ABSTRACT
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen that requires iron for virulence. Iron homeostasis is maintained in part by the PrrF1 and PrrF2 small RNAs (sRNAs), which block the expression of iron-containing proteins under iron-depleted conditions. The PrrF sRNAs also promote the production of the Pseudomonas quinolone signal (PQS), a quorum sensing molecule that activates the expression of several virulence genes. The tandem arrangement of the prrF genes allows for expression of a third sRNA, PrrH, which is predicted to regulate gene expression through its unique sequence derived from the prrF1-prrF2 intergenic (IG) sequence (the PrrHIG sequence). Previous studies showed that the prrF locus is required for acute lung infection. However, the individual functions of the PrrF and PrrH sRNAs were not determined. Here, we describe a system for differentiating PrrF and PrrH functions by deleting the PrrHIG sequence [prrF(ΔHIG)]. Our analyses of this construct indicate that the PrrF sRNAs, but not PrrH, are required for acute lung infection by P. aeruginosa. Moreover, we show that the virulence defect of the ΔprrF1-prrF2 mutant is due to decreased bacterial burden during acute lung infection. In vivo analysis of gene expression in lung homogenates shows that PrrF-mediated regulation of genes for iron-containing proteins is disrupted in the ΔprrF1-prrF2 mutant during infection, while the expression of genes that mediate PrrF-regulated PQS production are not affected by prrF deletion in vivo. Combined, these studies demonstrate that regulation of iron utilization plays a critical role in P. aeruginosa's ability to survive during infection.
KEYWORDS: Pseudomonas aeruginosa, sRNA, PrrF, PrrH, iron regulation, PQS, small RNA
INTRODUCTION
Pseudomonas aeruginosa is a Gram-negative bacterium that causes life-threatening infections in a variety of patient populations, including acute blood and lung infections in hospitalized patients and chronic lung infections in individuals with cystic fibrosis (CF) (1–4). Iron is an essential nutrient for virulence in P. aeruginosa (5–9), but it is sequestered by mammalian host proteins such as lactoferrin and transferrin (10). To overcome this barrier to infection, P. aeruginosa secretes two siderophores, pyoverdine and pyochelin, which scavenge ferric iron (Fe3+) from host proteins and are required for acute infections (5, 7–9). P. aeruginosa also obtains iron from host heme using outer membrane heme transporters and a cytosolic HemO heme oxygenase that degrades heme (11, 12). Ferrous iron (Fe2+) can also be obtained through the Feo system in microaerobic environments, such as those found within biofilms and the CF lung (13–15).
While required for growth and infection, iron reacts with oxygen in aerobic environments via Fenton chemistry, resulting in the production of reactive oxygen species that can damage cells. The expression of iron uptake proteins is therefore regulated in response to cytosolic iron levels (16). Under iron-replete conditions, the P. aeruginosa ferric uptake regulator (Fur) binds iron and represses the expression of genes for iron uptake proteins, preventing iron toxicity (17–19). Fur also mediates positive regulation of numerous genes through negative regulation of the PrrF1 and PrrF2 small regulatory RNAs (collectively referred to as the PrrF sRNAs) (20). The PrrF sRNAs are highly homologous to one another (94%), and both negatively affect the expression of over 50 genes for iron-containing proteins in P. aeruginosa, presumably by base pairing with and destabilizing their mRNA transcripts (21). PrrF regulation therefore limits iron use when this nutrient is limiting, an effect referred to as the iron-sparing response (22, 23). Iron-responsive sRNAs that mediate similar regulatory effects have been identified in diverse bacterial species, suggesting a central role for the iron-sparing response in bacterial iron homeostasis (23–32).
Among the known P. aeruginosa PrrF targets are mRNAs encoding the iron-cofactored superoxide dismutase (product of sodB), a putative bacterioferritin (PA4880), a heme-cofactored katalase (product of katA), and succinate dehydrogenase (product of sdh), all of which are iron-containing proteins (20). PrrF also promotes the production of the Pseudomonas quinolone signal (PQS) (21), an alkylquinolone (AQ) quorum sensing molecule that activates the expression of numerous virulence genes (33, 34). PrrF's effect on PQS production is achieved through repression of antR, encoding an activator of genes for degradation of anthranilate, the biosynthetic precursor for PQS. As such, the PrrF sRNAs have the potential to affect virulence through at least two distinct mechanisms: regulation of iron utilization, i.e., the iron-sparing response, and activation of virulence gene expression through PQS.
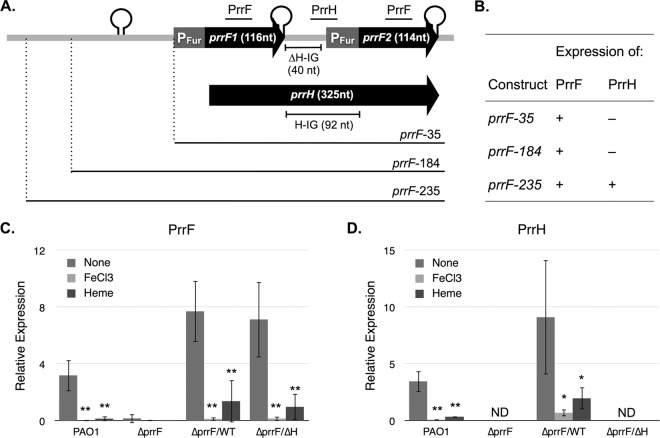
The PrrF sRNAs of P. aeruginosa are transcribed from two highly homologous genes, prrF1 and prrF2, which are arranged in tandem on the chromosome (20). This tandem arrangement allows the transcription of a distinct sRNA species that is responsive to heme, referred to as PrrH (35, 36). Transcription of PrrH initiates at the prrF1 promoter, reads through the PrrF1 Rho-independent terminator, and extends through the prrF1-prrF2 intergenic (IG) sequence and the prrF2 gene (35). PrrH is predicted to regulate the expression of a distinct regulon through the sequence derived from the prrF1-prrF2 intergenic sequence (PrrHIG) (Fig. 1A, H-IG). Several putative target mRNAs of PrrH have been identified based on complementarity with the PrrHIG sequence and deregulation in a prrF locus deletion mutant (22, 35). These include phuS, encoding a cytoplasmic heme binding protein involved in heme uptake (37), and vreR, encoding a regulator of virulence gene expression (38). However, no studies have confirmed if deregulation of these putative targets in the ΔprrF1-prrF2 mutant is specifically due to the PrrH sRNA.
FIG 1.
PrrF expression is maintained in the absence of the PrrHIG sequence. (A) Diagram of the prrF locus indicating the gene sequence transcribed into the PrrF1 (116 nt), PrrF2 (114 nt), and PrrH (325 nt) sRNAs. The sequence included in each of the prrF complementation constructs is shown below the diagram. Putative and confirmed Rho-independent terminators are indicated by a stem loop. The Fur-regulated promoters of each prrF gene (PFur) are indicated by a gray box. The prrF1-prrF2 intergenic (HIG) region is 92 nt long, the first 40 nt of which were deleted in the prrF(ΔH) complementation construct. The locations of primers and probes used for RT-PCR of the PrrF and PrrH sRNAs throughout this study are indicated above the diagram and described in more detail in the text. (B) Summary of RT-PCR results of the ΔprrF1-prrF2 mutant complemented with the prrF complementation constructs. Detailed RT-PCR data are shown in Fig. S2. (C and D) PAO1 and the ΔprrF1-prrF2 (ΔprrF) strain, transformed with either pUCP18, pUCP-prrF-235 (ΔprrF/WT strain), or pUCP-prrFΔH-235 (ΔprrF/ΔH strain), were grown overnight in DTSB to deplete intracellular iron stores, diluted into M9 medium with or without supplementation of FeCl3 (100 μM) or heme (5 μM) to an absorbance at 600 nm of 0.08, and then grown for an additional 8 h. RNA was extracted from cultures and analyzed by RT-PCR as described in Materials and Methods. Error bars represent the standard deviations from six independent experiments. The following P values were determined by a two-tailed Student's t test comparing expression levels under low-iron conditions to levels under iron-replete or heme-supplemented conditions: *, P < 0.05; **, P < 0.01; ND, not detected.
