Abstract
Osteoarticular tuberculosis (OAT) is an extrapulmonary tuberculosis and accounts for 1 to 3% of all tuberculosis cases. We used an rpoB PCR-plasmid TA cloning-sequencing method to detect and identify tubercle bacilli in surgical specimens from patients suspected of having OAT. By comparing the similarities of the rpoB sequences determined with those in GenBank, Mycobacterium tuberculosis was detected in 23 of 43 samples. Three of the 23 positive samples had mutations at codon 531, which are commonly observed in rifampin-resistant M. tuberculosis strains. Our results suggest that the rpoB PCR-TA cloning-sequencing method developed, which detects M. tuberculosis and which simultaneously determines its rifampin susceptibility, can also be used efficiently for the diagnosis of OAT.
The World Health Organization reported that nearly a third of the world's population suffers from tuberculosis (TB). Each year 8 million individuals have active disease, and 2 million deaths occur annually (28). With this resurgence, cases of extrapulmonary TB have also shown an increase. Approximately, 10 to 11% of extrapulmonary TB cases involve joints and bones (1 to 3% of all reported TB cases).
Thus, the estimated global prevalence of latent joint and bone TB is approximately 19 million to 38 million cases (17). Moreover, since osteoarticular tuberculosis (OAT) can cause functional disability, it should be accurately diagnosed and treated early.
As the identification of mycobacterial species from clinical samples usually requires culture (26), the diagnosis of OAT depends upon microbiologic testing (i.e., smear or culture) and the histologic examination of tissue samples. Although culture is the “gold standard,” it may take 6 to 8 weeks before a positive culture is detected (22), unless the radiometric BACTEC 460 method or the nonradiometric BACTEC 960/Mycobacteria Growth Indicator Tube method (4) is used.
In recent years, several nucleic acid-based techniques have been developed for the rapid detection of Mycobacterium tuberculosis in clinical samples. By testing sputa and bronchoalveolar lavage specimens, mycobacteria can be detected and identified by PCR or PCR-linked methods (1, 18). However, unlike pulmonary TB samples, such as sputa and bronchoalveolar lavage specimens, from which tubercle bacilli are concentrated for culture and further testing, joint biopsy samples usually contain only a small number of bacteria. This causes difficulties with culture and staining for acid-fast bacilli (8, 17, 19) that necessitate the use of molecular biology-based methods.
In the present study, rpoB PCR-plasmid TA cloning-sequencing for Mycobacterium species was applied directly to clinical specimens from patients suspected of having OAT without culture. rpoB encodes the β subunit of RNA polymerase (3), and recently, partial rpoB DNA sequences containing the Rifr region, which is related to rifampin resistance, have been used to identify Mycobacterium species (5, 10, 11) and non-Mycobacterium species (12, 13, 14, 15, 16).
M. tuberculosis is readily differentiated by its rpoB sequence. In addition, important information on rifampin susceptibility can be provided by rpoB sequence analysis, which is impossible by IS6110 PCR or 16S rRNA gene PCR. Moreover, because the amplified rpoB DNA of M. tuberculosis has one HindII restriction site, it can easily be differentiated from other mycobacteria (10). We used these characteristics of rpoB DNA to rapidly and accurately detect and identify M. tuberculosis in specimens from patients with OAT, and because PCR amplicons are usually weak due to the minimal number of mycobacteria in biopsy samples, TA cloning-sequencing analysis was used.
MATERIALS AND METHODS
Clinical specimens.
Specimens were obtained from 43 Korean patients who were clinically diagnosed with OAT and who underwent surgery during the 3-year period from 2001 to 2003. Other osteoarticular diseases had been ruled out by clinical history and laboratory testing. Some of the surgically removed specimens were kept frozen at −70°C or on ice and were transferred to the laboratory. After removing unnecessary tissue debris, specimens were divided into several fragments (approximate diameter, <5 mm) with scissors and used for DNA preparation.
DNA preparation and rpoB PCR.
DNA was extracted by using the previously described bead beater-phenol extraction method (11). Two or three fragmented specimens were suspended in 200 μl of distilled water in a screw-cap microcentrifuge tube filled with 200 μl (packed volume) of glass beads (diameter, 0.1 mm; Biospec Products; Bartlesville, Okla.) and 200 μl of phenol-chloroform-isopropyl alcohol (50:49:1). To disrupt the tissues and the bacteria, the tube was oscillated on a Mini-Bead Beater (Biospec Products) for 1 min and then centrifuged (12,000 ×g, 5 min).
After the aqueous phase had been transferred to another clean tube, 10 μl of 3 M sodium acetate and 250 μl of ice-cold ethanol were added, and the mixture was kept at −20°C for 10 min. The DNA pellet obtained was then washed with 70% ethanol, dissolved in 60 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]), and used as a template for PCR. rpoB PCR was carried out as described previously (11). Plasmid TA-cloned M. tuberculosis DNA and sterile distilled water were used as positive and negative (nontemplate) controls, respectively. The PCR products obtained were electrophoresed in a 1.5% agarose gel and purified by use of a QIAEX II gel extraction kit (Qiagen, Hilden, Germany). Separately, rpoB nested PCR (9), 16S rRNA gene PCR (7), and IS6110 PCR (23) were performed with those samples with negative rpoB PCR results. The potential effects of PCR inhibitors for the rpoB PCR-negative samples were also tested. Helicobacter pylori DNA (0.001 μg) and cagA-specific PCR primers (29) were added to the reaction mixtures containing each PCR-negative sample. Then, the cagA PCR products were compared with those obtained with a control, which contained only the H. pylori DNA.
TA cloning.
