Abstract
An inexpensive enzyme-linked immunosorbent assay method for human immunodeficiency virus type 1 quantitation, ultrasensitive p24 antigen assay (Up24), was compared with RNA viral load assay (VL). Up24 had 100% sensitivity of detection at a viral load of ≥30,000, with sensitivity of 46.4% at a viral load of <30,000 (232 specimens from 65 seropositive subjects). The assay was highly reproducible, with excellent correlation between duplicates and among three laboratories.
Human immunodeficiency virus type 1 (HIV-1) viral load has become the mainstay for monitoring antiretroviral (ARV) therapy for HIV infection. However, the routinely used viral load assays are based on amplification of nucleic acid and as a result require skilled technicians, dedicated laboratory space, and complex equipment and are generally expensive (3, 5, 8). As a result, these tests are not readily available in areas where resources are limited. An inexpensive and technically less demanding approach to quantify HIV-1 would be of great value for places where nucleic acid testing is impractical or prohibited because of resource limitations.
Two potential methods include an assay that detects virion-associated reverse transcriptase activity (2) and a “boosted” p24 antigen assay that uses heat dissociation to allow detection of HIV-1 p24 antigen with sensitivity and reproducibility reported to be comparable to those of RNA viral load testing (1, 3, 5, 10, 11, 12, 14). The assay has been evaluated for several applications, including pediatric diagnosis (7, 9, 11) and clinical monitoring of patients on therapy (6, 13, 14). Most studies to date have been carried out with HIV-1 subtype B-infected patients (1, 3, 5, 10, 12, 14), although a few studies suggest that the assay may also work with non-B subtypes (3, 5, 7, 10). Although technical challenges in transferring the technique due to the research nature of the assay have hindered the routine use of the boosted version of the p24 assay outside one laboratory, the availability of a simpler version based on commercial components could be a major asset for settings with limited resources if the assay produced results that correlated well with nucleic acid testing.
Perkin Elmer Life Sciences (Boston, Mass.) has developed an integrated kit and protocol using components from the experimental boosted p24 assay, termed the ultrasensitive p24 antigen assay (Up24). In this study Up24 was compared with RNA viral load assay (VL) in samples from HIV-1-seronegative and HIV-1-seropositive patients that were either drug naïve or receiving ARV treatment.
(This study was presented in part at the XIV International AIDS Conference, July 7 to 12, 2002, Barcelona, Spain [R. A. Respess, A. Cachafeiro, D. G. Withum, S. A. Fiscus, D. R. Newman, I. Cabruja, B. M. Branson, O. E. Varnier, T. J. Dondero, abstr. WeOrB1341, 2002].)
A total of 232 plasma specimens from 65 adult U.S. patients infected with HIV-1 subtype B (83 from untreated and 149 from ARV-treated patients) with previously determined RNA viral loads were tested under blinded conditions with Up24. Plasma samples from an additional 219 HIV-seronegative adult U.S. subjects were tested in a similar blinded manner. Up24 was performed in duplicate, and the average of the two results was used for our comparisons.
To determine interlaboratory concordance, a 19-member proficiency testing panel was prepared by the Centers for Disease Control and Prevention and tested in duplicate under identical conditions by three laboratories experienced with performing Up24.
Samples were tested as described in the protocol provided with the p24-specific viral load ELAST amplification system kit (catalog no. NEP116VL; Perkin Elmer Life Sciences), which is used in combination with a HIV-1 p24 enzyme-linked immunosorbent assay kit (catalog no. NEK050) for Up24. After the addition of orthopenylenediamine-HCl substrate the plate was read kinetically for 30 min by using Quanti-Kin detection system software (Rilab, Genoa, Italy) as described previously (4). The colorimetric reaction was stopped after 30 min, and the endpoint reading was determined for final calculations with the Quanti-Kin software.
HIV RNA levels for these specimens had been measured previously by using either version 2.0 or 3.0 of the Versant bDNA assay (Bayer Corporation, Berkeley, Calif.) as described in the product insert.
Log10 transformation was used for comparing the VL and Up24 determinations. Pearson correlation coefficients and linear regressions were determined by using SAS version 8.2 (SAS Institute, Cary, N.C.). Assay results below the limit of detection were assigned a value of 0 for ease of comparison. Results were then segregated and analyzed in viral load increments. Pearson correlation coefficients and linear regressions were also used to compare duplicate runs and results from different laboratories.
