VO2, VCO2, and SMABF became stable. Animals were then assigned to H/R (n = 10) or sham (n = 7) group. In the H/R group, three consecutive bleedings of 5–10 mL kg−1 were performed at 30-min intervals, until reaching reductions in systemic VO2 and increases in RQ. Then, shed blood was rapidly reinfused (~2 min) and sheep were followed during one additional hour. In the sham group, the same experimental preparation was carried out and 0.9% NaCl was infused to maintain hemodynamic variables at basal values, without further interventions. Measurements were performed at baseline (0′), during bleeding (30′, 60′, and 90′), and after retransfusion (2′, 30′, and 60′). Microcirculatory videos were only acquired at 0′, 30′, and 90′ of hemorrhage and 60′ of retransfusion, but in sublingual mucosa, images were also continuously obtained during blood reinfusion. Thereafter, the initial and the final portion of these videos (0′ and 2′) were analyzed. Blood temperature was kept constant throughout the study with a heating lamp.
At the end of the experiment, animals were killed with an additional dose of pentobarbital and a KCl bolus. A catheter was inserted in the superior mesenteric artery, and Indian ink was instilled through it. Dyed intestinal segments were dissected, washed, and weighed to calculate gut indexes.
Data analysis
Because of the small numbers of animals, nonparametric tests were used. Changes over time within each group were assessed with nonparametric analysis of variance for repeated measurements (Friedman test) followed by a post hoc test (Dunn’s multiple comparison test). Differences between groups at each time point were analyzed with Mann-Whitney U test. Correlations between variables were calculated with Spearman test. Data are expressed as median and interquartile range. A P value <0.05 was considered statistically significant.
Results
Effects on systemic and intestinal hemodynamics and oxygen transport
In the H/R group, mean arterial pressure decreased from the first step of bleeding, while reductions in CI and SMABF reached statistical significance during the last stage (Table 1). Each variable was normalized after retransfusion, but at 2′, CI and SMABF were higher than baseline. During bleeding, there were progressive reductions in systemic and intestinal DO2 which, in the last step, were associated with systemic and intestinal VO2 falls and RQ increases (Figs. 1 and 2). During retransfusion, all these variables were normalized. Bleeding induced lactic acidosis, which persisted after retransfusion (Fig. 3).
Table 1.
Hemoglobin, systemic and intestinal hemodynamic and oxygen transport variables, and arterial blood gases in hemorrhage/retransfusion (H/R) and sham groups
| Hemorrhage | Retransfusion | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 30′ | 60′ | 90′ | 2′ | 30′ | 60′ | ||
| Hemoglobin (g per 100 mL) | H/R | 8.3 [7.7–9.7] | 7.8 [7.3–9.1]† | 7.3 [7.0–9.1]*† | 6.5 [6.1–7.7]*† | 8.0 [6.9–9.3]† | 7.8 [7.7–9.7] | 8.1 [7.2–9.7] |