We previously showed that the P. aeruginosa prrF locus is required for virulence in an acute murine lung infection model (22), yet the specific contributions of the PrrF and PrrH sRNAs in this model remained unknown. Here, we describe the development of a genetic system intended to differentiate between the functions of the PrrF and PrrH sRNAs, based on the hypothesis that the PrrHIG sequence is required for PrrH function. Using this system, we show that the PrrHIG sequence is not required for expression of, or regulation by, the PrrF sRNAs. We further show that this sequence is not required for acute murine lung infection, suggesting that PrrH regulation is dispensable in this model of acute virulence. We further delineate PrrF regulatory pathways that are active during acute murine infection by analyzing in vivo expression of known and putative PrrF target mRNAs. The results of these studies indicate that the virulence defect of the ΔprrF1-prrF2 mutant during acute infection is largely, if not solely, due to the loss of the iron-sparing response, demonstrating the central role of iron homeostasis in P. aeruginosa pathogenesis.
RESULTS
Construction of a ΔPrrHIG mutant.
The PrrH sRNA is predicted to affect the expression of genes involved in heme homeostasis (phuS) and virulence (vreR) through the PrrHIG sequence derived from the prrF1-prrF2 intergenic region (22, 35). To begin addressing the role of PrrH in gene regulation and virulence, we sought to develop a clone of the prrF locus that lacked the PrrHIG sequence. Development of constructs for ΔprrF1-prrF2 mutant complementation has previously been plagued by mutations in cloned prrF fragments, preventing construction of mutants with targeted deletions in the PrrHIG sequence (22). To circumvent this issue, the prrF locus, including either 35, 184, or 235 nucleotides (nt) upstream of the prrF1 transcriptional start site (Fig. 1A and Table 1), was cloned into the broad-host-range vector pUCP18 by Genewiz. Isogenic plasmids, with a deletion of the first 40 nucleotides of the PrrHIG sequence to maintain the prrF2 Fur binding site and promoter intact, as indicated in Fig. 1A, were also generated. The resulting plasmids were shipped to our laboratory and transformed into the PAO1 ΔprrF1-prrF2 mutant.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| PAO1 | Human wound isolate of P. aeruginosa from Australia | 64 |
| ΔprrF1-prrF2 strain | PAO1 with a deletion of the prrF1-prrF2 locus | 20 |
| Plasmids | ||
| pUCP18 | pUC18 derivative carrying the origin of replication from the broad-host-range plasmid pRO1600; Apr | 63 |
| pUCP-prrFWT-35 | pUCP18 with the prrF locus and 35 bp upstream of prrF1 start site (bp 5283960–5284319) | This work |
| pUCP-prrFWT-184 | pUCP18 with the prrF locus and 184 bp upstream of prrF1 start site (bp 5283811–5284319) | This work |
| pUCP-prrFWT-235 | pUCP18 with the prrF locus and 235 bp upstream of prrF1 start site (bp 5283760–5284319) | This work |
| pUCP-prrFΔH-35 | pUCP-prrFWT-35 with a deletion of the PrrH intergenic sequence (bp 5284111–5284151) | This work |
| pUCP-prrFΔH-184 | pUCP-prrFWT-184 with a deletion of the PrrH intergenic sequence (bp 5284111–5284151) | This work |
| pUCP-prrFΔH-235 | pUCP-prrFWT-235 with a deletion of the PrrH intergenic sequence (bp 5284111–5284151) | This work |
We next analyzed expression of the PrrF and PrrH sRNAs from the ΔprrF1-prrF2 mutant carrying the wild-type [prrF(WT)] and ΔprrHIG [prrF(ΔH)] constructs. PAO1 and the ΔprrF1-prrF2 mutant, transformed with either the pUCP18 vector or one of the prrF complementation constructs, were grown overnight in dialyzed Trypticase soy broth (DTSB) to deplete intracellular iron stores and then subcultured into M9 minimal medium for an additional 8 h. RNA was extracted, and reverse transcription-PCR (RT-PCR) was performed to analyze expression of the PrrF and PrrH sRNAs using PCR primers and probes as indicated in Fig. 1A. While all of the wild-type prrF constructs allowed PrrF expression, PrrH expression was detected in the ΔprrF1-prrF2 mutant only when it was complemented with the construct including 235 bp upstream of the prrF1 start site [prrF(WT)-235] (Fig. 1B, prrF-235; see also Fig. S2 in the supplemental material). This was a surprising result as the promoter for prrH was previously presumed to be shared with the prrF1 promoter (35). Since the requirement for this sequence is restricted to PrrH expression, these data may reflect the role for this upstream sequence in regulating prrF1 antitermination. Moreover, expression of both the PrrF and PrrH sRNAs from the prrF(WT)-235 plasmid was regulated by iron and heme in a manner similar to that of the PAO1 strain (Fig. 1C and D), distinguishing this construct from earlier prrF complementation plasmids (22).
We next analyzed PrrF and PrrH expression from the prrF(ΔH) constructs lacking the PrrHIG sequence. As expected, no PrrH was detected in the ΔprrF1-prrF2 mutant when it was complemented with any of the prrF(ΔH) constructs (Fig. 1 and S2) as the RT-PCR primers and probe used to detect PrrH bind to the PrrHIG sequence. However, PrrF levels were restored to wild-type levels or higher when the ΔprrF1-prrF2 mutant was transformed with any of the prrF(ΔH) complementation plasmids, indicating that the PrrHIG sequence is not required for PrrF expression (Fig. 1 and S2). Sequencing analysis of both the prrF(WT) and prrF(ΔH) plasmids, purified from the complemented ΔprrF1-prrF2 mutant strains, demonstrated that no mutations were acquired in the complementation plasmids under these conditions.
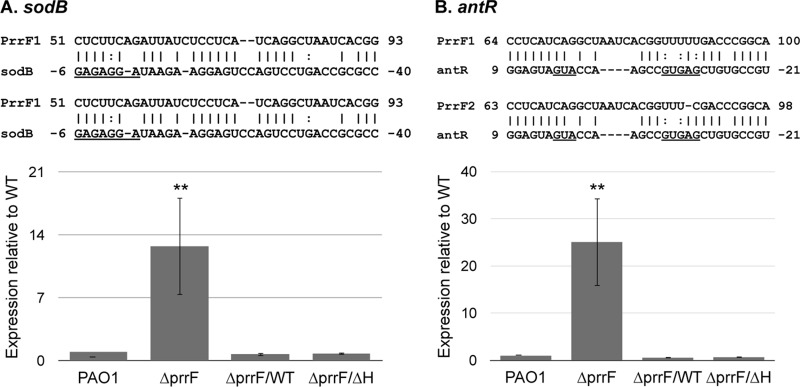
To further determine if the function of the PrrF sRNAs is dependent on the PrrHIG sequence, we analyzed expression of two known PrrF targets, sodB (20) and antR (21), by RT-PCR. Each of these targets shares extensive complementarity with both the PrrF1 and PrrF2 sRNAs, indicating the capacity for both PrrF sRNAs to anneal with each mRNA (Fig. 2). Accordingly, expression levels of both sodB and antR were restored to wild-type levels when the ΔprrF1-prrF2 mutant was complemented with either the prrF(WT)-235 or prrF(ΔH)-235 construct (Fig. 2), demonstrating that the PrrF sRNAs retain their regulatory functions in the absence of the PrrHIG sequence. Thus, these constructs provide a novel system to interrogate the role of the PrrHIG sequence in P. aeruginosa gene regulation and virulence.
FIG 2.
PrrF regulates expression of the sodB and antR target mRNAs. PAO1 and the ΔprrF1-prrF2 (ΔprrF) strain, transformed with either pUCP18, pUCP-prrF-235 (ΔprrF/WT strain), or pUCP-prrFΔH-235 (ΔprrF/ΔH strain), were grown overnight in DTSB to deplete intracellular iron stores, subcultured into M9 medium to a final an optical density (600 nm) of 0.08, and then grown for an additional 8 h. RNA was extracted from aerobic and microaerobic cultures and analyzed for expression of sodB (A) and antR (B) expression as described in Materials and Methods. Complementarity that was previously identified (21) between the PrrF sRNAs and the sodB and antR mRNAs is shown above each graph, and the Shine-Dalgarno and/or translational start sites of the mRNAs are underlined. Error bars indicate the standard deviations from six biological replicates. Significance was determined by a two-tailed Student's t test comparing results for each strain to the result with the wild-type vector control (**, P < 0.001).
The PrrF sRNAs mediate iron homeostasis under iron-depleted conditions.
