Abstract
Mycoplasma pneumoniae is the causative agent of primary atypical pneumonia in humans. Adherence of M. pneumoniae to host cells requires several adhesin proteins, such as P1, P30, and P116. A major limitation in developing a specific diagnostic test for M. pneumoniae is the inability to express adhesin proteins in heterologous expression systems due to unusual usage of the UGA stop codon, leading to premature termination of these proteins in Escherichia coli. In the present study, we successfully expressed the C-terminal (P1-C1) and N-terminal (P1-N1) regions of the P1 protein in E. coli. On screening these recombinant proteins with sera from M. pneumoniae-infected patients, only the P1-C1 protein was found to be immunogenic. This protein can be used as an antigen for immunodiagnosis of M. pneumoniae infection, as well as in adherence inhibition studies to understand the pathophysiology of the disease.
Mycoplasma pneumoniae is the causative agent of atypical pneumonia and is also responsible for other respiratory tract infections such as tracheobronchitis, bronchiolitis, croup, and less severe upper respiratory tract infections in older children and young adults (3, 14). It has been estimated that between 10 and 20% of X-ray-proven pneumonia cases that occur in the endemic period and that up to 50% of all cases that occur in the epidemic period are caused by M. pneumoniae (7, 23).
Adherence of M. pneumoniae to the human host respiratory epithelium is a prerequisite for colonization of the respiratory epithelium and subsequent induction of disease (12, 14). Cytadherence is mediated by a specialized tip-like attachment organelle found in M. pneumoniae (8, 21). It requires a complex interaction of several M. pneumoniae proteins present on the attachment organelle, including the major surface adhesins P1 (170 kDa), P30 (30 kDa), and P116 and proteins HMW1 to HMW5, as well as proteins A, B and C (1, 10, 14, 24, 29, 30). These proteins cooperate structurally and functionally so that M. pneumoniae major surface adhesins display polar clustering at the organelle tip assisted by HMW proteins. The major proteins P1 and P30 appear to be directly involved in receptor binding (5, 13, 21). The HMW proteins and proteins A, B, and C are accessory proteins since they are not adhesins but are required for proper functioning. The native adhesins P1 and P30 are also known to be strongly immunogenic in humans and experimental animals infected with M. pneumoniae.
The true incidence of M. pneumoniae associated infection is not clear because of the nonavailability of rapid and specific diagnostic tests. Being a fastidious organism, M. pneumoniae grows slowly and poorly. The standard methods for the diagnosis of M. pneumoniae are culture, serology, and PCR. Since M. pneumoniae can be difficult to isolate (11), laboratory diagnosis is based on serological tests, such as complement fixation and enzyme-linked immunosorbent assays (ELISAs) (31). PCR has also been used for its detection (6, 34). The complement fixation test has limited value and produces inconclusive results because it also measures antibodies from previous infections (26). The glycolipid antigen, which is not M. pneumoniae specific, cross-reacts with antigens of other microorganism and body tissues (3). Since serology is often used for the diagnosis of M. pneumoniae infections (15), it is important to identify specific antigen(s) that can distinguish between previous and current infections. Recently, the P1 protein has been used as an antigen for the immunodiagnosis of M. pneumoniae infection (19). However, technical difficulties in protein purification and its large-scale expression limits its diagnostic use. The P1 gene contains an open reading frame of 4,881 nucleotides coding for a protein of 1,627 amino acids with a calculated molecular weight of 176,888 and contains 21 UGA codons that code for tryptophan (29). The presence of these UGA codons makes it difficult to express the P1 protein in Escherichia coli as UGA codes for a stop codon. A number of attempts have been made to express this protein by using different expression systems (9, 27, 33). To circumvent these problems, we decided to express two different regions of the P1 protein in E. coli. This includes the N-terminal (P1-N1) and a C-terminal (P1-C1) region, which have been suggested to be immunodominant and to act as adhesins (17). The UGA codons in these fragments were modified to UGG, the genes were cloned and expressed in E. coli and immunological characterization of both the proteins were undertaken.