| Sham | 9.9 [9.3–10.3] | 9.9 [9.5–10.4] | 9.8 [9.3–10.5] | 9.6 [9.2–9.8] | 9.6 [9.2–9.8] | 9.3 [8.8–9.8] | 9.4 [8.6–10.0] | |
| Mean arterial pressure (mmHg) | H/R | 83 [72–96] | 42 [34–47]*† | 40 [32–44]*† | 28 [22–30]*† | 95 [90–100] | 90 [82–99] | 91 [79–97] |
| Sham | 91 [90–102] | 97 [80–109] | 92 [89–102] | 99 [91–109] | 98 [90–108] | 100 [92–112] | 100 [91–110] | |
| Central venous pressure (mmHg) | H/R | 3 [0–6] | 3 [1–6] | 4 [1–7] | 4 [0–7] | 6 [1–9] | 3 [1–5] | 3 [0–5] |
| Sham | 2 [1–2] | 2 [1–4] | 3 [1–3] | 2 [1–4] | 2 [1–4] | 2 [1–4] | 2 [1–4] | |
| Mean pulmonary pressure (mmHg) | H/R | 17 [10–20] | 12 [7–17] | 11 [8–13] | 10 [6–12]*† | 22 [17–32]† | 18 [12–20] | 16 [13–19] |
| Sham | 11 [10–13] | 13 [12–14] | 14 [13–15] | 13 [12–13] | 13 [12–13] | 14 [13–15] | 13 [11–16] | |
| Pulmonary occlusion pressure (mmHg) | H/R | 5 [4–7] | 6 [1–8] | 4 [1–8] | 6 [0–7] | 7 [4–9] | 5 [2–9] | 4 [2–7] |
| Sham | 3 [2–6] | 2 [2–4] | 2 [2–3] | 3 [3–4] | 3 [3–4] | 2 [1–4] | 2 [2–3] | |
| Cardiac index (mL min−1 kg−1) | H/R | 155 [136–177] | 89 [64–177] | 78 [70–119] | 53 [39–72]*† | 210 [158–340]† | 133 [116–179] | 134 [102–180] |
| Sham | 122 [101–149] | 122 [100–151] | 108 [97–167] | 99 [94–168] | 101 [96–112] | 110 [99–178] | 113 [96–174] | |
| Mesenteric flow (mL min−1 per 100 g) | H/R | 805 [489–903] | 451 [253–665] | 427 [264–576] | 236 [110–332]*† | 1052 [644–1406]† | 850 [550–1072] | 923 [523–986] |
| Sham | 457 [445–786] | 500 [457–992] | 445 [426–743] | 457 [379–714] | 448 [428–750] | 487 [457–892] | 502 [487–814] | |
| Arterial pH | H/R | 7.44 [7.35–7.49] | 7.44 [7.39–7.49] | 7.44 [7.40–7.49] | 7.42 [7.39–7.48] | 7.28 [7.20–7.37]*† | 7.37 [7.29–7.43]† | 7.41 [7.32–7.45]† |
| Sham | 7.49 [7.42–7.51] | 7.49 [7.39–7.56] | 7.52 [7.41–7.52] | 7.53 [7.42–54] | 7.52 [7.41–7.53] | 7.51 [7.44–55] | 7.55 [7.42–54] | |
| Arterial PCO2 (mmHg) | H/R | 38 [37–40] | 36 [35–39] | 36 [33–39] | 34 [31–36]* | 44 [41–52]*† | 38 [36–42] | 39 [34–41] |
| Sham | 38 [33–40] | 37 [35–40] | 36 [34–39] | 34 [33–39] | 34 [33–39] | 34 [32–38] | 34 [32–38] | |
| Arterial PO2 (mmHg) | H/R | 86 [77–91] | 83 [77–93] | 88 [77–95] | 92 [87–106] | 75 [69–84]*† | 86 [75–92] | 85 [77–94] |
| Sham | 87 [78–93] | 83 [81–87] | 87 [83–95] | 87 [78–99] | 91 [74–99] | 88 [87–100] | 89 [85–99] | |
| Arterial bicarbonate (mEq L−1) | H/R | 26 [21–28] | 25 [22–27] | 23 [20–26]* | 23 [18–25]*† | 23 [18–25]*† | 23 [19–25]*† | 24 [19–27]† |
| Sham | 26 [25–28] | 29 [23–31] | 28 [25–29] | 27 [25–29] | 27 [25–29] | 27 [25–28] | 28 [25–30] | |
| Arterial base excess (mEq L−1) | H/R | 2 [−4 to 4] | 1 [−2 to 5] | 2 [−2 to 3] | −1 [−5 to 3]*† | −3 [−9 to 0]*† | −2 [−6 to 1]*† | −1 [−6 to 2]† |
| Sham | 3 [−3 to 5] | 5 [−2 to 9] | 5 [1–6] | 4 [0–6] | 4 [0–6] | 4 [0–5] | 5 [1–8] | |
Data are shown as median [percentile 0.25–0.75]
*P < 0.05 vs. basal; †P < 0.05 vs. sham
Fig. 1.
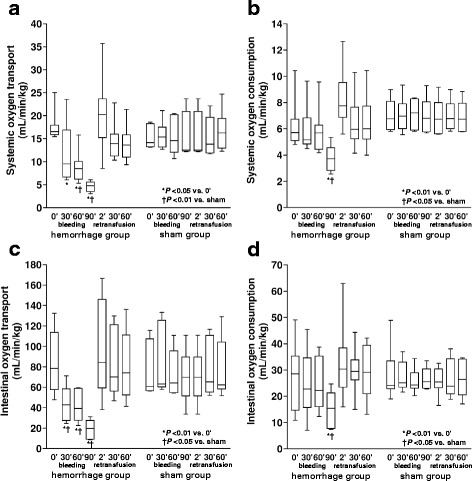
Behavior of systemic and intestinal O2 transport and consumption. a Systemic O2 transport. b Systemic O2 consumption. c Intestinal O2 transport. d Intestinal O2 consumption
Fig. 2.