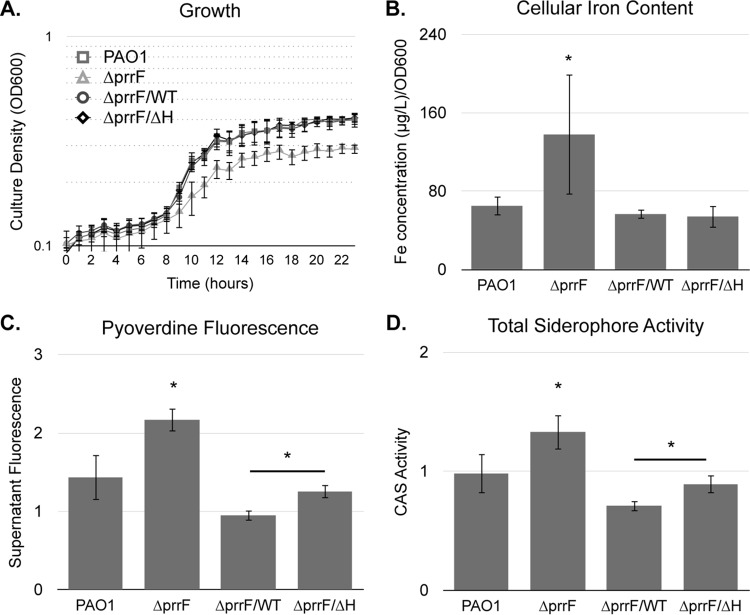
We previously showed that the ΔprrF1-prrF2 mutant is defective for growth in low-iron medium but that this growth defect is not due to an inability to acquire iron via siderophore- or heme-dependent pathways (22). In fact, the ΔprrF1-prrF2 mutant produces significantly more siderophore than its wild-type parent, and inductively coupled plasma mass spectrometry (ICP-MS) analysis shows a significant increase in cellular iron content in the ΔprrF1-prrF2 mutant compared to that of the wild type (22). Thus, we concluded that the ΔprrF1-prrF2 mutant growth defect in low-iron medium is due to constitutive expression of iron-utilizing proteins, in effect starving the cells for iron. To determine if PrrF regulation is solely responsible for this iron homeostasis defect, we repeated these analyses with the ΔprrF1-prrF2 mutant complemented with either the prrF(WT)-235 or prrF(ΔH)-235 construct. As previously observed (22), the ΔprrF1-prrF2 mutant vector control grew significantly less well than the wild-type vector control (Fig. 3A). Also consistent with previous studies (22), cellular iron content, pyoverdine fluorescence, and siderophore activity were significantly increased in the ΔprrF1-prrF2 mutant compared to levels in the wild type (Fig. 3B to D). Complementation of the ΔprrF1-prrF2 mutant with either the prrF(WT)-235 or prrF(ΔH)-235 construct restored growth, cellular iron content, pyoverdine fluorescence, and siderophore activity back to wild-type levels (Fig. 3). Curiously, supernatants from the prrF(ΔH)-235-complemented ΔprrF1-prrF2 mutant displayed a modest but significant increase in both pyoverdine fluorescence and siderophore activity compared to levels of the prrF(WT)-235-complemented mutant, suggesting that PrrH may play a role, either direct or indirect, in regulation of siderophore production. Overall, however, these results indicate that the iron homeostasis defect of the ΔprrF1-prrF2 mutant is largely due to loss of regulation by the PrrF sRNAs.
FIG 3.
PrrF mediates iron homeostasis under iron-depleted conditions. (A.) PAO1 and the ΔprrF1-prrF2 (ΔprrF) strain, transformed with either pUCP18, pUCP-prrF-235 (ΔprrF/WT strain), or pUCP-prrFΔH-235 (ΔprrF/ΔH strain), were grown in DTSB overnight and then subcultured into M9 minimal medium to a final optical density at 600 nm (OD600) of 0.08 for 24 h in a BioScreen multiwell plate reader. Error bars indicate the standard deviations from three independent experiments. (B to D) Strains were grown in DTSB to deplete intracellular iron stores, diluted to an OD of 0.08 in M9 medium, and then grown for an additional 8 h. Cultures were then analyzed for iron content, pyoverdine production (supernatant fluorescence at 410 nm normalized to culture absorbance at 600 nm), and total siderophore activity (CAS reactivity measured at 410 nm normalized to culture absorbance at 600 nm) as described in Materials and Methods. Error bars indicate the standard deviations of six independent experiments. Significance (*, P < 0.05) was determined by a two-tailed, paired Student's t test comparing results for each strain to those for the wild type.
PQS production is independent of the PrrHIG sequence.
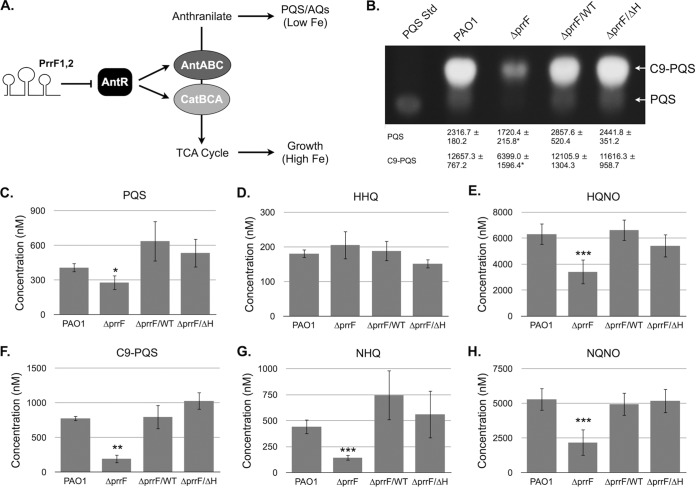
The ΔprrF1-prrF2 mutant is defective for PQS production in iron-depleted environments due to increased expression of genes that allow for anthranilate degradation (21). Expression of antABC and catBCA, encoding enzymes that mediate anthranilate catabolism, depends on an LysR-type transcriptional activator named AntR (Fig. 4A) (21). Substantial complementarity exists between the PrrF sRNAs and the antR ribosomal binding and translational start sites, and antR mRNA levels are derepressed in the ΔprrF1-prrF2 mutant when it is grown in iron-depleted medium (Fig. 2B) (21). Combined, these data indicate that PrrF-regulated expression of antR is required for optimal PQS production. To determine if the PrrHIG sequence affects PrrF-regulated production of PQS, we analyzed extracts from culture supernatants grown in dialyzed Trypticase soy broth (DTSB), a low-iron medium that allows for robust PQS production (21). As was observed in M9 minimal medium (Fig. 1), expression of the PrrF sRNAs was restored to the ΔprrF1-prrF2 mutant by both the prrF(WT)-235 and prrF(ΔH)-235 constructs in this medium (Fig. S3). We also noted strong derepression of sodB and antR in the ΔprrF1-prrF2 mutant when it was grown in this medium, and expression of these mRNAs was restored to wild-type levels by transformation with either complementation construct (Fig. S3). Consistent with these data, the ΔprrF1-prrF2 mutant produced significantly less PQS than the wild type, and complementation with either the prrF(WT)-235 or prrF(ΔH)-235 construct restored PQS to wild-type levels when production was analyzed by thin-layer chromatography (TLC) (Fig. 4B). Combined, these data demonstrate that the PrrHIG sequence is not required for PrrF regulation of anthranilate metabolism and PQS production.
FIG 4.