MATERIALS AND METHODS
Organisms and growth conditions.
The lyophilized ampoule of M. pneumoniae standard strain (FH lieu; National Collection of Type Cultures, London, United Kingdom) was reconstituted in Edward Hayflick medium containing PPLO basal broth that was supplemented with 1% glucose (Difco) as the carbon source and 0.0002% phenol red as the indicator. Tissue culture flasks (Nunc, Roskilde, Denmark) were incubated at 37°C aerobically and inspected daily. An exponential growth phase was indicated by a change in color of the medium from red to orange. Cells were harvested at this stage, washed in phosphate-buffered saline (PBS), and centrifuged, and the pellet was stored at −70°C. The organism was confirmed by subculturing 0.2 ml of the broth culture on PPLO agar plates (Borosil). Plates were incubated at 37°C in 5% CO2 in a candle jar and were examined at 3 day intervals. Suspected colonies were confirmed by Dienes staining and by growth inhibition test with M. pneumoniae polyclonal antisera (National Collection of Type Cultures).
PCR amplification of N-terminal (P1-N1) and C-terminal fragments (P1-C1) of the P1 gene.
To circumvent the translational barrier of UGA codon, we searched the P1 gene-encoded protein for functional domains involved in cytadherence and immunodominant regions. Based on published literature, we decided to express two of these regions, the N-terminal region [P1-N1] (18) and the C-terminal region [P1-C1] (4), encompassing amino acid residues 60 to 180 and 1160 to 1521, respectively.
Genomic DNA extracted from the standard strain of M. pneumoniae (FH lieu) was used as a template for amplification of N-terminal and C-terminal regions of the P1 gene. DNA was extracted according to the method of Stauffer et al. (28) and then subjected to PCR amplification.
For PCR amplification of the P1-N1 region, we used the following primer sets (Biobasic, Inc.). For the P1-N1 region the forward (P-1c) 5′-AGA TCT GAA TTC AAT GCC ATC AAC CCG CGC TTA ACC CCG TGG ACG TAT CG-3′ and reverse (P-1d) 5′-CCC AAG CTT GAC CTC GTT CCA CTG TTG GGG TGC AGC CCC-3′ primers were used, and for the P1-C1 region the forward (P-1e) 5′-AGA TCT GAA TTC GCG GCC TTT CGT GGC AGT TGG GTC-3′ and reverse (P-If) 5′-CAT TGG CTG CAG ATC AGG CCA CTG GTT AAA CGG ACT AAA CAA-3′ primers were used. These primers were designed so that the UGA codon was changed to UGG for an E. coli expression system.
PCR was performed in a total of 100 μl of reaction mixture containing 1× PCR buffer (100 mM Tris-HCl [pH 9.0], 500 mM KCl,15 mM MgCl2, and 0.1% gelatin), 200 μM deoxynucleoside triphosphates, 50 pmol of each primer, 1 U of Taq polymerase (3 U/μl; Bangalore Genei, Bangalore, India), and template DNA (100 ng). Amplification was carried out by denaturation at 94°C for 1 min, annealing at 62°C for 1 min, and extension at 72°C for 3 min for 35 cycles. PCR products were analyzed on 1.5% agarose gel, and the amplified fragments of 1,085 and 362 bp were purified by using gel extraction kit (Qiagen).
Cloning and expression of P1-N1 and P1-C1 regions of P1 gene.
PCR amplified fragments were first ligated into the pGEMT vector (Promega). The ligation mixtures were used to transform competent DH5α E. coli cells. Transformants were selected on Luria-Bertani (LB) agar containing ampicillin (100 μg/ml). Individual colonies were picked up and grown overnight in 5 ml of LB broth containing ampicillin (100 μg/ml). Plasmid DNA was isolated from overnight cultures by using Miniprep plasmid extraction kit (Qiagen) and digested with EcoRI to ensure the presence of the inserts. These inserts were then sequenced to rule out any mutations.
