ABSTRACT
There is evidence that mast cells, basophils, and IgE can contribute to immune responses to parasites; however, the relative levels of importance of these effector elements in parasite immunity are not fully understood. Previous work in Il3-deficient and c-kit mutant KitW/W-v mice indicated that interleukin-3 and c-Kit contribute to expulsion of the intestinal nematode Strongyloides venezuelensis during primary infection. Our findings in mast cell-deficient KitW-sh/W-sh mice and two types of mast cell-deficient mice that have normal c-kit (“Hello Kitty” and MasTRECK mice) confirmed prior work in KitW/W-v mice that suggested that mast cells play an important role in S. venezuelensis egg clearance in primary infections. We also assessed a possible contribution of basophils in immune responses to S. venezuelensis. By immunohistochemistry, we found that numbers of basophils and mast cells were markedly increased in the jejunal mucosa during primary infections with S. venezuelensis. Studies in basophil-deficient Mcpt8DTR mice revealed a small but significant contribution of basophils to S. venezuelensis egg clearance in primary infections. Studies in mice deficient in various components of immune responses showed that CD4+ T cells and ILC2 cells, IgG, FcRγ, and, to a lesser extent, IgE and FcεRI contribute to effective immunity in primary S. venezuelensis infections. These findings support the conclusion that the hierarchy of importance of immune effector mechanisms in primary S. venezuelensis infection is as follows: CD4+ T cells/ILC2 cells, IgG, and FcRγ>mast cells>IgE and FcεRI>basophils. In contrast, in secondary S. venezuelensis infection, our evidence indicates that the presence of CD4+ T cells is of critical importance but mast cells, antibodies, and basophils have few or no nonredundant roles.
KEYWORDS: Immunoglobulin E, basophils, mast cells, parasites
INTRODUCTION
Strongyloides stercoralis is endemic mainly in tropical and subtropical areas and is estimated to infect 30 million to 100 million people worldwide (1, 2). Strongyloides venezuelensis is a rodent parasite with a similar life cycle that is used as an experimental model of S. stercoralis (3). Prior work has implicated mast cells (MCs) (4–15) and interleukin-3 (IL-3) (5, 7, 12) in immune resistance to primary infections with S. venezuelensis. IL-3 is essential for the increases in levels of jejunal MCs and blood basophils observed after S. venezuelensis infection (7, 12), and KitW/W-v mice (markedly deficient in MCs) (16, 17) that were also deficient in Il3 exhibited a more pronounced defect in S. venezuelensis expulsion during primary infections than did either KitW/W-v or Il3-deficient mice, suggesting that basophils also might contribute to S. venezuelensis clearance (7).
However, the importance of MCs and basophils in S. venezuelensis immunity was not proven by such work. For example, the MC-deficient mice used in many of the studies (4, 6, 7, 9, 11, 14), KitW/W-v mice, have several c-Kit-related defects in addition to their MC deficiency (17–26) and, until recently, no basophil-deficient mice were available to assess specifically the roles of basophils in this setting. In the present study, we used basophil-deficient mice and three types of MC-deficient mice to examine the roles of basophils and MCs in primary and secondary infections with S. venezuelensis. Because it has been reported that IgG1 and IgE antibodies (Ab) can function in concert to enhance expulsion of S. venezuelensis during primary infections but that neither type of antibody had a nonredundant function in such clearance (14), we also tested mice deficient in antibodies, IgE, the FcR γ chain, and FcεRI alpha, as well as mice with deficiencies in T cells and B cells. These experiments revealed that MCs, basophils, IgG, and IgE have distinct roles in primary versus secondary infections with S. venezuelensis.
RESULTS
Mast cells and, to a lesser extent, basophils contribute to expulsion of S. venezuelensis in primary infections.
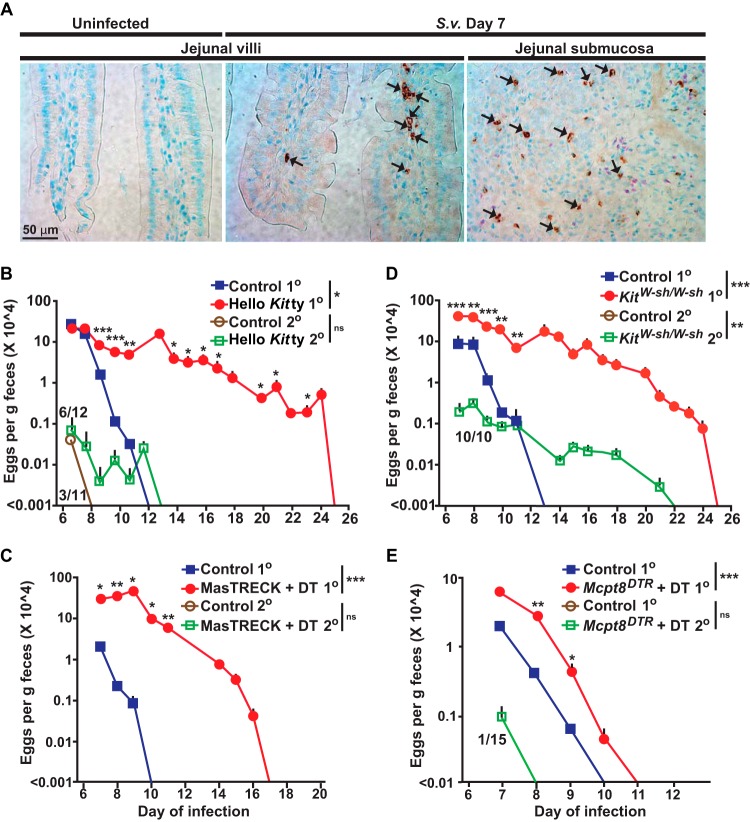
Both IL-3 and c-Kit can enhance clearance of a primary infection with S. venezuelensis (7). The role of c-Kit is consistent with other lines of evidence indicating possible contributions of MCs to host resistance to this nematode (4, 6, 11, 27). While it is well known that MC numbers increase greatly in the jejunum of wild-type mice during primary infections with S. venezuelensis (6–9, 11), it was not known to what extent basophils might also infiltrate such tissues. Using an anti-mouse mast cell protease 8 (mMCP-8) Ab (28) recognizing the basophil-specific marker mMCP-8, we found that basophils were undetectable by immunohistochemistry in the jejunum of uninfected wild-type mice (Fig. 1A, left panel) but that many basophils were present 7 days after the first S. venezuelensis inoculation (Fig. 1A, middle and right panels).
FIG 1.
Roles of mast cells and basophils in primary and secondary S. venezuelensis infections. (A) Immunohistochemical staining with an anti-mMCP8 Ab (DAB [3,3′-diaminobenzidine] substrate) to visualize basophils (some are indicated with small black arrows) and Giemsa counterstaining (4-μm-thick, paraffin-embedded sections) in sections of jejunum from wild-type C57BL/6 mice 7 days after inoculation with 10,000 S. venezuelensis L3. The left and middle panels are representative images from jejunal villi, and the right panel is a representative image from jejunal submucosa. S. v., S. venezuelensis. (B to E) Kinetics of S. venezuelensis egg excretion after inoculation of 10,000 S. venezuelensis L3 in primary infections (1o) or secondary infections (2o [in mice inoculated with 10,000 S. venezuelensis L3 28 days after the first inoculation]) in C57BL/6-Hello Kitty and control mice (n = 22 each) for 1o infections (7 experiments) and C57BL/6-Hello Kitty (n = 12) and control (n = 11) mice for 2o infections (4 experiments) (B), BALB/c-MasTRECK mice treated with DT (n = 5) and control mice (n = 4) for 1o infections (2 experiments) and BALB/c-MasTRECK mice treated with DT and control mice (n = 5 for each) for 2o infections (2 experiments) (C), C57BL/6-KitW-sh/W-sh and control mice (n = 9 each) for 1o infections (6 experiments) and C57BL/6-KitW-sh/W-sh (n = 10) and control (n = 3) mice for 2o infections (3 experiments) (D), and Mcpt8DTR/+ mice treated with DT (n = 26) and control mice (n = 27) for 1o infections (9 experiments) and Mcpt8DTR/+ mice treated with DT (n = 15) and control mice (n = 7) for 2o infections (2 experiments) (E). The numbers of eggs are shown as means + standard errors of the means (SEM) determined by pooling the data from all the experiments that were performed with each strain. Numerators in the fraction numbers for 2o infections indicate the number of mice for which eggs were detected, and denominators indicate the number of mice examined. Absence of fraction numbers or lines for 2o infections means that none of the mice produced detectable eggs. ***, P < 0.0001; **, P < 0.001; *, P < 0.05; no asterisks, P > 0.05 (for comparisons between each mutant mouse and the corresponding controls at each time point performed with the unpaired, two-tailed Student's t test and, as indicated next to the legend for groups of mice, with 2-way ANOVA to compare the time courses of the responses).
It has been suggested that MCs and basophils might have complementary and/or partially overlapping roles in certain immune responses to parasites (7, 27, 29–31). We used genetic approaches to investigate the relative contributions of MCs and basophils in primary and secondary infections with S. venezuelensis. MC-deficient WBB6F1-KitW/W-v mice exhibited delayed expulsion of S. venezuelensis in primary infections (6, 7, 11), but such mice have other abnormalities related to their c-kit mutations that may influence the response to this parasite by mechanisms unrelated to their MC deficiency (17, 32–35).
Therefore, we tested genetically engineered MC-deficient Cpa3-Cre;Mcl-1fl/fl “Hello Kitty” mice, in which a marked MC deficiency, and also a substantial reduction in basophils, occurs because of a mechanism independent of mutations affecting c-kit (36). Hello Kitty mice exhibited a 13-day delay in achieving cessation of S. venezuelensis egg excretion compared to the corresponding control Cpa3-Cre;Mcl-1+/+ MC- and basophil-sufficient mice (Fig. 1B). Another genetically engineered MC-deficient mouse (with normal c-kit), the MasTRECK (Tg/+) mouse (32), exhibited a 7-day delay in cessation of egg excretion compared to diphtheria toxin (DT)-treated littermate wild-type control (+/+) or phosphate-buffered saline (PBS)-treated MasTRECK mice (Fig. 1C; also see Fig. S1A in the supplemental material).