PrrF promotes the production of numerous AQ metabolites. (A) Model for PrrF regulation of alkylquinolone production. The PrrF sRNAs are predicted to interact with the antR mRNA, which encodes an LysR transcriptional activator of the antABC and catBCA operons. The enzyme complexes encoded by these two operons mediate the degradation of anthranilate for use as a carbon source. Anthranilate can alternatively serve as a biosynthetic precursor to the Pseudomonas quinolone signal (PQS) and other alkylquinolones (AQs). Thus, PrrF regulation of antR provides a molecular switch to promote AQ production under low-iron conditions. (B to H) PAO1 and the ΔprrF1-prrF2 (ΔprrF) strain, transformed with either pUCP18, pUCP-prrF-235 (ΔprrF/WT strain), or pUCP-prrFΔH-235 (ΔprrF/ΔH strain), were grown in DTSB for 16 h, and alkylquinolones were extracted from culture supernatants and analyzed by thin-layer chromatography (TLC) as described in Materials and Methods. (B) A representative image of TLC analysis of PQS and C9-PQS is shown, and densitometry results of each spot normalized to culture density (OD600) from three independent experiments are shown below the image (B). Relative intensity of the indicated alkylquinolones extracted from culture supernatants was determined by LC-M/MS as described in Materials and Methods and normalized to culture density (optical density at 600 nm) (C to H). Error bars indicate the standard deviations of three independent experiments. Significance was determined by a two-tailed Student's t test comparing results for the ΔprrF1-prrF2 mutants to those with the wild type: *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
The PQS biosynthetic pathway generates numerous additional alkylquinolone (AQ) metabolites, many of which are not detected by TLC, including a distinct quorum sensing molecule (2-heptyl-4-quinolone, or HHQ) and a cytochrome b inhibitor with antimicrobial activity (4-hydroxy-2-heptyl quinolone N-oxide, or HQNO) (reviewed in reference 39). The alkyl chains of these metabolites can vary in length and saturation, and we recently showed that iron-regulated production varied between related congeners of these AQs with distinct alkyl chain lengths (40). To determine if PrrF regulation of antR similarly impacts production of these metabolites, we employed a previously developed targeted liquid chromatography-tandem mass spectrometry (LC-MS/MS) protocol (40, 41). With the exception of HHQ, the immediate biosynthetic precursor of PQS (42), levels of all of the AQ metabolites that we analyzed were significantly reduced in the ΔprrF1-prrF2 mutant supernatants (Fig. 4C to H). Moreover, transformation of the ΔprrF1-prrF2 mutant with either the prrF(WT)-235 or prrF(ΔH)-235 construct restored production of each AQ back to the wild-type level (Fig. 4C to H). We assume that the lack of reduction in HHQ in the ΔprrF1-prrF2 mutant supernatants is due to the already low levels of this metabolite produced by the wild-type strain: we detected less than 200 nM HHQ in wild-type supernatants, while the concentration of the C9 alkyl chain congener, 2-nonyl-4-quinolone (NHQ), was nearly 500 nM in wild-type supernatants (Fig. 4D and G). This analysis also provided validation of the TLC analysis of PQS and its C9 congener (C9-PQS) as the changes observed by LC-MS/MS correlated very closely with the densitometry analysis of the PQS and C9-PQS spot intensities (Fig. 4B, C, and F). Combined, these data show that deletion of the prrF locus leads to an overall reduction in AQ production and that regulation of AQ production by the PrrF sRNAs is not dependent upon the PrrHIG sequence.
PrrF-regulated expression of phuS is independent of the PrrHIG sequence.
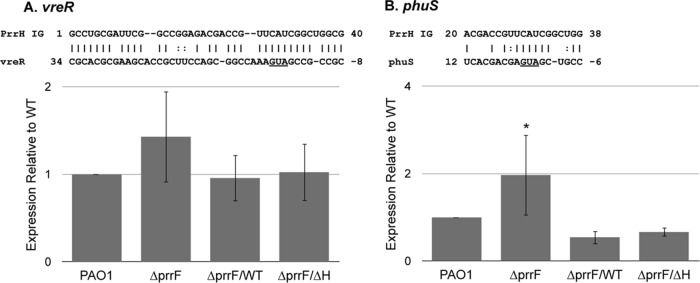
Our previous studies showed that the prrF locus negatively impacts the expression of genes involved in heme homeostasis (phuS) and virulence (vreR), presumably through the action of the PrrH sRNA (22, 35). This idea is supported by the observation that the PrrHIG sequence shares complementarity with regions of each of these mRNAs overlapping the ribosomal binding and translational start sites (Fig. 5A to C). We therefore hypothesized that PrrH binds to these mRNAs through the PrrHIG sequence, destabilizing and preventing the translation of each mRNA. To test this hypothesis, we determined if the prrF(ΔH)-235 construct was capable of restoring expression of two putative PrrH targets, phuS and vreR, to wild-type levels in the ΔprrF1-prrF2 mutant. We observed a modest increase in vreR expression in the ΔprrF1-prrF2 mutant, and expression was restored to wild-type levels when the mutant was complemented with either the prrF(WT)-235 or prrF(ΔH)-235 construct. However, the observed changes in vreR levels were not significant due to erratic expression or stability of this mRNA for these strains (Fig. 5A). In contrast, expression of phuS was significantly induced in the ΔprrF1-prrF2 mutant compared to the level in the wild type, and it was restored to wild-type levels when the ΔprrF1-prrF2 mutant was complemented with either the prrF(WT)-235 or prrF(ΔH)-235 construct (Fig. 5B). Thus, while the impact of PrrF and PrrH on vreR expression remains unclear at this time, our data indicate that the negative effect of the prrF locus on phuS expression is due to the PrrF sRNAs.
FIG 5.
PrrF regulation of phuS is independent of the PrrHIG. PAO1 and the ΔprrF1-prrF2 (ΔprrF) strain, transformed with either pUCP18, pUCP-prrF-235 (ΔprrF/WT strain), or pUCP-prrFΔH-235 (ΔprrF/ΔH strain), were grown overnight in DTSB to deplete intracellular iron stores, subcultured into M9 medium to a final optical density (600 nm) of 0.08, and then grown for an additional 8 h. RNA was extracted from cultures and analyzed for expression of vreR (A) and phuS (B) as described in Materials and Methods. Complementarity that was previously identified (22, 35) between the PrrHIG region and the phuS and vreR mRNAs is shown above each graph, and the Shine-Dalgarno and/or translational start sites of the mRNAs are underlined. Error bars indicate the standard deviations from six biological replicates. Significance was determined by a two-tailed Student's t test comparing results for each strain to those with the wild-type vector control. *, P < 0.05.
The PrrF sRNAs are required for growth during acute lung infection.
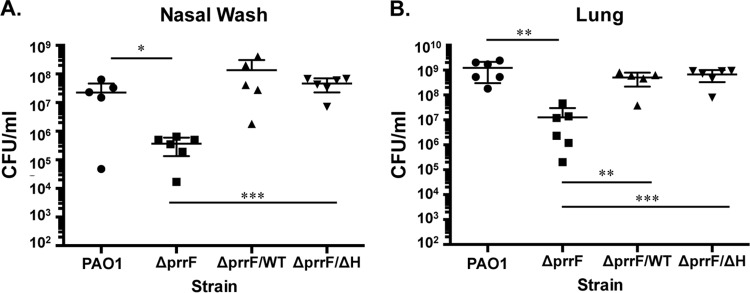
We previously found that the ΔprrF1-prrF2 mutant is attenuated for virulence in an acute murine lung infection model (22). To address whether this attenuation was due to loss of the PrrF or PrrH sRNA, we determined the virulence capacity of the ΔprrF1-prrF2 mutant complemented with either the prrF(WT)-235 or prrF(ΔH)-235 construct. CD-1 outbred mice were intranasally (i.n.) inoculated with 108 CFU of PAO1 or the ΔprrF1-prrF2 mutant vector control, as well as with the ΔprrF1-prrF2 mutant complemented with either the prrF(WT)-235 or prrF(ΔH)-235 construct. At 16 h postinoculation, two mice infected with the wild-type vector control and three infected with each of the complemented ΔprrF1-prrF2 mutant strains were deceased. The remaining mice were euthanized, and nasal washes and lungs were collected from all mice and plated to determine CFU counts. Bacterial counts in both the nasal washes and lung homogenates from the ΔprrF1-prrF2 mutant-infected mice showed a significant 2-log reduction compared to counts obtained from the wild-type-infected mice (Fig. 6). Moreover, bacterial counts were restored to wild-type levels in mice infected with the ΔprrF1-prrF2 mutant complemented with either the prrF(WT)-235 or prrF(ΔH)-235 construct (Fig. 6). Thus, deletion of the PrrHIG sequence did not reduce lung colonization, indicating that PrrH is not required for acute murine lung infection.
FIG 6.
PrrF is required for growth during acute murine lung infection. Six CD-1 mice were inoculated intranasally with 108 CFU of PAO1 or the ΔprrF1-prrF2 (ΔprrF) strain, transformed with either pUCP18, pUCP-prrF-235 (ΔprrF/WT strain), or pUCP-prrFΔH-235 (ΔprrF/ΔH strain), as described in Materials and Methods. At 16 h postinfection, mice were euthanized, and serial dilutions of nasal washes (A) and lung homogenates (B) were plated on PIA and incubated for 24 h. Significance was determined by a two-tailed Student's t test: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We next analyzed PrrF and PrrH expression levels in lung homogenates by RT-PCR to determine if lung colonization correlated with PrrF and/or PrrH sRNA expression. Both the PrrF and PrrH sRNAs were detectable in the wild-type-infected mouse lung homogenates, and detection of both sRNAs was significantly reduced in the ΔprrF1-prrF2 mutant (Table 2 and Fig. S1). Complementation of the ΔprrF1-prrF2 mutant with either the prrF(WT)-235 or prrF(ΔH)-235 construct restored in vivo expression of the PrrF sRNAs, while the PrrH sRNA was not detected in mice infected with the ΔprrF(ΔH)-complemented strain (Table 2 and Fig. S1). These data demonstrate that both the PrrF and PrrH sRNAs are expressed by bacteria growing in the lungs during this infection model but that lung colonization correlates most closely with expression of the PrrF sRNAs.