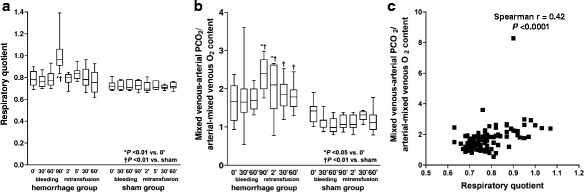
Behavior of the respiratory quotient and the venoarterial PCO2 to arteriovenous oxygen content difference ratio (Pv-aCO2/Ca-vO2). a Respiratory quotient. b Pv-aCO2/Ca-vO2. c Correlation between respiratory quotient and Pv-aCO2/Ca-vO2
Fig. 3.
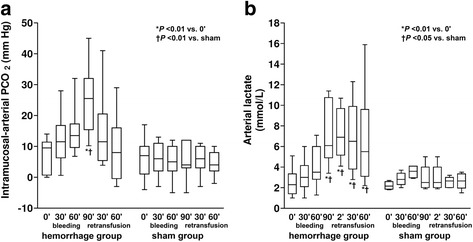
Behavior of intramucosal-arterial PCO2 difference and arterial lactate. a Intramucosal-arterial PCO2 difference. b Arterial lactate
Effects on CO2 metabolism
Compared to baseline, VCO2 decreased in the last step of bleeding and increased at 2′ of retransfusion. Systemic and intestinal Pv-aCO2 and ΔPCO2 augmented during bleeding and were normalized during retransfusion (Fig. 2 and Table 2). ΔPCO2 correlated with intestinal mucosal total vascular density (R = −0.44, P = 0.0002), perfused vascular density (R = −0.43, P = 0.0003), proportion of perfused vessels (R = −0.48, P < 0.0001), RBC velocity (R = −0.35, P < 0.001), MFI (R = −0.52, P < 0.0001), and heterogeneity flow index (R = 0.52, P < 0.0001).
Table 2.
Systemic CO2 production and venoarterial PCO2 differences in hemorrhage/retransfusion (H/R) and sham groups
| Hemorrhage | Retransfusion | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 30′ | 60′ | 90′ | 2′ | 30′ | 60′ | ||
| CO2 production (mL min−1 kg−1) | H/R | 4.5 [4.2–5.0] | 4.1 [3.6–5.3] | 4.3 [3.8–4.8] | 3.6 [3.0–4.4]*† | 6.2 [5.0–7.5]*† | 4.7 [4.2–6.4] | 4.7 [4.0–5.3] |
| Sham | 4.9 [4.5–5.4] | 4.8 [4.7–5.8] | 4.9 [4.4–6.0] | 4.8 [4.5–5.3] | 4.8 [4.5–5.4] | 4.8 [4.3–5.8] | 4.5 [4.3–5.9] | |
| Mixed venoarterial PCO2 (mmHg) | H/R | 6 [5–7]† | 10 [7–12]* | 11 [9–12]*† | 16 [14–20]*† | 9 [4–12] | 8 [6–10] | 8 [7–10] |
| Sham | 8 [7–10] | 7 [6–8] | 6 [6–8] | 6 [6–8] | 7 [6–8] | 7 [6–8] | 6 [5–7] | |
| Mesenteric venoarterial PCO2 (mmHg) | H/R | 5 [5–6] | 8 [8–10]*† | 11 [10–12]*† | 15 [12–22]*† | 6 [4–13] | 6 [5–11] | 6 [5–7] |
| Sham | 6 [4–12] | 5 [4–6] | 6 [5–7] | 6 [5–7] | 6 [5–7] | 6 [4–9] | 5 [4–7] | |
Data are shown as median [percentile 0.25–0.75]
*P < 0.05 vs. basal; †P < 0.05 vs. sham
Pv-aCO2/Ca-vO2 increased during bleeding and, during retransfusion, remained higher than that of the sham group. This ratio correlated with RQ (R = 0.42, P < 0.0001) (Fig. 2).