Beyond a profound MC deficiency, both Hello Kitty mice (36) and, at early times after DT treatment, DT-treated MasTRECK mice (32) have significantly reduced numbers of basophils. We therefore also tested MC-deficient C57BL/6J-KitW-sh/W-sh mice (37), which have Kit-related MC deficiency but have slightly increased numbers of basophils (37). Like the Hello Kitty mice, KitW-sh/W-sh mice exhibited a 12-day delay in cessation of S. venezuelensis egg excretion compared to the corresponding wild-type mice (Fig. 1D). Finally, we tested Mcpt8DTR/+ mice (38), which can be rendered deficient specifically in basophils. DT-treated Mcpt8DTR/+ mice exhibited significantly more eggs in their feces on days 8 and 9 (and a 1-day delay in cessation of egg excretion) than the corresponding control mice (i.e., the DT-treated basophil-sufficient littermate wild-type Mcpt8+/+ mice and PBS-treated Mcpt8DTR/+ mice; see Fig. S1B in the supplemental material) (Fig. 1E). It has been shown that, during primary infection with S. venezuelensis, KitW/W-v mice (4–7, 11) and IL-3-deficient KitW/W-v mice (7) harbored more adult worms in the intestines than the corresponding control mice, as well as exhibiting a more extensively delayed cessation of egg excretion. We found that basophil-depleted DT-treated Mcpt8DTR/+ mice also exhibited increased numbers of worms in the intestines at day 8 of primary S. venezuelensis infection compared to the corresponding wild-type mice (Fig. S2A in the supplemental material), in addition to exhibiting increased numbers of eggs in the feces on that day and also a subsequent delay in cessation of egg excretion (Fig. 1E).
In addition to monitoring primary responses to S. venezuelensis by counting egg excretion, we measured changes in numbers of basophils and MCs. The various control MC- and basophil-sufficient mice exhibited substantial increases in blood basophil levels during primary infections (Fig. 2A; also see Fig. S3 in the supplemental material) which peaked around days 10 to 12. Hello Kitty mice, DT-treated MasTRECK mice, and DT-treated Mcpt8DTR/+ mice each exhibited slight increases in blood basophil levels that were detectable ∼7 to ∼11 days after initiation of S. venezuelensis infection; however, all but the DT-treated MasTRECK mice maintained substantial basophil depletion (Fig. 2A; also see Fig. S3 in the supplemental material). The temporary basophil depletion that we saw in DT-treated MasTRECK mice is consistent with previous observations (32).
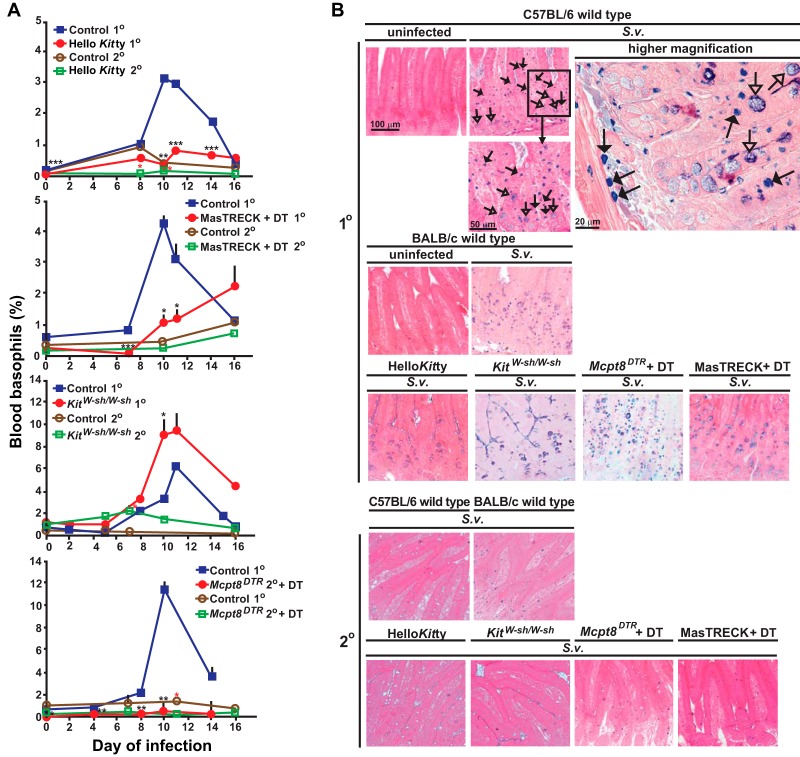
FIG 2.
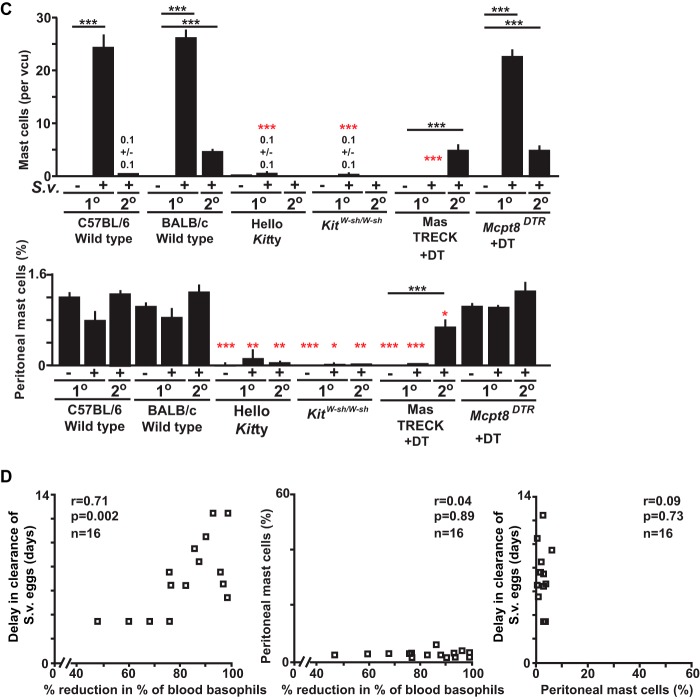
Changes in numbers of basophils, mast cells, and epithelial goblet cells in wild-type and mast cell- and/or basophil-deficient mice during primary and secondary S. venezuelensis infections. (A) Blood was collected from each mouse at the indicated points after S. venezuelensis infection and stained with anti-IgE and anti-CD49b. Percentages of basophils in live cells were plotted. C57BL/6-Hello Kitty (n = 21) and control (n = 22) mice were analyzed in both the 1o and 2o infections (separate groups of 21 and 22 mice, for a total of 86 mice). Data for 1o infections include those from 16/21 Hello Kitty mice and all 22 control mice in the same 7 experiments performed as described for Fig. 1, as well as 5 additional Hello Kitty mice with 1o infections which were not used as described for Fig. 1. For basophil counts in 2o infections, data are shown from all of the Hello Kitty mice (n = 11) and control mice (n = 12) reported as described for Fig. 1 plus an additional 10 Hello Kitty and 10 control mice with 2o infections. Data represent results for BALB/c-MasTRECK mice treated with DT and control mice (a mixture of DT-treated wild-type mice [n = 5] and PBS-treated BALB/c-MasTRECK mice [n = 5]) (n = 10 each for both 1o and 2o infections, for a total of 40 mice), including 5 BALB/c-MasTRECK mice treated with DT and 4 control mice for the 1o infections and 5 BALB/c-MasTRECK mice treated with DT and 5 control mice for the 2o infections. Data represent results for C57BL/6-KitW-sh/W-sh (n = 14) and control (n = 18) mice (separate groups of 14 and 18 mice for the 1o and 2o infections, for a total of 64 mice), including 9 C57BL/6-KitW-sh/W-sh and 9 control mice from the 6 experiments performed as described for Fig. 1 for the 1o infections and 3 C57BL/6-KitW-sh/W-sh and 10 control mice from the 3 experiments performed as described for Fig. 1 for the 2o infections. Data for Mcpt8DTR mice treated with DT and control mice (a mixture of DT-treated wild-type mice [n = 3] and PBS-treated Mcpt8DTR mice [n = 3]) (n = 6 each) were from the 2 experiments performed as described for Fig. 1. ***, P < 0.0005; **, P < 0.005; *, P < 0.05; no asterisks, P > 0.05. Black asterisks (for 1o infections) or red asterisks (for 2o infections) indicate the statistical significance of results of comparisons between each type of mutant mouse and the corresponding control mice for that time point. (B and C) Jejunums were collected 14 days after 1o or 2o infections. (B) Mast cells (some indicated with filled arrows) were stained with alcian blue after fixation with Carnoy's solution (open arrows indicate goblet cells). Representative tissue images are shown in panel B, and numbers of mast cells per villus crypt unit (vcu) are shown as bar graphs in panel C (upper panel, means + SEM, n = 9 to 10 each; data pooled from 3 experiments). Peritoneal fluid was collected 14 days after the start of the 1o or 2o infections, and mast cells in live cells were assessed using anti-IgE and anti-c-Kit antibodies. Percentages of peritoneal mast cells in live cells are shown as bar graphs in panel C (lower panel, means + SEM, n = 3 to 10 each; data pooled from 2 or 3 experiments). Wild-type values for comparisons to those from mutant mice were combined from corresponding controls of the same strain (C57BL/6 or BALB/c). Black asterisks, statistical significance of results of comparisons of values for uninfected mice versus 1o or 2o infected mice in the same group (as indicated by bars). Red asterisks, statistical significance of results of comparisons of values for each type of mutant mouse and the corresponding wild-type mice for that condition (i.e., uninfected or 1o or 2o infected). Actual values are noted (means ± SEM) for low numbers; absence of a bar or of numbers means that none were detected. (D) (Left panel) Correlation between the delay in achieving cessation of egg excretion (defined as 0 egg count) in each Hello Kitty mouse (compared to the average number of days for cessation of egg excretion in the corresponding wild-type mice in each experiment) (y axis) and percent reduction in the percentage of blood basophils of individual Hello Kitty mice (calculated based on the average percentage of blood basophils in the corresponding control wild-type mice in each experiment) 10 to 12 days after initiation of 1o infection with S. venezuelensis (e.g., an 80% reduction means that that Hello Kitty mouse had 20% the average percentage of basophils in the corresponding wild-type mice in that experiment]) (x axis) (n = 16). (Middle panel) Correlation between the percentage of peritoneal mast cells in that Hello Kitty mouse 18 to 20 days postinitiation of 1o infection with S. venezuelensis (y axis) and the percentage of reduction in the percentage of blood basophils of individual Hello Kitty mice (defined as for left panel) (x axis) (n = 16). (Right panel) Correlation between the delay in achieving cessation of egg excretion in each Hello Kitty mouse (defined as for left panel) (y axis) and the percentage of peritoneal mast cells in that Hello Kitty mouse 18 to 20 days after initiation of 1o infection with S. venezuelensis (x axis) (n = 16). r, correlation coefficient. The data in each panel are from the same 16 Hello Kitty mice, all of them pooled from the data for the 16 mice in the 7 experiments performed as described for Fig. 1 for which data on basophils and peritoneal mast cells were obtained.
