ABSTRACT
Coxiella burnetii, the causative agent of Q fever, establishes a unique lysosome-derived intracellular niche termed the Coxiella-containing vacuole (CCV). The Dot/Icm-type IVB secretion system is essential for the biogenesis of the CCV and the intracellular replication of Coxiella. Effector proteins, translocated into the host cell through this apparatus, act to modulate host trafficking and signaling processes to facilitate CCV development. Here we investigated the role of CBU0077, a conserved Coxiella effector that had previously been observed to localize to lysosomal membranes. CBU0077 was dispensable for the intracellular replication of Coxiella in HeLa and THP-1 cells and did not appear to participate in CCV biogenesis. Intriguingly, native and epitope-tagged CBU0077 produced by Coxiella displayed specific punctate localization at host cell mitochondria. As such, we designated CBU0077 MceA (mitochondrial Coxiella effector protein A). Analysis of ectopically expressed MceA truncations revealed that the capacity to traffic to mitochondria is encoded within the first 84 amino acids of this protein. MceA is farnesylated by the host cell; however, this does not impact mitochondrial localization. Examination of mitochondria isolated from infected cells revealed that MceA is specifically integrated into the mitochondrial outer membrane and forms a complex of approximately 120 kDa. Engineering Coxiella to express either MceA tagged with 3×FLAG or MceA tagged with 2×hemagglutinin allowed us to perform immunoprecipitation experiments that showed that MceA forms a homo-oligomeric species at the mitochondrial outer membrane during infection. This research reveals that mitochondria are a bona fide target of Coxiella effectors and MceA is a complex-forming effector at the mitochondrial outer membrane during Coxiella infection.
KEYWORDS: Coxiella burnetii, bacterial effector, mitochondria, prenylation, host-pathogen interactions
INTRODUCTION
Coxiella burnetii is the causative agent of human Q fever, a zoonotic infection with a worldwide distribution. The extremely low infectious dose, the ease of aerosol dissemination, and the environmental stability of this pathogen all contribute to Coxiella being classified as a category B select agent by the U.S. Centers for Disease Control and Prevention (1, 2).
During human infection, inhaled Coxiella bacteria predominantly infect alveolar macrophages, where they develop a unique intracellular replicative niche termed the Coxiella-containing vacuole (CCV). Internalized bacteria are trafficked through the endocytic pathway until they reach the acidic and hydrolytic confines of the lysosome. Here, environmental cues stimulate the metabolism of Coxiella, leading to the initiation of pathogen-directed changes to the CCV (3). The active and replicating Coxiella bacteria remodel the CCV into a highly fusogenic vacuole that expands to occupy the majority of the host cell space (4). The CCV promiscuously fuses with endosomal vesicles and mature autophagosomes to maintain an autolysosomal stage of maturation that further promotes the homotypic fusion of CCVs (5).
The recent development of axenic culture conditions for Coxiella has allowed researchers to genetically manipulate this pathogen and conduct investigations into the contribution of individual bacterial genes to Coxiella pathogenesis (6, 7). As such, in a short space of time, much has been revealed about the pathogenesis of this organism. Initial analysis of transposon mutants led to confirmation that a Dot/Icm-type IVB secretion system is essential for intracellular replication (8, 9). This secretion system is homologous and functionally analogous to the well-studied Legionella Dot/Icm system that delivers a large cohort of effector proteins into the host cytosol to manipulate host cell processes, enabling intracellular replication of the pathogen (10; reviewed in reference 11). Significantly, unlike Legionella, where the Dot/Icm system begins translocating effectors upon contact with a host cell (12), the Coxiella Dot/Icm system is not activated until the pathogen is trafficked to the lysosome (13).
In recent years, over 130 Coxiella Dot/Icm effector proteins have been identified, although the function of the majority of these effectors remains unknown. Importantly, several mutant screens have identified a number of Dot/Icm effectors that are required for efficient intracellular replication and CCV biogenesis (14–17). The function of these effectors is an expanding area of significant investigation. Much of the characterization of bacterial effector proteins relies on examination of the effector when it is ectopically expressed in eukaryotic host cells. This approach usually involves the use of an epitope tag to monitor the effector and significantly simplifies the context of effector activity by eliminating both host responses to infection and the presence of other bacterial factors that may influence the effector under investigation. Despite these caveats, ectopic expression has been used to demonstrate that Coxiella effectors direct an array of subcellular localizations within eukaryotic cells (8, 16, 18).
A small cohort of the investigated Coxiella effectors displays mitochondrial localization when ectopically expressed in human cell lines. Epitope-tagged and ectopically expressed CBUA0020, CBU1825, CBU1425, and AnkJ colocalize with mitochondrial markers (8, 16, 18). Additionally, the antiapoptotic effectors CaeB (CBU1532) and AnkG (CBU0781) associate with mitochondria (8, 19, 20). AnkG is believed to sense apoptotic stress at mitochondria, leading to interaction with p32 and subsequent translocation to the nucleus, where it exerts a strong, physiologically relevant, antiapoptotic phenotype (19, 21). Thus, mitochondria represent an important effector target for the control of host viability. However, mitochondria also participate in a range of other important cellular functions, including energy production, lipid metabolism, calcium homeostasis, and immune signaling. Coxiella effectors targeting the mitochondria could potentially have a significant influence on multiple aspects of the infection environment (reviewed in reference 22).
Here we describe the characterization of the mitochondrial Coxiella effector protein A (MceA; CBU0077), which we previously observed to be localized to lysosomal membranes during ectopic expression in HeLa cells (8). Our investigation of native and tagged MceA, expressed by Coxiella and translocated into host cells via the Dot/Icm system, demonstrates specific and distinct localization to the mitochondrial outer membrane, where MceA interacts with itself to form a multimeric complex. In addition, the host cell farnesylates MceA, which may contribute to the stability of the mitochondrial localization. Characterization of Coxiella mceA mutants demonstrated that this effector is dispensable for CCV biogenesis and intracellular replication. This work has progressed our understanding of the interactions between Coxiella and human host cells and identified the mitochondrion to be a genuine target of Coxiella effectors during infection. In addition, we have highlighted the importance of characterizing effector localization in the context of infection.
RESULTS
MceA is dispensable for intracellular growth of Coxiella.
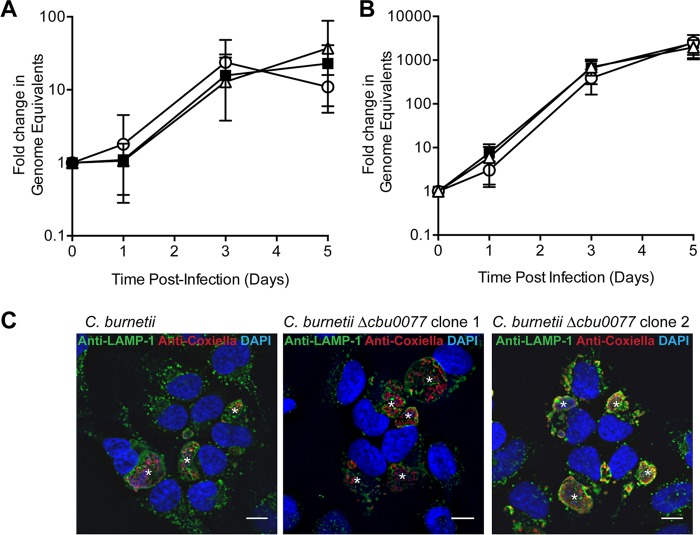
A nonbiased screen to identify Coxiella proteins that could be translocated by the Legionella pneumophila Dot/Icm type IV secretion system (T4SS) identified 18 novel Coxiella effectors (8). Of the effectors in this cohort, we were drawn to CBU0077 since this was the only effector conserved in all sequenced Coxiella genomes, suggesting that there is likely an evolutionary advantage for Coxiella to maintain functional CBU0077. We constructed a cbu0077 mutant of C. burnetii RSA439 Nine Mile phase II (NM PhII), replacing cbu0077 with a kanamycin resistance cassette, using a recently described homologous recombination protocol (23). Two independent mutants were isolated and confirmed using both Southern hybridization and PCR analysis. We examined any requirement for CBU0077 for the intracellular growth of Coxiella by quantifying the replication of both Δcbu0077 clones in HeLa epithelial cells and THP-1 macrophage-like cells. Intracellular growth curves were performed, and the number of Coxiella bacteria present was enumerated via quantitative PCR (qPCR) at days 0, 1, 3, and 5 (Fig. 1A and B). Both Δcbu0077 clones showed a level of replication equivalent to that of the parental strain in both infection models, indicating that CBU0077 is not required for the intracellular growth of Coxiella. When HeLa cells were stained with anti-LAMP-1 and anti-Coxiella to examine the morphology of the CCV (Fig. 1C), the CCVs formed by both Coxiella Δcbu0077 clones were comparable to those formed by the wild type, indicating that CBU0077 is not required for CCV biogenesis.
FIG 1.