Effects on microcirculation
From the first step of bleeding, each intestinal and sublingual microcirculatory variable was compromised.
During retransfusion, all the variables improved in the three territories. However, proportion of perfused vessels, microvascular flow index, and heterogeneity flow index could not be normalized. RBC velocity returned to basal values in intestinal mucosa and serosa and persisted diminished in sublingual mucosa. Conversely, total and perfused vascular density remained low in intestinal mucosa and serosa and were restored to baseline in sublingual mucosa.
In sublingual mucosa, each microvascular variable improved after 2′ of blood reinfusion. Such variables were similar at 2′ and 60′ of retransfusion (Figs. 4, 5, and 6 and the video in Additional file 1).
Fig. 4.
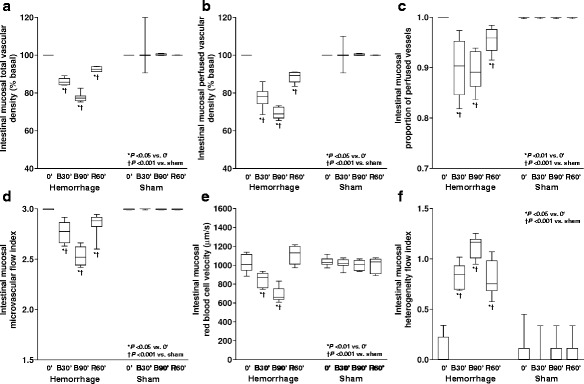
Behavior of the intestinal mucosal microcirculatory variables. a Total vascular density. b Perfused vascular density. c Proportion of perfused vessels. d Microvascular flow index. e Red blood cell velocity. f Heterogeneity flow index
Fig. 5.
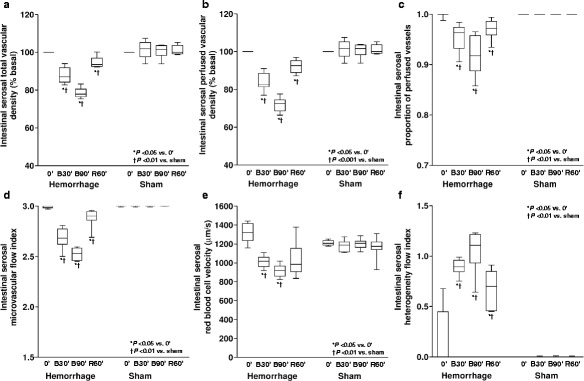
Behavior of the intestinal serosal microcirculatory variables. a Total vascular density. b Perfused vascular density. c Proportion of perfused vessels. d Microvascular flow index. e Red blood cell velocity. f Heterogeneity flow index
Fig. 6.
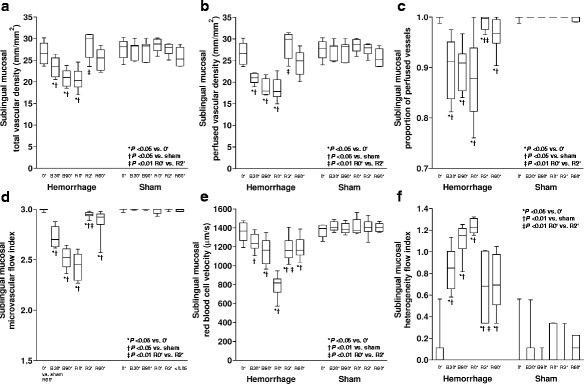
Behavior of the sublingual microcirculatory variables. a Total vascular density. b Perfused vascular density. c Proportion of perfused vessels. d Microvascular flow index. e Red blood cell velocity. f Heterogeneity flow index
Additional file 1: Video sublingual microcirculation during retransfusion. The video was continuously acquired during the period of shed blood reinfusion. Segments of the whole video were cut and edited. The left lower corner shows the actual time. There were sharp and fast increases in flow velocity and density. (WMV 13046 kb)
Discussion
Our main finding was that retransfusion improved the microcirculatory alterations that developed in hemorrhagic shock. However, subtle abnormalities persisted in the face of the normalization of aerobic metabolism. Microvascular reperfusion injury was present in the three studied vascular beds, with minor differences among them. In addition, Pv-aCO2/Ca-vO2 only showed a weak correlation with RQ and, mainly, remained elevated during retransfusion.