For the expression, the inserts were released from pGEMT clones by restriction digestion with BglII and PstI for the 1,085-bp P1-C1 insert and with BglII and HindIII for the 362-bp P1-N1 insert. After gel purifications, the inserts were ligated into the expression vector pQE-30 (Qiagen) digested at corresponding sites. Ligation mixtures were used to transform M15 cells, and transformants were selected on kanamycin (25 μg/ml) and ampicillin (100 μg/ml) plates. Plasmid DNA was extracted from overnight cultures and subjected to restriction digestion to check the inserts.
M15 cells containing the recombinant plasmids were cultivated in 5 ml of LB broth (containing ampicillin and kanamycin as described above) at 37°C with shaking until the optical density (OD) reached 0.5 to 0.6. Protein expression was induced by 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside; Sigma). After 3 h of shaking incubation at 37°C, bacteria were pelleted by centrifugation and further subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to check the expression of recombinant proteins (25).
SDS-PAGE and Western blotting:.
For SDS-PAGE analysis, crude E. coli lysates expressing the recombinant P1 fragments (50 μg) were dissolved in 30 μl of SDS sample buffer (62.5 mM Tris-HCl [pH 6.8], 10% glycerol, 2.3% [wt/vol] SDS, 5% [vol/vol] β-mercaptoethanol, and 0.05% [wt/vol] bromophenol blue) and boiled for 2 min. The proteins were separated by SDS-10% PAGE and subsequently stained with Coomassie brilliant blue R-250. To ascertain the expression of the recombinant proteins, Western blotting was performed for E. coli cell extracts by using Penta-His monoclonal antibodies (Qiagen). Purified recombinant protein P1-C1 was also studied for its reactivity with anti-M. pneumoniae infected pooled human sera and individual sera (collected from patients with community-acquired pneumonia who tested positive for immunoglobulin G [IgG] antibodies to M. pneumoniae [Serion Classic ELISA kit; Serion GmbH, Wurzburg, Germany]). For immunoblotting, E. coli extracts expressing recombinant P1-C1/P1-N1 and purified recombinant P1-C1 protein were separated by SDS-PAGE, and proteins were transferred to nitrocellulose membrane by standard procedures (32). The membranes were blocked with 5% casein at room temperature for 1 h. After being washed with PBS-Tween 20, the blots were incubated with Penta-His monoclonal antibodies at a dilution of (1:2,000) or anti-M. pneumoniae antibody-positive pooled human sera (n = 20; the same individual patients sera were mixed equally to make pooled sera) at a dilution of 1:100 in two separate experiments. The dilution used for the pooled sera, as well as for the individual sera, was the same. The negative control was negative individual sera (tested negative by commercial kit), and it was also diluted to the same extent.
The blots were reacted with peroxidase-conjugated anti-mouse IgG (1:1,000 dilution) or anti-human IgG (1:1,000 dilution), respectively. After being washed, the final enzyme reaction was developed with 4-chloro-1-naphthol and H2O2.
Purification of recombinant P1-C1 protein:.
Once we ascertained that the expressed proteins were of M. pneumoniae origin, we purified recombinant protein P1-C1 by using an Ni-nitrilotriacetic acid (NTA) column (Qiagen). For large-scale production of P1-C1 protein, 100 ml of culture of E. coli cells expressing P1-C1 were grown and induced with 1 mM IPTG. After the induction, the bacterial pellet was obtained by centrifugation at 5,000 × g for 30 min at 4°C and then resuspended in 10 ml of sonication buffer (50 mM Na2HPO4 [pH 7.0], 120 mM NaCl, 1 mM phenylmethylsulfonyl fluoride). Cells were sonicated with a 5-min pulse at 2-min intervals. The supernatant was incubated at 4°C with Ni-NTA agarose resin (Qiagen) with constant shaking. After 1 h, the resin was packed to a column and extensively washed with the sonication buffer, followed by wash buffer (57 mM Na2HPO4 [pH 6.0], 128 mM NaCl, and 10% glycerol). Bound proteins were eluted with Tris-HCl (pH 8.0) containing 0.25 M imidazole (Sigma). Fractions containing the recombinant protein with a high degree of purity were pooled and extensively dialyzed against PBS, and the protein concentration was determined by Lowry method (22).