In S. venezuelensis-infected MC- and basophil-sufficient mice, MC levels were increased in the jejunal mucosa during the primary infection, and numbers of goblet cells also increased (Fig. 2B and C; see also Fig. 4D). In contrast, while all 3 types of MC-deficient mice exhibited increased levels of goblet cells in the jejunum (reaching levels similar to those in the corresponding MC-sufficient mice), numbers of mucosal MCs and peritoneal MCs were drastically reduced (Fig. 2B and C; see also Fig. 4D and Fig. S3 in the supplemental material). In S. venezuelensis-infected basophil-deficient mice, levels of both mucosal and peritoneal MCs were similar to those in the corresponding control mice (Fig. 2B and C; also see Fig. S3 in the supplemental material).
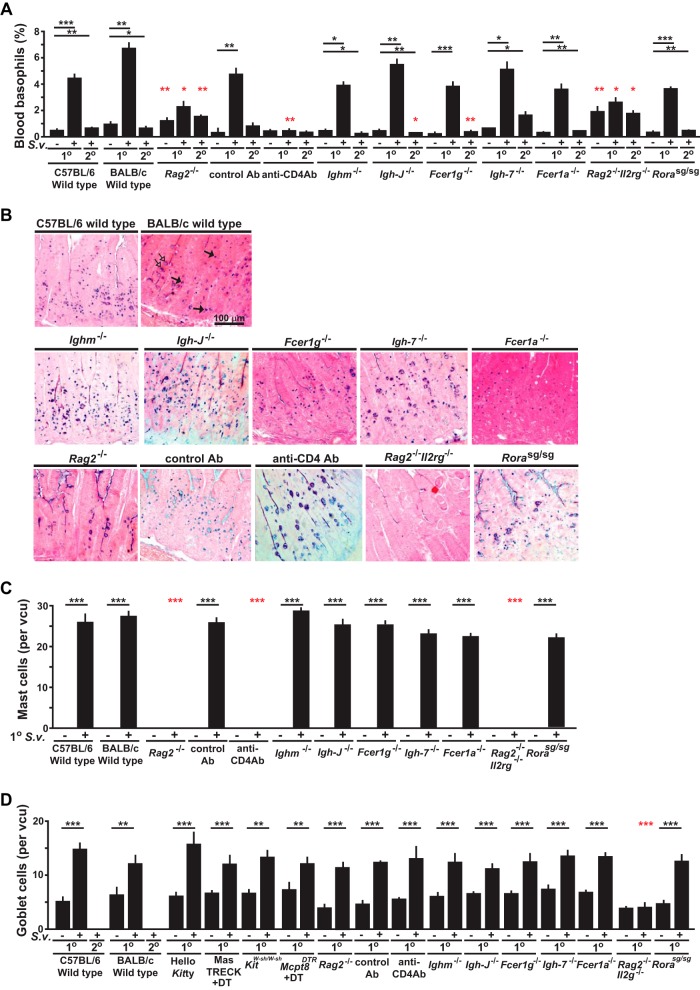
FIG 4.
Changes in numbers of basophils, mast cells, and epithelial goblet cells in mice deficient in various components of immune responses during primary and secondary S. venezuelensis infections. (A) Blood was collected from each mouse during all of the experiments performed as described for Fig. 3A to J before infection or 10 to 12 days after 1o or 2o S. venezuelensis infections and stained with anti-IgE and anti-CD49b. Percentages of basophils in live cells are shown as bar graphs with means + SEM. ***, P < 0.0005; **, P < 0.005; *, P > 0.05; no asterisks, P > 0.05. Black asterisks indicate the statistical significance of results of comparisons between values for uninfected mice and 1o or 2o infected mice in the same group (as indicated by bars). Red asterisks indicate the statistical significance of results of comparisons between each type of mutant or anti-CD4 Ab-treated mouse and the corresponding control mice for that condition. (B to D) Jejunums were collected 14 days after the start of 1o infections, and sections of jejunum were stained as described for Fig. 2B (in the panel showing the histology in BALB/c wild-type mice, some of the mast cells are indicated with filled arrows and some of the goblet cells are indicated with open arrows) (B), and numbers of mast cells (C) or goblet cells (D) per villus crypt unit (vcu) are shown as bar graphs (n = 4 to 12 each; data were combined from 2 or 3 experiments). Wild-type values for comparisons to those from mutant mice were combined from corresponding controls of the same strain (C57BL/6 or BALB/c).
These results establish that MC deficiencies were sustained during primary S. venezuelensis infections in the three kinds of MC-deficient mice tested and that the basophil deficiencies are maintained during such infections in both DT-treated Mcpt8DTR/+ mice and Hello Kitty mice. These findings also indicate that the expansion of MC numbers observed in this setting appears not to require an important nonredundant contribution of basophils and that the jejunal goblet cell hyperplasia associated with primary infections with S. venezuelensis can occur largely independently of contributions of MCs or basophils.
Compared to the consistent basophil depletion observed before day 7 in DT-treated Mcpt8DTR/+ and MasTRECK mice, Hello Kitty mice had a larger variation in the percentage of blood basophil deficiency and also exhibited more variation in the delay in cessation of S. venezuelensis egg production than DT-treated MasTRECK and Mcpt8DTR/+ mice. Notably, in individual Hello Kitty mice, there was a strong correlation between the delay in cessation of S. venezuelensis egg excretion and the magnitude of their blood basophil deficiency (Fig. 2D, left panel). No such correlation was observed between the delay in S. venezuelensis egg clearance and the reduction in peritoneal MCs (Fig. 2D, middle panel) or between the reductions in the levels of blood basophils and peritoneal MCs (Fig. 2D, right panel). Also, all individual Hello Kitty mice remained substantially MC deficient during S. venezuelensis infection. The strong correlation in individual Hello Kitty mice between the extent of their reduction in blood basophils and the length of delay in cessation of parasite egg excretion supports the conclusion that basophils, as well as MCs, contribute to protective immunity during a primary infection with S. venezuelensis.
Minor or no roles of mast cells and basophils in S. venezuelensis expulsion during secondary infections.
We also examined whether MCs and/or basophils contribute to cessation of S. venezuelensis egg excretion during secondary infections. Several studies indicated that, in secondary nematode infections of wild-type mice, the larvae can be trapped in the skin and lungs before reaching the intestines, as reported for S. venezuelensis (39–41), Strongyloides ratti (41), and Nippostrongylus brasiliensis (42–44). In this study, we assessed fecal egg numbers as an indicator of whether larvae could reach the intestines and then produce eggs. Only 3 of 11 Cpa3-Cre;Mcl-1+/+ mice (the MC- and basophil-sufficient controls for Hello Kitty mice) and none of the C57BL/6J-Kit+/+ mice (the MC-sufficient controls for the MC-deficient KitW-sh/W-sh mice) excreted any eggs during secondary infections (Fig. 1B and D). Even when eggs were detected, the amounts were ∼1,000-fold lower than during primary infections and eggs were detected only on day 7 (Fig. 1B). No eggs at all were detected during secondary infections in control (MC- and basophil-sufficient) mice on the BALB/cJ background (Fig. 1C and E).
Compared to the corresponding control mice, half of the Hello Kitty mice and all of the KitW-sh/W-sh mice exhibited delayed cessation of S. venezuelensis egg excretion during secondary infections, although the amounts of eggs in the feces were ∼1,000-fold lower than during primary infections (Fig. 1B and D). However, the differences between the MC-deficient and corresponding control mice were not statistically significant. DT-treated MasTRECK mice and the corresponding control mice did not differ in their response to secondary infection with S. venezuelensis, in that none of the mice in either group exhibited any eggs in the feces (Fig. 1C). Only 1 of 15 DT-treated basophil-deficient Mcpt8DTR/+ mice excreted any eggs during the secondary infection, and that excretion was seen only on day 7 (Fig. 1E).
Blood basophil levels were not increased in the various basophil-sufficient control mice in secondary infections to the same extent as in primary infections (Fig. 2A; also see Fig. S3 in the supplemental material). Moreover, such mice exhibited substantially fewer MCs in the jejunal mucosa than in the primary infections, especially in mice on the C57BL/6J background (Fig. 2B and C). Peritoneal MCs were not detectable after secondary infections in Hello Kitty mice or KitW-sh/W-sh mice (Fig. 2B and C; also see Fig. S3 in the supplemental material). DT-treated Mcpt8DTR/+ mice and MasTRECK mice (each on the BALB/cJ background) had numbers of mucosal MCs in the jejunums after secondary infections that were similar to those in the corresponding control mice (Fig. 2B and C; also see Fig. S3 in the supplemental material). Importantly, we found that DT-treated MasTRECK mice had not only mucosal MCs but also peritoneal MCs after secondary infections, at levels similar to those in the corresponding control mice (Fig. 2C). The fact that DT-based MC depletion was no longer effective in this setting (Fig. 2C) might, in part, explain why no eggs were detected during secondary infections in DT-treated MasTRECK mice.
Taken together, these results suggest that MCs played only a minor (if any) role in resistance to S. venezuelensis during secondary infections in the mice analyzed and that basophils did not provide a critical nonredundant function in orchestrating cessation of S. venezuelensis egg excretion in these mice during the secondary infection.
Roles of CD4+ T cells, ILC2 cells, and IgG and IgE antibodies in immune responses to S. venezuelensis.
