ABSTRACT
Bacterial pathogens have evolved sophisticated mechanisms to sense and adapt to redox stress in nature and within the host. However, deciphering the redox environment encountered by intracellular pathogens in the mammalian cytosol is challenging, and that environment remains poorly understood. In this study, we assessed the contributions of the two redox-responsive, Spx-family transcriptional regulators to the virulence of Listeria monocytogenes, a Gram-positive facultative intracellular pathogen. Spx-family proteins are highly conserved in Firmicutes, and the L. monocytogenes genome contains two paralogues, spxA1 and spxA2. Here, we demonstrate that spxA1, but not spxA2, is required for the oxidative stress response and pathogenesis. SpxA1 function appeared to be conserved with the Bacillus subtilis homologue, and resistance to oxidative stress required the canonical CXXC redox-sensing motif. Remarkably, spxA1 was essential for aerobic growth, demonstrating that L. monocytogenes SpxA1 likely regulates a distinct set of genes. Although the ΔspxA1 mutant did not grow in the presence of oxygen in the laboratory, it was able to replicate in macrophages and colonize the spleens, but not the livers, of infected mice. These data suggest that the redox state of bacteria during infection differs significantly from that of bacteria growing in vitro. Further, the host cell cytosol may resemble an anaerobic environment, with tissue-specific variations in redox stress and oxygen concentration.
KEYWORDS: intracellular bacteria, pathogenesis, redox signaling, virulence regulation
INTRODUCTION
Bacteria adapt and respond to a wide variety of stressors. One of these is oxidative stress (also referred to here as redox stress), which is an imbalance in electrons that can damage DNA, iron-sulfur clusters, lipids, and proteins (1). Redox stress is both produced by the bacteria (endogenous) and encountered in the environment (exogenous) (2). Endogenous redox stressors, such as reactive oxygen species (ROS) generated from the incomplete reduction of oxygen, are constitutively produced during aerobic respiration (3). Therefore, bacteria have evolved diverse detoxification mechanisms to survive in oxygen-rich environments, including production of antioxidants and enzymes that consume damaging ROS. The same detoxification strategies that bacteria use to survive endogenous oxidative stress have been adopted by bacterial pathogens to thrive under the exogenous stress conditions encountered during infection (as reviewed elsewhere [1, 2, 4, 5]). Exogenous sources of redox stress are abundant within a mammalian host, most notably, during the respiratory burst generated by phagocytes and aimed at defending against invading pathogens (2). During this assault, ROS, such as superoxide anions, hydroxyl radicals, and hydrogen peroxide, as well as reactive nitrogen species (RNS), such as nitric oxide and peroxynitrite, are produced (6). To survive the hostile host environment and cause disease, bacterial pathogens have developed mechanisms to detect and adapt to the myriad redox stressors.
The Gram-positive facultative intracellular pathogen Listeria monocytogenes is an excellent model for studying redox regulation during pathogenesis, as it is able to adapt to a wide variety of conditions and has a well-characterized infectious life cycle (7). The intracellular life cycle of L. monocytogenes begins when the bacterium is phagocytosed by a host cell, where it transiently resides within the oxidizing environment of the phagosome (8). L. monocytogenes then secretes the pore-forming toxin listeriolysin O (LLO; encoded by hly) to escape from the phagosome and enter into the cytosol (9). The host cytosol is a highly reducing environment containing millimolar concentrations of the low-molecular-weight thiol antioxidant glutathione (10). In this reducing environment, L. monocytogenes replicates and expresses ActA, which mediates host actin polymerization and allows the bacterium to move within the cell as well as spread to neighboring cells without entering the extracellular space (11). Within 30 min, the bacteria transit from the oxidizing phagosome to the reducing cytosol (8), making L. monocytogenes an ideal model for studying adaptive responses to redox changes during pathogenesis.
To sense and adapt to redox stress, the genomes of many Firmicutes, including the genome of L. monocytogenes, encode one or more copies of an arsenate reductase (ArsC)-family protein, named Spx in the model organism Bacillus subtilis (12). Spx is a global regulator that activates and represses transcription in response to oxidative stress via direct interaction with the α subunit of RNA polymerase (RNAP) (12–15). Oxidative stress is sensed through the conserved cysteine-X-X-cysteine (CXXC) motif, which is reduced under normal growth conditions. Upon encountering oxidative stress, the cysteine residues of the Spx CXXC motif form an intramolecular disulfide bond that stabilizes the Spx-RNAP-DNA interaction and allows the Spx-mediated activation of transcription (16). In B. subtilis, over 100 genes are activated in an Spx-dependent manner, including those important for thiol homeostasis, such as thioredoxin, thioredoxin reductase, and bacillithiol biosynthesis (15, 17, 18). In addition to maintaining redox homeostasis, Spx homologues in related organisms regulate organosulfur metabolism (19), cell wall homeostasis (20, 21), competence (22), and biofilm formation (23). Spx also has anti-alpha factor activity, as it represses over 170 genes, including the biosynthetic machinery for amino acids, vitamins, and nucleic acids (15). Spx-family proteins have been demonstrated to be important for the virulence of Enterococcus faecalis (24), Streptococcus mutans (25, 26), and Streptococcus sanguinis (27).
The L. monocytogenes genome contains two spx orthologues: spxA1 (lmo2191) and spxA2 (lmo2426). SpxA1 shares 83% amino acid sequence identity with B. subtilis Spx and 25% amino acid sequence identity with SpxA2. spxA1 is reported to be essential (28), but we recently identified a transposon insertion in a promoter of spxA1 that results in a 10-fold reduction in spxA1 expression (29). The spxA1 knockdown strain is more sensitive to oxidative stress, impaired for growth, and significantly attenuated in a murine model of infection, suggesting an important role for spxA1 and redox homeostasis in virulence (29). Here, we further examined the role of spxA1 and spxA2 in L. monocytogenes pathogenesis. Specifically, we demonstrated that spxA1 is essential for aerobic growth and report that spxA1 can be deleted only under anaerobic conditions. Surprisingly, the ΔspxA1 mutant was capable of replicating in the host cytosol and colonizing the spleens of infected mice, although it was significantly attenuated compared to the wild-type strain. In contrast, spxA2 was not required for virulence, demonstrating that spxA1 is the dominant Spx-family member required for the oxidative stress response during L. monocytogenes infection.