Comparative ELISA with purified P1-C1 epitope and commercial IgG assay.
Serum samples were collected from adult patients with community-acquired pneumonia and then screened for anti-M. pneumoniae IgG antibodies by using the commercial Serion Classic ELISA kit, which has a sensitivity of 100% and a specificity of 90% according to the manufacturer's instructions. A total of 33 IgG-positive sera and 32 IgG-negative sera were included in the present study.
Antibody responses to recombinant protein P1-C1 were evaluated by ELISA. Flat-bottom 96-well Immulon plates (Dynatech) were coated with 100 ng of the recombinant protein per well in 0.06 M carbonate-bicarbonate buffer (pH 9.6) and then incubated overnight at 4°C and a further 1 h at 37°C. The uncovered reactive sites were blocked with 2% skimmed milk in PBS at 37°C for 2 h. The antigen-coated wells were incubated sequentially for 1 h with 1:100-diluted test sera and a 1:10,000 dilution of peroxidase-labeled goat anti-human IgG. In between these incubations, the plates were washed thrice with PBS containing a 0.05% Tween 20. The enzyme reaction was developed by the addition of 1 mg of ortho-phenylenediamine (Sigma)/ml diluted in phosphate-citrate buffer (pH 5.0) containing 0.03% (vol/vol) hydrogen peroxide. The enzymatic reaction was stopped with 8 N H2SO4, and the OD at 492 nm (OD492) of the reaction product in the wells was recorded with a Bio-Tek microplate reader. The sample was considered positive if the OD492 was >0.5.
RESULTS
Generation of recombinant fragments of P1 protein and their characterization.
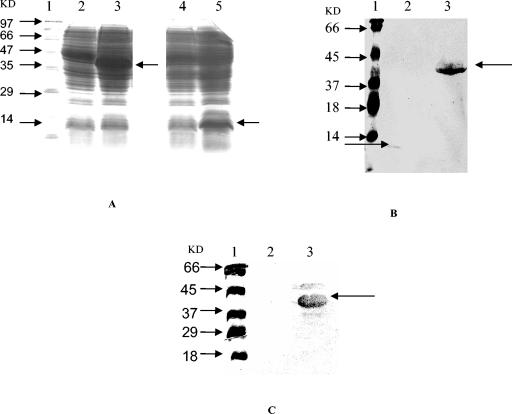
Fragments of the P1 gene, i.e., P1-N1 and P1-C1 (Fig. 1), were amplified by PCR and then expressed in E. coli M15 cells by using pQE-30 vector. Both of the recombinant protein fragments were successfully expressed in E. coli, and the expression level of P1-C1 was found to be quite high. Figure 2A shows the expression levels of these proteins as detected by SDS-PAGE. The apparent molecular masses were 13.9 kDa for P1-N1 and ∼39.7 kDa for P1-C1. Upon immunoblotting, both of the recombinant proteins in E. coli cell lysates reacted nicely with Penta-His monoclonal antibody (Fig. 2B). However, with pooled patient sera only P1-C1 fragment was recognized (Fig. 2C). Recombinant purified P1-C1 reacted nicely with individual and pooled positive sera and also with anti-histidine antibodies (see Fig. 4).
FIG. 1.
Schematic diagram showing P1 gene sequence and location of the P1-C1 and P1-N1 regions.
FIG. 2.