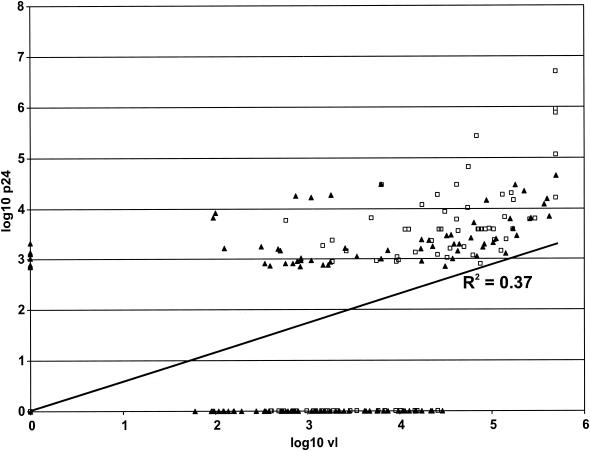
There was a correlation overall between VL and Up24 for the combined (n = 232, r = 0.60 [P < 0.0001], R2 = 0.37), untreated (n = 83, r = 0.69 [P < 0.0001], R2 = 0.47), and ARV-treated (n = 149, r = 0.48 [P < 0.0001], R2 = 0.23) HIV-1-seropositive samples (Fig. 1). However, there was no statistical correlation between VL and Up24 for combined, untreated, or ARV-treated specimens with viral loads of <5,000 copies/ml (n = 127). At viral loads of >5,000 copies/ml, there was a correlation between VL and Up24 for combined (n = 105, r = 0.65 [P < 0.0001], R2 = 0.42), untreated (n = 55, r = 0.62 [P < 0.0001], R2 = 0.38), and ARV-treated specimens (n = 50, r = 0.69 [P < 0.0001], R2 = 0.47).
FIG. 1.
Correlation of Up24 and VL for 65 seropositive adult U.S. patients, either untreated (n = 83; ) or ARV treated (n = 149; ▴).
Both VL and Up24 were negative for all 219 HIV-1-seronegative specimens. Of the 37 VL-negative HIV-1-seropositive specimens, 8 from ARV-treated patients were Up24 positive. Conversely, 79 VL-positive specimens were Up24 negative. For specimens with viral loads of 1,000 to 10,000 copies/ml, Up24 sensitivity of detection was 43.6% (24 of 55) for combined, 56.5% (13 of 23) for untreated, and 34.4% (11 of 32) for ARV-treated groups. For specimens with viral loads between 10,000 and 20,000 copies/ml, Up24 sensitivity of detection was 38.9% (7 of 18) for combined, 57.1% (4 of 7) for untreated, and 27.3% (3 of 11) for ARV-treated groups. For specimens with viral loads of 20,000 to 30,000 copies/ml, Up24 sensitivity of detection was 72.7% (8 of 11) for combined, 100% (6 of 6) for untreated, and 40.0% (2 of 5) for ARV-treated groups. For specimens with viral loads of ≥30,000 copies/ml, Up24 sensitivity of detection was 100% for combined (60 of 60), untreated (34 of 34), and ARV-treated (26 of 26) groups compared to VL.
In all analyses, each specimen was tested in duplicate. There was a significant correlation between replicates (r = 0.90 [P < 0.0001], R2 = 0.80). Of the 19 proficiency samples tested for Up24, 2 were negative in all three laboratories and 17 were positive (Table 1). For the 17 positives, the correlation was good among all three laboratories: for laboratory A compared to B (r = 0.87 [P < 0.0001], R2 = 0.76) and C (r = 0.89 [P < 0.0001], R2 = 0.79) and between laboratory B and C (r = 0.98 [P < 0.0001], R2 = 0.96).
TABLE 1.