CBU0077 is not required for CCV biogenesis or the intracellular replication of Coxiella. (A, B) Two independent Coxiella Δcbu0077 clones were assessed for their ability to replicate in HeLa cells (A) and THP-1 cells (B) over a 5-day infection period. (A) HeLa cells were infected at an MOI of 100, and lysates of infected cells were collected at days 0, 1, 3, and 5 postinfection to enumerate the Coxiella genome equivalents using qPCR. (B) THP-1 cells were infected at an MOI of 25, and samples were collected at the same time points described above. Graphs represent the fold change in genome equivalents (± standard deviation) relative to those on day 0 for the Coxiella NM PhII wild type (black squares), Δcbu0077 clone 1 (white circles), and Δcbu0077 clone 2 (white triangles) from at least five independent experiments. (C) At day 3 postinfection, HeLa cells were fixed and stained with anti-LAMP-1 (green) and anti-Coxiella (red). *, CCVs. Bars, 10 μm.
Translocated CBU0077 localizes to mitochondria.
Previously, we observed that transfected 3×FLAG-tagged CBU0077 (3×FLAGCBU0077), expressed from pFLAG-0077, colocalized with the lysosomal marker LAMP-1 (8). Thus, it was not surprising to observe that ectopically expressed 3×FLAGCBU0077 specifically colocalized with LAMP-1 on the CCV membrane of infected cells (Fig. 2A). In order to validate this localization, we introduced pJB-CAT:3×FLAG-0077 into Coxiella cells and examined the subcellular localization of the 3×FLAGCBU0077 that was produced by Coxiella bacteria and translocated into the host cell via the Dot/Icm secretion system (Fig. 2B). Translocated 3×FLAGCBU0077 did not localize with LAMP-1 and instead was observed as puncta associated with mitochondria. This punctate mitochondrial localization represents the true localization of CBU0077 during infection, as shown with C. burnetii NM PhII using specific antiserum raised against recombinant CBU0077 and MitoTracker Red (Thermo Fisher) (Fig. 2C). Given this consistent observation of the mitochondrial localization of CBU0077, we propose to name CBU0077 MceA, for mitochondrial Coxiella effector protein A. We were able to confirm the specificity of our anti-MceA antibody by comparing infection of HeLa cells by wild-type Coxiella with infection of HeLa cells by our two mceA mutants (Fig. 2D). Here we consistently observed that HeLa cells infected with wild-type Coxiella demonstrated punctate MceA staining that colocalized with MitoTracker Red, and this signal was absent in the cells infected with the mceA mutants.
FIG 2.
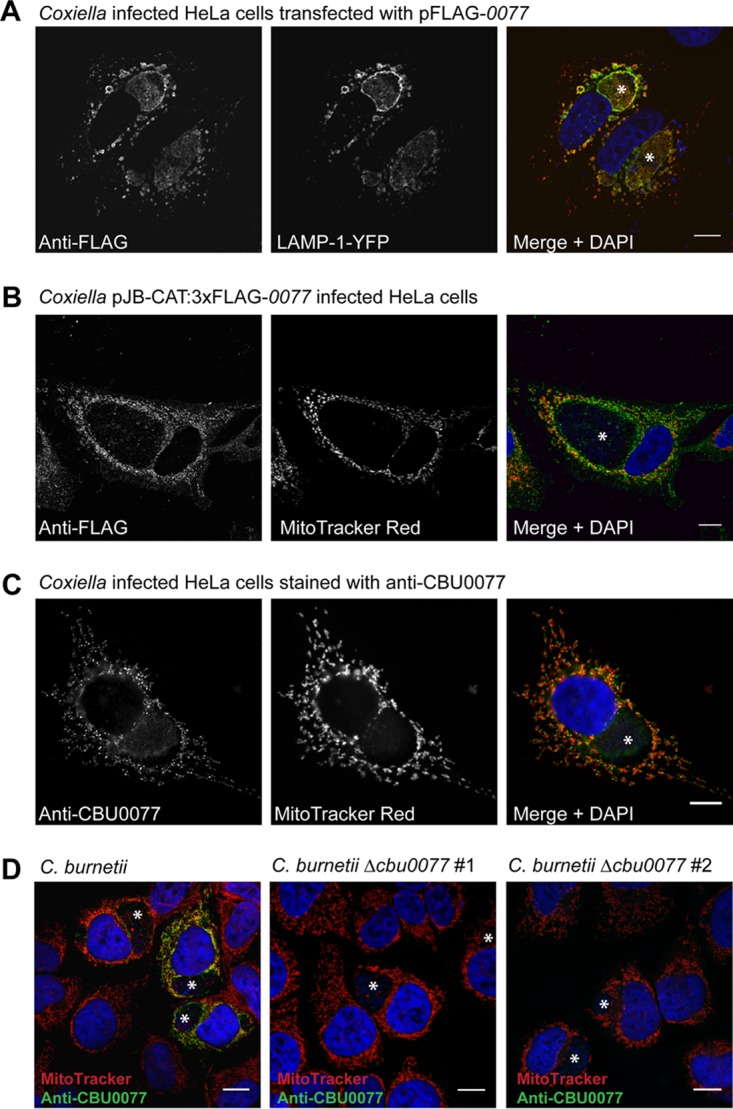
Transfected and translocated CBU0077 proteins demonstrate distinct subcellular localizations. (A) HeLa cells, infected with Coxiella NM PhII and transfected with pFLAG-0077 and pYFP-LAMP-1, demonstrate that ectopically expressed CBU0077 shows lysosomal membrane localization. (B, C) In contrast, 3×FLAGCBU0077 (B) and the native CBU0077 (C) expressed by Coxiella colocalize with mitochondria stained with MitoTracker Red. (D) This specific mitochondrial localization of CBU0077 was further verified by observing no anti-CBU0077 signal for HeLa cells infected with two independent Coxiella Δcbu0077 strains. These confocal microscopy images are representative of images showing consistently observed localization. YFP, yellow fluorescent protein; *, CCV. Bars, 10 μm.
The N terminus of MceA mediates mitochondrial localization.
Bioinformatic analysis of MceA, using the TMPred server, suggested four possible regions of hydrophobicity that could represent transmembrane domains (Fig. 3A). We asked if ectopic expression of truncated versions of MceA would also result in mislocalization, as was observed for the full-length protein. We assessed the localization of different MceA truncations in Coxiella-infected HeLa cells (Fig. 3B to E) and were able to demonstrate that, unlike full-length MceA, a truncated version of the protein consisting of amino acids 1 to 144 (MceA1–144) specifically localized to the mitochondria and was observed as mitochondrial puncta, reminiscent of the localization of the native effector (Fig. 3D). The region of MceA responsible for mitochondrial localization was further defined to be amino acids 1 to 84 (MceA1–84), indicating that this N-terminal region, which encodes two predicted transmembrane domains, contains the targeting elements necessary for mitochondrial localization (Fig. 3E).
FIG 3.
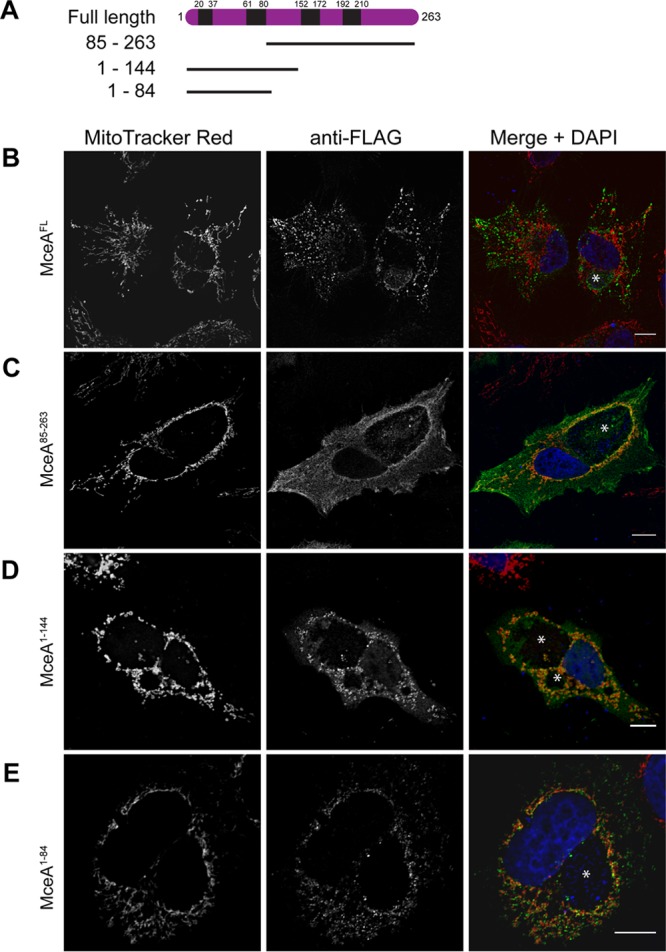
The N-terminal 84 amino acids of MceA can direct mitochondrial localization. (A) The schematic representation demonstrates that MceA is a 263-amino-acid protein with four predicted transmembrane domains (black). Several truncated versions incorporating either the N-terminal or C-terminal transmembrane domain were created and examined for localization. (B to E) HeLa cells persistently infected with Coxiella were transfected with pFLAG constructs to produce 3×FLAG-tagged full-length MceA (MceAFL) (B), MceA85–263 (C), MceA1–144 (D), or MceA1–84 (E). At 24 h posttransfection, cells were incubated for 30 min with MitoTracker Red before being fixed and stained with anti-FLAG and examined with a confocal microscope. The representative images shown here demonstrate that MceAFL and MceA85–263 do not associate with mitochondria; however, the N-terminal fragments of MceA from residues 1 to 144 and 1 to 84 do direct mitochondrial localization. *, CCVs. Bars, 10 μm.