(A) SDS-PAGE showing the expression of P1-C1 and P1-N1 proteins in E. coli extracts. Lanes: 1, standard protein marker; 2, uninduced P1-C1; 3, induced P1-C1; 4, uninduced P1-N1; 5, induced P1-N1. (B and C) Western blot analysis of E. coli extracts showing the expression of P1-C1 and P1-N1 proteins with anti-histidine antibody (B) and with pooled patient sera (C). Lanes: 1, prestained protein marker; 2, induced P1-N1 (∼13.9 kDa); 3, induced P1-C1 (∼39.7 kDa).
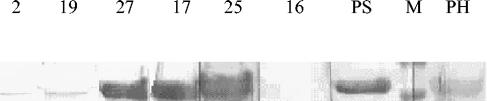
FIG. 4.
Immunoblot analysis of purified P1-C1 protein with individual and pooled IgG. Results with positive patient sera and with anti-histidine antibodies are shown. Lanes 2, 19, 27, 17, and 25 represent positive individual sera. Lane 16 represents a negative control (negative individual sera, tested negative by commercial kit). PS, P1-C1 reacted with positive pooled sera; M, standard protein marker; PH, P1-C1 reacted with anti-histidine antibodies.
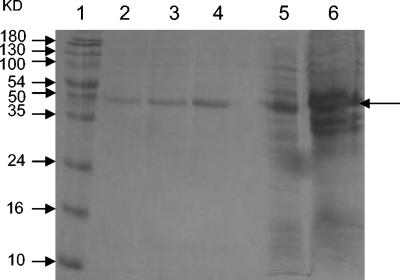
Purification of recombinant P1-C1 fragment.
Purification of P1-C1 was initiated by lysis of the E. coli pellet and then by analysis of protein in the soluble fraction as well as in the E. coli pellet. The protein was found to be expressed in the soluble fraction. This was purified by Ni-NTA affinity chromatography to apparent homogeneity (Fig. 3). Fractions that showed the single P1-C1 band were pooled, dialyzed, and used for Western blot and comparative ELISA analyses. On immunoblotting, the purified recombinant P1-C1 protein reacted well with anti-histidine antibodies and with individual and pooled patient sera. The protein did not show any reactivity with negative human sera (Fig. 4).
FIG. 3.
SDS-PAGE showing purification of P1-C1 protein on Ni-NTA column. Lanes: 1, standard protein marker; 2, 3, and 4, 0.25 mM imidazole elutions; 5, flowthrough; 6, induced E. coli cell pellet sample expressing P1-C1.
Comparative ELISA analysis.
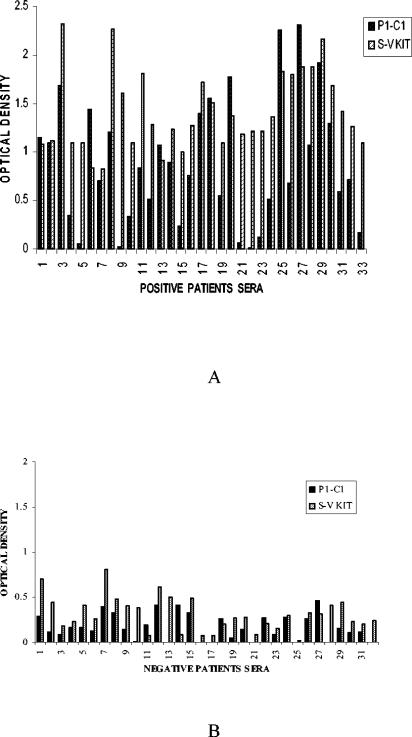
Comparative evaluation of the reactivity of the patient sera with P1-C1, as well as with a Serion Classic ELISA kit, was carried out. In our ELISA experiment with purified P1-C1 protein as an antigen, of the 33 positive sera selected based on the reactivity with Serion-Virion kit, 24 reacted with P1-C1 (Fig. 5A). A total of 32 patients sera who showed no reactivity when tested with the Serion-Virion ELISA kit also did not react with the P1-C1 fragment (Fig. 5B).