RESULTS
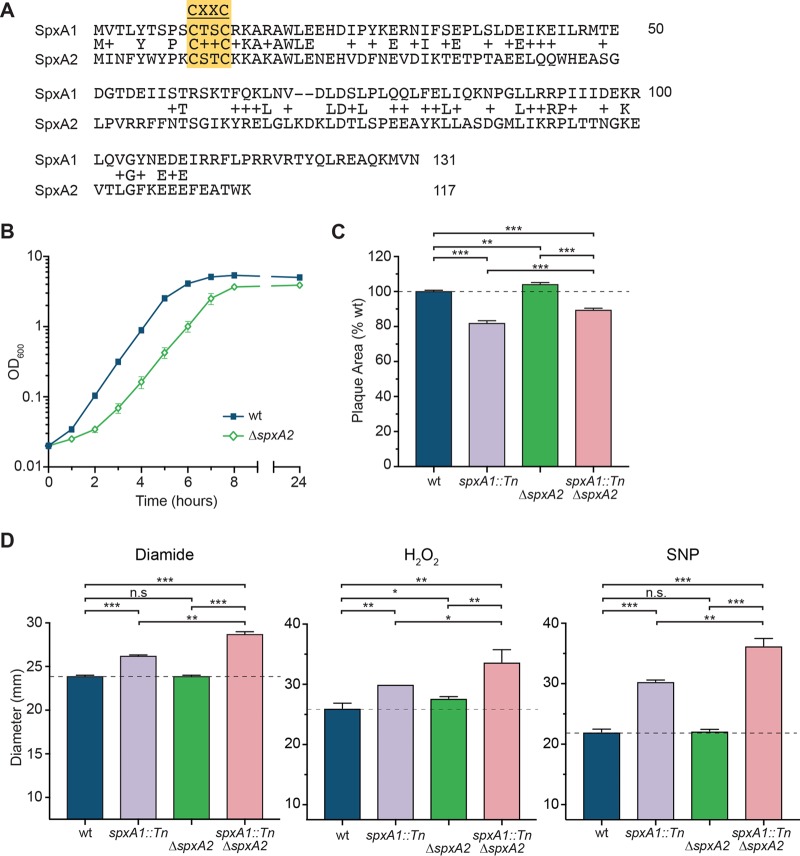
SpxA2 is not required for the oxidative stress response or intracellular growth.
Previous work suggested a connection between the L. monocytogenes redox response and virulence (29). These observations prompted us to investigate the role of the Spx family of global redox-responsive transcription regulators in L. monocytogenes. Like the genomes of many other Firmicutes, the genome of L. monocytogenes contains two Spx-family paralogues, spxA1 and spxA2, which share 56% similarity, 25% amino acid sequence identity, and the canonical CXXC motif that is required to sense oxidative stress (Fig. 1A) (16). spxA1 was reported to be an essential gene (28), and accordingly, we were unable to delete spxA1 using conventional methods. In contrast, spxA2 could be deleted by standard allelic exchange. The ΔspxA2 strain exhibited a slight growth defect in broth, with a doubling time of 44.2 ± 1.75 min (standard error of the mean [SEM]), whereas the doubling time for the wild type (wt) was 39.9 ± 0.32 min (Fig. 1B).
FIG 1.
SpxA2 is not required for the disulfide stress response or cell-to-cell spread. (A) Alignment of L. monocytogenes SpxA1 and SpxA2. Yellow box, the CXXC motif. Identical residues are indicated, and amino acids of similar charge are marked with a plus sign. (B) Growth obtained in flasks with shaking at 37°C. Data are the means and SEMs from three independent experiments. (C) Plaque area measured as a percentage of that of the wt strain. Data are the means and SEMs from three independent experiments. (D) Sensitivity to disulfide stress was measured by growth inhibition in a disk diffusion assay using diamide (1 M solution), hydrogen peroxide (H2O2; 5% solution), or sodium nitroprusside (SNP; 2 M solution). The diameters of the zone of clearance, including the disks (diameter, 7.5 mm; thus, the minimum value is provided), were measured after 24 h of growth in tryptic soy agar. Data are the means and SEMs from three independent experiments. In all panels, P values were calculated using a heteroscedastic Student's t test. n.s., not significant (P > 0.05); *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To investigate the role of spxA2 in intracellular growth and cell-to-cell spread, we utilized a plaque assay, an in vitro model of infection that is correlated with L. monocytogenes virulence in vivo. In this assay, a monolayer of L2 murine fibroblasts is infected and immobilized in agarose containing gentamicin to kill the extracellular bacteria. At 3 days postinfection, live cells are imaged and the area of each plaque formed by L. monocytogenes is a measure of cell-to-cell spread. The ΔspxA2 mutant formed a plaque similar in size to that formed by the wt (Fig. 1C), indicating that the ΔspxA2 mutant is not deficient for intracellular growth or cell-to-cell spread. In some Gram-positive bacteria containing two spx paralogues, the Spx proteins function together to modulate the transcriptional response to oxidative stress (30). To test if SpxA1 and SpxA2 cooperate in L. monocytogenes, a double mutant in which spxA2 was deleted and spxA1 expression was significantly reduced via a transposon in the spxA1 promoter was constructed (the spxA1::Tn ΔspxA2 mutant) (29). The spxA1 knockdown strain forms a plaque significantly smaller than that formed by the wt. However, the deletion of spxA2 in this background did not reduce the ability of L. monocytogenes to grow intracellularly or spread from cell to cell (Fig. 1C). Together, these data suggest that spxA2 is not required for pathogenesis.
In other Firmicutes, Spx-family proteins are required to survive the oxidative stress imposed by the thiol-oxidizing compound diamide (15, 23, 26). Therefore, we used a disk diffusion assay to analyze the role of spxA2 in L. monocytogenes resistance to thiol stress. Whereas spxA1 depletion was associated with increased sensitivity to diamide (29), the ΔspxA2 mutant and the wt were similarly resistant (Fig. 1D), indicating that spxA2 is not required for the L. monocytogenes response to disulfide stress. In addition to disulfide stress, spxA1 was required for the response to peroxide and nitrosative stress, while spxA2 had only a minor effect on the resistance to peroxide (Fig. 1D). Interestingly, the double mutant was significantly more sensitive than the single mutants to all three oxidative stressors, demonstrating that the function of SpxA2 may be revealed only in the absence of SpxA1. These data suggest that SpxA1 and SpxA2 may function cooperatively in response to a variety of redox stressors.
SpxA1 function is conserved between L. monocytogenes and B. subtilis.
SpxA1 homologues in related organisms are often not essential, and we postulated that (i) SpxA1 in L. monocytogenes has evolved additional protein functions, (ii) SpxA1 regulates a distinct set of genes, or (iii) L. monocytogenes is uniquely sensitive to redox stress. To overcome the challenges of investigating an essential gene, a strain that was merodiploid for spxA1 was constructed (Fig. 2A). This bacterium harbored a second copy of spxA1, expressed from its predicted promoters at a neutral locus in the chromosome (tRNAArg) via a tetracycline-resistant version of the integration vector pPL2 (31, 32). The endogenous spxA1 gene was then deleted using standard allelic exchange techniques. Subsequently, various alleles of spxA1 could replace the remaining wild-type copy of spxA1 via generalized transduction using bacteriophage and a chloramphenicol-resistant pPL2 vector (Fig. 2A). This approach enabled interrogation of multiple SpxA1 alleles; however, attempts to generate an spxA1-deficient strain by transducing an empty chloramphenicol-resistant pPL2 vector into the ΔspxA1 pPL2t.spxA1 strain under conventional growth conditions were repeatedly unsuccessful.