Translocated MceA is farnesylated during infection, but this does not alter mitochondrial localization.
Analysis of the amino acid sequence of MceA revealed a C-terminal CAAX motif. In eukaryotic cells, this C-terminal signature of a cysteine residue that is followed by two aliphatic residues and that terminates with any amino acid directs the posttranslational lipid modification termed prenylation. Protein prenylation involves addition of either farnesyl (15-carbon) or geranylgeranyl (20-carbon) isoprenoids to the terminal cysteine residue. For many eukaryotic proteins, this process is important for anchoring the protein into cellular membranes. Given that MceA terminates with the amino acids CSIM, we examined whether the host cell modified this motif and if this modification impacted the mitochondrial localization of MceA.
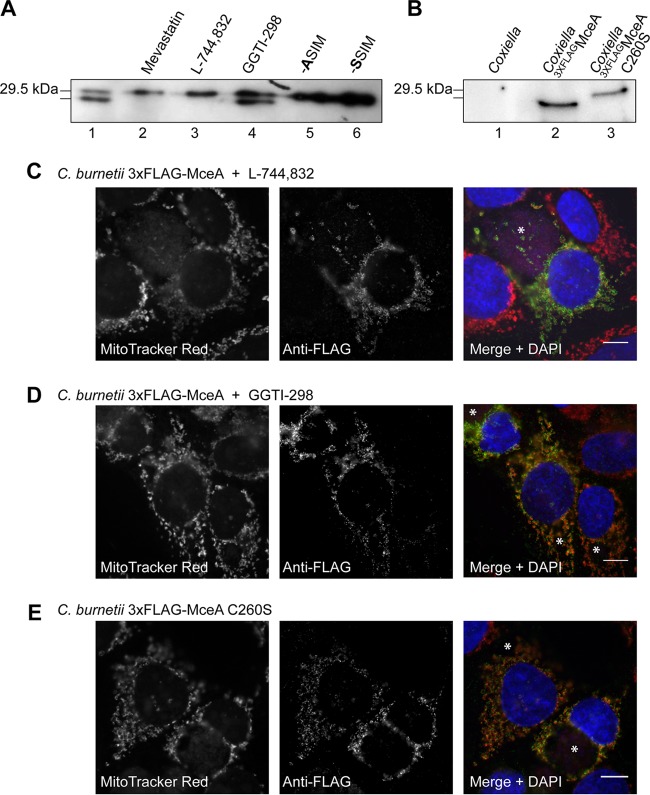
Initial observations of ectopically expressed MceA tagged with 3×FLAG (3×FLAGMceA) consistently showed a doublet during anti-FLAG immunodetection. The lower band was absent in the presence of 10 μM the prenylation inhibitor mevastatin (Sigma-Aldrich), indicating that this may represent a prenylated form of 3×FLAGMceA (Fig. 4A). Increased electrophoretic mobility has long been observed for prenylated eukaryotic proteins, including Rab GTPases, and is thought to represent the increased hydrophobicity of the lipidated form of the protein (24). This was further validated when only the higher band was observed for site-directed mutants of 3×FLAGMceA in which the cysteine residue at position 260 was altered to alanine (C260A) or serine (C260S) (Fig. 4A). These data indicate that MceA is prenylated by eukaryotic cells, and immunodetection of ectopically expressed 3×FLAGMceA in the presence of the farnesyltransferase inhibitor L-744,832 (Sigma-Aldrich) or the geranylgeranyltransferase I inhibitor GGTI-298 (Sigma-Aldrich) further characterized this modification as farnesylation (Fig. 4A). To determine if MceA is farnesylated during infection, we compared the size of 3×FLAGMceA and 3×FLAGMceA C260S expressed by Coxiella and translocated into HeLa cells. These proteins showed a distinct size difference, indicating that translocated 3×FLAGMceA is farnesylated by the host during infection (Fig. 4B). In order to determine whether the farnesylation of MceA is important for mitochondrial localization, we compared the localization of MceA in the presence of 1 μM GGTI-298, which does not impact the farnesylation of MceA; MceA in the presence of 5 μg/ml L-744,832, which blocks farnesylation; and MceA C260S, which was unable to be farnesylated (Fig. 4C to E). These experiments demonstrate that translocated MceA localizes to mitochondrial puncta independently of the addition of a farnesyl group.
FIG 4.
MceA is farnesylated in HeLa cells, but this does not influence mitochondrial localization. (A) HeLa cells were transfected with pFLAG-MceA for 24 h before being treated with mevastatin (10 μM), L-744,832 (5 μg/ml), or GGTI-298 (1 μM) for 16 h. Cell lysates were collected, separated by SDS-PAGE, and transferred to a nitrocellulose membrane before being probed with anti-FLAG. Ectopically expressed 3×FLAGMceA forms two distinct bands when detected with anti-FLAG. The lower band represents a prenylated form of 3×FLAGMceA, as both the prenylation inhibitor mevastatin and the farnesyltransferase inhibitor L-744,832, but not the geranylgeranyltransferase I inhibitor GGTI-298, abolished the presence of this form of 3×FLAGMceA. Prenylation was confirmed by the absence of the lower band when 3×FLAGMceA C260A or 3×FLAGMceA C260S was ectopically expressed. (B) MceA prenylation occurs during infection, as the unmodified 3×FLAGMceA C260S expressed and translocated by Coxiella is observed as a band higher than the band for translocated 3×FLAGMceA. Lysate was collected from infected HeLa cells, and proteins were detected with anti-FLAG. (C to E) Host modification does not alter the mitochondrial localization of MceA. HeLa cells were persistently infected with Coxiella expressing 3×FLAGMceA and treated with either L-744,832 (C) or GGTI-298 (D) for 16 h before being stained with MitoTracker Red and anti-FLAG. Under both treatment conditions, MceA still demonstrated specific punctate mitochondrial localization. (E) Similarly, unmodified 3×FLAGMceA C260S expressed by Coxiella was consistently observed in the same pattern. *, CCVs. Bars, 10 μm.
MceA localizes to the mitochondrial outer membrane.
Given the observed mitochondrial localization and potential membrane-spanning nature of MceA, we set out to establish if the protein is integrated into mitochondrial membranes and, if so, whether it is localized to the outer or inner membrane. Mitochondria were isolated from HeLa cells persistently infected with Coxiella using magnetic beads labeled with antibodies against the mitochondrial outer membrane protein Tom22, to ensure the specific enrichment of mitochondria. This approach has previously been reported to produce a high yield of pure and intact mitochondria (25, 26). Immunoblotting for a mitochondrial protein, Bak, demonstrated that this protocol significantly enriched for mitochondria, and probing for the endoplasmic reticulum (ER) protein PDI showed that minimal ER was present in these mitochondrial preparations (see Fig. S1 in the supplemental material). Isolated mitochondria were treated with sodium carbonate for the separation of membrane-integrated proteins from peripherally associated proteins. This alkaline treatment disrupts electrostatic interactions and the hydrogen bonds of peripheral membrane proteins, while integral membrane proteins remain associated with the lipid bilayer (27–29). Detection of the peripheral membrane component of complex II, succinate dehydrogenase complex subunit A (SDHA), and the membrane-integrated outer membrane protein Bak served as controls for this experiment. The 3×FLAGMceA appearing in the pellet fraction was resistant to carbonate treatment, like the membrane-integrated protein Bak, indicating that it is likely an integral membrane protein (Fig. 5A).
FIG 5.
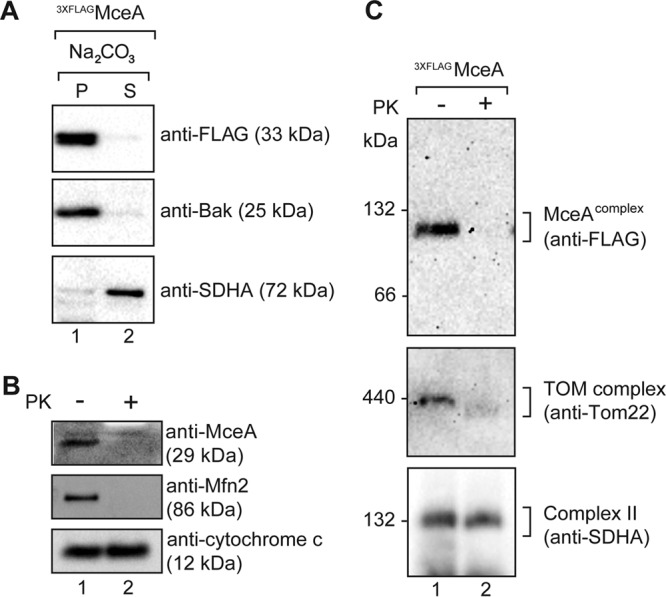
MceA is localized to the mitochondrial outer membrane. (A) Mitochondria isolated from cells infected with Coxiella expressing 3×FLAGMceA were subjected to alkaline extraction using 100 mM Na2CO3 (pH 11). The membrane-integrated protein fraction (the pellet fraction [P]) and the peripheral membrane protein fraction (the supernatant fraction [S]) were obtained by ultracentrifugation and subsequently analyzed by SDS-PAGE and immunoblotting using the indicated antibodies. (B and C) Mitochondria isolated from cells infected with Coxiella expressing 3×FLAGMceA were either left untreated (lanes 1) or treated with PK (50 μg/ml) (lanes 2) and either analyzed by SDS-PAGE (B) or solubilized in digitonin-containing buffer and analyzed by BN-PAGE (C). Immunoblotting was performed with the indicated antibodies.