FIG. 5.
Comparative ELISA analysis with recombinant P1-C1 and Serion-Virion test with 65 patients. (A) Positive patient sera; (B) negative patient sera.
Statistical analysis.
Comparative statistical analysis of P1-C1 ELISA, taking the Serion-Virion ELISA as the standard, has been done by applying the diagnostic test. For P1-C1, the cutoff value was taken as 0.5 absorbance. At 0.5 absorbance, the cutoff points for the sensitivity and specificity of P1-C1 were determined to be 72.7% (24 of 33) and 100% (32 of 32), respectively.
DISCUSSION
The major limitation of developing a specific diagnostic test for M. pneumoniae is the inability to express the specific proteins of M. pneumoniae in heterologous expression systems due to the unusual codon usage of UGA for stop codon. Although in other systems this is a stop codon, in M. pneumoniae it codes for tryptophan. We were interested in identifying the antigenic sites of the P1 gene, which may serve as candidate antigens in developing a diagnostic assay. In the present study, a simple cloning strategy was adopted to express stretches of the P1 gene that are known to be immunogenic and are also involved in cytadherence.
Toward this end, we expressed two domains of P1 gene, one in the N-terminal region and another in the C-terminal region. Both the fragments were expressed in E. coli by using the pQE-30 expression system. In our study, the C-terminal region was found to be immunogenic since it showed strong reactivity on immunoblotting with M. pneumoniae IgG antibody-positive patient sera, whereas the region corresponding to N-terminal position showed no reactivity with these sera. We purified the C-terminal domain of the P1 adhesin by using an Ni-NTA affinity column and then used this purified protein for comparative ELISA analysis with the Serion Classic ELISA kit. This kit has been reported to be 100% sensitive and 90% specific as described in the manufacturer's instructions (20) and was found to be useful in our previous study (2) for the diagnosis of M. pneumoniae infection.
During the course of our study, we came across a report by Svenstrup et al. (30), who expressed three different regions of P1 protein spanning N-terminal, middle, and C-terminal parts in an E. coli expression system. One of the regions, P116, overlapped with the fragment P1-C1 expressed in the present study. Svenstrup et al. raised monoclonal antibodies to these fragments and showed that anti-P116 antibody blocked attachment of M. pneumoniae to the respiratory epithelial cells. However, they did not check the immunoreactivity of this protein against patient sera. Interestingly, the immunodominant epitope found in the C-terminal part of P1 gene in our study was also shown to be immunodominant as well as cytadherent by Dallo et al. (4). This author detected a region of P1 gene spanning 4067 to 4185 bp while screening a λgt11 library with patient sera.
A recombinant protein corresponding to the N-terminal region of P1 gene in this study codes for a P1 protein that has been shown to be immunogenic by Jacobs et al. (18) by using a chemically synthesized peptide. However, in our study this region was found to be nonreactive with patient sera. The reason for the nonimmunogenicity of the P1-N1 portion may be that immunoreactive epitopes may be hidden in the expressed P1-N1 fragment or it may be due to the biological mimicry between P1 epitopes and mammalian antigens, as suggested in another study (16).
In conclusion, the results from the present study indicate that the C-terminal region of the P1 protein is an important antigen and can be used along with two other adhesin molecules, P30 and P116, for the development of a sensitive assay for the diagnosis of M. pneumoniae infection. The use of this protein to study cytadherence may also provide insight into the pathophysiology of this disease.
Acknowledgments
This study was supported by grants from the Department of Science and Technology and the Indian Council of Medical Research.
We thank Irum Tabassum for help in collecting clinical samples, statistical analysis, and modifying the manuscript. A. B. Dey for help in providing clinical samples. We also thank Shahid Jamil for critically reviewing the manuscript. We also acknowledge Guresh Kumar for statistical analysis and Avanish K. Varshney and Pramod Kumar for their assistance.