FIG 2.
SpxA1 function is conserved with B. subtilis Spx. (A) Schematic of the spxA1 merodiploid strain. spxA1 is predicted to have two transcription start sites (32), pictured as thin black arrows with the labels P2 and P1. pPL2t.spxA1 is integrated at the tRNAArg site and can be replaced with a chloramphenicol-resistant pPL2 vector expressing other alleles of spxA1 via generalized transduction (31). (B) Alignment of L. monocytogenes SpxA1 (Lm) and B. subtilis Spx (Bs). Asterisks, residues that differ; yellow box, the CXXC motif; boldface, other functional Spx residues (G52, R60, R91, and R92) (13, 33, 34). (C) Growth obtained in flasks with shaking at 37°C. Data are the means and SEMs from three independent experiments. (D) Sensitivity to disulfide stress was measured by growth inhibition in a disk diffusion assay using a 1 M diamide solution, as described in the legend to Fig. 1D. Data are the means and SEMs from three independent experiments. P values were calculated using a heteroscedastic Student's t test. **, P < 0.01. (E and F) Female CD-1 mice were infected with 105 CFU intravenously, and organs were harvested at 48 h postinfection. Each symbol represents an individual mouse, and the horizontal lines indicate the median. Data are for 5 mice per strain. P values were calculated using a heteroscedastic Student's t test.
The Spx family of proteins is defined by an N-terminal CXXC redox switch, which is required for Spx-mediated transcriptional activation in B. subtilis (16). In addition, several residues of B. subtilis Spx are required for its function and interaction with RNAP, including G52, R60, R90, and R91 (13, 33, 34). All of these residues are conserved between B. subtilis Spx and L. monocytogenes SpxA1, which share 95% similarity and 83% amino acid sequence identity overall (Fig. 2B). This high degree of similarity suggested that the molecular function(s) of Spx may be conserved, so we next tested if spx from B. subtilis (spxBs) was sufficient to complement ΔspxA1. Using the approach described above (Fig. 2A), the ΔspxA1 strain was complemented with B. subtilis spx under the control of the L. monocytogenes spxA1 promoters (ΔspxA1 + spxBs), spxA1 in which the cysteine residues of the CXXC motif were mutated to alanine (AXXA; spxA1AXXA), or the wt spxA1 allele. We obtained equivalent numbers of transductants during construction of these strains, suggesting that spxBs and spxA1AXXA were sufficient to complement the essential functions of spxA1 and were unlikely to harbor suppressor mutations (data not shown). The strain expressing spxBs grew like the wt in rich brain heart infusion (BHI) broth (Fig. 2C). However, the strain expressing the spxA1AXXA allele grew very poorly, with a doubling time of 119.6 ± 4.8 min (Fig. 2C). Further, in the strains expressing spxA1 and spxBs but not the strain expressing spxA1AXXA, resistance to disulfide stress was restored and the strains exhibited a level of resistance to diamide similar to that of the wt (Fig. 2D). Together, these data demonstrate the importance of the SpxA1 CXXC motif for L. monocytogenes growth and the disulfide stress response.
We next assessed the complementation of ΔspxA1 by L. monocytogenes spxA1 or spxBs and the role of spxA2 during infection. Both of the ΔspxA1 strains complemented with spxA1 or spxBs were capable of colonizing the spleens and livers of infected mice similarly to the wt (Fig. 2E and F). Consistent with the plaque data, the ΔspxA2 mutant was also fully virulent in vivo (Fig. 2E and F), demonstrating that spxA2 is not required for pathogenesis. Together, these data underscore the functional conservation of Spx-family proteins across the Firmicutes and demonstrate that spxA1 may be essential due to the divergence of the SpxA1 regulon or the physiology of L. monocytogenes, rather than a novel function of the SpxA1 protein.
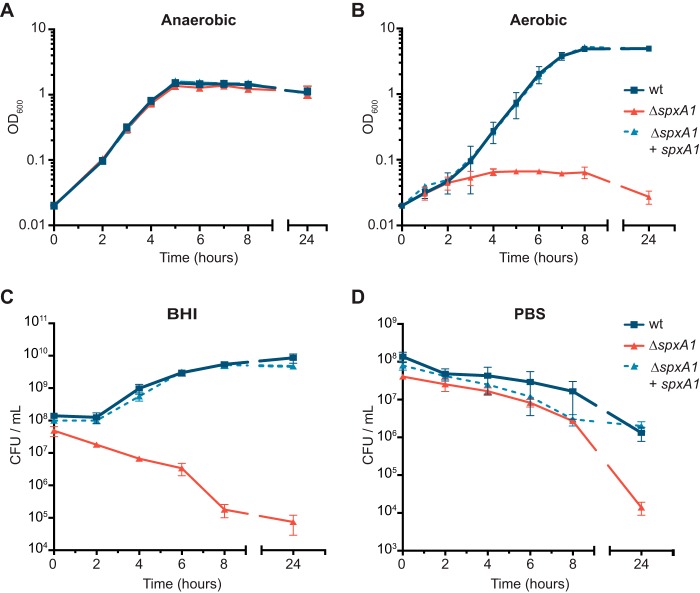
SpxA1 is essential for aerobic growth.
SpxA1 appeared to be critical to the redox stress response of L. monocytogenes (Fig. 1D) (29). We reasoned that this hypersensitivity to oxidative stress might render spxA1-deficient strains unable to cope with the endogenous redox stress generated during aerobic respiration. In support of this hypothesis, E. faecalis Δspx mutants were obtained only during anaerobic growth (24). Using the approach described above (Fig. 2A), we generated spxA1-null mutants by transducing an empty pPL2 vector and growing the bacteria anaerobically. Transductants incubated anaerobically at 37°C appeared within the same time frame as the wt, and the colonies were similar in size (data not shown). When these ΔspxA1 mutants were inoculated anaerobically into deoxygenated BHI broth, they grew similarly to the wt (Fig. 3A). However, after dilution into aerobic broth, the ΔspxA1 mutant was unable to grow aerobically in a shaking flask (Fig. 3B). To investigate the kinetics of oxygen toxicity to the ΔspxA1 mutant, anaerobic stationary-phase cultures were diluted into BHI or phosphate-buffered saline (PBS) and incubated at 37°C with shaking. Samples were removed, and serial dilutions were plated and incubated anaerobically to enumerate the surviving bacteria. In rich medium, the wt and the complemented strain grew with normal logarithmic kinetics, while the growth of the ΔspxA1 mutant decreased ∼10-fold in 6 h and approximately 1,000-fold after 24 h (Fig. 3C). All three strains survived similarly in PBS for 8 h, while the growth of the ΔspxA1 mutant compared to that of the wt was reduced ∼2 log units 24 h after inoculation (Fig. 3D). These data demonstrate that spxA1 is essential for the aerobic growth of L. monocytogenes but that oxygen is not rapidly toxic in the absence of spxA1.