The punctate nature of the MceA mitochondrial localization observed by fluorescence microscopy (Fig. 2B to D) suggested that the protein is likely associated with the outer membrane. To confirm this localization, mitochondria isolated from infected cells were either left untreated or treated with external proteinase (proteinase K [PK]). The localization of endogenous MceA was assessed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and immunoblotting with anti-MceA serum. Indeed, MceA was degraded by external protease, like the outer membrane protein Mfn2 (Fig. 5B). To the contrary, the inner membrane-localized protein cytochrome c remained protected from external protease, confirming that the integrity of the mitochondria was not compromised. Thus, we conclude that MceA is an integral membrane protein of the mitochondrial outer membrane.
MceA interacts with itself at the mitochondrial outer membrane.
To gauge some insight into potential mitochondrial interactions of MceA, we assessed if the protein forms any high-molecular-mass complexes within the outer membrane. Mitochondria were isolated from cells infected with Coxiella expressing 3×FLAGMceA, and the assembly of MceA was monitored by blue native (BN) electrophoresis upon solubilization of the mitochondria in digitonin. MceA was observed in a complex of approximately 120 kDa (Fig. 5C, top), which was sensitive to external protease, further supporting the outer membrane localization of the protein. In a similar manner, the outer membrane TOM complex displayed sensitivity (Fig. 5C, middle) to protease, as expected, while complex II of the inner membrane remained protected (Fig. 5C, bottom). Importantly, we also observed the 120-kDa complex with endogenous, untagged MceA (data not shown).
To confirm that the 120-kDa complex was a bona fide complex of the mitochondrial outer membrane, mitochondria were isolated from HeLa cells infected with Coxiella expressing 3×FLAGMceA or the previously described prenylation-deficient 3×FLAGMceA C260S protein and were solubilized in digitonin in the presence of SDS (Fig. 6A). Such treatment can be used to dissociate mitochondrial complexes, such as translocation machineries (30), and aid in distinguishing between genuine complexes and monomeric protein species. Importantly, 3×FLAGMceA C260S was also observed in the 120-kDa complex, and SDS treatment dissociated both the wild-type and mutant MceA complexes (Fig. 6A). The SDS-mediated dissociation of complex I, monitored with anti-NDUFA9, was used as a positive control here. It should be noted that we observed the MceA complex to be more resistant to SDS treatment than complex I, indicating that MceA likely forms a much more stable complex that is integrated into the mitochondrial outer membrane. These data strongly indicate that the 120-kDa MceA complex is a genuine complex of the mitochondrial outer membrane.
FIG 6.
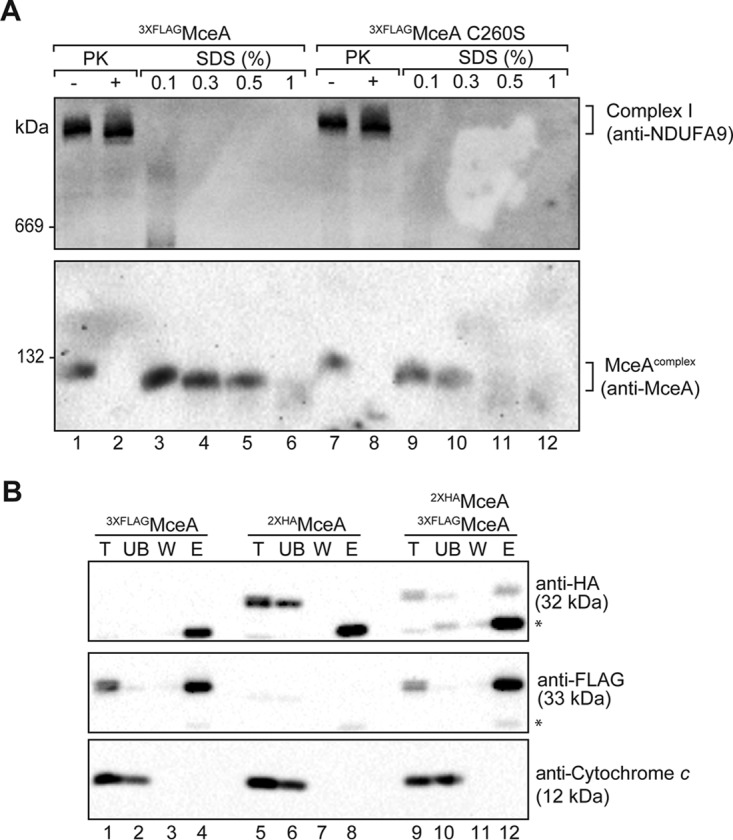
MceA interacts with itself at the mitochondrial outer membrane. (A) Mitochondria isolated from cells infected with Coxiella expressing 3×FLAGMceA or 3×FLAGMceA C260S were isolated and either left untreated (lanes 1 and 7) or treated with PK (50 μg/ml) (lanes 2 and 8), reisolated, and solubilized in digitonin-containing buffer. For SDS treatment (lanes 3 to 6 and 9 to 12), mitochondria were solubilized in digitonin-containing buffer before addition of SDS at the indicated concentration (in percent [wt/vol]). Samples were analyzed by BN-PAGE and immunoblotting with anti-MceA and anti-NDUFA9 antibodies. (B) Mitochondria isolated from cells infected with Coxiella expressing either 3×FLAGMceA (lanes 1 to 4) or 2×HAMceA (lanes 5 to 8) or cells coinfected with Coxiella expressing both 3×FLAGMceA and 2×HAMceA (lanes 9 to 12) were solubilized in digitonin-containing buffer and incubated with anti-FLAG resin. Bound proteins were eluted in 0.2 M glycine following washing. The collected fractions were analyzed by SDS-PAGE and immunoblotting with anti-FLAG, anti-HA, and anti-cytochrome c antibodies. Five percent of each of the total (T), unbound (UB), and wash (W) fractions and 100% of the elution (E) fraction were loaded for SDS-PAGE analysis. *, a nonspecific band that is likely the immunoglobulin light chain.
We made numerous attempts to verify potential interacting partners of MceA by immunoprecipitation and mass spectrometry; however, we were unable to obtain any reproducible candidate interacting partners. This raised the possibility that MceA could be associating within a homo-oligomeric species within the 120-kDa complex. To address this, HeLa cells were coinfected with Coxiella expressing either 3×FLAGMceA or MceA tagged with 2× hemagglutinin (HA) (2×HAMceA) from a plasmid. We reasoned that if MceA forms a homo-oligomeric species, we should be able to immunoprecipitate HA-tagged MceA with the FLAG-tagged protein, or vice versa. Indeed, when we performed immunoprecipitations using anti-FLAG resin, we consistently observed the 2×HAMceA to coelute with the 3×FLAGMceA protein (Fig. 6B). Thus, our data suggest that MceA is a protein that assembles into a 120-kDa homo-oligomeric complex at the mitochondrial outer membrane.
DISCUSSION
In recent years, research into the function of effector proteins produced by bacterial pathogens has proven an exciting area of discovery in terms of understanding host-pathogen interactions and in providing novel insight and elucidating the biology of eukaryotic host cells. A common approach employed to understand effector function is to observe the localization and functional impact of ectopically expressed effectors in model systems, such as yeast, Saccharomyces cerevisiae, and mammalian tissue culture cells. This methodology has yielded substantial insight and remains a standard tactic to resolve information about effector functions. There are, however, significant caveats associated with these experiments, in that they do not incorporate the altered host state and the impact of other effectors during infection, particularly given that there is increasing evidence that bacterial effector proteins can influence the function of each other (reviewed in reference 31). Even if transfections are performed in the context of tissue culture infection, the impact of significantly overexpressing one effector, usually with an epitope or fluorescent tag, may lead to findings that are not relevant during natural infections.
Here we provide a profound example of how the ectopic expression of bacterial proteins in eukaryotic cells can be misleading. The Coxiella effector MceA specifically localizes to the outer membrane of mitochondria during infection, but when it is transfected into mammalian cells, MceA is associated with lysosomal membranes. Interestingly, a recent publication demonstrated that green fluorescent protein-tagged MceA shows some mitochondrial localization when it is expressed in HeLa cells (32). The spatial arrangement of effector proteins may depend on the presence and/or action of other effectors; however, this does not appear to be the case for MceA, given both the inability of ectopically expressed MceA to correctly localize in infected cells and the observation that eukaryotically expressed truncated MceA encoding the N-terminal region localizes to mitochondria. It is more likely that the different folding states of MceA produced within the eukaryotic cell rather than the introduction of MceA into the cell through the Dot/Icm secretion system contribute to altered membrane localizations.