REFERENCES
- 1.Baseman, J. B., J. Morrison-Plummer, D. Drouillard, B. Puleoscheppke, V. V. Tryon, and S. C. Holt. 1987. Identification of 30-kilodalton protein of Mycoplasma pneumoniae associated with hemadsorption. Israel J. Med. Sci. 23:474-479. [PubMed] [Google Scholar]
- 2.Chaudhry, R., A. B. Dey, et al. 2002. Community-acquired pneumonia in the elderly. ICMR Report. Personal communication.
- 3.Clyde, W. A. 1993. Clinical overview of atypical Mycoplasma pneumoniae infections. Clin. Infect. Dis. 17(Suppl.):S32-S37. [PubMed] [Google Scholar]
- 4.Dallo, S. F., C. J. Su, J. R. Horton, and J. B. Baseman. 1988. Identification of P1 gene domain containing epitopes mediating Mycoplasma pneumoniae cytadherence. J. Exp. Med. 167:718-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David, D., et al. 1989. Sialic acid-dependent adhesion of Mycoplasma pneumoniae to purified glycoproteins. J. Biol. Chem. 264:9289-9293. [PubMed] [Google Scholar]
- 6.Dorigo-Zetsma, J. W., S. A. Zaat, P. M. Wertheim van Dillen, L. Spanjaard, J. Rijintjes, G. Van Waveren, J. S. Jensen, A. F. Angulo, and J. Dankert. 1999. Comparison of PCR, culture, and serological tests for diagnosis of Mycoplasma pneumoniae respiratory tract infection in children. J. Clin. Microbiol. 37:14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dybvig, K. 1990. Mycoplasma genetics. Annu. Rev. Microbiol. 44:81-104. [DOI] [PubMed] [Google Scholar]
- 8.Franzoso, G., P. C. Hu, G. A. Meloni, and M. F. Barile. 1993. The immunodominant 90-kilodalton protein is localized on the terminal tip structure of Mycoplasma pneumoniae. Infect. Immun. 61:1523-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frydenberg, J., K. Lind, and P. C. Hu. 1987. Cloning of Mycoplasma pneumoniae DNA and expression of P1 epitopes in Escherichia coli. Israel J. Med. Sci. 23:759-762. [PubMed] [Google Scholar]
- 10.Gerstenecker, B., and E. Jacobs. 1990. Topological mapping of the P1 adhesin of Mycoplasma pneumoniae with adherence inhibiting monoclonal antibodies. J. Gen. Microbiol. 136:471-476. [DOI] [PubMed] [Google Scholar]
- 11.Harris, R., B. P. Marmion, G. Varkanis, T. Kok, B. Lunn, and J. Martin. 1988. Laboratory diagnosis of Mycoplasma pneumoniae infection: comparison of methods for the direct detection of specific antigen or nucleic acid sequences in respiratory exudates. Epidemiol. Infect. 101:685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayflick, L. 1965. Tissue cultures and mycoplasmas. Text. Rep. Biol. Med. 23:285-303. [PubMed] [Google Scholar]
- 13.Howard, C., et al. 1989. Adhesin of Mycoplasma pneumoniae to sulfated glycolipids and inhibition by dextran sulfate. J. Biol. Chem. 264:9283-9288. [PubMed] [Google Scholar]
- 14.Hu, P. C., A. M. Collier, and J. B. Baseman. 1977. Surface parasitism by Mycoplasma pneumoniae in respiratory epithelium. J. Exp. Med. 145:1328-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs, E. 1993. Serological diagnosis of Mycoplasma pneumoniae infections: a critical review of current procedures. Clin. Infect. Dis. Suppl. 1:S79-S82. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs, E., A. Bartl, K. Oberle, and E. Schiltz. 1995. Molecular mimicry by Mycoplasma pneumoniae to evade the induction of adherence inhibiting antibodies. J. Med. Microbiol. 43:422-429. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs, E., A. Pilatschek, and B. Gerstenecker. 1990. Immunodominant epitopes of the adhesin of Mycoplasma pneumoniae. J. Clin. Microbiol. 28:1194-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs, E., K. Fuchte, and W. Bredt. 1987. Amino acid sequence and antigenicity of the amino terminus of the 168-kDa adherence protein of Mycoplasma pneumoniae. J. Gen. Microbiol. 133:2233-2236. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs, E., A. Buchholz, B. Kleinmann, and W. Bredt. 1987. Use of adherence protein of Mycoplasma pneumoniae as antigen for enzyme linked immunosorbent assay (ELISA). Israel J. Med. Sci. 23:709-712. [PubMed] [Google Scholar]
- 20.Kleiner, C., K. Oberle, and D. Huzly. 1999. Diagnosis of acute Mycoplasma infections using ELISA IgG, IgM, and IgA detection in comparison to CFT and antigen detection or culture. German Congress for Infections and Tropical Medicine, Munich, Germany. 24 November to 27 November 1999.