FIG 3.
spxA1 is essential for aerobic growth. (A) Anaerobic growth under static conditions at 37°C was measured by determination of the OD600. The definitions of the symbols identified in the key are the same in all panels. (B) Curve of aerobic growth obtained in flasks with shaking at 37°C. (C and D) Oxygen toxicity was measured by diluting anaerobic stationary-phase cultures into BHI or PBS and incubating aerobically at 37°C with shaking. The number of CFU per milliliter was measured over time by plating serial dilutions anaerobically on BHI. The data in all panels represent the means and SEMs from three independent experiments.
The ΔspxA1 mutant grows intracellularly and colonizes the spleens of infected mice.
The role of SpxA1 in virulence could now be directly addressed by constructing and culturing ΔspxA1 mutants anaerobically. Bone marrow-derived macrophages (BMMs) were infected with anaerobic cultures of the wt or the ΔspxA1 mutant, and bacteria harvested at each time point were plated anaerobically to enumerate the CFU. Remarkably, the ΔspxA1 strain was able to replicate in the host cytosol and grew ∼20-fold during the 8 h of infection (Fig. 4A). In a plaque assay of cell-to-cell spread, the ΔspxA1 mutant formed plaques 25% of the size of wt plaques (Fig. 4B). We previously observed that an spxA1 knockdown strain was deficient for vacuolar escape and that its plaque defect was restored by overexpression of hly, encoding the pore-forming toxin LLO (29). To test the hypothesis that the ΔspxA1 mutant may also be impaired in vacuolar escape and therefore unable to form a plaque, LLO abundance was analyzed by immunoblotting after anaerobic growth at 30°C, the conditions under which the bacteria were incubated prior to infection. However, under these growth conditions, the ΔspxA1 mutant did not exhibit a defect in LLO secretion (see Fig. S1A in the supplemental material). Further, the overexpression of hly in the ΔspxA1 mutant did not alter the overall kinetics of intracellular growth in BMMs (Fig. S1B).
FIG 4.
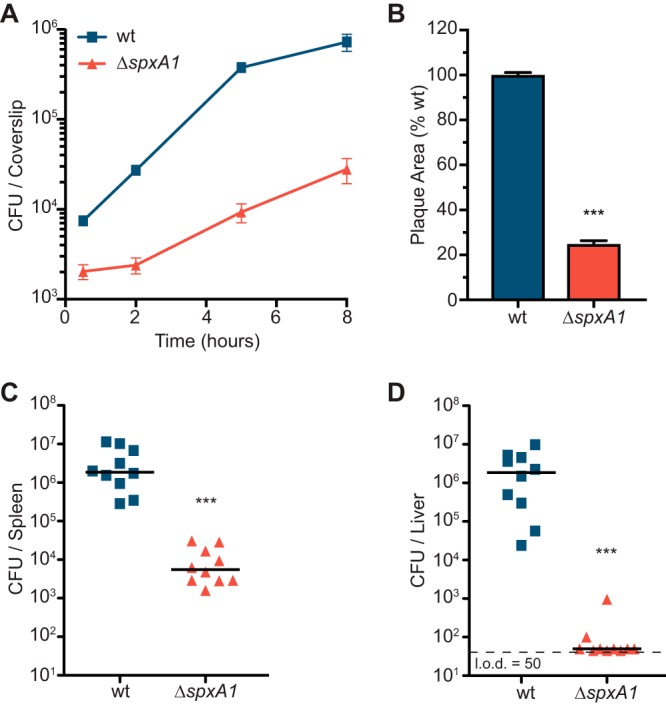
ΔspxA1 mutants grow in macrophages and colonize mice. (A) Curves of intracellular growth of L. monocytogenes strains in BMMs. Data are the means and SEMs from three independent experiments. (B) Plaque area measured as a percentage of that for the wt strain. Data are the means and SEMs from three independent experiments. (C and D) Female CD-1 mice were infected with 105 CFU intravenously, and organs were harvested at 48 h postinfection. Each symbol represents an individual mouse, and the horizontal lines are the medians. Data are for 10 mice per strain. P values were calculated using a heteroscedastic Student's t test. ***, P < 0.001. The limit of detection (l.o.d.) is indicated with a dashed line.
To examine the role of spxA1 during infection of a mammalian host, bacteria were cultured anaerobically before 105 CFU of the bacteria was used to infect female CD-1 mice intravenously via the tail vein. At 48 h postinfection, the spleens and livers were harvested and homogenized and the bacteria were incubated on BHI agar anaerobically. The ΔspxA1 strain was able to colonize the spleens of infected mice, although it was attenuated over 500-fold compared to the wt (Fig. 4C). In contrast, only 2 of the 10 mice had recoverable numbers of CFU of the ΔspxA1 mutant in their livers (Fig. 4D). Overexpression of hly during infection did not significantly increase the virulence of the ΔspxA1 mutant (Fig. S1C and D), demonstrating that the attenuated virulence of this mutant is not simply due to an inability to escape the vacuole.
DISCUSSION
In this study, we investigated the adaptation of L. monocytogenes to diverse redox environments, including those found during infection. The L. monocytogenes genome contains two Spx-family transcriptional regulators, spxA1 and spxA2, that are predicted to modulate gene expression in response to redox stress. Only spxA1 and not spxA2 was required for the oxidative stress response, aerobic growth, and virulence. However, SpxA1 and SpxA2 may have overlapping functions during oxidative stress in vitro, as the double mutant (spxA1::Tn ΔspxA2) was more sensitive to a variety of stressors than either single mutant.
The role of the ΔspxA1 mutant during infection was directly interrogated by constructing and maintaining the mutant anaerobically. The ΔspxA1 mutant failed to replicate in nutrient broth in the presence of oxygen, but it was able to access the host cytosol and replicate intracellularly in macrophages, suggesting that the redox environment of the host cytosol may be less stressful than the growth environment in aerobic broth. These data were also consistent with the findings obtained of a mouse model of infection, in which the ΔspxA1 mutant colonized the spleens of infected mice. However, the ΔspxA1 mutant replicated at a reduced rate compared to the wt both in cell culture and in vivo, and the livers of infected mice were nearly sterilized of the ΔspxA1 mutant, a defect that may be a result of organ-specific differences in the oxygen concentration or redox stress. Our previous study found that an spxA1 knockdown strain is impaired in cell-to-cell spread, a phenotype that is rescued by the constitutive expression of hly, which encodes LLO and is absolutely required for phagosomal escape (29). These data suggest a role for spxA1 in escape from the primary vacuole. Here, we found that a strain lacking spxA1 produced an amount of LLO equivalent to that produced by the wt in vitro, and increased expression of hly did not improve the growth of the ΔspxA1 mutant in macrophages or virulence in mice. Together, these data indicate that vacuolar escape is not the rate-limiting step for the intracellular growth of the ΔspxA1 mutant in vivo.