In experimental hemorrhagic shock, the reports of the microcirculatory effects of blood transfusion are controversial. For example, in rats, transfusion deteriorated perfused capillary density of gastric mucosa [15]. Conversely, in another study, RBC administration restored conjunctival microcirculation and muscle tissue oxygenation [16]. In the hamster window chamber model, packed fresh RBC improved but not completely normalized functional capillary density and flow [17, 18]. Partial beneficial effects were also found on rat hepatic and ileum microcirculation [19, 20].
These inconsistent results might be related to differences in the studied species, in the severity of shock, and in the particular microvascular bed evaluated. Accordingly, it has been suggested that the gut might be less responsive to blood resuscitation than the heart [21]. We found beneficial effects of blood resuscitation on intestinal and sublingual microcirculation. The recovery of microvascular perfusion, however, was incomplete, and regional deficits persisted. The novelty of our study consists in the assessment of different and relevant microvascular beds, along with a comprehensive evaluation of systemic and regional hemodynamics and oxygenation.
Microcirculatory reperfusion injury exhibited different manifestations in sublingual and intestinal territories. Although in the three areas blood reinfusion improved all the microvascular variables, only sublingual densities and intestinal RBC velocity were completely normalized. Taking into account these minor regional differences, the sublingual mucosa might be an adequate window for the monitoring of reperfusion microvascular injury. This situation might differ in other forms of distributive shock. After the resuscitation of septic shock, microcirculatory derangements are more severe in intestinal than in sublingual mucosa [22–24]. In a sheep model of endotoxic shock, the hemodynamic normalization by means of fluids corrected sublingual microcirculation whereas intestinal villi remained hypoperfused [22]. The reasons for this higher heterogeneity between microvascular beds in sepsis than in our model reperfusion injury are uncertain but might be related to different mechanisms of damage as well as the modality of resuscitation (colloid or crystalloid solutions vs. blood).
The subtle villi abnormalities could trigger mechanisms of tissue damage such as the alteration in the mucosa barrier dysfunction and systemic translocation of bacteria and their products [25]. What is more, in patients with traumatic hemorrhagic shock, the persistence of microcirculatory alterations after resuscitation predicted the development of multiorgan failure [26]. The microcirculatory dysfunction has also been proposed as the link between trauma and coagulopathy [27]. In addition, the presence of sublingual microvascular alterations in trauma patients might help to select patients who will benefit from blood transfusion. In patients with hemorrhagic shock, blood transfusion improved sublingual microcirculation independently of macrocirculation and hemoglobin level. The change in microvascular perfusion, however, was negatively correlated with the basal microvascular perfusion [28]. A similar response was described in trauma patients with hemodynamic stability [29]. The identification of microcirculatory disorders might thus contribute to the evaluation of patients with hemorrhagic shock.
Another original approach of this study was the continuous video acquisition in the sublingual area during retransfusion. This allowed a thorough assessment of the timing of capillary recruitment. While many microvascular parameters did not reach basal values, all microcirculatory variables improved sharply in the 2-min period of observation. In addition, the values at 2′ were similar to those observed at the end of the reperfusion phase, evidencing that the changes in systemic hemodynamics rapidly recruited the microcirculation. Hence, we found a coherence between macro- and microcirculation, which was only partial, since most of the microvascular variables stayed altered.
The ΔPCO2 is considered a sensitive marker of mucosal perfusion [22]. This study confirms the dependency of ΔPCO2 on microcirculation. Nevertheless, ΔPCO2 normalized at the end of the retransfusion period when microcirculatory alterations were still present. Consequently, our results suggest that tissue capnometry is less sensitive than videomicroscopy to disclose the presence of mucosal hypoperfusion.