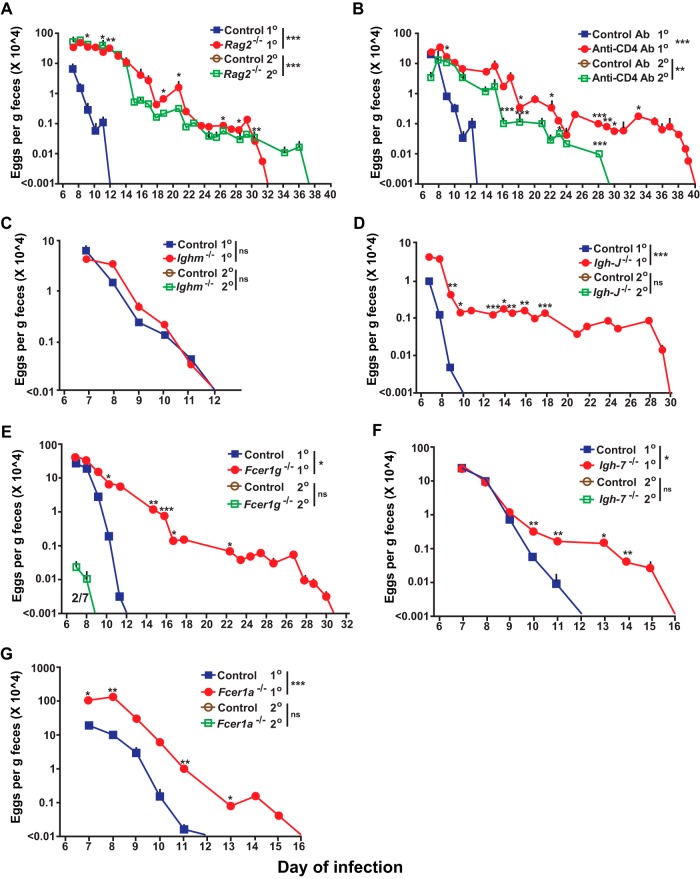
The more rapidly effective expulsion of S. venezuelensis observed in wild-type mice in the secondary infection is consistent with prior work indicating that adaptive immunity enhances host resistance to this nematode (39, 40, 45). To investigate the importance of various potential effector mechanisms in orchestrating immune responses to S. venezuelensis, we examined primary and secondary infections in mice deficient in different components of innate or adaptive immunity. We found that T and B cell-deficient Rag2-/- mice exhibited a 20-day delay (i.e., requiring 32 days in total) in cessation of S. venezuelensis egg excretion during primary infections and a 25-day delay (i.e., requiring 37 days in total) in the secondary infection (versus no eggs detected during secondary infections in any of the wild-type mice) (Fig. 3A), underscoring the importance of an adaptive immune response for effective secondary immune responses to this nematode (8, 11, 13, 14, 41, 46–48). In addition, we confirmed that the number of adult worms in the intestines on day 8 of the primary infection was significantly higher in the Rag2-/- mice than in the corresponding wild-type mice (Fig. S2B in the supplemental material), a finding consistent with the differences in the levels of egg excretion observed in these groups (Fig. 3A).
FIG 3.
Roles of T cells, ILC2 cells, antibodies, and antibody receptors in primary and secondary S. venezuelensis infections. Analyses of kinetics of S. venezuelensis egg excretion during 1o or 2o infections after inoculation with 10,000 S. venezuelensis L3 (second inoculations with S. venezuelensis were performed 42 days [panels A, B, D, E, and I] or 28 days [C, F, G, and J] after the first inoculation) in C57BL/6-Rag2-deficient (n = 13) and control C57BL/6 (n = 4) mice for 1o infection (3 experiments) and C57BL/6-Rag2-deficient (n = 6) and control C57BL/6 (n = 4) mice for 2o infection (2 experiments) (A), C57BL/6 wild-type mice treated with an anti-CD4 antibody or an isotype-matched control antibody (n = 3 each) (2 experiments) (B), C57BL/6-Ighm-deficient (n = 9) and control C57BL/6 (n = 7) mice for 1o infection (4 experiments) and C57BL/6-Ighm-deficient and control mice for 2o infection (n = 5 each) (2 experiments) (C), BALB/c-Igh-J-deficient (n = 8) and control BALB/c (n = 5) mice for 1o infection (3 experiments) and BALB/c-Igh-J-deficient and control BALB/c mice (n = 5 each) for 2o infection (2 experiments) (D), C57BL/6-Fcer1g-deficient (n = 7) and control C57BL/6 (n = 6) mice for 1o infection (3 experiments) and C57BL/6-Fcer1g-deficient (n = 3) and control C57BL/6 (n = 7) mice for 2o infection (3 experiments) (E), BALB/c-Igh-7-deficient (n = 17) and BALB/c-Igh-7-sufficient (n = 15) mice for 1o infection (6 experiments) and BALB/c-Igh-7-deficient (n = 11) and control BALB/c (n = 3) mice for 2o infection (3 experiments) (F), C57BL/6-Fcer1a-deficient (n = 10) and control C57BL/6 (n = 8) mice for 1o infection (3 experiments) and C57BL/6-Fcer1a-deficient and control C57BL/6 mice (n = 5 each) for 2o infection (2 experiments) (G), BALB/c-Rag2Il2g doubly deficient (n = 12) and control BALB/c (n = 10) mice for 1o infection (2 experiments) (H), C57BL/6-Rag2-deficient mice injected with an anti-NK1.1 antibody or an isotype-matched control antibody (n = 3 each) for 1o infection (2 experiments) (I), C57BL/6-Rorasg/sg (n = 3) and control C57BL/6 (n = 4) mice for 1o infection (2 experiments) and C57BL/6-Rorasg/sg (n = 4) and control C57BL/6 (n = 3) mice for 2o infection (2 experiments) (J), C57BL/6-Rag2-deficient mice injected with an anti-Thy1.2 antibody or an isotype-matched control antibody (n = 3 each) for 1o infection (2 experiments) (K), C57BL/6-Rag2-deficient mice (n = 5) and C57BL/6-Rag2-deficient Rorasg/sg mice (n = 3) and the corresponding wild-type C57BL/6 (n = 3) mice for 1o infection (2 experiments) (L), and BALB/c-Rag2Il2g doubly deficient mice treated with PBS (n = 3) and BALB/c-Rag2Il2g doubly deficient mice which received splenic T and B cells from wild-type BALB/c mice (n = 3) and the corresponding control BALB/c mice treated with PBS (n = 3) for 1o infection (2 experiments) (M). The numbers of eggs are shown as means + SEM, with data derived by pooling results from all experiments performed (as indicated for each strain). The fraction number in panel E is displayed as described for Fig. 1. ***, P < 0.0001; **, P < 0.001; *, P < 0.05; no asterisks, P > 0.05 (for comparisons between each type of mutant or antibody-treated mouse or mutant mouse that received splenic T and B cells and the corresponding control mice at each time point performed with the unpaired, two-tailed Student's t test and, as indicated next to the legend for groups of mice, with 2-way ANOVA to compare the time courses of the responses).
To assess contributions of CD4+ T cells, we injected anti-CD4 or isotype control antibodies into wild-type C57BL/6J mice. Compared to the control group, during the primary infection mice injected with the anti-CD4 antibody exhibited a 27-day delay in cessation of S. venezuelensis egg excretion (Fig. 3B). During secondary infections, no eggs were detected in any of the wild-type mice injected with the isotype control antibody but eggs persisted until day 29 in the anti-CD4 antibody-treated mice (Fig. 3B). While both Rag2-deficient mice and anti-CD4 antibody-treated wild-type mice exhibited an elevated percentage of blood basophils at baseline compared to the corresponding control mice (reflecting the depletion of lymphocytes in the blood of such mice), neither the primary nor the secondary S. venezuelensis infection induced a significant increase in the percentage of blood basophils (Fig. 4A; also see Fig. S4 in the supplemental material). As previously reported (11), jejunal mucosal MCs were essentially absent in Rag2-/- mice at baseline and did not appear in such mice after a primary infection (Fig. 4B and C). The low levels of expression of IgE on the surface of blood basophils in anti-CD4 antibody-treated wild-type mice during primary infections with S. venezuelensis (see Fig. S4 in the supplemental material) are consistent with an important role of CD4+ T cells in the induction of an IgE response in this setting (49, 50), with resulting upregulation of surface expression of FcεRI on blood basophils (51–53). However, these Rag2-deficient mice and anti-CD4 antibody-treated wild-type mice exhibited levels of jejunal goblet cell hyperplasia similar to those in the corresponding control mice (Fig. 4B to D; also see Fig. S5 in the supplemental material). These results confirm that, during S. venezuelensis infection, CD4+ T cells are essential for the expansion of jejunal mucosal MCs (11), elevation of IgE levels, and effective clearance of S. venezuelensis eggs (14) but are dispensable for jejunal goblet cell hyperplasia (Fig. 4B to D; also see Fig. S4 and S5 in the supplemental material).
To investigate the role of B cells in immune responses to S. venezuelensis, we assessed the infections in Ighm-deficient mice (known as μMT mice), which lack conventional B cells due to a deficiency in membrane μ heavy chain and exhibit markedly reduced levels of immunoglobulins (54). Surprisingly, Ighm-deficient mice did not exhibit a delay in cessation of S. venezuelensis egg excretion in the primary infection and, like the corresponding control mice, exhibited no detectable egg production during secondary infections. However, while Ighm-deficient mice had no detectable IgE on the surface of basophils before S. venezuelensis infection, IgE was detected on basophils on day 10 of S. venezuelensis infection (see Fig. S4 in the supplemental material), a finding in accord with the previously reported observation of IgE production in these mice infected with Heligmosomoides polygyrus, Trichuris muris, and Schistosoma mansoni (55). IgE was detected by flow cytometry on the surface of basophils after primary infections in all Ighm-deficient mice. On the other hand, with enzyme-linked immunosorbent assay (ELISA), which is not as sensitive as flow cytometry, total IgE was detected in 2 of 6 Ighm-deficient mice after secondary infections (see Fig. S6A in the supplemental material). Total IgG1 levels were elevated after primary infections in all Ighm-deficient mice (see Fig. S6B in the supplemental material). S. venezuelensis-specific IgG1 was detected in 1 of 6 Ighm-deficient mice after secondary infections, but much higher titers were detected in all wild-type mice after primary infections (see Fig. S6C in the supplemental material). Moreover, during primary infection with S. venezuelensis, Ighm-deficient mice and the corresponding wild-type mice developed similarly expanded populations of blood basophils, jejunal mucosal MCs, and jejunal goblet cells (Fig. 4; also see Fig. S4 in the supplemental material).