Experiments demonstrating that B. subtilis Spx can functionally replace L. monocytogenes SpxA1 during infection suggest that the physical mechanism by which these Spx-family proteins interact with RNAP to regulate transcription is conserved. However, the regulons of SpxA1 and B. subtilis Spx are likely distinct because spxA1 is essential for L. monocytogenes aerobic growth, while the B. subtilis Δspx mutant does not display a growth defect (35). Spx-dependent transcriptional activation in B. subtilis requires the formation of an intramolecular disulfide bond at the N-terminal CXXC motif (16). Consequently, genes required for the response to disulfide stress (e.g., the thioredoxin gene) are activated only upon oxidation of Spx. However, Spx-dependent transcriptional repression does not require Spx oxidation (36). The function of L. monocytogenes redox sensing via the SpxA1 CXXC motif is not yet known. Our results demonstrated that the CXXC motif is not absolutely required for aerobic growth, although mutants expressing the AXXA allele were severely impaired for growth and diamide sensitivity. These data suggest that SpxA1-mediated transcriptional repression is required for aerobic growth, while SpxA1-dependent transcriptional activation is required to respond to oxidative stress. Ongoing experiments aim to clarify these findings to determine the role of SpxA1 redox sensing in L. monocytogenes pathogenesis.
The first attempt to generate an L. monocytogenes spxA1 mutant reported that the spxA1 gene is essential (28), and we advanced this observation by demonstrating that spxA1 can be deleted anaerobically, similarly to spx in E. faecalis (24). The Staphylococcus aureus spx orthologue was also recently reported to be essential, but suppressor mutations that allow the aerobic culture of Δspx mutants under normal laboratory conditions arise (37). The S. aureus suppressor mutations are located in rpoB and are postulated to alter the way in which the RNAP holoenzyme interacts with the promoter sequences of essential genes. We were unable to isolate suppressor mutants of the L. monocytogenes ΔspxA1 strain and do not yet know if similar rpoB mutations would alleviate spxA1 essentiality.
Several other Firmicutes are similar to L. monocytogenes, in that their genomes encode multiple Spx-family proteins that function either cooperatively or independently. For example, in Streptococcus pneumoniae, spxA1 and spxA2 can each be deleted independently, but the simultaneous deletion of both is lethal, suggesting that they may have overlapping regulons (22). In Bacillus anthracis, transcriptomics revealed that spxA1 and spxA2 are expressed at distinct phases of growth and largely regulate the same genes (38, 39). L. monocytogenes SpxA1 is clearly the dominant Spx-family protein required for virulence and aerobic growth. However, deletion of spxA2 in the spxA1 knockdown strain significantly increased the sensitivity of L. monocytogenes to disulfide, peroxide, and nitrosative stressors in vitro. These data suggest that SpxA1 and SpxA2 may cooperate in coordinating the response to oxidative stress and may regulate common genes. More research is required to determine the function of spxA2 in L. monocytogenes physiology.
Spx homologues positively and negatively regulate over 200 genes (15, 18). In L. monocytogenes, SpxA1-dependent transcriptional changes enable aerobic growth and virulence, though it is unclear if these activities require regulation of the same genes. Ongoing studies to characterize the SpxA1 regulon aim to identify the genes that are required for growth and define why spxA1 is not essential during infection. These analyses will allow us to differentiate whether changes in bacterial metabolism, the relative abundance of oxygen, or the extracellular abundance of reducing agents allow ΔspxA1 mutants to replicate in vivo.
MATERIALS AND METHODS
Ethics statement.
This study was carried out in strict accordance with the recommendations in the Public Health Service Policy on Humane Care and Use of Laboratory Animals (40). All protocols were reviewed and approved by the Animal Care and Use Committee at the University of California, Berkeley (AUP-2016-05-8811).
Bacterial strains and culture conditions.
L. monocytogenes mutants were derived from wild-type strain 10403S and cultured in brain heart infusion (BHI) at 37°C with shaking, unless otherwise stated. All chemicals were purchased from Sigma-Aldrich unless otherwise stated. Antibiotics were used at the following concentrations: streptomycin, 200 μg ml−1; chloramphenicol, 10 μg ml−1 (Escherichia coli) and 7.5 μg ml−1 (L. monocytogenes); tetracycline, 2 μg ml−1; carbenicillin, 100 μg ml−1; and erythromycin, 1 μg ml−1. The L. monocytogenes strains used in this study are listed in Table 1, and the E. coli strains and plasmids used in this study are listed in Table 2. Plasmids were introduced into E. coli via chemical competence and heat shock and introduced into L. monocytogenes via trans-conjugation from E. coli SM10 (41).
TABLE 1.
L. monocytogenes strains used in this study
| Strain | Description | Reference or source |
|---|---|---|
| 10403S | wt | 54 |
| MLR-L470 | ΔspxA2 | This study |
| MLR-L232 | ΔspxA1 pPL2t.spxA1 | This study |
| MLR-L472 | ΔspxA1 pPL2.spxA1 | This study |
| MLR-L637 | ΔspxA1 pPL2a | This study |
| MLR-L473 | ΔspxA1 pPL2.spxBs | This study |
| MLR-L613 | ΔspxA1 pPL2.spxA1AXXA | This study |
| MLR-L609 | wt pH-hly | 49 |
| MLR-L614 | ΔspxA1 pH-hly | This study |
Referred to here as the ΔspxA1 mutant.
TABLE 2.
Plasmids and E. coli strains used in this study
| Strain | Description | Reference or source |
|---|---|---|
| XL1 | For vector construction | Stratagene |
| SM10 | For trans-conjugation | 41 |
| MLR-E011 | pKSV7-oriT | 42 |
| MLR-E006 | pPL2 | 31 |
| MLR-E234 | pPL2t | 43 |
| MLR-E480 | SM10/pKSV7ΔspxA2 | This study |
| MLR-E184 | SM10/pKSV7ΔspxA1 | This study |
| MLR-E228 | SM10/pPL2t.spxA1 | This study |
| MLR-E481 | SM10/pPL2.spxA1 | This study |
| MLR-E482 | SM10/pPL2.spxBs | This study |
| MLR-E607 | SM10/pPL2.spxA1AXXA | This study |
| MLR-E530 | SM10/pH-hly | 49 |
Vector construction and cloning.