Here we evaluated the contribution of MceA to CCV biogenesis and the intracellular replication of Coxiella by constructing a mutant in which mceA was replaced with a kanamycin resistance gene. We observed no defect in the intracellular replication of Coxiella in the absence of MceA, confirming the findings of a previous study that observed that the disruption of mceA with a transposon does not impact the intracellular replication of Coxiella (14). These studies do not rule out the possibility that MceA impacts the intracellular fitness of Coxiella in a manner that cannot be measured by our assays, for example, dissemination of the bacteria in a multicellular organism, and/or that MceA contributes to an important function that is redundant among multiple mitochondrial effectors.
We also demonstrated that host cells posttranslationally modify MceA during infection. Using inhibitors of prenylation and site-directed mutants of MceA, we have shown that the cysteine residue of the C-terminal CAAX (CSIM) motif is farnesylated. Protein prenylation increases the hydrophobicity of a protein and can contribute to membrane association, often in conjunction with additional modifications or a polybasic domain (reviewed in reference 33). This is the first report of host cell lipid posttranslational modification of a Coxiella effector; however, host prenylation of bacterial effectors has been observed in the context of other infections. For example, the Salmonella effector SifA is prenylated, aiding the membrane localization of this important effector, and prenylation of several Legionella pneumophila effectors with the C-terminal CAAX motif has been demonstrated (34). Prenylation enhances the membrane affinity for most of these effectors, facilitating their specific localization during infection (34, 35). Prenylation of MceA does not modify the mitochondrial outer membrane localization or its capacity to self-assemble, but it may be important to facilitate the function of MceA at this subcellular location.
The use of chemical inhibitors to disrupt the host prenylation machinery negatively impacts the biogenesis of the Legionella-containing vacuole, suggesting that this modification of bacterial and/or host proteins is important for the intracellular success of L. pneumophila (34). Conversely, as shown by the large CCVs in Fig. 4, we observed no significant alterations in CCV biogenesis during a 16-h treatment with L-744,832 or GGTI-298, the peptidomimetic inhibitors that target farnesyltransferase and geranylgeranyltransferase I, respectively. However, this observation was not quantified; thus, it remains possible that this posttranslational modification system plays a minor role in maintenance of the mature CCV.
Both epitope-tagged and endogenous MceA are integrated into the mitochondrial outer membrane during Coxiella infection. The outer membrane of mitochondria forms the barrier between the organelle and the rest of the cell, and proteins of varied functions, including translocation components, morphology mediators, and key players in cell death, reside in the outer membrane (22). This collection of protein functions is accommodated by unique protein topologies in which the proteins are categorized broadly into three distinct classes: (i) peripheral proteins, (ii) membrane proteins with β-barrel transmembrane segments, and (iii) membrane proteins with α-helical transmembrane domains. MceA is predicted to contain four α-helical transmembrane domains (Fig. 4A), and there is significant variation in the topology of proteins with α-helical transmembrane domains as well as their mechanism of integration into the membrane. For example, the ubiquitin ligase MARCH5 and translocator protein (TSPO), which comprise four and five helical transmembrane domains, respectively (36–39), require the Tom70 receptor for integration into the outer membrane (40). To the contrary, another multispanning membrane protein, Ugo1, appears to rely on the lipid composition of the outer membrane for its biogenesis (41). Further investigation is required to determine if MceA follows one of these established pathways or a completely unique biogenesis pathway. However, we have verified that the first 84 amino acids (incorporating the first two predicted transmembrane domains) of MceA contain all targeting elements necessary for mitochondrial localization. This is not surprising, considering that the C terminus of the protein is predicted to encode a signal for translocation via the Dot/Icm T4SS into the host cell. Thus, positioning of the MceA mitochondrial localization signal at the N terminus may represent adaptation to the multiple membranes that the protein must cross during its biogenesis.
Although the precise function and contribution of MceA to Coxiella pathogenesis remain to be established, the identification of the protein within an oligomeric complex at the outer membrane represents an exciting avenue to explore in future research. Our data suggest that host cell prenylation is not necessary for the formation of the 120-kDa MceA complex, but it is feasible to suggest that the function of MceA is reliant on the protein's oligomeric state. For instance, the mitochondrial outer membrane protein insertase Mim1 contains a single α-helical transmembrane segment and does not function as a monomer (13 kDa) but, rather, functions within a 200-kDa oligomeric complex (42–44). The transmembrane segment of Mim1 is critical for oligomerization and the function of the protein and occurs via two helix dimerization GXXX(G/A) motifs (45). The GXXXG motif (in which two glycine residues are separated by any three residues) and GXXXG-like motifs (in which one or both glycine residues are replaced by other small residues, such as alanine) are often found to be important for mediating the interaction of transmembrane helices (46). Proteins with these sequences have been proposed to have a strong potential for oligomerization, likely promoted by additional specificity elements (47). Indeed, closer examination of MceA transmembrane sequences reveals two GXXXA motifs encompassing residues 69 to 73 (predicted transmembrane 2 [TM 2]) and 195 to 199 (predicted TM 4) and representing exciting candidates for future mutagenesis work.
This research has demonstrated that the Coxiella effector protein MceA is capable of assembly at the outer membrane of mitochondria, highlighting that mitochondria are a bona fide target during Coxiella infection. The unique trafficking of this protein may serve as a tool to study protein trafficking in mammalian mitochondria, which could reveal unique biogenesis pathways. Further functional investigation of mitochondria during Coxiella infection may reveal the collective impact of targeting multiple effector proteins to this important organelle and how this contributes to bacterial survival within the host cell.
MATERIALS AND METHODS
Bacterial strains and host cell lines.
Plaque-purified C. burnetii Nine Mile phase II (NMII) strain RSA439 clone 4 was axenically grown in liquid ACCM-2 or in ACCM-2 agarose at 37°C in 5% CO2 and 2.5% O2 as previously described (48). When required, kanamycin and chloramphenicol were added to ACCM-2 at 350 μg/ml and 3 μg/ml, respectively. Escherichia coli DH5α, used for plasmid propagation and construction, was cultivated on Luria-Bertani (LB) agar plates or broth with the addition of chloramphenicol (25 μg/ml), ampicillin (100 μg/ml), or kanamycin (100 μg/ml) as appropriate.
HeLa CCL2 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5 to 10% heat-inactivated fetal calf serum (FCS) at 37°C in 5% CO2. THP-1 cells were maintained in RPMI 1640 supplemented with 10% heat-inactivated FCS at 37°C in 5% CO2.
Plasmid construction.
The oligonucleotides used in this study are listed in Table 1. We previously constructed pFLAG-0077 to express full-length MceA with an N-terminal 3×FLAG tag (8). Truncations of cbu0077 were cloned into the BamHI/XhoI sites of pcDNA4/TO (Invitrogen) with an N-terminal 3×FLAG tag (pFLAG). In order to express tagged versions of MceA in Coxiella, cbu0077 was cloned into the SalI site of both pJB-CAT:3×FLAG and pJB-CAT:2×HA. Prenylation-deficient versions of MceA were engineered by using alternative C-terminal oligonucleotides (Table 1) to modify the cysteine at position 260 to serine or alanine.
TABLE 1.