Results of proficiency testing panel between three laboratories routinely running Up24
| CDC panel | Result (pg/ml) in laboratory
|
||
|---|---|---|---|
| A | B | C | |
| 31 | 16.3 | 47.8 | 14 |
| 33 | 14 | 36.8 | 5.6 |
| 34 | 5.6 | 12.6 | 2.2 |
| 35 | 322.4 | 313.8 | 84.8 |
| 36 | 3.1 | 8.2 | 1.5 |
| 37 | 70.7 | 171.9 | 51.9 |
| 38 | 2.5 | 7.4 | 0.9 |
| 39 | Negative | Negative | Negative |
| 310 | 28.8 | 106.7 | 13.4 |
| 311 | 4.9 | 15.5 | 1 |
| 312 | 21.3 | 51.7 | 9.9 |
| 313 | 3.2 | 6.9 | 1.3 |
| 314 | 2.7 | 6.5 | 1.5 |
| 315 | 2.6 | 8 | 1.6 |
| 316 | 21.9 | 65.9 | 18.9 |
| 317 | 48.5 | 143.1 | 37.6 |
| 318 | 48.5 | 185.4 | 37.3 |
| 319 | Negative | Negative | Negative |
| 320 | 38.3 | 109.1 | 29.2 |
Antiretroviral therapy has become increasingly available to larger numbers of HIV-infected patients worldwide through reduced pricing and other programs such as the UNAIDS Drug Initiative (16). However, the expense and complexity of the RNA viral load assay make its use for patient monitoring difficult to successfully implement in resource-limited settings. With all reagents required to perform the assay now available in a commercial kit configuration, with easy-to-follow instructions and dedicated software, some of the difficulties in running this boosted version of the p24 assay (e.g., requirement to prepare in-house relevant buffers, need to titrate the ELAST for each new lot of streptavidin conjugate, and lack of standardized software for calculation purposes) appear to have been addressed. Our study found good concordance among Up24 results from three laboratories, as have others (C. L. Jennings et al., Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. H-1944, 2003). However, the actual significance of the absolute differences between the laboratories remains to be determined. The price per assay ($5 to 10) is lower than that of nucleic acid testing ($17 to 80 per assay for reagents). An important feature is its enzyme-linked immunosorbent assay-based format, which allows Up24 to be run with equipment and staff already present in many laboratories performing routine serologic assays. In our evaluation, there was good correlation and detection between Up24 for adult samples with viral loads of >30,000 copies, whether patients were drug naïve or ARV treated. Although these results suggest that the assay in its current configuration does not have the sensitivity required for use in routine monitoring of patients on ARV therapy, a new sample preparation buffer may improve the sensitivity of the assay to a more useful range (C. L. Jennings et al., Abstr. 43rd ICAAC; Fiscus et al., Abstr. 11th Conference on Retroviruses and Opportunistic Infections, abstr. 957, 2004). However, the current Up24 appears to have sufficient sensitivity to be useful in qualitative pediatric diagnosis (15), where viral load on average is very high. Further evaluation is necessary to confirm reports that Up24 performance in testing non-B subtypes is similar to that reported here for subtype B (R. A. Respess et al., Abstr. 10th Conference on Retroviruses and Opportunistic Infections, abstr. R-21, 2003). Because VL detects intact viral particles while Up24 detects both virus-associated and nonvirion p24, a rigorous evaluation of Up24 needs to be done before the test can routinely be used for clinical management.
Acknowledgments
We acknowledge Frederick (Rick) M. Hecht, San Francisco General Hospital, San Francisco, Calif., and Robert A. Weinstein, Rush University School of Medicine and Cook County Hospital, Chicago, Ill., for patient specimens and Isabel Cabruja, Perkin Elmer Life Sciences, for technical support.
Use of trade names is for identification only and does not constitute endorsement by the Public Health Service, U.S. Department of Health and Human Services.
REFERENCES
- 1.Boni, J., O. Opravil, Z. Tomasik, M. Rothen, L. Bisset, P. J. Grob, R. Luthy, and J. Schupbach. 1997. Simple monitoring of antiretroviral therapy with a signal-amplification-boosted HIV-1 p24 antigen assay with heat-denatured plasma. AIDS 11:F47-F52. [DOI] [PubMed] [Google Scholar]
- 2.Braun, J., J-C Plantier, M-F Hellot, E. Tuaillon, M. Gueudin, F. Damond, A. Malmsten, G. E. Corrigan, and F. Simon. 2003. A new quantitative HIV load assay based on plasma virion reverse transcriptase activity for the different types, groups and subtypes. AIDS 17:331-336. [DOI] [PubMed] [Google Scholar]