The oligonucleotides used in this study are listed in Table S1 in the supplemental material. To delete spxA2, pKSV7ΔspxA2 was constructed by amplifying a 5′ homologous region with primers ΔspxA2-H1-f/r and a 3′ homologous region with primers ΔspxA2-H2-f/r. Synthesis by overlapping extension (SOE) PCR was used to join the fragments together. This cassette was restriction digested and ligated into pKSV7-oriT (42). An analogous protocol was performed to delete spxA1, using primers ΔspxA1-H1-f/r and ΔspxA1-H2-f/r.
The spxA1 complementation vector pPL2.spxA1 was constructed by amplifying spxA1 and its endogenous promoters with primers spxA1-f/r. This fragment was digested and ligated into pPL2 (chloramphenicol resistant) or pPL2t (tetracycline resistant) (31, 43). The B. subtilis spx complementation vector pPL2.spxBs was constructed by amplifying spx from B. subtilis (strain 168) with primers Bs-spx-f/r and the L. monocytogenes spxA1 promoter region with primers P-spxA1-f/r. SOE PCR joined the two products together, and this construct was digested and ligated into pPL2 (31). pPL2.spxA1 C10AC13A (spxA1AXXA) was constructed by site-directed mutagenesis (inverse PCR) of pPL2.spxA1 with primers CXXC-f/r. The PCR product was treated with DpnI and transformed into E. coli. The sequences of all plasmids were confirmed by Sanger DNA sequencing. The nomenclature used for genetic loci is that for the EGD-e strain of L. monocytogenes by convention. However, all genetic material used here was derived from 10403S strains of L. monocytogenes, and the homologous loci are listed as follows: spxA1, lmo2191, LMRG_01641 and spxA2, lmo2426, LMRG_01822.
L. monocytogenes strain construction.
In-frame deletions were carried out by allelic exchange using a conjugation-proficient version of the suicide vector pKSV7 (42). Vectors bearing the mutant ΔspxA1 and ΔspxA2 alleles were introduced into L. monocytogenes via trans-conjugation and integrated into the chromosome, and colonies were purified on selective nutrient agar and subsequently cured of the plasmid by conventional methods (29). Chromosomal mutations were confirmed by PCR and Sanger DNA sequencing when necessary. The knock-in of genes into L. monocytogenes was carried out using the pPL2 and pPL2t integration plasmids (31, 43). Integration was confirmed by antibiotic resistance.
Disk diffusion assays.
The zone of inhibition was measured as previously described (29, 44, 45). Briefly, L. monocytogenes was grown overnight in tryptic soy broth (TSB) at 37°C with shaking. The stationary-phase cultures (∼6 × 107 CFU) were added to molten top agar (0.8% NaCl, 0.8% Bacto agar) and spread evenly over tryptic soy agar (TSA) plates. After the agar solidified, Whatman paper disks soaked in 10 μl of diamide (a 1 M solution), hydrogen peroxide (a 5% [vol/vol] solution), or sodium nitroprusside (a 2 M solution) were placed on the immobilized lawn of bacteria, and the plates were incubated at 37°C for 24 h. The diameter of the zone of inhibition was then measured with a ruler.
Plaque assays.
Plaque assays were carried out by conventional methods (46, 55). In brief, 6-well tissue culture-treated dishes were seeded with 1.2 × 106 L2 murine fibroblasts per well. The L. monocytogenes strains were incubated overnight at 30°C in BHI in a stationary culture. Overnight cultures were diluted 1:10 in sterile PBS, and 5 μl was used to infect each well of L2 cells. At 1 h postinfection, the cells were washed twice with PBS, followed by addition of 3 ml of molten agarose-Dulbecco modified Eagle medium (DMEM) solution. This solution consisted of gentamicin at 10 μg ml−1 and a 1:1 mixture of 2× DMEM (Gibco) and 1.4% SuperPure agarose LE (U.S. Biotech Sources, LLC). At 3 days postinfection, 2 ml of molten agarose-DMEM solution containing neutral red (Sigma) was added to each well to visualize the plaques. After 24 h, the plaques were scanned and the area was measured using ImageJ software (47).
Growth curves.
For anaerobic growth, L. monocytogenes colonies were inoculated into broth and incubated in closed containers containing anaerobic gas-generating pouches (GasPak EZ; BD). For curves of the growth in broth, the optical density at 600 nm (OD600) of the overnight cultures was measured, and the cultures were normalized to an optical density of 0.02 in either 25 ml BHI in 250-ml flasks (for aerobic growth) incubated with shaking (220 rpm) at 37°C or 10 ml BHI in culture tubes (for anaerobic growth) placed in an anaerobic chamber and incubated statically at 37°C. The OD600 was measured every hour.
Intracellular growth curves were performed as previously described (48, 49). Briefly, bone marrow-derived macrophages (BMMs) were harvested as previously reported (50) and seeded at a concentration of 3 × 106 cells in 5 ml of medium in a 60-mm dish containing sterilized tissue culture-treated coverslips. Bacteria were incubated in a stationary manner at 30°C, washed, and used to infect BMMs at a multiplicity of infection (MOI) of one bacterium for every 10 cells. At 30 min postinfection, the cells were washed and medium containing gentamicin (50 μg ml−1) was added. At each time point, three coverslips were removed, BMMs were lysed in water, and dilutions were plated on BHI agar to enumerate the CFU.
Virulence assays.
Infections were performed as previously described (51, 52), with the following modifications. Aerobic strains were incubated at 37°C with shaking (220 rpm), while anaerobic strains were incubated at 37°C in closed containers containing anaerobic gas-generating pouches. All strains were diluted in PBS to a concentration of 5 × 105 CFU ml−1, and 200 μl was injected into the tail vein of 6- to 8-week-old female CD-1 mice (Charles River Laboratories). The inocula were plated after infection and incubated anaerobically to ensure consistent doses across strains. The ΔspxA1 mutant inoculum was within ±3-fold of the wt inoculum. At 48 h postinfection, the mice were euthanized and the livers and spleens were harvested. Organs were homogenized in 0.1% (vol/vol) NP-40 in water, and serial dilutions were made in PBS and plated on BHI agar to enumerate the CFU. Plates with tissue samples from ΔspxA1 mutant-infected mice were incubated anaerobically.
Generalized transduction.
The U153 phage was utilized for generalized transduction as previously described (53). Briefly, transducing lysates from donor strains were constructed by mixing donors with phage at an MOI of approximately 1, incubated overnight at 30°C in LB soft agar, filter sterilized, and mixed with recipient L. monocytogenes at an MOI of 0.1 for 30 min, and transductants were selected on antibiotic-containing BHI agar at 37°C.
Supplementary Material
ACKNOWLEDGMENTS
We thank Daniel A. Portnoy (UC Berkeley) for his advice and generosity. We are also grateful to Dan Wolter and Lucas Hoffman (University of Washington) for use of their anaerobic chamber, which is funded by P30 DK08950.