We also examined primary and secondary S. venezuelensis infections in Igh-J-deficient mice (also known as Jh-deficient mice), which lack all detectable antibodies from either conventional or nonconventional B cells (56). Igh-J-deficient mice exhibited a 20-day delay in cessation of S. venezuelensis egg excretion during the primary infection, suggesting an indispensable role of antibodies in the response to S. venezuelensis; however, like the corresponding control mice, Igh-J-deficient mice exhibited no detectable egg excretion during the secondary infection (Fig. 4D). Antibodies remained undetectable by ELISA (or by fluorescence-activated cell sorter [FACS] analysis of basophils) after either primary or secondary S. venezuelensis infection in Igh-J-deficient mice (data not shown), but Igh-J-deficient mice and the corresponding wild-type mice developed similarly expanded populations of blood basophils, jejunal mucosal MCs, and jejunal goblet cells during the primary infection (Fig. 4; also see Fig. S4 in the supplemental material).
The important role of antibodies in the primary infection with S. venezuelensis appears to depend on the binding of antibodies to FcRs that signal via the FcR common γ-chain (8, 10, 14), and in our model, we found that Fcer1g-deficient mice exhibited a 19-day delay in cessation of egg excretion (Fig. 3E), a delay very similar to that seen in Igh-J-deficient mice (Fig. 3D). Like Igh-J-deficient mice, Fcer1g-deficient mice exhibited levels of expanded populations of blood basophils, jejunal mucosal MCs, and jejunal goblet cells similar to those seen with the corresponding wild-type mice during primary S. venezuelensis infections (Fig. 4; also see Fig. S4 in the supplemental material). In secondary S. venezuelensis infections, 2 of 7 Fcer1g-deficient but none of the corresponding wild-type mice exhibited some egg excretion, but not after day 8 (Fig. 3E), but these differences were not statistically significant. Other groups showed that the numbers of adult worms in the intestines, as well as levels of S. venezuelensis egg excretion, were increased in antibody-deficient Aicda−/− mice and Fcer1g-deficient mice compared to the corresponding wild-type mice (8, 14).
Because FcRγ is a component of receptors for IgG and IgE, we searched for any nonredundant contribution of IgE by testing mice deficient in IgE (i.e., Igh-7-deficient mice) or the FcεRI α chain (i.e., Fcer1a−/− mice). IgE-deficient and FcεRI-deficient mice each had a 4-day delay to egg clearance in primary infections (Fig. 3F and G) and exhibited expanded populations of blood basophils, jejunal mucosal MCs, and jejunal goblet cells similar to those seen with the corresponding wild-type mice (Fig. 4; also see Fig. S4 in the supplemental material). Neither of these mutant mouse strains exhibited any egg production during secondary infections. These findings confirm prior evidence (14) indicating that both IgG and IgE antibodies can contribute to resistance to S. venezuelensis during primary infections and identify for the first time a small but nonredundant role for IgE, functioning via FcεRI, in the cessation of egg excretion in this setting. In contrast, there was no evidence of a nonredundant function of IgE or FcεRIα in egg clearance in secondary infections with S. venezuelensis.
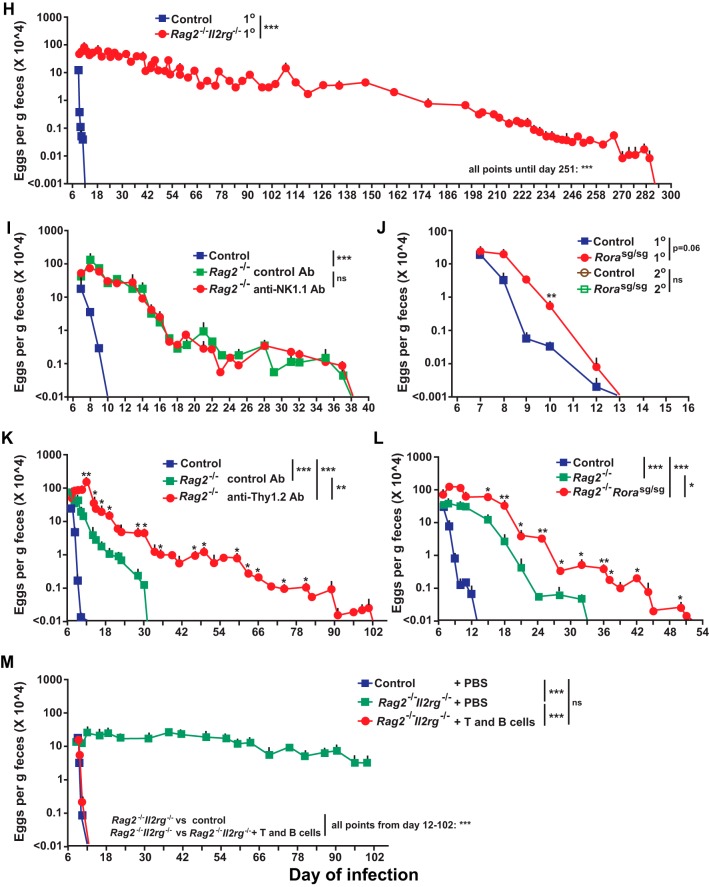
We also examined Rag2 and Il2g doubly deficient mice, which lack T cells, B cells, NK cells, and ILC2 cells (57), and found that they had a delay of about 270 days in egg clearance during primary infection with S. venezuelensis (Fig. 3H), as well as significantly increased numbers of adult worms in the intestines on day 8 of the infection (see Fig. S2C in the supplemental material). As in the Rag2−/− mice, Rag2 and Il2g doubly deficient mice exhibited no significant increase in the percentage of blood basophils and virtually lacked jejunal mucosal MCs (Fig. 4A to C; also see Fig. S4 in the supplemental material). However, unlike Rag2−/− mice, Rag2 and Il2g doubly deficient mice exhibited no increase in the levels of jejunal goblet cells during the primary infection (Fig. 4B and D).
Because Rag2 and Il2g doubly deficient mice lack NK cells and ILC2 cells (57), in addition to analyses of T and B cells, we examined Rag2-deficient mice depleted of NK cells using an anti-NK1.1 antibody (see Fig. S5 in the supplemental material). Such treatment did not influence the time to egg clearance in the primary infection (Fig. 3I). We then tested ILC2-deficient Rorasg/sg mice (58). Rorasg/sg mice excreted significantly more eggs on day10 of the primary infection than the corresponding wild-type mice, but the two strains of mice did not differ in time to cessation of egg excretion (Fig. 3J). Nor did such S. venezuelensis-infected Rorasg/sg mice differ from the corresponding wild-type mice in S. venezuelensis-induced expansion of blood basophil or jejunal mucosal MC populations or in exhibiting increases in levels of jejunal goblet cells (Fig. 4; also see Fig. S3 in the supplemental material).
Finally, we used 3 approaches to assess the effect of combined deficiencies in T cells, B cells, and ILC2 cells on primary infection with S. venezuelensis. First, we injected anti-Thy1.2 antibodies into Rag2-deficient mice to deplete ILC2 cells. Compared to the wild-type control group, the isotype control antibody-injected Rag2-deficient mice had a 20-day delay in cessation of egg excretion (to a total of 31 days) but the Thy1.2 antibody-injected Rag2-deficient mice exhibited an additional 71-day delay (to a total of 102 days) (Fig. 3K). Second, we found that, compared to the 20-day delay seen in Rag2-deficient mice (to a total of 33 days), Rorasg/sg Rag2-deficient mice exhibited an additional 19-day delay (to a total of 52 days) (Fig. 3L). Third, we found that the adoptive transfer of purified (>98%) splenic T cells and B cells from wild-type mice into Rag2;Il2g doubly deficient mice fully normalized the time to cessation of egg clearance (to a total of 13 days) (Fig. 3M). Taken together, the results shown in Fig. 3H to M suggest that NK cells made little detectable difference in the time to cessation of egg excretion in primary S. venezuelensis infection (Fig. 3H) but support the conclusion that both T cells and ILC2 cells can contribute to egg clearance (Fig. 3J to M). However, compared to the extraordinarily long delay in egg clearance seen in Rag2;Il2g doubly deficient mice (Fig. 3H), the shorter delays seen in mice deficient in T cells and/or ILC2 cells suggest that there might be an additional mechanism(s) contributing to the very long delay in egg clearance in Rag2;Il2g doubly deficient mice.
DISCUSSION
S. venezuelensis has been used for many years as an animal model for investigating the roles of various immune cells and antibodies in the control of infections with Strongyloides species. Previous work suggested that MCs can contribute to control of the primary infection, but most of those studies were done with KitW/W-v mice, which have a profound MC deficiency but also express other Kit-related abnormalities that might influence their response to S. venezuelensis (17–26). To our knowledge, there have been no prior reports of studies investigating the role of basophils in this infection.
By studying 3 types of MC-deficient mice and Mcpt8DTR mice, which can be selectively depleted of basophils, we provide additional evidence that MCs can contribute substantially to S. venezuelensis egg clearance during primary infections but make minor (if any) contributions during secondary infections, at least in the model of infection analyzed, whereas basophils can make a minor albeit statistically significant contribution during the primary infection but have no detectable role in the secondary infection (see Table S1 and Fig. S7 in the supplemental material).