Oligonucleotides used in this study
| Namea | Use (restriction enzyme) | Sequenceb (5′-3′) |
|---|---|---|
| FLAG-0077 Fc | Amplify cbu0077 for pFLAG-0077 (BamHI) | AAGGATCCATGAGACAACTCGTTTCAATTAAA |
| FLAG-0077 Rc | Amplify cbu0077 for pFLAG-0077 (XhoI) | AAGAGCTCTTACATAATAGAACACCCACGA |
| FLAG-0077 R C-A | Amplify cbu0077 C260A (XhoI) | AAGAGCTCTTACATAATAGAAGCCCCACGA |
| FLAG-0077 R C-S | Amplify cbu0077 C260S (XhoI) | AAGAGCTCTTACATAATAGAAGACCCACGA |
| FLAG-0077 F@85 | Amplify cbu0077 from amino acid 85 (BamHI) | AAGGATCCAATGATTGGCAACTAAGAAAG |
| FLAG-0077 R@84 | Amplify cbu0077 up to amino acid 84 (XhoI) | CACTCGAGGAAAATAGTTCGAGCCGAAGT |
| FLAG-0077 R@144 | Amplify cbu0077 up to amino acid 144 (XhoI) | CCCTCGAGTTACGGATGTTTTTCAAAAAAGGC |
| pJB-0077 F | Amplify cbu0077 for pJB-Cm:3 × FLAG (SalI) | AAGGTCGACATGAGACAACTCGTTTCAATTAAA |
| pJB-0077 R | Amplify cbu0077 for pJB-Cm:3× FLAG (SalI) | TCAGTCGACTTACATAATAGAACACCCACGA |
| pJB-0077 R C-A | Amplify cbu0077 C260A (SalI) | TCAGTCGACTTACATAATAGAAGCCCCACGA |
| pJB-0077 R C-S | Amplify cbu0077 C260S (SalI) | TCAGTCGACTTACATAATAGAAGACCCACGA |
| cbu0077 up F | Amplify 2 kb upstream of cbu0077 (XbaI) | AATCTAGACGCCGCTTATTAAAAATTCC |
| cbu0077 up R | Amplify 2 kb upstream of cbu0077 | AGTATCCTTACATAAAAATCTCCTTATAATCTT |
| cbu0077 down F | Amplify 2 kb downstream of cbu0077 | GATTTTTATGTAAGGATACTGACGCAATTT |
| cbu0077 down R | Amplify 2 kb downstream of cbu0077 (SalI) | AAGTCGACGCTGTTTAATAGTTCGAACG |
| pJC-0077 F | Amplify pJC-0077 (NotI) | AGTATCCTTACATAAGCGGCCGCGCATGCAAATCTCCTTATAATCTT |
| pJC-0077 R | Amplify pJC-0077 (NotI) | GATTTTTATGTGCATGCGCGGCCGCAAGGATACTGACGCAATTT |
| KanF | Amplify kanamycin resistance cassette from pJB-Kan (NotI) | GACGCGGCCGCAGCTTATGGCTTCGTTTCGCAG |
| KanR | Amplify kanamycin resistance cassette from pJB-Kan (NotI) | GACGCGGCCGCTCAGAAGAACTCGTCAAGAAGGCG |
| Kan probe F | Amplify fragment of kanamycin resistance cassette for Southern hybridization probe | GTGGAGAGGCTATTCGGCTATG |
| Kan probe R | Amplify fragment of kanamycin resistance cassette for Southern hybridization probe | TTGAGCCTGGCGAACAGTT |
| cbu0077 probe F | Amplify 461 bp of cbu0077 for Southern hybridization probe | ATAGATTTGACGGCTGTGC |
| cbu0077 probe R | Amplify 461 bp of cbu0077 for Southern hybridization probe | AAGGGAGGACTCATCGTTGC |
| ompA qPCR F | Quantification of Coxiella genomesd | CAGAGCCGGGAGTCAAGCT |
| ompA qPCR R | Quantification of Coxiella genomesd | CTGAGTAGGAGATTTGAATCGC |
Genetic manipulation of Coxiella.
Plasmid DNA was introduced into Coxiella as previously described (8). However, bacteria were clonally isolated via serial dilution in ACCM-2 agarose plates containing appropriate antibiotics at 24 h postelectroporation. Individual colonies were selected and expanded in ACCM-2, and Western blotting was used to confirm the expression of FLAG-tagged or HA-tagged proteins.
The cbu0077 deletion strain was created through homologous recombination in accordance with a previously described method (23). The PCR products of the 2 kb upstream and downstream of cbu0077 were amplified from genomic DNA and joined together using PfuUltra II Fusion Hotstart DNA polymerase (Agilent Technology). The 4-kb product was ligated into the XbaI and SalI sites of pJC-CAT (49). The resulting construct, pJC-CAT-0077, was then PCR amplified, using oligonucleotides pJC-0077 F and pJC-0077 R, digested with NotI, and ligated with the kanamycin resistance gene amplified from pJB-Kan. The resulting plasmid was introduced into Coxiella to create the cbu0077 deletion strain. The replacement of cbu0077 with a kanamycin resistance gene was confirmed by PCR and Southern hybridization using probes to both cbu0077 and the kanamycin resistance cassette.
Coxiella infection and intracellular growth curves.
Curves of the intracellular growth of Coxiella in both HeLa and THP-1 cells were performed. For HeLa cell experiments, cells were plated at a density of 5 × 104 cells/well in 24-well tissue culture trays on the day before infection, with or without 10-mm glass coverslips, in DMEM with 10% FCS. THP-1 cells were seeded into 24-well tissue culture trays at a density of 5 × 105 cells/well and treated with 10 nM phorbol 12-myristate 13-acetate for 72 h before infection. This treatment induced differentiation into macrophage-like adherent cells. Axenically grown Coxiella strains were quantified using a Quant-iT PicoGreen double-stranded DNA assay kit (Thermo Fisher Scientific) (50) to provide a multiplicity of infection (MOI) of 100 for HeLa cell infections and an MOI of 25 for THP-1 cell infections. Following addition of the bacteria, the cells were incubated at 37°C with 5% CO2 for 4 h and then washed once with phosphate-buffered saline (PBS) and incubated with fresh DMEM with 5% FCS or RPMI 1640 with 10% FCS. At defined time points, cells were either fixed with 4% paraformaldehyde for microscopy analysis or lysed with H2O and collected for Coxiella quantification. The bacteria and cell debris were pelleted from lysed samples and resuspended in 50 or 100 μl of H2O. The number of Coxiella genomes was calculated by comparison to the number on a known standard curve obtained using ompA-specific primers for qPCR (51, 52).
Transfection and immunofluorescence microscopy.
HeLa cells, either uninfected or persistently infected with Coxiella, were seeded at a density of 105 cells/well onto glass coverslips in 24-well tissue culture trays. Approximately 24 h later, the cells were transfected with plasmids expressing MceA and/or pYFP-LAMP-1 using the Lipofectamine 3000 reagent per the manufacturer's instructions (Thermo Fisher Scientific). At the desired time points, cells were fixed with PBS containing 4% (wt/vol) paraformaldehyde for 15 min. For staining of mitochondria, the cells were incubated with 500 nM MitoTracker Red CMXRos (Thermo Fisher Scientific) for 30 min at 37°C with 5% CO2 prior to fixation. Samples were blocked and permeabilized in blocking buffer (PBS containing 2% [wt/vol] bovine serum albumin and 0.05% [wt/vol] saponin) before being stained with the following primary antibodies at the indicated concentrations in blocking buffer: mouse monoclonal anti-FLAG (1:250; Sigma), rabbit polyclonal anti-MceA (1:50; raised in-house against recombinant protein by Cocalico Biologicals, Inc., and previously used to demonstrate Dot/Icm translocation of MceA [8]), mouse monoclonal anti-LAMP-1 (1:200; Developmental Studies Hybridoma Bank), and rabbit polyclonal anti-Coxiella (1:5,000). Secondary antibodies conjugated to Alexa Fluor 488 and 568 (Invitrogen) were used at 1:2,000 in blocking buffer. During the final PBS washes, bacterial and host cell DNAs were stained with 4′,6′-diamidino-2-phenylindole (DAPI) diluted 1:10,000. Glass coverslips were mounted onto glass slides with Prolong Gold antifade reagent (Thermo Fisher Scientific). Images were acquired with a Zeiss LSM700 confocal laser scanning microscope and processed using ImageJ software.
Mitochondrial isolation and mitochondrial treatments.
Mitochondria from HeLa cells persistently infected with Coxiella were enriched using a MACS mitochondrial isolation kit according to the manufacturer's instructions (Miltenyi Biotec). Briefly, HeLa cells infected with Coxiella were harvested and isolated by centrifugation (800 × g, 10 min, 4°C) before washing in ice-cold PBS. Approximately 107 HeLa cells were resuspended in cell lysis buffer (as per the instructions accompanying the Miltenyi Biotec kit) and lysed using a Dounce homogenizer. The lysate was clarified by centrifugation (800 × g, 5 min, 4°C) and then incubated for 1 h with anti-Tom22 microbeads before being separated in an LS column (Miltenyi Biotec). Mitochondria were flushed out in separation buffer, collected by centrifugation (13,000 × g, 3 min, 4°C), washed in buffer A (20 mM HEPES-KOH, pH 7.6, 220 mM mannitol, 70 mM sucrose, 1 mM EDTA), and reisolated. Samples to be treated with proteinase K (PK) were incubated with the protease (50 μg/ml) for 10 min on ice, followed by incubation with 0.5 mM phenylmethylsulfonyl fluoride for 5 min on ice. For carbonate extraction of mitochondrial proteins, mitochondrial pellets (100 μg of mitochondrial protein) were resuspended in fresh Na2CO3 (0.1 M, pH 11) at 0.5 mg/ml and incubated on ice for 30 min. Samples were subsequently centrifuged at 100,000 × g for 30 min, which separated the pellet (integral membrane proteins) and supernatant (peripheral and soluble proteins) fractions. Both fractions were precipitated with trichloroacetic acid and analyzed by SDS-PAGE and immunoblotting.
PAGE and immunoblotting.