This project was initiated in the lab of Daniel A. Portnoy, which is supported by National Institutes of Health grants 1R01 AI27655 and 1P01 AI063302.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00978-16.
REFERENCES
- 1.Imlay JA. 2003. Pathways of oxidative damage. Annu Rev Microbiol 57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 2.Hassett DJ, Cohen MS. 1989. Bacterial adaptation to oxidative stress: implications for pathogenesis and interaction with phagocytic cells. FASEB J 3:2574–2582. [DOI] [PubMed] [Google Scholar]
- 3.Cabiscol E, Tamarit J, Ros J. 2000. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol 3:3–8. [PubMed] [Google Scholar]
- 4.Hillion M, Antelmann H. 2015. Thiol-based redox switches in prokaryotes. Biol Chem 396:415–444. doi: 10.1515/hsz-2015-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson AR, Somerville GA, Sonenshein AL. 2015. Regulating the intersection of metabolism and pathogenesis in Gram-positive bacteria. Microbiol Spectr 3(3):MBP-0004-2014. doi: 10.1128/microbiolspec.MBP-0004-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flannagan RS, Jaumouillé V, Grinstein S. 2012. The cell biology of phagocytosis. Annu Rev Pathol 7:61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- 7.Tilney LG, Portnoy DA. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol 109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers JT, Tsang AW, Swanson JA. 2003. Localized reactive oxygen and nitrogen intermediates inhibit escape of Listeria monocytogenes from vacuoles in activated macrophages. J Immunol 171:5447–5453. doi: 10.4049/jimmunol.171.10.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnupf P, Portnoy DA. 2007. Listeriolysin O: a phagosome-specific lysin. Microbes Infect 9:1176–1187. doi: 10.1016/j.micinf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Meister A, Anderson ME. 1983. Glutathione. Annu Rev Biochem 52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 11.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68:521–531. [DOI] [PubMed] [Google Scholar]
- 12.Zuber P. 2004. Spx-RNA polymerase interaction and global transcriptional control during oxidative stress. J Bacteriol 186:1911–1918. doi: 10.1128/JB.186.7.1911-1918.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano S, Nakano MM, Zhang Y, Leelakriangsak M, Zuber P. 2003. A regulatory protein that interferes with activator-stimulated transcription in bacteria. Proc Natl Acad Sci U S A 100:4233–4238. doi: 10.1073/pnas.0637648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newberry KJ, Nakano S, Zuber P, Brennan RG. 2005. Crystal structure of the Bacillus subtilis anti-alpha, global transcriptional regulator, Spx, in complex with the alpha C-terminal domain of RNA polymerase. Proc Natl Acad Sci U S A 102:15839–15844. doi: 10.1073/pnas.0506592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakano S, Küster-Schöck E, Grossman AD, Zuber P. 2003. Spx-dependent global transcriptional control is induced by thiol-specific oxidative stress in Bacillus subtilis. Proc Natl Acad Sci U S A 100:13603–13608. doi: 10.1073/pnas.2235180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano S, Erwin KN, Ralle M, Zuber P. 2005. Redox-sensitive transcriptional control by a thiol/disulphide switch in the global regulator, Spx. Mol Microbiol 55:498–510. [DOI] [PubMed] [Google Scholar]
- 17.Gaballa A, Antelmann H, Hamilton CJ, Helmann JD. 2013. Regulation of Bacillus subtilis bacillithiol biosynthesis operons by Spx. Microbiology 159:2025–2035. doi: 10.1099/mic.0.070482-0. [DOI] [PubMed] [Google Scholar]
- 18.Rochat T, Nicolas P, Delumeau O, Rabatinová A, Korelusová J, Leduc A, Bessières P, Dervyn E, Krásny L, Noirot P. 2012. Genome-wide identification of genes directly regulated by the pleiotropic transcription factor Spx in Bacillus subtilis. Nucleic Acids Res 40:9571–9583. doi: 10.1093/nar/gks755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi S-Y, Reyes D, Leelakriangsak M, Zuber P. 2006. The global regulator Spx functions in the control of organosulfur metabolism in Bacillus subtilis. J Bacteriol 188:5741–5751. doi: 10.1128/JB.00443-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veiga P, Bulbarela-Sampieri C, Furlan S, Maisons A, Chapot-Chartier M-P, Erkelenz M, Mervelet P, Noirot P, Frees D, Kuipers OP, Kok J, Gruss A, Buist G, Kulakauskas S. 2007. SpxB regulates O-acetylation-dependent resistance of Lactococcus lactis peptidoglycan to hydrolysis. J Biol Chem 282:19342–19354. doi: 10.1074/jbc.M611308200. [DOI] [PubMed] [Google Scholar]
- 21.Jousselin A, Kelley WL, Barras C, Lew DP, Renzoni A. 2013. The Staphylococcus aureus thiol/oxidative stress global regulator Spx controls trfA, a gene implicated in cell wall antibiotic resistance. Antimicrob Agents Chemother 57:3283–3292. doi: 10.1128/AAC.00220-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turlan C, Prudhomme M, Fichant G, Martin B, Gutierrez C. 2009. SpxA1, a novel transcriptional regulator involved in X-state (competence) development in Streptococcus pneumoniae. Mol Microbiol 73:492–506. doi: 10.1111/j.1365-2958.2009.06789.x. [DOI] [PubMed] [Google Scholar]
- 23.Pamp SJ, Frees D, Engelmann S, Hecker M, Ingmer H. 2006. Spx is a global effector impacting stress tolerance and biofilm formation in Staphylococcus aureus. J Bacteriol 188:4861–4870. doi: 10.1128/JB.00194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kajfasz JK, Mendoza JE, Gaca AO, Miller JH, Koselny KA, Giambiagi-Demarval M, Wellington M, Abranches J, Lemos JA. 2012. The Spx regulator modulates stress responses and virulence in Enterococcus faecalis. Infect Immun 80:2265–2275. doi: 10.1128/IAI.00026-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galvão LCC, Rosalen PL, Rivera-Ramos I, Franco GCN, Kajfasz JK, Abranches J, Bueno Silva B, Koo H, Lemos JA. 1 April 2016. Inactivation of the spxA1 or spxA2 gene of Streptococcus mutans decreases virulence in the rat caries model. Mol Oral Microbiol. doi: 10.1111/omi.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kajfasz JK, Rivera-Ramos I, Abranches J, Martinez AR, Rosalen PL, Derr AM, Quivey RG, Lemos JA. 2010. Two Spx proteins modulate stress tolerance, survival, and virulence in Streptococcus mutans. J Bacteriol 192:2546–2556. doi: 10.1128/JB.00028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Ge X, Wang X, Patel JR, Xu P. 2012. SpxA1 involved in hydrogen peroxide production, stress tolerance and endocarditis virulence in Streptococcus sanguinis. PLoS One 7:e40034. doi: 10.1371/journal.pone.0040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borezee E, Msadek T, Durant L, Berche P. 2000. Identification in Listeria monocytogenes of MecA, a homologue of the Bacillus subtilis competence regulatory protein. J Bacteriol 182:5931–5934. doi: 10.1128/JB.182.20.5931-5934.