Some studies have suggested that Pv-aCO2/Ca-vO2 could be an adequate surrogate for RQ [9, 10]. Moreover, a cutoff of 1.4 might point out the presence of anaerobic metabolism. Nevertheless, Pv-aCO2/Ca-vO2 has never been compared to RQ. Our results showed that the correlation between both variables was weak, and more importantly, the physiological behavior was different. Given that RQ normalized after retransfusion, the increased Pv-aCO2/Ca-vO2 should be ascribed to increased release of CO2 from hemoglobin, not to tissue hypoxia. The persistent Pv-aCO2/Ca-vO2 elevation might result from changes in venous oxygen saturation (Haldane effect) and hemoglobin levels and mostly from persistent hyperlactatemia [30, 31]. All these factors can shift the CO2Hb dissociation curve. Since Pv-aCO2/Ca-vO2 might be a misleading indicator of RQ and anaerobic metabolism, its values should be carefully interpreted. This is additionally emphasized by the fact that ongoing anaerobic metabolism is identified by acute increases in RQ, not by isolated values [6–8]. Actually, the normal range of RQ is 0.67 to 1.30 [32].
Our study has some limitations. First, capillary reperfusion failure has been attributed to several mechanisms, including microthrombosis, leukocyte plugging, endothelial cell swelling, vasomotor dysfunction, and capillary narrowing due to edema [3]. Our study was only descriptive and did not address such mechanisms. In addition, we only studied three microvascular territories and the microcirculation might have behaved differently in other vascular beds. Finally, the assessment of retransfusion was limited to 60 min. Longer observation periods might have produced different results.
Conclusions
Our main findings were that reperfusion microvascular injury developed in intestinal and sublingual areas, despite the complete restoration of aerobic metabolism. Since differences among microvascular beds were minor, sublingual mucosa might be an adequate window for the monitoring of intestinal reperfusion injury. Finally, given that Pv-aCO2/Ca-vO2 only had a poor correlation with RQ and a different physiologic behavior, it seems to be an inadequate surrogate for RQ.
Acknowledgements
Data from this manuscript were presented at the ESICM Annual Congress in 2016.
This work was performed in Facultad de Ciencias Médicas, Universidad Nacional de La Plata, Cátedra de Farmacología Aplicada, La Plata, Argentina.
Funding
This study was supported by the grant PICT 2010-00495, Agencia Nacional de Promoción Científica y Tecnológica, Argentina.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional file. The datasets supporting the conclusions of this article are available in the Open Science Framework repository [https://osf.io/r7pfq/].
Authors’ contributions
GF, VSKE, HSC, EM, CC, GM, MOP, JFCE, MGB, and AD carried out the animal experiments and participated in the design of the study. AD performed the statistical analysis and drafted the manuscript. CI participated in the study design and interpretation of the data. All authors discussed the results, participated in the writing, and approved the final the manuscript.
Competing interests
Dr Ince has developed SDF imaging and is listed as inventor on related patents commercialized by MicroVision Medical (MVM) under a license from the Academic Medical Center (AMC). He has been a consultant for MVM in the past, but has not been involved with this company for more than 5 years now, except that he still holds shares. Braedius Medical, a company owned by a relative of Dr Ince, has developed and designed a handheld microscope called CytoCam-IDF imaging. Dr Ince has no financial relation with Braedius Medical of any sort - i.e., he never owned shares or received consultancy or speaker fees from Braedius Medical. All other authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
The local research committee (Comité de Bioética y Ética de la Investigación) approved this study (reference number 0800-009634/11-000). Care of animals was in accordance with the National Institutes of Health (United States).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- Ca-vO2
Arteriovenous oxygen content difference
- CI
Cardiac index
- DO2
Oxygen transport
- H/R
Hemorrhagic shock and retransfusion
- MFI
Microvascular flow index
- Pv-aCO2
Venoarterial PCO2 difference
- Pv-aCO2/Ca-vO2
Ratio of venoarterial PCO2 to arteriovenous oxygen content difference
- RBC
Red blood cell
- RQ
Respiratory quotient
- SDF
Sidestream dark field
- SMABF
Superior mesenteric artery blood flow
- VCO2
Carbon dioxide production
- VO2
Oxygen consumption
- ΔPCO2
Intramucosal-arterial PCO2
Contributor Information
Gonzalo Ferrara, Email: gonzaloferrara@gmail.com.
Vanina S. Kanoore Edul, Email: vaninaedul@gmail.com.
Héctor S. Canales, Email: canaleshector@hotmail.com
Enrique Martins, Email: enriqueflmartins@gmail.com.
Carlos Canullán, Email: carloscanullan@yahoo.com.ar.