In addition to confirming a prior report (14) indicating that IgG and IgE antibodies (in that report, acting in concert) can contribute to cessation of S. venezuelensis egg excretion during primary infections and prior reports that FcRγ also has an important role in that setting (8, 10, 14), we identified a small but statistically significant contribution of IgE and FcεRI as well (see Table S1 and Fig. S7 in the supplemental material), perhaps because we inoculated our mice with a larger number of cells of third-stage infective S. venezuelensis larvae (L3) than did Matsumoto et al. (14). While it seems likely that parasite-specific IgE and IgG antibodies bind to Fc receptors on effector cells and that their cross-linking by parasite antigens (especially for IgE) or the activation of effector cells by antigen-IgG immune complexes then results in expulsion of the parasites (14, 59, 60), the actual roles of parasite-specific and nonspecific antibodies in this setting are still controversial (61, 62). Ighm-deficient mice that lack antibodies at baseline were able to produce IgG1 and IgE during primary S. venezuelensis infections, and S. venezuelensis eggs were cleared on the same day as in wild-type mice (Fig. 3C; also see Fig. S4 and S6 in the supplemental material). Total IgE (detected by flow cytometry) and total IgG1 (measured by ELISA) levels were elevated in all Ighm-deficient mice (see Fig. S4 and S6 in the supplemental material). S. venezuelensis-specific IgG1 levels were elevated in all wild-type mice; however, only 1 of 6 Ighm-deficient mice had detectable S. venezuelensis-specific IgG1 (see Fig. S6 in the supplemental material). While the latter result does not strongly support the conclusion that parasite-specific antibodies have an essential role in cessation of egg excretion during primary S. venezuelensis infections, it is possible that some important S. venezuelensis antigens (e.g., those associated with adults worms but not larvae) were not included in our extract from L3. Similarly, it would be helpful to have more-sensitive assays to detect S. venezuelensis-specific IgE. Clearly, more research needs to be done to understand better the roles of parasite-specific antibodies in this setting.
In confirmation of findings reported by others (11), we found that CD4+ T cells contributed importantly to cessation of S. venezuelensis egg excretion in both primary and secondary infections. They contributed to the expansion of mucosal MCs and blood basophils in the primary infection and also to the development of an IgE response, as inferred based on increased levels of IgE on the surface of basophils (see Fig. S4 in the supplemental material and data not shown). Moreover, our evidence indicates that CD4+ T cells are largely if not entirely responsible for orchestrating effective immunity during secondary infections (see Table S1 in the supplemental material).
Notably, Rag2;Il2g doubly deficient mice exhibited a much longer delay in cessation of S. venezuelensis egg production than did Rag2-deficient mice. In addition to lacking cytokine responses requiring Il2g (which encodes a component of the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21), Rag2;Il2g doubly deficient mice lack NK cells, ILC2 cells, and T cells. Since NK cell depletion in Rag2-deficient mice did not have a detectable effect on S. venezuelensis expulsion, NK cells are unlikely to contribute substantially to S. venezuelensis expulsion. However, ILC2 cell-depleted Rag2-deficient mice took 102 days to achieve egg clearance (71 days longer than ILC2-sufficient Rag2-deficient mice), and Rag2-deficient Rorasg/sg mice took 51 days to achieve egg clearance (19 days longer than ILC2-sufficient Rag2-deficient mice), suggesting that there is cooperation between T cells and ILC2 cells in achieving cessation of S. venezuelensis egg production. This result also suggests that additional factors can contribute to the 270-day delay in egg clearance exhibited by Rag2;Il2g doubly deficient mice, such as effects related to the lack of cytokine signaling dependent on Il2g. Our work with Rorasg/sg mice, which lack ILC2 cells, also suggests that ILC2 can have a supportive role in cessation of S. venezuelensis egg production, which is in contrast to what appears to be a major role for these cells in immune responses to Nippostrongylus brasiliensis (63). However, jejunal goblet cell hyperplasia was observed during primary infections with S. venezuelensis in Rag2-deficient mice but not in Rag2;Il2g doubly deficient mice, raising the possibility that mucus production by goblet cells can contribute to cessation of S. venezuelensis egg excretion, as it does during immune responses to Nippostrongylus brasiliensis (64–66).
MATERIALS AND METHODS
Mice.
All animal experiments were carried out following protocols approved by the Stanford University Administrative Panel on Laboratory Animal Care. C57BL/6J (B6J)-KitW-sh/W-sh mice (37, 67) and littermate control B6J-Kit+/+ mice (wild-type control mice), C57BL/6J-Cpa3-Cre;Mcl-1fl/fl “Hello Kitty” mice (36) and the corresponding control (Cpa3-Cre;Mcl-1+/+) mice, BALB/cJ-Mcpt8DTR/+ mice (38) and littermate BALB/cJ-Mcpt8+/+ control mice, and BALB/cJ-MasTRECK (“MasTRECK” in this paper means heterozygous: Tg/+) mice (32) and littermate wild-type control mice (+/+; no Tg gene) were all from stocks bred in our laboratory. C57BL/6J wild-type, BALB/cJ wild-type, C57BL/6J-Fcer1g−/− (68), C57BL/6J-Rag2−/− (69), C57BL/6J-Ighm−/− (54), C57BL/6J-Rorasg/sg (70, 71), and C57BL/6J-Fcer1a−/− (72) mice were purchased from The Jackson Laboratory (Sacramento, CA). BALB/cAnNTac-Igh-J−/− mice (56) were purchased from Taconic (Germantown, NY). BALB/cJ-Rag2−/−Il2g−/− mice (73) and BALB/c-Igh-7−/− mice (74) (and the corresponding control BALB/c-Igh-7+/+ mice) (74) were kindly provided by I. Weissman (Stanford University School of Medicine) and H. Oettgen (Harvard Medical School, Boston, MA), respectively.
Infection with Strongyloides venezuelensis.
As previously described (7, 12), mice were infected by subcutaneous inoculation with 10,000 third-stage infective larvae (L3). For secondary infections, mice were infected by subcutaneous inoculation with 10,000 L3 28 or 42 days (as indicated) after the primary inoculation. We monitored infections in individual mice by counting the numbers of eggs (per gram of feces) excreted daily. Each type of immune cell was identified by flow cytometry except for tissue mast cells, which were counted as described previously (36). Mcpt8DTR/+ mice received intravenous injections of diphtheria toxin (DT; Sigma-Aldrich, St. Louis, MO) (200 ng/mouse) every 5 days starting 2 days before infection, and MasTRECK mice received intraperitoneal injections of DT (250 ng/mouse) every 5 days starting 2 days before infection. Control groups were a combination of DT-treated wild-type (Mcpt8+/+ or littermate wild-type control for MasTRECK) mice and PBS-treated Mcpt8DTR/+ mice or MasTRECK mice, because the two groups behaved similarly (see Fig. S1 in the supplemental material). For CD4 T cell depletion, 100 μg of anti-CD4 or isotype-matched control rat IgG2b κ antibodies (clone GK1.5 or clone RTK4530 from BioLegend, San Diego, CA) was injected intraperitoneally once a week starting at day −3. For NK cell depletion, 300 μg of anti-NK1.1 or isotype-matched control mouse IgG2a κ antibodies (clone PK136 or clone MOPC-173 from BioLegend) was injected intraperitoneally once a week starting at day −3. For depletion of ILC2 cells, 200 μg of anti-Thy1.2 or isotype-matched control rat IgG2b κ antibodies (clone 30-H12 or clone RTK4530 from BioLegend, San Diego, CA) was injected intraperitoneally into C57BL/6J-Rag2-deficient mice starting at day −1 and then every 3 days until the end of the experiment. Both male and female mice, which were 6 to 8 weeks old on the day of infection (day 0), were tested in each of the groups analyzed, and we found no statistically significant differences between male and female mice in the results obtained in any of the experimental groups. By direct observation at least until the time of cessation of S. venezuelensis egg excretion, all mice appeared to be healthy during the entire course of S. venezuelensis infection.
Antibodies and flow cytometry.
All reagents used for cytometry were from BioLegend and were as follows: mouse IgE-fluorescein isothiocyanate (IgE-FITC) or mouse IgE-biotin (R35-72), CD49b-Alexa 647 or -FITC (DX5), c-Kit-FITC or -phycoerythrin (PE) (2B8), FcεRIα-PE or -allophycocyanin (APC) (MAR-1), CD200R3-PE (Ba13), and APC-streptavidin. Basophils were identified as CD49b positive and mIgE, FcεRIα, or CD200R3 positive (depending on the type of mutant mouse). Purified anti-mMCP-8 (TUG8) (for tissue staining) was from Biolegend.
Histological analysis.
Sections (4 μm thick) of Carnoy's solution-fixed, paraffin-embedded jejunum were stained with 0.5% alcian blue. Mast cells and goblet cells were quantified per villus crypt unit (vcu) (75). To visualize tissue basophils, sections were stained with anti-mMCP-8 (10 μg/ml) (28, 36). Images were captured with an Olympus BX60 microscope using a Retiga-2000R QImaging camera run by Image-Pro Plus Version 6.3 software (Media Cybernetics).
ELISA.
Serum was collected before infections, 14 days after primary infections, and 14 days after secondary infections from wild-type C57BL/6J mice or C57BL/6J-Ighm-deficient mice. For detection of S. venezuelensis-specific IgG1 antibodies, S. venezuelensis antigens were prepared from L3 by an alkaline digestion method (76) and coated onto multiwell plates. Serum was added to the antigen-coated plates, followed by incubation with biotinylated anti-mouse IgG1 (clone R85-1) and then incubation with TMB (3,3′,5,5′-tetramethylbenzidine) as a substrate; the reaction then was stopped with 2 N sulfuric acid, and the optical density (OD) (405 nm) was measured. Total IgG1 was measured with a standard sandwich ELISA (clone A85-3 for coating and clone A85-1 for detection, with TMB as a substrate). Total IgE was measured with a standard sandwich ELISA (clone R35-72 for coating and clone R35-118 for detection, with TMB as a substrate).
Adult worm count.
At 8 days after inoculation with 10,000 S. venezuelensis L3 to induce a primary infection, the mice were sacrificed and the small intestines were collected and cut open longitudinally and then cut into small pieces and incubated in PBS at 37C° for 4 h and worms were counted under the microscope. By direct observation, none of the mice sacrificed for analysis of adult worm burdens exhibited evidence of adverse effects of such worms, such as weight loss, reduced spontaneous activity, or evidence of discomfort or loss of appetite.
Cell transfer.