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reniere ML, Whiteley AT, Portnoy DA. 2016. An in vivo selection identifies Listeria monocytogenes genes required to sense the intracellular environment and activate virulence factor expression. PLoS Pathog 12:e1005741. doi: 10.1371/journal.ppat.1005741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kajfasz JK, Rivera-Ramos I, Scott-Anne K, Gregoire S, Abranches J, Lemos JA. 2015. Transcription of oxidative stress genes is directly activated by SpxA1 and, to a lesser extent, by SpxA2 in Streptococcus mutans. J Bacteriol 197:2160–2170. doi: 10.1128/JB.00118-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauer P, Chow MYN, Loessner MJ, Portnoy DA, Calendar R. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J Bacteriol 184:4177–4186. doi: 10.1128/JB.184.15.4177-4186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wurtzel O, Sesto N, Mellin JR, Karunker I, Edelheit S, Bécavin C, Archambaud C, Cossart P, Sorek R. 2012. Comparative transcriptomics of pathogenic and non-pathogenic Listeria species. Mol Syst Biol 8:583. doi: 10.1038/msb.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin AA, Walthers D, Zuber P. 2013. Residue substitutions near the redox center of Bacillus subtilis Spx affect RNA polymerase interaction, redox control, and Spx-DNA contact at a conserved cis-acting element. J Bacteriol 195:3967–3978. doi: 10.1128/JB.00645-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakano MM, Lin A, Zuber CS, Newberry KJ, Brennan RG, Zuber P. 2010. Promoter recognition by a complex of Spx and the C-terminal domain of the RNA polymerase alpha subunit. PLoS One 5:e8664. doi: 10.1371/journal.pone.0008664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakano MM, Hajarizadeh F, Zhu Y, Zuber P. 2001. Loss-of-function mutations in yjbD result in ClpX- and ClpP-independent competence development of Bacillus subtilis. Mol Microbiol 42:383–394. doi: 10.1046/j.1365-2958.2001.02639.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Nakano S, Choi S-Y, Zuber P. 2006. Mutational analysis of the Bacillus subtilis RNA polymerase alpha C-terminal domain supports the interference model of Spx-dependent repression. J Bacteriol 188:4300–4311. doi: 10.1128/JB.00220-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villanueva M, Jousselin A, Baek KT, Prados J, Andrey DO, Renzoni A, Ingmer H, Frees D, Kelley WL. 2016. Rifampin resistance rpoB alleles or multicopy thioredoxin/thioredoxin reductase suppresses the lethality of disruption of the global stress regulator spx in Staphylococcus aureus. J Bacteriol 198:2719–2731. doi: 10.1128/JB.00261-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergman NH, Anderson EC, Swenson EE, Niemeyer MM, Miyoshi AD, Hanna PC. 2006. Transcriptional profiling of the Bacillus anthracis life cycle in vitro and an implied model for regulation of spore formation. J Bacteriol 188:6092–6100. doi: 10.1128/JB.00723-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barendt S, Lee H, Birch C, Nakano MM, Jones M, Zuber P. 2013. Transcriptomic and phenotypic analysis of paralogous spx gene function in Bacillus anthracis Sterne. Microbiologyopen 2:695–714. doi: 10.1002/mbo3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Institutes of Health. 2002. Public Health Service policy on humane care and use of laboratory animals. Office of Laboratory Animal Welfare, National Institutes of Health, Bethesda, MD. [Google Scholar]
- 41.Simon R, Priefer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat Biotechnol 1:784–791. [Google Scholar]
- 42.Camilli A, Tilney LG, Portnoy DA. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol 8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whiteley AT, Pollock AJ, Portnoy DA. 2015. The PAMP c-di-AMP Is essential for Listeria monocytogenes growth in rich but not minimal media due to a toxic increase in (p)ppGpp. Cell Host Microbe 17:788–798. doi: 10.1016/j.chom.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Durack J, Burke TP, Portnoy DA. 2015. A prl mutation in SecY suppresses secretion and virulence defects of Listeria monocytogenes secA2 mutants. J Bacteriol 197:932–942. doi: 10.1128/JB.02284-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burke TP, Loukitcheva A, Zemansky J, Wheeler R, Boneca IG, Portnoy DA. 2014. Listeria monocytogenes is resistant to lysozyme through the regulation, not the acquisition, of cell wall-modifying enzymes. J Bacteriol 196:3756–3767. doi: 10.1128/JB.02053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun AN, Camilli A, Portnoy DA. 1990. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun 58:3770–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sauer J-D, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA. 2010. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 7:412–419. doi: 10.1016/j.chom.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell G, Ge L, Huang Q, Chen C, Kianian S, Roberts MF, Schekman R, Portnoy DA. 2015. Avoidance of autophagy mediated by PlcA or ActA is required for Listeria monocytogenes growth in macrophages. Infect Immun 83:2175–2184. doi: 10.1128/IAI.00110-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sauer J-D, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, Hyodo M, Hayakawa Y, Woodward JJ, Portnoy DA, Vance RE. 2011. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect Immun 79:688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Archer KA, Durack J, Portnoy DA. 2014. STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes. PLoS Pathog 10:e1003861. doi: 10.1371/journal.ppat.1003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burke TP, Portnoy DA. 2016. SpoVG is a conserved RNA-binding protein that regulates Listeria monocytogenes lysozyme resistance, virulence, and swarming motility. mBio 7:e00240-16. doi: 10.1128/mBio.00240-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zemansky J, Kline BC, Woodward JJ, Leber JH, Marquis H, Portnoy DA. 2009. Development of a mariner-based transposon and identification of Listeria monocytogenes determinants, including the peptidyl-prolyl isomerase PrsA2, that contribute to its hemolytic phenotype. J Bacteriol 191:3950–3964. doi: 10.1128/JB.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bécavin C, Bouchier C, Lechat P, Archambaud C, Creno S, Gouin E, Wu Z, Kühbacher A, Brisse S, Pucciarelli MG, García-Del Portillo F, Hain T, Portnoy DA, Chakraborty T, Lecuit M, Pizarro-Cerda J, Moszer I, Bierne H, Cossart P. 2014. Comparison of widely used Listeria monocytogenes strains EGD, 10403S, and EGD-e highlights genomic variations underlying differences in pathogenicity. mBio 5:e00969-14. doi: 10.1128/mBio.00969-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reniere ML, Whiteley AT, Hamilton KL, John SM, Lauer P, Brennan RG, Portnoy DA. 2015. Glutathione activates virulence gene expression of an intracellular pathogen. Nature 517:170–173. doi: 10.1038/nature14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.