TABLE 2.
In vivo expression of PrrF and PrrH sRNAs
| Strain | Relative expressiona |
|
|---|---|---|
| PrrF | PrrH | |
| ΔprrF1-prrF2 strain | 0.01 ± 0.01* | 0.28 ± 0.21* |
| ∆prrF1-prrF2/prrF(WT) strain | 4.43 ± 2.61 | 8.12 ± 6.03 |
| ∆prrF1-prrF2/prrF(∆H) strain | 4.48 ± 5.18 | ND |
Expression level in the indicated strain compared to that of PAO1, determined by RT-PCR of mouse lung homogenates as described in Materials and Methods. Significance was determined by a paired, two-tailed Student's t test (*, P < 0.01). ND, not detected.
PrrF-mediated iron homeostasis is likely critical for murine lung infection.
While the above data indicate that the PrrF sRNAs are critical for acute lung infection, the requirement for a specific regulatory pathway(s) mediated by the PrrF sRNAs during infection remains unknown. To begin to understand how the PrrF sRNAs function in vivo, we analyzed expression of several putative PrrF target mRNAs in lung homogenates. We began by analyzing expression of sodB and antR, which are negatively regulated by the PrrF sRNAs (Fig. 2) (20, 22). Expression of sodB was significantly induced in mice infected with the ΔprrF1-prrF2 mutant compared to the level in the wild type, and expression was restored to wild-type levels when the ΔprrF1-prrF2 mutant was complemented with either the prrF(WT)-235 or prrF(ΔH)-235 construct (Table 3). Surprisingly, antR expression was not affected by prrF1-prrF2 deletion in vivo (Table 3). Since PrrF-mediated regulation of antR is important for promoting PQS production in vitro (Fig. 4) (21), these data suggest that PrrF-mediated iron homeostasis plays a more prominent role than PrrF-mediated PQS production during acute lung infection.
TABLE 3.
In vivo expression of putative PrrF and PrrH regulatory targets
| Strain | Relative expressiona |
|||
|---|---|---|---|---|
| sodB | antR | phuS | vreR | |
| ΔprrF1-prrF2 strain | 3.85 ± 1.30*** | 1.17 ± 0.42 | 4.30 ± 3.08* | 4.16 ± 2.86 |
| ∆prrF1-prrF2/prrF(WT) strain | 0.81 ± 0.14* | 1.57 ± 0.56 | 0.60 ± 0.45 | 1.15 ± 0.52 |
| ∆prrF1-prrF2/prrF(∆H) strain | 0.65 ± 0.24** | 1.26 ± 0.70 | 1.17 ± 1.49 | 1.16 ± 1.28 |
Expression level in the indicated strain compared to that in PAO1, determined by RT-PCR of mouse lung homogenates as described in Materials and Methods. Significance was determined by a paired, two-tailed Student's t test: *, P < 0.05; **, P < 0.01; ***, P < 0.005.
We next determined if putative PrrH targets (phuS and vreR) were deregulated in ΔprrF1-prrF2 mutant-infected mice. The vreR mRNAs showed no significant increase in expression in either the ΔprrF1-prrF2 mutant compared to PAO1 or the prrF(ΔH)-235-complemented strain compared to the prrF(WT)-235 strain (Table 3). Thus, these data indicate that neither PrrF nor PrrH has an effect on expression of vreR in vivo. We did note a significant 4-fold induction of phuS expression in mice infected with the ΔprrF1-prrF2 mutant compared to the wild-type level, and expression was restored to wild-type levels upon complementation of the ΔprrF1-prrF2 mutant with either the prrF(WT)-235 or prrF(ΔH)-235 construct (Table 3). Our above results indicate that phuS is induced in the ΔprrF1-prrF2 mutant in a PrrF-dependent manner (Fig. 5B). Thus, this result is likely reflective of altered iron homeostasis in the ΔprrF1-prrF2 mutant due to loss of the PrrF sRNAs. Combined, these results indicate that the PrrF-mediated iron-sparing response is critical for P. aeruginosa survival during acute lung infection.
DISCUSSION
The prrF locus of P. aeruginosa has the capacity to impact virulence by affecting any number of physiological processes, including iron homeostasis, central and intermediary metabolism, quorum sensing, and heme metabolism (20, 21, 35). However, the individual contributions of the PrrF and PrrH sRNAs to each of these processes have remained elusive, making it difficult to assign the role of specific regulatory functions in pathogenesis. In this report, we describe a genetic system that allows such differentiation by deleting the first 40 nucleotides of the PrrHIG region, putatively ablating PrrH function. We show that deletion of the PrrHIG sequence does not impact the expression or regulatory functions of the PrrF sRNAs. We further show that this sequence, which is presumed to be important for PrrH function, is not required for growth in iron-depleted environments, for PQS production, or for acute lung infection. These results therefore indicate that the PrrF sRNAs play a more prominent role than PrrH during acute P. aeruginosa lung infections in mice.
This study further delineated PrrF-mediated regulatory pathways that are active during acute lung infection, providing our first insights into the mechanism(s) by which the prrF locus affects virulence. RT-PCR analyses of lung homogenates from infected mice showed that sodB, encoding an iron-containing superoxide dismutase, is repressed by the PrrF sRNAs during acute lung infection. In contrast to current (Fig. 2B) and previous (43) in vitro expression studies, expression of the antR mRNA was not upregulated in the ΔprrF1-prrF2 mutant during infection. PQS and its biosynthetic precursor, HHQ, are quorum sensing molecules that activate the expression of several virulence traits (33, 34, 44), and a variety of AQ metabolites are thought to contribute to interactions of P. aeruginosa with other bacterial pathogens during chronic, polymicrobial infections (45–47). To the best of our knowledge, though, the role of these metabolites in acute mammalian infections has not been addressed. While our data do not directly speak to the role of PQS and other AQs during acute mammalian infection, they do suggest that PrrF-mediated production of PQS is not required for acute lung infection.
In the course of generating the prrF complementation constructs for these studies, we identified a region, spanning 235 bp upstream of the prrF1 transcriptional start site, that is required for PrrH expression (Fig. 1). As this region is not needed for PrrF expression, we predict that this upstream sequence affects the predicted PrrF1 antitermination event that is necessary for PrrH transcription. To date, the mechanism of how the RNA polymerase complex is stabilized at the PrrF1 Rho-independent terminator, allowing for PrrH transcription, remains unknown. One possible explanation for these data is that this newly identified upstream region codes for a small protein or RNA molecule that acts as a PrrH antiterminator. This hypothesis is consistent with a previous study, which detected a transcript that initiates upstream of the prrF locus (48). Analysis of this sequence revealed a putative hairpin structure that may function as a transcriptional terminator (Fig. 1A). Alternatively, this upstream region may serve as a binding site for a heme-responsive protein that promotes PrrH expression by altering DNA structure. Studies investigating the capacity of this sequence to affect PrrH transcription are under way.
The tandem arrangement of the prrF genes in P. aeruginosa is distinct from that of other pseudomonads, most of which are not significant contributors to human disease (35). This arrangement is conserved in all P. aeruginosa strains that have thus far been sequenced, as is the PrrHIG sequence, consistent with a role for this sequence in regulation of target mRNAs by complementary base pairing. Using in silico analysis, we previously identified the phuS and vreR mRNAs as sharing complementarity with the PrrHIG sequence (22, 35). However, the current study indicates that these genes are not regulated by PrrH, at least not under the in vitro and in vivo conditions used in this study. The putative PrrH target vreR was unaffected by prrF1-prrF2 deletion either in vitro or in vivo. Moreover, our work indicates that induction of phuS in the ΔprrF1-prrF2 mutant is due to the loss of the PrrF sRNAs, which leads to an iron homeostasis defect and upregulation of multiple Fur-repressed iron uptake pathways. Thus, the biological role of the PrrH sRNA remains unknown. However, we expect that the strains constructed in this study will allow future experimental identification of PrrH-regulated genes. Targeted mutations of the PrrH intergenic sequence designed to disrupt putative interactions with PrrH-regulated genes will help provide information on the role of the PrrHIG sequence in gene expression.