CD4+ splenic T cells (4 × 106) and B220+ splenic B cells (1 × 106) were purified (>98% purity) from wild-type BALB/cJ mice with biotinylated anti-CD4 (clone GK1.5) and anti-B220 (clone RA3-6B2) antibodies (both from Biolegend), followed by incubation with streptavidin microbeads with a magnetically activated cell sorting (MACS) system (Miltenyi Biotec, Germany) and a single wash in PBS, and the purified splenic T and B cells in PBS were injected intravenously into BALB/cJ-Rag2Il2 doubly deficient mice on day −1.
Statistics.
Data were examined for statistical significance using the unpaired two-tailed Student's t test to compare values at each time point and by 2-way analysis of variance (ANOVA) for the time course of responses.
Supplementary Material
ACKNOWLEDGMENTS
We thank Chen Liu and Mariola Liebersbach for excellent technical assistance, Hans C. Oettgen for providing IgE-deficient mice and for his critical reading of the manuscript, and the members of the Galli laboratory for helpful discussions.
This work was supported by National Institutes of Health grants AI023990, CA072074, AI070813, and AR067145 and by the Department of Pathology, Stanford University School of Medicine (to S.J.G.).
We have no conflicting financial interests.
ADDENDUM IN PROOF
Shortly after our accepted manuscript was posted online, it was brought to our attention that a similar study (M. Reitz, M. L. Brunn, H. R. Rodewald, T. B. Feyerabend, A. Roers, et al., Mucosal Immunol 10:481–492, 2017, https://doi.org/10.1038/mi.2016.56) was posted online about the time we submitted the first version of our manuscript on 13 July 2016. Reitz et al., using c-Kit-independent mast cell deficient-mice and genetically basophil-deficient mice, detected roles for mast cells and basophils in primary infections with Strongyloides ratti. Although Reitz et al. and we used different species of Strongyloides, numbers of L3 to establish infection, types of c-Kit-independent mast cell- or genetically basophil-deficient mice, and protocols to establish secondary infections, the findings of the two studies support the following conclusions regarding mast cells and basophils: (i) mast cells can have an important role and basophils a smaller role in host defense against Strongyloides venezuelensis or Strongyloides ratti in primary infections, and (ii) while mast cells also may contribute to host defense in secondary infections with Strongyloides, their role is minor compared to that in primary infections, and the importance of such roles may vary according to the species of Strongyloides, the model of secondary infection analyzed, and/or other factors.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00053-17.
REFERENCES
- 1.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. 2006. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 2.Taylor MJ, Hoerauf A, Bockarie M. 2010. Lymphatic filariasis and onchocerciasis. Lancet 376:1175–1185. doi: 10.1016/S0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- 3.Grove DI. 1996. Human strongyloidiasis. Adv Parasitol 38:251–309. doi: 10.1016/S0065-308X(08)60036-6. [DOI] [PubMed] [Google Scholar]
- 4.Nawa Y, Kiyota M, Korenaga M. 1985. Defective protective capacity of W/Wv mice against Strongyloides ratti infection and its reconstitution with bone marrow cells. Parasite Immunol 7:429–438. doi: 10.1111/j.1365-3024.1985.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 5.Abe T, Sugaya H, Ishida K, Khan WI, Tasdemir I, Yoshimura K. 1993. Intestinal protection against Strongyloides ratti and mastocytosis induced by administration of interleukin-3 in mice. Immunology 80:116–121. [PMC free article] [PubMed] [Google Scholar]
- 6.Khan AI, Horii Y, Tiuria R, Sato Y, Nawa Y. 1993. Mucosal mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis. Int J Parasitol 23:551–555. doi: 10.1016/0020-7519(93)90159-V. [DOI] [PubMed] [Google Scholar]
- 7.Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, Nawa Y, Dranoff G, Galli SJ. 1998. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature 392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 8.Onah DN, Uchiyama F, Nagakui Y, Ono M, Takai T, Nawa Y. 2000. Mucosal defense against gastrointestinal nematodes: responses of mucosal mast cells and mouse mast cell protease 1 during primary Strongyloides venezuelensis infection in FcRγ-knockout mice. Infect Immun 68:4968–4971. doi: 10.1128/IAI.68.9.4968-4971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukao T, Yamada T, Tanabe M, Terauchi Y, Ota T, Takayama T, Asano T, Takeuchi T, Kadowaki T, Hata Ji J, Koyasu S. 2002. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol 3:295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- 10.Onah DN, Nawa Y. 2004. Mucosal mast cell-derived chondroitin sulphate levels in and worm expulsion from FcRγ-knockout mice following oral challenge with Strongyloides venezuelensis. J Vet Sci 5:221–226. [PubMed] [Google Scholar]
- 11.Sasaki Y, Yoshimoto T, Maruyama H, Tegoshi T, Ohta N, Arizono N, Nakanishi K. 2005. IL-18 with IL-2 protects against Strongyloides venezuelensis infection by activating mucosal mast cell-dependent type 2 innate immunity. J Exp Med 202:607–616. doi: 10.1084/jem.20042202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lantz CS, Min B, Tsai M, Chatterjea D, Dranoff G, Galli SJ. 2008. IL-3 is required for increases in blood basophils in nematode infection in mice and can enhance IgE-dependent IL-4 production by basophils in vitro. Lab Invest 88:1134–1142. doi: 10.1038/labinvest.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasuda K, Muto T, Kawagoe T, Matsumoto M, Sasaki Y, Matsushita K, Taki Y, Futatsugi-Yumikura S, Tsutsui H, Ishii KJ, Yoshimoto T, Akira S, Nakanishi K. 2012. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci U S A 109:3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto M, Sasaki Y, Yasuda K, Takai T, Muramatsu M, Yoshimoto T, Nakanishi K. 2013. IgG and IgE collaboratively accelerate expulsion of Strongyloides venezuelensis in a primary infection. Infect Immun 81:2518–2527. doi: 10.1128/IAI.00285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blankenhaus B, Reitz M, Brenz Y, Eschbach ML, Hartmann W, Haben I, Sparwasser T, Huehn J, Kühl A, Feyerabend TB, Rodewald HR, Breloer M. 2014. Foxp3+ regulatory T cells delay expulsion of intestinal nematodes by suppression of IL-9-driven mast cell activation in BALB/c but not in C57BL/6 mice. PLoS Pathog 10:e1003913. doi: 10.1371/journal.ppat.1003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitamura Y, Go S, Hatanaka K. 1978. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood 52:447–452. [PubMed] [Google Scholar]
- 17.Galli SJ, Zsebo KM, Geissler EN. 1994. The kit ligand, stem cell factor. Adv Immunol 55:1–96. doi: 10.1016/S0065-2776(08)60508-8. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura Y, Yokoyama M, Matsuda H, Shimada M. 1980. Coincidental development of forestomach papilloma and prepyloric ulcer in nontreated mutant mice of W/Wv and SI/SId genotypes. Cancer Res 40:3392–3397. [PubMed] [Google Scholar]
- 19.Shimada M, Kitamura Y, Yokoyama M, Miyano Y, Maeyama K, Yamatodani A, Takahashi Y, Tatsuta M. 1980. Spontaneous stomach ulcer in genetically mast-cell depleted W/Wv mice. Nature 283:662–664. doi: 10.1038/283662a0. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama M, Tatsuta M, Baba M, Kitamura Y. 1982. Bile reflux: a possible cause of stomach ulcer in nontreated mutant mice of W/Wv genotype. Gastroenterology 82:857–863. [PubMed] [Google Scholar]
- 21.Galli SJ, Arizono N, Murakami T, Dvorak AM, Fox JG. 1987. Development of large numbers of mast cells at sites of idiopathic chronic dermatitis in genetically mast cell-deficient WBB6F1-W/Wv mice. Blood 69:1661–1666. [PubMed] [Google Scholar]
- 22.Nakano T, Waki N, Asai H, Kitamura Y. 1989. Lymphoid differentiation of the hematopoietic stem cell that reconstitutes total erythropoiesis of a genetically anemic W/Wv mouse. Blood 73:1175–1179. [PubMed] [Google Scholar]
- 23.Nakano T, Waki N, Asai H, Kitamura Y. 1989. Different repopulation profile between erythroid and nonerythroid progenitor cells in genetically anemic W/Wv mice after bone marrow transplantation. Blood 74:1552–1556. [PubMed] [Google Scholar]
- 24.Puddington L, Olson S, Lefrançois L. 1994. Interactions between stem cell factor and c-Kit are required for intestinal immune system homeostasis. Immunity 1:733–739. doi: 10.1016/S1074-7613(94)80015-4. [DOI] [PubMed] [Google Scholar]
- 25.Huizinga JD, Thuneberg L, Klüppel M. 1995. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 26.Tsai M, Tam SY, Wedemeyer J, Galli SJ. 2002. Mast cells derived from embryonic stem cells: a model system for studying the effects of genetic manipulations on mast cell development, phenotype, and function in vitro and in vivo. Int J Hematol 75:345–349. doi: 10.1007/BF02982122. [DOI] [PubMed] [Google Scholar]
- 27.Mukai K, Tsai M, Starkl P, Marichal T, Galli SJ. 25 May 2016, posting date IgE and mast cells in host defense against parasites and venoms. Semin Immunopathol doi: 10.1007/s00281-016-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ugajin T, Kojima T, Mukai K, Obata K, Kawano Y, Minegishi Y, Eishi Y, Yokozeki H, Karasuyama H. 2009. Basophils preferentially express mouse Mast Cell Protease 11 among the mast cell tryptase family in contrast to mast cells. J Leukoc Biol 86:1417–1425. doi: 10.1189/jlb.0609400. [DOI] [PubMed] [Google Scholar]
- 29.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. 2010. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CD, Locksley RM. 2011. Genetic analysis of basophil function in vivo. Nat Immunol 12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eberle JU, Voehringer D. 26 April 2016, posting date Role of basophils in protective immunity to parasitic infections. Semin Immunopathol doi: 10.1007/s00281-016-0563-3. [DOI] [PubMed] [Google Scholar]
- 32.Sawaguchi M, Tanaka S, Nakatani Y, Harada Y, Mukai K, Matsunaga Y, Ishiwata K, Oboki K, Kambayashi T, Watanabe N, Karasuyama H, Nakae S, Inoue H, Kubo M. 2012. Role of mast cells and basophils in IgE responses and allergic airway hyperresponsiveness. J Immunol 188:1809–1818. doi: 10.4049/jimmunol.1101746. [DOI] [PubMed] [Google Scholar]