Gastón Murias, Email: gmurias@gmail.com.
Mario O. Pozo, Email: pozomario@gmail.com
Juan F. Caminos Eguillor, Email: Peguillor@hotmail.com
María G. Buscetti, Email: guillerminabuscetti@hotmail.com
Can Ince, Email: c.ince@amc.uva.nl.
Arnaldo Dubin, Phone: +5491150102431, Email: arnaldodubin@gmail.com.
References
- 1.Crowell JW, Smith EE. Oxygen deficit and irreversible hemorrhagic shock. Am J Physiol. 1964;206:313–316. doi: 10.1152/ajplegacy.1964.206.2.313. [DOI] [PubMed] [Google Scholar]
- 2.Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menger MD, Rücker M, Vollmar B. Capillary dysfunction in striated muscle ischemia/reperfusion: on the mechanisms of capillary “no-reflow”. Shock. 1997;8:2–7. doi: 10.1097/00024382-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima Y, Baudry N, Duranteau J, et al. Microcirculation in intestinal villi: a comparison between hemorrhagic and endotoxin shock. Am J Respir Crit Care Med. 2001;164:1526–1530. doi: 10.1164/ajrccm.164.8.2009065. [DOI] [PubMed] [Google Scholar]
- 5.Dubin A, Pozo MO, Ferrara G, et al. Systemic and microcirculatory responses to progressive hemorrhage. Intensive Care Med. 2009;35:556–564. doi: 10.1007/s00134-008-1385-0. [DOI] [PubMed] [Google Scholar]
- 6.Wasserman K, Whipp BJ, Koyl SN, et al. Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol. 1973;35:236–243. doi: 10.1152/jappl.1973.35.2.236. [DOI] [PubMed] [Google Scholar]
- 7.Cohen IL, Sheikh FM, Perkins RJ, et al. Effect of hemorrhagic shock and reperfusion on the respiratory quotient in swine. Crit Care Med. 1995;23:545–552. doi: 10.1097/00003246-199503000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara G, Kanoore Edul VS, Martins E, et al. Intestinal and sublingual microcirculation are more severely compromised in hemodilution than in hemorrhage. J Appl Physiol (1985) 2016;120:1132–1140. doi: 10.1152/japplphysiol.00007.2016. [DOI] [PubMed] [Google Scholar]
- 9.Mekontso-Dessap A, Castelain V, Anguel N, et al. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med. 2002;28:272–277. doi: 10.1007/s00134-002-1215-8. [DOI] [PubMed] [Google Scholar]
- 10.Monnet X, Julien F, Ait-Hamou N, et al. Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med. 2013;41:1412–1420. doi: 10.1097/CCM.0b013e318275cece. [DOI] [PubMed] [Google Scholar]
- 11.Goedhart PT, Khalilzada M, Bezemer R, et al. Sidestream Dark Field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express. 2007;15:15101–15104. doi: 10.1364/OE.15.015101. [DOI] [PubMed] [Google Scholar]
- 12.Dobbe JG, Streekstra GJ, Atasever B, et al. Measurement of functional microcirculatory geometry and velocity distributions using automated image analysis. Med Biol Eng Comput. 2008;46:659–670. doi: 10.1007/s11517-008-0349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pozo MO, Kanoore Edul VS, Ince C, et al. Comparison of different methods for the calculation of the microvascular flow index. Crit Care Res Pract. 2012;2012:102483. doi: 10.1155/2012/102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trzeciak S, Dellinger RP, Parrillo JE, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007;49:88–98. doi: 10.1016/j.annemergmed.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Ekman T, Risberg B, Soussi B, et al. Gastric mucosal microcirculation and purine nucleotide metabolism after retransfusion of rats in hemorrhagic shock. Scand J Gastroenterol. 1993;28:173–178. doi: 10.3109/00365529309096066. [DOI] [PubMed] [Google Scholar]
- 16.Cheung AT, Duong PL, Driessen B, et al. Systemic function, oxygenation and microvascular correlation during treatment of hemorrhagic shock with blood substitutes. Clin Hemorheol Microcirc. 2006;34:325–334. [PubMed] [Google Scholar]