Tricine SDS-PAGE was performed as previously described (53). Blue native (BN) polyacrylamide gel electrophoresis (BN-PAGE) was performed as previously described (54). Briefly, mitochondrial pellets (typically, 50 to 100 μg of protein) were solubilized in ice-cold digitonin-containing buffer (1% [wt/vol] digitonin, 20 mM Tris-HCl, pH 7.0, 0.1 mM EDTA, 50 mM NaCl, 10% [wt/vol] glycerol) at 1 mg/ml and incubated on ice for 15 to 20 min. Those samples requiring SDS treatment were incubated in a similar manner, but in the presence of SDS (0.1%, 0.3%, 0.5%, or 1%). Samples were clarified by centrifugation and subsequently added to 1/10 the volume of sample buffer (5% [wt/vol] Coomassie brilliant blue G-250, 100 mM bis-Tris, pH 7.0, 500 mM ε-amino-n-caproic acid). Samples were separated on a 4 to 16% polyacrylamide gradient. Thyroglobulin (669 kDa), ferritin (440 kDa), and bovine serum albumin (132 and 66 kDa) were used as molecular mass markers. Detection of various human and Coxiella proteins was achieved by immunoblotting with the following antibodies at the indicated concentrations: anti-FLAG (1:2,000; Sigma-Aldrich), anti-Bak (1:1,000; generated and kindly provided by Michael Ryan, Monash University), anti-SDHA (1:1,000; Abcam), anti-Mfn2 (1:200; Michael Ryan laboratory), anti-NDUFA9 (1:500; Michael Ryan Laboratory), anti-Tom22 (1:1,000; Michael Ryan laboratory), anti-cytochrome c (1:300; BD Biosciences), anti-PDI (1:1,000; Enzo Life Sciences), anti-HA (1:500; BioLegend), and anti-MceA (1:300 for BN-PAGE and 1:600 for SDS-PAGE [8]).
Immunoprecipitation.
Mitochondria isolated from HeLa cells infected with C. burnetii expressing (i) 3×FLAGCBU0077, (ii) 2×HA-tagged CBU0077 (2×HACBU0077), or (iii) 3×FLAGCBU0077 and 2×HACBU0077 were solubilized in digitonin-containing buffer (1% [wt/vol] digitonin, 20 mM Tris, pH 7.0, 0.1 mM EDTA, 50 mM NaCl, 10% [wt/vol] glycerol) supplemented with 1× cOmplete protease inhibitor (Roche) at 1 mg/ml end over end on a rotary wheel for 1.5 h at 4°C. Mitochondrial lysates were cleared by centrifugation at 16,000 × g at 4°C for 30 min and diluted in buffer (20 mM Tris-HCl, pH 7.0, 0.1 mM EDTA, 50 mM NaCl, 10% [wt/vol] glycerol) to a final detergent concentration of 0.1%. Cleared lysates were applied to preequilibrated anti-FLAG agarose (Sigma-Aldrich) and incubated at 4°C for 1 h end over end on a rotary wheel. Unbound material was collected, and the anti-FLAG resin and bound proteins were washed 3 times in a buffer containing a lower concentration of digitonin (0.1% [wt/vol] digitonin, 20 mM Tris-HCl, pH 7.0, 0.1 mM EDTA, 50 mM NaCl, 10% [wt/vol] glycerol, 1× cOmplete protease inhibitor). Bound proteins were eluted with 0.2 M glycine, pH 2.5. Fractions eluted with glycine were separated by SDS-PAGE, and proteins were visualized by immunoblotting.
Supplementary Material
ACKNOWLEDGMENTS
The yellow fluorescent protein-tagged LAMP-1 construct was kindly provided by Jenny Stow, The University of Queensland. Confocal imaging was performed at the Biological Optical Microscopy Platform, The University of Melbourne (www.microscopy.unimelb.edu.au). We thank Michael Ryan (Monash University) for generously providing us with antibodies.
This research was supported by project grants awarded to H.J.N. from the Australian National Health and Medical Research Council (grant numbers 1062383 and 1063646). D.S. is supported by a Biochemistry Fund fellowship through the Department of Biochemistry and Molecular Biology, The University of Melbourne. L.F.F. is supported by a postgraduate research scholarship through the Department of Biochemistry and Molecular Biology, The University of Melbourne. Y.K. is supported by a Melbourne International Research Scholarship and Melbourne International Fee Remission Scholarship.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.01046-16.
REFERENCES
- 1.Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. 2002. Public health assessment of potential biological terrorism agents. Emerg Infect Dis 8:225–230. doi: 10.3201/eid0802.010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madariaga MG, Rezai K, Trenholme GM, Weinstein RA. 2003. Q fever: a biological weapon in your backyard. Lancet Infect Dis 3:709–721. doi: 10.1016/S1473-3099(03)00804-1. [DOI] [PubMed] [Google Scholar]
- 3.Hackstadt T, Williams JC. 1981. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci U S A 78:3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howe D, Melnicakova J, Barak I, Heinzen RA. 2003. Fusogenicity of the Coxiella burnetii parasitophorous vacuole. Ann N Y Acad Sci 990:556–562. doi: 10.1111/j.1749-6632.2003.tb07426.x. [DOI] [PubMed] [Google Scholar]
- 5.Kohler LJ, Reed SR, Sarraf SA, Arteaga DD, Newton HJ, Roy CR. 2016. Effector protein Cig2 decreases host tolerance of infection by directing constitutive fusion of autophagosomes with the Coxiella-containing vacuole. mBio 7:e01127-16. doi: 10.1128/mBio.01127-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omsland A, Cockrell DC, Howe D, Fischer ER, Virtaneva K, Sturdevant DE, Porcella SF, Heinzen RA. 2009. Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc Natl Acad Sci U S A 106:4430–4434. doi: 10.1073/pnas.0812074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moffatt JH, Newton P, Newton HJ. 2015. Coxiella burnetii: turning hostility into a home. Cell Microbiol 17:621–631. doi: 10.1111/cmi.12432. [DOI] [PubMed] [Google Scholar]
- 8.Carey KL, Newton HJ, Luhrmann A, Roy CR. 2011. The Coxiella burnetii Dot/Icm system delivers a unique repertoire of type IV effectors into host cells and is required for intracellular replication. PLoS Pathog 7:e1002056. doi: 10.1371/journal.ppat.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beare PA, Gilk SD, Larson CL, Hill J, Stead CM, Omsland A, Cockrell DC, Howe D, Voth DE, Heinzen RA. 2011. Dot/Icm type IVB secretion system requirements for Coxiella burnetii growth in human macrophages. mBio 2:e00175-11. doi: 10.1128/mBio.00175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamboni DS, McGrath S, Rabinovitch M, Roy CR. 2003. Coxiella burnetii express type IV secretion system proteins that function similarly to components of the Legionella pneumophila Dot/Icm system. Mol Microbiol 49:965–976. doi: 10.1046/j.1365-2958.2003.03626.x. [DOI] [PubMed] [Google Scholar]
- 11.Hubber A, Roy CR. 2010. Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol 26:261–283. doi: 10.1146/annurev-cellbio-100109-104034. [DOI] [PubMed] [Google Scholar]
- 12.Nagai H, Cambronne ED, Kagan JC, Amor JC, Kahn RA, Roy CR. 2005. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc Natl Acad Sci U S A 102:826–831. doi: 10.1073/pnas.0406239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton HJ, McDonough JA, Roy CR. 2013. Effector protein translocation by the Coxiella burnetii Dot/Icm type IV secretion system requires endocytic maturation of the pathogen-occupied vacuole. PLoS One 8:e54566. doi: 10.1371/journal.pone.0054566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez E, Cantet F, Fava L, Norville I, Bonazzi M. 2014. Identification of OmpA, a Coxiella burnetii protein involved in host cell invasion, by multi-phenotypic high-content screening. PLoS Pathog 10:e1004013. doi: 10.1371/journal.ppat.1004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton HJ, Kohler LJ, McDonough JA, Temoche-Diaz M, Crabill E, Hartland EL, Roy CR. 2014. A screen of Coxiella burnetii mutants reveals important roles for Dot/Icm effectors and host autophagy in vacuole biogenesis. PLoS Pathog 10:e1004286. doi: 10.1371/journal.ppat.1004286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber MM, Chen C, Rowin K, Mertens K, Galvan G, Zhi H, Dealing CM, Roman VA, Banga S, Tan Y, Luo ZQ, Samuel JE. 2013. Identification of Coxiella burnetii type IV secretion substrates required for intracellular replication and Coxiella-containing vacuole formation. J Bacteriol 195:3914–3924. doi: 10.1128/JB.00071-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larson CL, Beare PA, Voth DE, Howe D, Cockrell DC, Bastidas RJ, Valdivia RH, Heinzen RA. 2015. Coxiella burnetii effector proteins that localize to the parasitophorous vacuole membrane promote intracellular replication. Infect Immun 83:661–670. doi: 10.1128/IAI.02763-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voth DE, Howe D, Beare PA, Vogel JP, Unsworth N, Samuel JE, Heinzen RA. 2009. The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J Bacteriol 191:4232–4242. doi: 10.1128/JB.01656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luhrmann A, Nogueira CV, Carey KL, Roy CR. 2010. Inhibition of pathogen-induced apoptosis by a Coxiella burnetii type IV effector protein. Proc Natl Acad Sci U S A 107:18997–19001. doi: 10.1073/pnas.1004380107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klingenbeck L, Eckart RA, Berens C, Luhrmann A. 2013. The Coxiella burnetii type IV secretion system substrate CaeB inhibits intrinsic apoptosis at the mitochondrial level. Cell Microbiol 15:675–687. doi: 10.1111/cmi.12066. [DOI] [PubMed] [Google Scholar]