Given the broad conservation of the PrrHIG sequence in multiple clinical isolates from acute and chronic infections (22, 43), we were surprised to find that it plays no obvious role in growth either in vitro or during acute infection. Previous studies showed that RNA containing this sequence was detectable in sputum samples from several patients, indicating a role for PrrH during chronic lung infections (43). It is therefore possible that PrrH plays a more prominent role in long-term survival of P. aeruginosa, which may be important in the CF lung and other chronic Pseudomonas infections. Alternatively, PrrH may be important for environmental survival of P. aeruginosa although this raises the question of why other pseudomonads, which all produce the PrrF sRNAs, do not share the tandem arrangement of the prrF genes. It is also possible that the PrrH sRNA is simply a nonfunctional read-through product of the PrrF1 sRNA as the PrrH sRNA is present at much lower levels than the PrrF sRNAs (35). However, conservation of the PrrHIG sequence across P. aeruginosa strains suggests the presence of positive selective pressure to maintain this sequence. Further analysis of the strains developed in this work using alternative infection models will help determine if the PrrH sRNA, or the PrrHIG DNA sequence itself, plays a role in P. aeruginosa physiology.
Iron is a critical component for multiple cellular processes and is required for virulence in many bacterial species (49–51). While iron uptake has been known for decades to be a major contributor to bacterial pathogenesis, how iron is utilized has only recently become appreciated as being critical for virulence (22, 25). One recent example, the iron-responsive RyhB sRNA, contributes to virulence of uropathogenic Escherichia coli (UPEC) by promoting siderophore production (25). In contrast, the PrrF sRNAs do not appear to play any role in activating the expression of iron uptake systems. Instead, the current work indicates that the iron-sparing response is the most critical role that the PrrF sRNAs play during acute infection. In addition to numerous findings that iron-responsive sRNAs regulate the expression of virulence traits (26, 27, 29–31), this study suggests that sRNA-mediated iron-sparing responses are widespread mediators of bacterial pathogenesis.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains used in this work are listed in Table 1. Sequences for the prrF complementation constructs were obtained from the Pseudomonas Genome Database (52) and were provided, along with the pUCP18 broad-host-range vector, to Genewiz, LLC, for construction. Fragments of the entire prrF locus, including 35, 184, or 235 nucleotides upstream of the prrF1 transcriptional start site, were cloned by Genewiz into the multicloning site (MCS) of pUCP18 using the restriction endonucleases BamHI and HindIII. Deletions of the first 40 nucleotides of the PrrHIG sequence in each construct as indicated in Table 1 were also synthesized by Genewiz. Purified plasmids were received from Genewiz and transformed into the P. aeruginosa prrF1-prrF2 mutant, and transformants were selected by plating on 150 μg/ml of carbenicillin.
Growth conditions.
P. aeruginosa strains were maintained in brain heart infusion (BHI) broth or on BHI agar plates. For enumeration of CFU during animal studies, P. aeruginosa strains were grown on either L broth or L agar plates, prepared as described by Miller (65), or on Pseudomonas isolation agar (PIA). For aerobic iron depletion studies, strains were grown overnight in dialyzed Trypticase soy broth (DTSB), prepared as previously described (22), to deplete intracellular iron stores, and then subcultured into M9 minimal medium purchased from Teknova. For microaerobic iron-depletion studies, strains were grown overnight in BHI broth and subcultured into synthetic cystic fibrosis sputum medium (SCFM) prepared as previously described (53, 54) with the following modifications: FeCl3 was added to 1 μM, and KNO3 was added to 10 mM. Microaerobic cultures were prepared in serum bottles as previously described (55). Briefly, cultures were purged of oxygen with a gas mixture of 95% N2 and 5% CO2 for 1 h per eight bottles, and O2 was injected into the serum bottles for a final percentage of 2.5%. FeCl3 was added to a final concentration of 100 μM for high-iron conditions as indicated in Fig. 1. Heme stocks were freshly prepared prior to use in 0.1 N NaOH–20 mM Tris and adjusted to pH 7.0 with HCl, and stock concentration was determined by pyridine hemochrome assay as previously described (56), using the following extinction coefficients: ε = 170.0 mM−1 cm−1 at A418, ε = 17.5 mM−1 cm−1 at A525, and ε = 34.5 mM−1cm−1 at A555. Heme was immediately diluted into M9 medium to a concentration of 5 μM as indicated in Fig. 1. Carbenicillin was used at 150 μg/ml for growth of P. aeruginosa strains carrying the pUCP18 vector or its derivatives.
Determination of cellular iron content.
Cellular iron content was determined by inductively coupled plasma mass spectrometry (ICP-MS) as previously described (22). Briefly, bacterial cells were harvested by centrifugation, dissolved in 20% nitric acid, and boiled overnight at 100°C. ICP-MS was then used to determine the iron content of the dissolved bacteria using an Agilent 7700 ICP-MS instrument (Agilent Technologies). Raw ICP-MS data (μg/liter) were corrected for drift using values of internal controls (indium, scandium, and germanium) that were added to each sample during processing. Corrected values were then normalized to culture density as determined by the absorbance at 600 nm and analyzed with a seven-point standard curve of elemental Fe in 2% nitric acid (purchased from CPI International).
Detection of siderophores.
Production of the pyoverdine chromophore was determined spectroscopically by reading the absorbance of culture supernatants at 410 nm as previously described (57). Total iron chelator production in culture supernatants was quantified by a chrome azurol S (CAS) assay as previously described (58). All readings were normalized to culture density as determined by the absorbance at 600 nm.
Real-time PCR.
Microaerobic cultures were treated with RNAlater (Sigma) prior to RNA extraction. RNA was extracted using an RNeasy minikit (Qiagen) according to the manufacturer's directions. A total of 50 ng/μl of RNA was used to generate cDNA with an ImProm-II cDNA synthesis kit (Promega) as previously described (22), and cDNA was analyzed using a StepOnePlus instrument (Applied Biosystems) and TaqMan reagents (Life Technologies). Standard curves were produced for each primer-probe set listed in Table S1 in the supplemental material by analyzing cDNA generated from serial dilutions of RNA and used to determine relative amounts of the RNAs, as described previously (22). Relative RNA levels were then normalized to the levels of the oprF mRNA as previously described (22, 59, 60) for aerobic expression studies or to the omlA level as previously described (21) for microaerobic expression studies. The expression of each of these genes was constant under these respective conditions (Fig. S1). As the consistency of expression of each of these genes could not be determined in vivo, expression data from lung homogenates were normalized to both oprF and omlA (Fig. S1).
Analysis of alkylquinolones.
Strains were grown for 18 h in DTSB, and cells were harvested for alkylquinolone (AQ) extraction as previously described by Collier et al. (61). For thin-layer chromatography analysis, one-half of the resulting organic extract was transferred to a clean tube and evaporated to dryness. Samples were resuspended in 1:1 acidified ethyl acetate-acetonitrile and analyzed by thin-layer chromatography (TLC) with a synthetic PQS standard (62). For liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, nalidixic acid and deuterated N-pentanoyl-l-homoserine lactone (C5-HSL) were added as internal standards. LC-MS/MS analysis was performed as previously described (40, 41).
Mouse infections.
Studies were performed under a protocol approved by the West Virginia University Animal Care and Use Committee. CD-1 mice (4 to 6 weeks old) were purchased from Charles River. P. aeruginosa strains were grown overnight on PIA plates with 300 μg/ml of carbenicillin. Cells were collected off the plate and resuspended in phosphate-buffered saline (PBS), and appropriate dilutions were prepared according to the absorbance of the suspensions at 600 nm. Mice were anesthetized by intraperitoneal injection of 200 μl of ketamine (6.7 mg/ml) and xylazine (1.3 mg/ml) and were inoculated intranasally (i.n.) with 20 μl of inoculum (10 μl per nostril) (n = 6 mice for each bacterial strain). Inocula were also serially diluted and plated on L agar to enumerate the delivered dose. Mice were sacrificed using Euthasol and cardiac puncture at 16 h postinfection. Lungs and nasal washes were collected, and lungs were homogenized and divided for analysis of gene expression and determination of CFU counts on PIA medium containing irgasan.
Supplementary Material
ACKNOWLEDGMENTS
We thank Miguel Cámara and Paul Williams (University of Nottingham, United Kingdom) for supplying standards for LC-MS/MS quantification of AQs.
This work was supported by NIH grant R01AI123320 (to A.G.O.-S.), start-up funding from the University of Maryland School of Pharmacy (to A.G.O.-S. and M.A.K.), bridge funding from the University of Maryland Graduate Program in Life Sciences (to A.G.O.-S.), NIH-NIAID contract HHSN272201000046C (to M.A.K.), the funding from University of Maryland School of Pharmacy Mass Spectrometry Center (SOP1841-IQB2014 to M.A.K.), a Graduate Research Award from the University of Maryland Graduate School (to A.A.R.), and start-up funding from West Virginia University School of Medicine (to F.H.D. and M.B.).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00764-16.