- 3.Burgisser, P. P. Vernazza, M. Flepp, J. Boni, Z. Tomasik, U. Hummel, G. Pantaleo, and J. Schupbach. 2000. Performance of five different assays for the quantification of viral load in subjects infected with various subtypes of HIV-1. J. Acquired Immune Defic. Syndr. 23:138-144. [DOI] [PubMed] [Google Scholar]
- 4.Giacomini, M., J. L. McDermott, A. A. Giri, I. Martini, F. B. Lillo, and O. E. Varnier. 1998. A novel and innovative quantitative kinetic software for virologic colorimetric assays. J. Virol. Methods 73:201-209. [DOI] [PubMed] [Google Scholar]
- 5.Goldschmidt, P. L., A. Devillechabrolle, Z. Ai-Arkoub, and J. T. Aubin. 1998. Comparison of an amplified enzyme-linked immunosorbent assay with procedures based on molecular biology for assessing human immunodeficiency virus type 1 viral load. Clin. Diagn. Lab. Immunol. 5:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ledergerber, B., M. Flepp, J. Boni, Z. Tomasik, R. W. Cone, R. Luthy, and J. Schupbach. 2000. Human immunodeficiency virus type 1 p24 concentration measured by boosted ELISA of heat-denatured plasma correlates with decline in CD4 cells, progression to AIDS, and survival: comparison with viral RNA measurement. J. Infect. Dis. 181:1280-1288. [DOI] [PubMed] [Google Scholar]
- 7.Lyamuya, E., U. Bredberg-Raden, A. Massawe, E. Urassa, G. Kawo, G. Msemo, T. Kazimoto, A. Ostborn, K. Karlsson, F. Mhalu, and G. Biberfeld. 1996. Performance of a modified HIV-1 p24 antigen assay for early diagnosis of HIV-1 infection in infants and prediction of mother-to-infant transmission of HIV-1 in Dar es Salaam, Tanzania. J. Acquired Immune Defic. Syndr. 12:421-426. [DOI] [PubMed] [Google Scholar]
- 8.Michael, N. L., S. A. Herman, S. Kwok, K. Dreyer, J. Wang, C. Christopherson, J. P. Spadoro, K. K. Y. Young, V. Polonis, F. E. McCutchan, J. Carr, J. R. Mascola, L. L. Jagodzinski, and M. L. Robb. 1999. Development of calibrated viral load standards for group M subtypes of human immunodeficiency virus type 1 and performance of an improved Amplicor HIV-1 Monitor test with isolates of diverse subtypes. J. Clin. Microbiol. 37:2557-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadal, D., J. Boni, C. Kind, O. E. Varnier, F. Steiner, Z. Tomasik, and J. Schupbach. 1999. Prospective evaluation of amplification boosted ELISA for heat-denatured p24 antigen for diagnosis and monitoring of pediatric human immunodeficiency virus type 1 infection. J. Infect. Dis. 180:1089-1095. [DOI] [PubMed] [Google Scholar]
- 10.Pascual, A., A. Cachafeiro, M. L. Funk, and S. A. Fiscus. 2002. Comparison of heat-dissociated “boosted” p24 antigen with the Roche monitor human immunodeficiency virus (HIV) RNA assay. J. Clin. Microbiol. 40:2472-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schupbach, J., J. Boni, Z. Tomasik, J. Jendis, R. Seger, C. Kind, and the Swiss Neonatal HIV Study Group. 1994. Sensitive detection and early prognostic significance of p24 antigen in heat-denatured plasma of human immunodeficiency virus type 1-infected infants. J. Infect. Dis. 170:318-324. [DOI] [PubMed] [Google Scholar]
- 12.Schupbach, J., M. Flepp, D. Pontelli, Z. Tomasik, R. Luthy, and J. Boni. 1996. Heat-mediated immune complex dissociation and enzyme-linked immunosorbent assay signal amplification render p24 antigen detection in plasma as sensitive as HIV-1 RNA detection by polymerase chain reaction. AIDS 10:1085-1090. [PubMed] [Google Scholar]
- 13.Schupbach, J., Z. Tomasik, D. Nadal, B. Ledergerber, M. Flepp, M. Opravil, and J. Boni. 2000. Use of HIV-1 p24 as a sensitive, precise, and inexpensive marker for infection, disease progression and treatment failure. Int. J. Antimicrob. Agents 16:441-445. [DOI] [PubMed] [Google Scholar]
- 14.Schupbach, J., J. Boni, M. Flepp, Z. Tomasik, H. Joller, and M. Opravil. 2001. Antiretroviral treatment monitoring with an improved HIV-1 p24 antigen test: an inexpensive alternative to tests for viral RNA. J. Med. Virol. 65:225-231. [DOI] [PubMed] [Google Scholar]
- 15.Sherman, G. G., G. Stevens, and W. S. Stevens. 2004. Affordable diagnosis of human immunodeficiency virus infection in infants by p24 antigen detection. Pediatr. Infect. Dis. J. 23:173-175. [DOI] [PubMed] [Google Scholar]
- 16.Weidle, P. J., S. Malamba, R. Mwebaze, C. Sozi, G. Rukundo, R. Downing, D. Hanson, D. Ochola, P. Mugyenyi, J. Mermin, B. Samb, and E. Lackritz. 2002. Assessment of a pilot antiretroviral drug therapy programme in Uganda: patients' response, survival, and drug resistance. Lancet 359:2261-2267. [DOI] [PubMed] [Google Scholar]