- 21.Schafer W, Eckart RA, Schmid B, Cagkoylu H, Hof K, Muller YA, Amin B, Luhrmann A. 2017. Nuclear trafficking of the anti-apoptotic Coxiella burnetii effector protein AnkG requires binding to p32 and Importin-alpha1. Cell Microbiol 19:e12634. doi: 10.1111/cmi.12634. [DOI] [PubMed] [Google Scholar]
- 22.Fielden LF, Kang Y, Newton HJ, Stojanovski D. 2017. Targeting mitochondria: how intravacuolar bacterial pathogens manipulate mitochondria. Cell Tissue Res 367:141–154. doi: 10.1007/s00441-016-2475-x. [DOI] [PubMed] [Google Scholar]
- 23.Beare PA, Heinzen RA. 2014. Gene inactivation in Coxiella burnetii. Methods Mol Biol 1197:329–345. doi: 10.1007/978-1-4939-1261-2_19. [DOI] [PubMed] [Google Scholar]
- 24.Sanford JC, Foster L, Kapadia Z, Wessling-Resnick M. 1995. Analysis of the stoichiometry of Rab protein prenylation. Anal Biochem 224:547–556. doi: 10.1006/abio.1995.1086. [DOI] [PubMed] [Google Scholar]
- 25.Hornig-Do HT, Gunther G, Bust M, Lehnartz P, Bosio A, Wiesner RJ. 2009. Isolation of functional pure mitochondria by superparamagnetic microbeads. Anal Biochem 389:1–5. doi: 10.1016/j.ab.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 26.Franko A, Baris OR, Bergschneider E, von Toerne C, Hauck SM, Aichler M, Walch AK, Wurst W, Wiesner RJ, Johnston IC, de Angelis MH. 2013. Efficient isolation of pure and functional mitochondria from mouse tissues using automated tissue disruption and enrichment with anti-TOM22 magnetic beads. PLoS One 8:e82392. doi: 10.1371/journal.pone.0082392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujiki Y, Hubbard AL, Fowler S, Lazarow PB. 1982. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol 93:97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burri L, Vascotto K, Gentle IE, Chan NC, Beilharz T, Stapleton DI, Ramage L, Lithgow T. 2006. Integral membrane proteins in the mitochondrial outer membrane of Saccharomyces cerevisiae. FEBS J 273:1507–1515. doi: 10.1111/j.1742-4658.2006.05171.x. [DOI] [PubMed] [Google Scholar]
- 29.Stojanovski D, Pfanner N, Wiedemann N. 2007. Import of proteins into mitochondria. Methods Cell Biol 80:783–806. doi: 10.1016/S0091-679X(06)80036-1. [DOI] [PubMed] [Google Scholar]
- 30.Stojanovski D, Guiard B, Kozjak-Pavlovic V, Pfanner N, Meisinger C. 2007. Alternative function for the mitochondrial SAM complex in biogenesis of alpha-helical TOM proteins. J Cell Biol 179:881–893. doi: 10.1083/jcb.200706043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ensminger AW. 2016. Legionella pneumophila, armed to the hilt: justifying the largest arsenal of effectors in the bacterial world. Curr Opin Microbiol 29:74–80. doi: 10.1016/j.mib.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Escudero M, Cid VJ, Molina M, Schulze-Luehrmann J, Luhrmann A, Rodriguez-Escudero I. 2016. Studying Coxiella burnetii type IV substrates in the yeast Saccharomyces cerevisiae: focus on subcellular localization and protein aggregation. PLoS One 11:e0148032. doi: 10.1371/journal.pone.0148032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Resh MD. 2006. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat Chem Biol 2:584–590. doi: 10.1038/nchembio834. [DOI] [PubMed] [Google Scholar]
- 34.Ivanov SS, Charron G, Hang HC, Roy CR. 2010. Lipidation by the host prenyltransferase machinery facilitates membrane localization of Legionella pneumophila effector proteins. J Biol Chem 285:34686–34698. doi: 10.1074/jbc.M110.170746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanov SS, Roy C. 2013. Host lipidation: a mechanism for spatial regulation of Legionella effectors. Curr Top Microbiol Immunol 376:135–154. doi: 10.1007/82_2013_344. [DOI] [PubMed] [Google Scholar]
- 36.Yonashiro R, Ishido S, Kyo S, Fukuda T, Goto E, Matsuki Y, Ohmura-Hoshino M, Sada K, Hotta H, Yamamura H, Inatome R, Yanagi S. 2006. A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J 25:3618–3626. doi: 10.1038/sj.emboj.7601249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Culty M, Li H, Boujrad N, Amri H, Vidic B, Bernassau JM, Reversat JL, Papadopoulos V. 1999. In vitro studies on the role of the peripheral-type benzodiazepine receptor in steroidogenesis. J Steroid Biochem Mol Biol 69:123–130. doi: 10.1016/S0960-0760(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 38.Joseph-Liauzun E, Delmas P, Shire D, Ferrara P. 1998. Topological analysis of the peripheral benzodiazepine receptor in yeast mitochondrial membranes supports a five-transmembrane structure. J Biol Chem 273:2146–2152. doi: 10.1074/jbc.273.4.2146. [DOI] [PubMed] [Google Scholar]
- 39.Korkhov VM, Sachse C, Short JM, Tate CG. 2010. Three-dimensional structure of TspO by electron cryomicroscopy of helical crystals. Structure 18:677–687. doi: 10.1016/j.str.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otera H, Taira Y, Horie C, Suzuki Y, Suzuki H, Setoguchi K, Kato H, Oka T, Mihara K. 2007. A novel insertion pathway of mitochondrial outer membrane proteins with multiple transmembrane segments. J Cell Biol 179:1355–1363. doi: 10.1083/jcb.200702143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogtle FN, Keller M, Taskin AA, Horvath SE, Guan XL, Prinz C, Opalinska M, Zorzin C, van der Laan M, Wenk MR, Schubert R, Wiedemann N, Holzer M, Meisinger C. 2015. The fusogenic lipid phosphatidic acid promotes the biogenesis of mitochondrial outer membrane protein Ugo1. J Cell Biol 210:951–960. doi: 10.1083/jcb.201506085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishikawa D, Yamamoto H, Tamura Y, Moritoh K, Endo T. 2004. Two novel proteins in the mitochondrial outer membrane mediate beta-barrel protein assembly. J Cell Biol 166:621–627. doi: 10.1083/jcb.200405138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waizenegger T, Schmitt S, Zivkovic J, Neupert W, Rapaport D. 2005. Mim1, a protein required for the assembly of the TOM complex of mitochondria. EMBO Rep 6:57–62. doi: 10.1038/sj.embor.7400318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hulett JM, Lueder F, Chan NC, Perry AJ, Wolynec P, Likic VA, Gooley PR, Lithgow T. 2008. The transmembrane segment of Tom20 is recognized by Mim1 for docking to the mitochondrial TOM complex. J Mol Biol 376:694–704. doi: 10.1016/j.jmb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 45.Popov-Celeketic J, Waizenegger T, Rapaport D. 2008. Mim1 functions in an oligomeric form to facilitate the integration of Tom20 into the mitochondrial outer membrane. J Mol Biol 376:671–680. doi: 10.1016/j.jmb.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Senes A, Engel DE, DeGrado WF. 2004. Folding of helical membrane proteins: the role of polar, GXXXG-like and proline motifs. Curr Opin Struct Biol 14:465–479. doi: 10.1016/j.sbi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Russ WP, Engelman DM. 2000. The GXXXG motif: a framework for transmembrane helix-helix association. J Mol Biol 296:911–919. doi: 10.1006/jmbi.1999.3489. [DOI] [PubMed] [Google Scholar]
- 48.Omsland A, Beare PA, Hill J, Cockrell DC, Howe D, Hansen B, Samuel JE, Heinzen RA. 2011. Isolation from animal tissue and genetic transformation of Coxiella burnetii are facilitated by an improved axenic growth medium. Appl Environ Microbiol 77:3720–3725. doi: 10.1128/AEM.02826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beare PA, Larson CL, Gilk SD, Heinzen RA. 2012. Two systems for targeted gene deletion in Coxiella burnetii. Appl Environ Microbiol 78:4580–4589. doi: 10.1128/AEM.00881-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez E, Cantet F, Bonazzi M. 2015. Generation and multi-phenotypic high-content screening of Coxiella burnetii transposon mutants. J Vis Exp 2015:e52851. doi: 10.3791/52851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaton K, Peter O, Raoult D, Tissot JD, Greub G. 2013. Development of a high throughput PCR to detect Coxiella burnetii and its application in a diagnostic laboratory over a 7-year period. New Microbes New Infect 1:6–12. doi: 10.1002/2052-2975.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newton P, Latomanski EA, Newton HJ. 2016. Applying fluorescence resonance energy transfer (FRET) to examine effector translocation efficiency by Coxiella burnetii during siRNA silencing. J Vis Exp 2016:e54210. doi: 10.3791/54210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schagger H, von Jagow G. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 54.Schagger H, von Jagow G. 1991. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 199:223–231. doi: 10.1016/0003-2697(91)90094-A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.