REFERENCES
- 1.Vento S, Cainelli F, Temesgen Z. 2008. Lung infections after cancer chemotherapy. Lancet Oncol 9:982–992. doi: 10.1016/S1470-2045(08)70255-9. [DOI] [PubMed] [Google Scholar]
- 2.Klastersky J, Ameye L, Maertens J, Georgala A, Muanza F, Aoun M, Ferrant A, Rapoport B, Rolston K, Paesmans M. 2007. Bacteraemia in febrile neutropenic cancer patients. Int J Antimicrob Agents 30(Suppl 1):S51–S59. doi: 10.1016/j.ijantimicag.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Chatzinikolaou I, Abi-Said D, Bodey GP, Rolston KV, Tarrand JJ, Samonis G. 2000. Recent experience with Pseudomonas aeruginosa bacteremia in patients with cancer: retrospective analysis of 245 episodes. Arch Intern Med 160:501–509. doi: 10.1001/archinte.160.4.501. [DOI] [PubMed] [Google Scholar]
- 4.FitzSimmons SC. 1993. The changing epidemiology of cystic fibrosis. J Pediatr 122:1–9. doi: 10.1016/S0022-3476(05)83478-X. [DOI] [PubMed] [Google Scholar]
- 5.Meyer JM, Neely A, Stintzi A, Georges C, Holder IA. 1996. Pyoverdine is essential for virulence of Pseudomonas aeruginosa. Infect Immun 64:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takase H, Nitanai H, Hoshino K, Otani T. 2000. Requirement of the Pseudomonas aeruginosa tonB gene for high-affinity iron acquisition and infection. Infect Immun 68:4498–4504. doi: 10.1128/IAI.68.8.4498-4504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takase H, Nitanai H, Hoshino K, Otani T. 2000. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect Immun 68:1834–1839. doi: 10.1128/IAI.68.4.1834-1839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nadal Jimenez P, Koch G, Papaioannou E, Wahjudi M, Krzeslak J, Coenye T, Cool RH, Quax WJ. 2010. Role of PvdQ in Pseudomonas aeruginosa virulence under iron-limiting conditions. Microbiology 156:49–59. doi: 10.1099/mic.0.030973-0. [DOI] [PubMed] [Google Scholar]
- 9.Xiong YQ, Vasil ML, Johnson Z, Ochsner UA, Bayer AS. 2000. The oxygen- and iron-dependent sigma factor pvdS of Pseudomonas aeruginosa is an important virulence factor in experimental infective endocarditis. J Infect Dis 181:1020–1026. doi: 10.1086/315338. [DOI] [PubMed] [Google Scholar]
- 10.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker KD, Barkovits K, Wilks A. 2012. Metabolic flux of extracellular heme uptake in Pseudomonas aeruginosa is driven by the iron-regulated heme oxygenase (HemO). J Biol Chem 287:18342–18350. doi: 10.1074/jbc.M112.359265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratliff M, Zhu W, Deshmukh R, Wilks A, Stojiljkovic I. 2001. Homologues of neisserial heme oxygenase in gram-negative bacteria: degradation of heme by the product of the pigA gene of Pseudomonas aeruginosa. J Bacteriol 183:6394–6403. doi: 10.1128/JB.183.21.6394-6403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter RC, Asfour F, Dingemans J, Osuna BL, Samad T, Malfroot A, Cornelis P, Newman DK. 2013. Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways. mBio 4:e00557-13. doi: 10.1128/mBio.00557-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Wilks JC, Danhorn T, Ramos I, Croal L, Newman DK. 2011. Phenazine-1-carboxylic acid promotes bacterial biofilm development via ferrous iron acquisition. J Bacteriol 193:3606–3617. doi: 10.1128/JB.00396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cartron ML, Maddocks S, Gillingham P, Craven CJ, Andrews SC. 2006. Feo—transport of ferrous iron into bacteria. Biometals 19:143–157. doi: 10.1007/s10534-006-0003-2. [DOI] [PubMed] [Google Scholar]
- 16.Hantke K. 2001. Iron and metal regulation in bacteria. Curr Opin Microbiol 4:172–177. doi: 10.1016/S1369-5274(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 17.Prince RW, Storey DG, Vasil AI, Vasil ML. 1991. Regulation of toxA and regA by the Escherichia coli fur gene and identification of a Fur homologue in Pseudomonas aeruginosa PA103 and PAO1. Mol Microbiol 5:2823–2831. doi: 10.1111/j.1365-2958.1991.tb01991.x. [DOI] [PubMed] [Google Scholar]
- 18.Prince RW, Cox CD, Vasil ML. 1993. Coordinate regulation of siderophore and exotoxin A production: molecular cloning and sequencing of the Pseudomonas aeruginosa fur gene. J Bacteriol 175:2589–2598. doi: 10.1128/jb.175.9.2589-2598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochsner UA, Vasil AI, Vasil ML. 1995. Role of the ferric uptake regulator of Pseudomonas aeruginosa in the regulation of siderophores and exotoxin A expression: purification and activity on iron-regulated promoters. J Bacteriol 177:7194–7201. doi: 10.1128/jb.177.24.7194-7201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilderman PJ, Sowa NA, FitzGerald DJ, FitzGerald PC, Gottesman S, Ochsner UA, Vasil ML. 2004. Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc Natl Acad Sci U S A 101:9792–9797. doi: 10.1073/pnas.0403423101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oglesby AG, Farrow JM III, Lee JH, Tomaras AP, Greenberg EP, Pesci EC, Vasil ML. 2008. The influence of iron on Pseudomonas aeruginosa physiology: a regulatory link between iron and quorum sensing. J Biol Chem 283:15558–15567. doi: 10.1074/jbc.M707840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinhart AA, Powell DA, Nguyen AT, O'Neill M, Djapgne L, Wilks A, Ernst RK, Oglesby-Sherrouse AG. 2015. The prrF-encoded small regulatory RNAs are required for iron homeostasis and virulence of Pseudomonas aeruginosa. Infect Immun 83:863–875. doi: 10.1128/IAI.02707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masse E, Gottesman S. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci U S A 99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellin JR, Goswami S, Grogan S, Tjaden B, Genco CA. 2007. A novel fur- and iron-regulated small RNA, NrrF, is required for indirect fur-mediated regulation of the sdhA and sdhC genes in Neisseria meningitidis. J Bacteriol 189:3686–3694. doi: 10.1128/JB.01890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porcheron G, Habib R, Houle S, Caza M, Lepine F, Daigle F, Masse E, Dozois CM. 2014. The small RNA RyhB contributes to siderophore production and virulence of uropathogenic Escherichia coli. Infect Immun 82:5056–5068. doi: 10.1128/IAI.02287-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JN, Kwon YM. 2013. Identification of target transcripts regulated by small RNA RyhB homologs in Salmonella: RyhB-2 regulates motility phenotype. Microbiol Res 168:621–629. doi: 10.1016/j.micres.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Huang SH, Wang CK, Peng HL, Wu CC, Chen YT, Hong YM, Lin CT. 2012. Role of the small RNA RyhB in the Fur regulon in mediating the capsular polysaccharide biosynthesis and iron acquisition systems in Klebsiella pneumoniae. BMC Microbiol 12:148. doi: 10.1186/1471-2180-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng Z, Meng X, Su S, Liu Z, Ji X, Zhang Y, Zhao X, Wang X, Yang R, Han Y. 2012. Two sRNA RyhB homologs from Yersinia pestis biovar microtus expressed in vivo have differential Hfq-dependent stability. Res Microbiol 163:413–418. doi: 10.1016/j.resmic.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Murphy ER, Payne SM. 2007. RyhB, an iron-responsive small RNA molecule, regulates Shigella dysenteriae virulence. Infect Immun 75:3470–3477. doi: 10.1128/IAI.00112-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mey AR, Craig SA, Payne SM. 2005. Characterization of Vibrio cholerae RyhB: the RyhB regulon and role of ryhB in biofilm formation. Infect Immun 73:5706–5719. doi: 10.1128/IAI.73.9.5706-5719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oglesby AG, Murphy ER, Iyer VR, Payne SM. 2005. Fur regulates acid resistance in Shigella flexneri via RyhB and ydeP. Mol Microbiol 58:1354–1367. doi: 10.1111/j.1365-2958.2005.04920.x. [DOI] [PubMed] [Google Scholar]