- 33.Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Köhler A, Peschke K, Vöhringer D, Waskow C, Krieg T, Müller W, Waisman A, Hartmann K, Gunzer M, Roers A. 2011. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 34:973–984. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 34.Feyerabend TB, Weiser A, Tietz A, Stassen M, Harris N, Kopf M, Radermacher P, Möller P, Benoist C, Mathis D, Fehling HJ, Rodewald HR. 2011. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity 35:832–844. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Rodewald HR, Feyerabend TB. 2012. Widespread immunological functions of mast cells: fact or fiction? Immunity 37:13–24. doi: 10.1016/j.immuni.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Lilla JN, Chen CC, Mukai K, BenBarak MJ, Franco CB, Kalesnikoff J, Yu M, Tsai M, Piliponsky AM, Galli SJ. 2011. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood 118:6930–6938. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piliponsky AM, Chen CC, Grimbaldeston MA, Burns-Guydish SM, Hardy J, Kalesnikoff J, Contag CH, Tsai M, Galli SJ. 2010. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am J Pathol 176:926–938. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, Obata K, Ishikawa R, Yoshikawa S, Mukai K, Kawano Y, Minegishi Y, Yokozeki H, Watanabe N, Karasuyama H. 2010. Selective ablation of basophils in mice reveals nonredundant role in acquired immunity against ticks. J Clin Invest 120:2867–2875. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato Y, Toma H. 1990. Strongyloides venezuelensis infections in mice. Int J Parasitol 20:57–62. doi: 10.1016/0020-7519(90)90173-K. [DOI] [PubMed] [Google Scholar]
- 40.Fernandes A, Pereira AT, Eschenazi PD, Schilter HC, Sousa AL, Teixeira MM, Negrão-Corrêa D. 2008. Evaluation of the immune response against Strongyloides venezuelensis in antigen-immunized or previously infected mice. Parasite Immunol 30:139–149. doi: 10.1111/j.1365-3024.2007.01009.x. [DOI] [PubMed] [Google Scholar]
- 41.Breloer M, Abraham D. 24 February 2016, posting date Strongyloides infection in rodents: immune response and immune regulation. Parasitology doi: 10.1017/S0031182016000111. [DOI] [PubMed] [Google Scholar]
- 42.Knott ML, Matthaei KI, Giacomin PR, Wang H, Foster PS, Dent LA. 2007. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol 37:1367–1378. doi: 10.1016/j.ijpara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Harvie M, Camberis M, Tang SC, Delahunt B, Paul W, Le Gros G. 2010. The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect Immun 78:3753–3762. doi: 10.1128/IAI.00502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obata-Ninomiya K, Ishiwata K, Tsutsui H, Nei Y, Yoshikawa S, Kawano Y, Minegishi Y, Ohta N, Watanabe N, Kanuka H, Karasuyama H. 2013. The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med 210:2583–2595. doi: 10.1084/jem.20130761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korenaga M, Hitoshi Y, Yamaguchi N, Sato Y, Takatsu K, Tada I. 1991. The role of interleukin-5 in protective immunity to Strongyloides venezuelensis infection in mice. Immunology 72:502–507. [PMC free article] [PubMed] [Google Scholar]
- 46.Gonçalves AL, Rodrigues RM, Silva NM, Gonçalves FA, Cardoso CR, Beletti ME, Ueta MT, Silva JS, Costa-Cruz JM. 2008. Immunolocalization and pathological alterations following Strongyloides venezuelensis infection in the lungs and the intestine of MHC class I or II deficient mice. Vet Parasitol 158:319–328. doi: 10.1016/j.vetpar.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 47.Rodrigues RM, Silva NM, Gonçalves AL, Cardoso CR, Alves R, Gonçalves FA, Beletti ME, Ueta MT, Silva JS, Costa-Cruz JM. 2009. Major histocompatibility complex (MHC) class II but not MHC class I molecules are required for efficient control of Strongyloides venezuelensis infection in mice. Immunology 128:e432–e441. doi: 10.1111/j.1365-2567.2008.02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodrigues RM, Cardoso CR, Gonçalves AL, Silva NM, Massa V, Alves R, Ueta MT, Silva JS, Costa-Cruz JM. 2013. Increased susceptibility to Strongyloides venezuelensis infection is related to the parasite load and absence of major histocompatibility complex (MHC) class II molecules. Exp Parasitol 135:580–586. doi: 10.1016/j.exppara.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Geha RS, Jabara HH, Brodeur SR. 2003. The regulation of immunoglobulin E class-switch recombination. Nat Rev Immunol 3:721–732. doi: 10.1038/nri1181. [DOI] [PubMed] [Google Scholar]
- 50.Wu LC, Zarrin AA. 2014. The production and regulation of IgE by the immune system. Nat Rev Immunol 14:247–259. doi: 10.1038/nri3632. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi M, Lantz CS, Oettgen HC, Katona IM, Fleming T, Miyajima I, Kinet JP, Galli SJ. 1997. IgE enhances mouse mast cell FcεRI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J Exp Med 185:663–672. doi: 10.1084/jem.185.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lantz CS, Yamaguchi M, Oettgen HC, Katona IM, Miyajima I, Kinet JP, Galli SJ. 1997. IgE regulates mouse basophil FcεRI expression in vivo. J Immunol 158:2517–2521. [PubMed] [Google Scholar]
- 53.Kubo S, Matsuoka K, Taya C, Kitamura F, Takai T, Yonekawa H, Karasuyama H. 2001. Drastic up-regulation of FcεRI on mast cells is induced by IgE binding through stabilization and accumulation of FcεRI on the cell surface. J Immunol 167:3427–3434. doi: 10.4049/jimmunol.167.6.3427. [DOI] [PubMed] [Google Scholar]
- 54.Kitamura D, Roes J, Kühn R, Rajewsky K. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature 350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 55.Perona-Wright G, Mohrs K, Taylor J, Zaph C, Artis D, Pearce EJ, Mohrs M. 2008. Cutting edge: helminth infection induces IgE in the absence of μ- or δ-chain expression. J Immunol 181:6697–6701. doi: 10.4049/jimmunol.181.10.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J, Trounstine M, Alt FW, Young F, Kurahara C, Loring JF, Huszar D. 1993. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol 5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 57.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. 2010. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 58.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, Radtke F, Hardman CS, Hwang YY, Fallon PG, McKenzie AN. 2012. Transcription factor RORα is critical for nuocyte development. Nat Immunol 13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anthony RM, Rutitzky LI, Urban JF Jr, Stadecker MJ, Gause WC. 2007. Protective immune mechanisms in helminth infection. Nat Rev Immunol 7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fitzsimmons CM, Falcone FH, Dunne DW. 14 February 2014, posting date Helminth allergens, parasite-specific IgE, and its protective role in human immunity. Front Immunol doi: 10.3389/fimmu.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pritchard DI. 1993. Immunity to helminths: is too much IgE parasite- rather than host-protective? Parasite Immunol 15:5–9. doi: 10.1111/j.1365-3024.1993.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 62.Pritchard DI, Hewitt C, Moqbel R. 1997. The relationship between immunological responsiveness controlled by T-helper 2 lymphocytes and infections with parasitic helminths. Parasitology 115:S33–44. doi: 10.1017/S0031182097001996. [DOI] [PubMed] [Google Scholar]
- 63.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. 2010. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishikawa N, Horii Y, Oinuma T, Suganuma T, Nawa Y. 1994. Goblet cell mucins as the selective barrier for the intestinal helminths: T-cell-independent alteration of goblet cell mucins by immunologically ‘damaged’ Nippostrongylus brasiliensis worms and its significance on the challenge infection with homologous and heterologous parasites. Immunology 81:480–486. [PMC free article] [PubMed] [Google Scholar]
- 65.Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, Orekov T, Perkins C, Wang Q, Brombacher F, Urban JF Jr, Rothenberg ME, Finkelman FD. 2009. Intestinal epithelial cell secretion of RELM-β protects against gastrointestinal worm infection. J Exp Med 206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, Dickey BF, Wilson MS, Wynn TA, Grencis RK, Thornton DJ. 2011. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med 208:893–900. doi: 10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. 2005. Mast cell-deficient W-sash c-kit mutant KitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol 167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. 1994. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell 76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 69.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855–867. doi: 10.1016/0092-8674(92)90029-C. [DOI] [PubMed] [Google Scholar]
- 70.Sidman RL, Lane PW, Dickie MM. 1962. Staggerer, a new mutation in the mouse affecting the cerebellum. Science 137:610–612. doi: 10.1126/science.137.3530.610. [DOI] [PubMed] [Google Scholar]
- 71.Mamontova A, Séguret-Macé S, Esposito B, Chaniale C, Bouly M, Delhaye-Bouchaud N, Luc G, Staels B, Duverger N, Mariani J, Tedgui A. 1998. Severe atherosclerosis and hypoalphalipoproteinemia in the staggerer mouse, a mutant of the nuclear receptor RORα. Circulation 98:2738–2743. doi: 10.1161/01.CIR.98.24.2738. [DOI] [PubMed] [Google Scholar]
- 72.Dombrowicz D, Flamand V, Brigman KK, Koller BH, Kinet JP. 1993. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell 75:969–976. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- 73.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, Paul WE, Katz SI, Love PE, Leonard WJ. 1995. Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity 2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 74.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. 1994. Active anaphylaxis in IgE-deficient mice. Nature 370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 75.Tsai M, Shih LS, Newlands GF, Takeishi T, Langley KE, Zsebo KM, Miller HR, Geissler EN, Galli SJ. 1991. The rat c-kit ligand, stem cell factor, induces the development of connective tissue-type and mucosal mast cells in vivo. Analysis by anatomical distribution, histochemistry, and protease phenotype. J Exp Med 174:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Machado ER, Ueta MT, de Fátima Gonçalves-Pires MDR, Alves de Oliveira JB, Faccioli LH, Costa-Cruz JM. 2003. Strongyloides venezuelensis alkaline extract for the diagnosis of human strongyloidiasis by enzyme-linked immunosorbent assay. Mem Inst Oswaldo Cruz 98:849–851. doi: 10.1590/S0074-02762003000600024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.