- 21.Krause, D. C. 1996. Mycoplasma pneumoniae cytadherence: unraveling the tie that binds. Mol. Microbiol. 20:247-253. [DOI] [PubMed] [Google Scholar]
- 22.Lowry,O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 23.Matas, L., J. Dominguez, F. D. Ory, N. Garcia, N. Gali, P. J. Cardona, A. Hernandez, C. Rodrigo, and V. Ausina. 1998. Evaluation of Meridian Immunocard Mycoplasma test for the detection of Mycoplasma pneumoniae specific IgM in paediatric patients. Scand. J. Infect. Dis. 30:289-293. [DOI] [PubMed] [Google Scholar]
- 24.Razin, S., and E. Jacobs. 1992. Mycoplasma adhesion. J. Gen. Microbiol. 138:407-422. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Sillis, M. 1990. The limitation of IgM assays in the serological diagnosis of Mycoplasma pneumoniae infections. J. Med. Microbiol. 33:253-258. [DOI] [PubMed] [Google Scholar]
- 27.Smiley, B. K., and F. C. Minion. 1993. Enhanced readthrough of opal (UGA) stop codons and production of Mycoplasma pneumoniae P1 epitopes in Escherichia coli. Gene 134:33-40. [DOI] [PubMed] [Google Scholar]
- 28.Stauffer, G. V., M. D. Plamann, and L. T. Stauffer. 1981. Construction and expression of hybrid plasmids containing the Escherichia coli Gly A genes. Gene 14:63-72. [DOI] [PubMed] [Google Scholar]
- 29.Su, C. J., V. V. Tryon, and J. B. Baseman. 1987. Cloning and sequence analysis of cytadhesin P1 gene from Mycoplasma pneumoniae. Infect. Immun. 55:3023-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svenstrup, H. F., P. K. Nielsen, M. Drasbek, S. Birkelund, and G. Christiansen. 2002. Adhesion and inhibition assay of Mycoplasma genitalium and M. pneumoniae by immunofluorescence microscopy. J. Med. Microbiol. 51:361-373. [DOI] [PubMed] [Google Scholar]
- 31.Thaker, W. L., and D. F. Talkington. 2000. Analysis of complement fixation and commercial enzyme immunoassays for detection of antibodies to Mycoplasma pneumoniae in human serum. Clin. Diagn. Lab. Immunol. 7:778-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electroelution transfer of proteins from polyacrylamide gels to nitrocellulose sheets, procedures and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trevino, L. B., W. G. Haldenwang, and J. B. Baseman. 1986. Expression of Mycoplasma pneumoniae antigens in Escherichia coli. Infect. Immun. 53:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waring, A. L., T. A. Halse, C. K. Csiza, C. J. Carlyn, K. Arruda-Musser, and R. J. Limberger. 2001. Development of a genomics-based PCR assay for detection of Mycoplasma pneumoniae in a large out break in New York State. J. Clin. Microbiol. 39:1385-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]