- 17.Cabrales P, Intaglietta M, Tsai AG. Transfusion restores blood viscosity and reinstates microvascular conditions from hemorrhagic shock independent of oxygen carrying capacity. Resuscitation. 2007;75:124–134. doi: 10.1016/j.resuscitation.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortiz D, Barros M, Yan S, et al. Resuscitation from hemorrhagic shock using polymerized hemoglobin compared to blood. Am J Emerg Med. 2014;32:248–255. doi: 10.1016/j.ajem.2013.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paxian M, Keller SA, Huynh TT, et al. Perflubron emulsion improves hepatic microvascular integrity and mitochondrial redox state after hemorrhagic shock. Shock. 2003;20:449–457. doi: 10.1097/01.shk.0000090601.26659.87. [DOI] [PubMed] [Google Scholar]
- 20.Scalia S, Burton H, Van Wylen D, et al. Persistent arteriolar constriction in microcirculation of the terminal ileum following moderate hemorrhagic hypovolemia and volume restoration. J Trauma. 1990;30:713–718. doi: 10.1097/00005373-199006000-00010. [DOI] [PubMed] [Google Scholar]
- 21.van Iterson M, Bezemer R, Heger M, et al. Microcirculation follows macrocirculation in heart and gut in the acute phase of hemorrhagic shock and isovolemic autologous whole blood resuscitation in pigs. Transfusion. 2012;52:1552–1559. doi: 10.1111/j.1537-2995.2011.03471.x. [DOI] [PubMed] [Google Scholar]
- 22.Dubin A, Edul VS, Pozo MO, et al. Persistent villi hypoperfusion explains intramucosal acidosis in sheep endotoxemia. Crit Care Med. 2008;36:535–542. doi: 10.1097/01.CCM.0000300083.74726.43. [DOI] [PubMed] [Google Scholar]
- 23.Boerma EC, van der Voort PH, Spronk PE, et al. Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis. Crit Care Med. 2007;35:1055–1060. doi: 10.1097/01.CCM.0000259527.89927.F9. [DOI] [PubMed] [Google Scholar]
- 24.Edul VS, Ince C, Navarro N, et al. Dissociation between sublingual and gut microcirculation in the response to a fluid challenge in postoperative patients with abdominal sepsis. Ann Intensive Care. 2014;4(4):39. doi: 10.1186/s13613-014-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szabó A, Vollmar B, Boros M, et al. Gender differences in ischemia-reperfusion-induced microcirculatory and epithelial dysfunctions in the small intestine. Life Sci. 2006;78:3058–3065. doi: 10.1016/j.lfs.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Tachon G, Harrois A, Tanaka S, et al. Microcirculatory alterations in traumatic hemorrhagic shock. Crit Care Med. 2014;42:1433–1441. doi: 10.1097/CCM.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 27.Ward KR. The microcirculation: linking trauma and coagulopathy. Transfusion. 2013;53(Suppl 1):38S–47S. doi: 10.1111/trf.12034. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka S, Escudier E, Hamada S, et al. Effect of RBC transfusion on sublingual microcirculation in hemorrhagic shock patients: a pilot study. Crit Care Med. 2017;45:e154–e160. doi: 10.1097/CCM.0000000000002064. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg JA, MacLennan PA, Vandromme-Cusick MJ, et al. Microvascular response to red blood cell transfusion in trauma patients. Shock. 2012;37:276–281. doi: 10.1097/SHK.0b013e318241b739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hachamovitch R, Brown HV, Rubin SA. Respiratory and circulatory analysis of CO2 output during exercise in chronic heart failure. Circulation. 1991;84:605–612. doi: 10.1161/01.CIR.84.2.605. [DOI] [PubMed] [Google Scholar]
- 31.Sun XG, Hansen JE, Stringer WW, et al. Carbon dioxide pressure-concentration relationship in arterial and mixed venous blood during exercise. J Appl Physiol (1985) 2001;90:1798–1810. doi: 10.1152/jappl.2001.90.5.1798. [DOI] [PubMed] [Google Scholar]
- 32.McClave SA, Lowen CC, Kleber MJ, et al. Clinical use of the respiratory quotient obtained from indirect calorimetry. JPEN J Parenter Enteral Nutr. 2003;27:21–26. doi: 10.1177/014860710302700121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional file. The datasets supporting the conclusions of this article are available in the Open Science Framework repository [https://osf.io/r7pfq/].