- 32.Gaballa A, Antelmann H, Aguilar C, Khakh SK, Song KB, Smaldone GT, Helmann JD. 2008. The Bacillus subtilis iron-sparing response is mediated by a Fur-regulated small RNA and three small, basic proteins. Proc Natl Acad Sci U S A 105:11927–11932. doi: 10.1073/pnas.0711752105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deziel E, Gopalan S, Tampakaki AP, Lepine F, Padfield KE, Saucier M, Xiao G, Rahme LG. 2005. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-l-homoserine lactones. Mol Microbiol 55:998–1014. doi: 10.1111/j.1365-2958.2004.04448.x. [DOI] [PubMed] [Google Scholar]
- 34.Calfee MW, Coleman JP, Pesci EC. 2001. Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 98:11633–11637. doi: 10.1073/pnas.201328498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oglesby-Sherrouse AG, Vasil ML. 2010. Characterization of a heme-regulated non-coding RNA encoded by the prrF locus of Pseudomonas aeruginosa. PLoS One 5:e9930. doi: 10.1371/journal.pone.0009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochsner UA, Johnson Z, Vasil ML. 2000. Genetics and regulation of two distinct haem-uptake systems, phu and has, in Pseudomonas aeruginosa. Microbiology 146:185–198. doi: 10.1099/00221287-146-1-185. [DOI] [PubMed] [Google Scholar]
- 37.Kaur AP, Lansky IB, Wilks A. 2009. The role of the cytoplasmic heme-binding protein (PhuS) of Pseudomonas aeruginosa in intracellular heme trafficking and iron homeostasis. J Biol Chem 284:56–66. doi: 10.1074/jbc.M806068200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llamas MA, van der Sar A, Chu BC, Sparrius M, Vogel HJ, Bitter W. 2009. A novel extracytoplasmic function (ECF) sigma factor regulates virulence in Pseudomonas aeruginosa. PLoS Pathog 5:e1000572. doi: 10.1371/journal.ppat.1000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heeb S, Fletcher MP, Chhabra SR, Diggle SP, Williams P, Camara M. 2011. Quinolones: from antibiotics to autoinducers. FEMS Microbiol Rev 35:247–274. doi: 10.1111/j.1574-6976.2010.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen AT, Jones JW, Camara M, Williams P, Kane MA, Oglesby-Sherrouse AG. 2016. Cystic fibrosis isolates of Pseudomonas aeruginosa retain iron-regulated antimicrobial activity against Staphylococcus aureus through the action of multiple alkylquinolones. Front Microbiol 7:1171. doi: 10.3389/fmicb.2016.01171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortori CA, Halliday N, Camara M, Williams P, Barrett DA. 2014. LC-MS/MS quantitative analysis of quorum sensing signal molecules. Methods Mol Biol 1149:255–270. doi: 10.1007/978-1-4939-0473-0_21. [DOI] [PubMed] [Google Scholar]
- 42.Dulcey CE, Dekimpe V, Fauvelle DA, Milot S, Groleau MC, Doucet N, Rahme LG, Lepine F, Deziel E. 2013. The end of an old hypothesis: the pseudomonas signaling molecules 4-hydroxy-2-alkylquinolines derive from fatty acids, not 3-ketofatty acids. Chem Biol 20:1481–1491. doi: 10.1016/j.chembiol.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen AT, O'Neill MJ, Watts AM, Robson CL, Lamont IL, Wilks A, Oglesby-Sherrouse AG. 2014. Adaptation of iron homeostasis pathways by a Pseudomonas aeruginosa pyoverdine mutant in the cystic fibrosis lung. J Bacteriol 196:2265–2276. doi: 10.1128/JB.01491-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deziel E, Lepine F, Milot S, He J, Mindrinos MN, Tompkins RG, Rahme LG. 2004. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc Natl Acad Sci U S A 101:1339–1344. doi: 10.1073/pnas.0307694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen AT, Jones JW, Ruge MA, Kane MA, Oglesby-Sherrouse AG. 2015. Iron depletion enhances production of antimicrobials by Pseudomonas aeruginosa. J Bacteriol 197:2265–2275. doi: 10.1128/JB.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filkins LM, Graber JA, Olson DG, Dolben EL, Lynd LR, Bhuju S, O'Toole GA. 2015. Coculture of Staphylococcus aureus with Pseudomonas aeruginosa drives S. aureus towards fermentative metabolism and reduced viability in a cystic fibrosis model. J Bacteriol 197:2252–2264. doi: 10.1128/JB.00059-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mashburn LM, Jett AM, Akins DR, Whiteley M. 2005. Staphylococcus aureus serves as an iron source for Pseudomonas aeruginosa during in vivo coculture. J Bacteriol 187:554–566. doi: 10.1128/JB.187.2.554-566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osborne J, Djapgne L, Tran BQ, Goo YA, Oglesby-Sherrouse AG. 2014. A method for in vivo identification of bacterial small RNA-binding proteins. Microbiologyopen 3:950–960. doi: 10.1002/mbo3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Payne SM. 1993. Iron acquisition in microbial pathogenesis. Trends Microbiol 1:66–69. doi: 10.1016/0966-842X(93)90036-Q. [DOI] [PubMed] [Google Scholar]
- 50.Braun V. 2001. Iron uptake mechanisms and their regulation in pathogenic bacteria. Int J Med Microbiol 291:67–79. doi: 10.1078/1438-4221-00103. [DOI] [PubMed] [Google Scholar]
- 51.Anzaldi LL, Skaar EP. 2010. Overcoming the heme paradox: heme toxicity and tolerance in bacterial pathogens. Infect Immun 78:4977–4989. doi: 10.1128/IAI.00613-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winsor GL GE, Lo R, Dhillon BK, Shay JA, Brinkman FS. 2016. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res 44:D646–D653. doi: 10.1093/nar/gkv1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palmer KL, Mashburn LM, Singh PK, Whiteley M. 2005. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J Bacteriol 187:5267–5277. doi: 10.1128/JB.187.15.5267-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmer KL, Aye LM, Whiteley M. 2007. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol 189:8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barton HA, Johnson Z, Cox CD, Vasil AI, Vasil ML. 1996. Ferric uptake regulator mutants of Pseudomonas aeruginosa with distinct alterations in the iron-dependent repression of exotoxin A and siderophores in aerobic and microaerobic environments. Mol Microbiol 21:1001–1017. doi: 10.1046/j.1365-2958.1996.381426.x. [DOI] [PubMed] [Google Scholar]
- 56.Sinclair PR, Gorman N, Jacobs JM. 2001. Measurement of heme concentration. Curr Protoc Toxicol Chapter 8:Unit 8.3. doi: 10.1002/0471140856.tx0803s00. [DOI] [PubMed] [Google Scholar]
- 57.Meyer JM. 2000. Pyoverdines: pigments, siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch Microbiol 174:135–142. doi: 10.1007/s002030000188. [DOI] [PubMed] [Google Scholar]
- 58.Schwyn B, Neilands JB. 1987. Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 59.Mourino S, Giardina BJ, Reyes-Caballero H, Wilks A. 2016. Metabolite-driven regulation of heme uptake by the biliverdin IXβ/δ selective heme oxygenase (HemO) of Pseudomonas aeruginosa. J Biol Chem 291:20503–20515. doi: 10.1074/jbc.M116.728527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rao J, DiGiandomenico A, Unger J, Bao Y, Polanowska-Grabowska RK, Goldberg JB. 2008. A novel oxidized low-density lipoprotein-binding protein from Pseudomonas aeruginosa. Microbiology 154:654–665. doi: 10.1099/mic.0.2007/011429-0. [DOI] [PubMed] [Google Scholar]
- 61.Collier DN, Anderson L, McKnight SL, Noah TL, Knowles M, Boucher R, Schwab U, Gilligan P, Pesci EC. 2002. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol Lett 215:41–46. doi: 10.1111/j.1574-6968.2002.tb11367.x. [DOI] [PubMed] [Google Scholar]
- 62.Pesci EC, Milbank JB, Pearson JP, McKnight S, Kende AS, Greenberg EP, Iglewski BH. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.West SE, Schweizer HP, Dall C, Sample AK, Runyen-Janecky LJ. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148:81–86. doi: 10.1016/0378-1119(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 64.Holloway BW. 1955. Genetic recombination in Pseudomonas aeruginosa. J Gen Microbiol 13:572–581. [DOI] [PubMed] [Google Scholar]
- 65.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.