The purified PCR product (5 to 10 ng) was cloned by use of a TA cloning kit (Invitrogen, Carlsbad, Calif.), according to the manual provided by the supplier. Three to 10 colonies of transformed Escherichia coli were picked in each reaction, cultured, and used to prepare plasmid DNA by use of a High Pure Plasmid Isolation kit (Roche, Mannheim, Germany).
The EcoRI restriction site in the plasmid and the HindII restriction site in the amplified M. tuberculosis rpoB DNA were used to confirm the presence of the rpoB DNA insert.
Double digestion with EcoRI and HindII (TAKARA, Shiga, Japan) generated a large plasmid fragment and either an uncleaved insert fragment (368 bp) or two cleaved insert fragments (248 and 120 bp). Constructs possessing the M. tuberculosis rpoB DNA insert produced cleaved DNA fragments. Specimens yielding more than one positive clone were regarded as positive for M. tuberculosis infection.
Nucleotide sequencing.
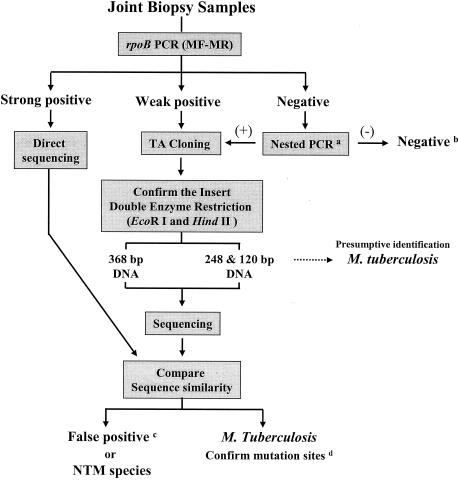
The nucleotide sequences of the cloned rpoB DNAs were directly determined from the purified plasmid by using M13 forward and reverse primers, which were supplied in the TA cloning kit, by using a 373A automatic sequencer and a BigDye Terminator Cycle Sequencing kit (PE Applied Biosystems, Warrington, United Kingdom). For the sequencing reaction, 60 ng of PCR-amplified DNA, 3.2 pmol of either the forward or the reverse primer, and 4 μl of BigDye Terminator RR mix (part no. 4303153; PE Applied Biosystems) were mixed and adjusted to a final volume of 20 μl by adding distilled water. The reaction was run with 5% (vol/vol) dimethyl sulfoxide for 30 cycles of 15 s at 95°C, 10 s at 50°C, and 4 min at 60°C. Both strands were sequenced as a cross-check. The sequences determined (306 bp) were aligned and compared to sequences in GenBank by using the multiple-alignment algorithms in the MegAlign package (Windows version 3.12e; DNASTAR, Madison, Wis.). The procedures are summarized in the flow sheet (Fig. 1).
FIG. 1.
Flow sheet of the rpoB PCR-TA cloning-sequencing protocol used to detect and identify M. tuberculosis in joint biopsy samples. If the rpoB PCR result was negative, nested PCR (a) (9), 16S rRNA gene PCR (b) (26), or IS6110 PCR was used for confirmation. c, false positive indicates that nonspecific human DNAs or other unknown sequences were detected; d, the sequences determined were compared to those in GenBank, which confirmed the presence of mutation sites (10). MF-MR, PCR primers.
RESULTS
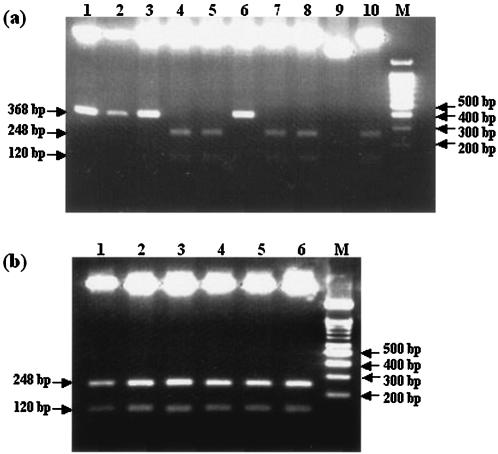
Of the 43 samples examined, 23 samples (53.5%) produced a positive rpoB PCR result. However, because of the weak amplification, PCR-direct sequencing was not possible for most of the positive samples; actually, it was possible for only 1 (2.3%) of the 43 samples. Therefore, the PCR products obtained were cloned into the cloning site of the TA plasmid for sequencing. The M. tuberculosis DNA insert was easily differentiated from other nonspecific inserts, such as human DNA, by double enzyme restriction (Fig. 2).
FIG. 2.
Presumptive identification of M. tuberculosis by double digestion (EcoRI and HindII) of cloned constructs. Lanes M, marker DNA (100-bp ladder). (a) Of the 10 plasmid constructs, only the constructs in lanes 4, 5, 7, 8, and 10 harbored the M. tuberculosis rpoB DNA insert. Lanes 1 to 10, plasmids purified from 10 colonies of transformed E. coli. (b) The plasmid constructs in all lanes harbored the M. tuberculosis rpoB DNA insert.
Thus, the 23 cloned constructs could be tentatively confirmed to harbor the M. tuberculosis rpoB DNA insert by restriction analysis. Finally, the insert DNA sequences of the 23 samples determined were compared with the sequences in the GenBank database. They showed 99.3 to 100% homology with M. tuberculosis H37Rv (GenBank accession no. AF057454), whereas inserts uncleaved by double digestion were human DNAs or unidentifiable DNAs; the rpoB DNA of nontuberculous mycobacteria (NTM) was not found. Thus, we were able to demonstrate the existence of M. tuberculosis in the 23 samples from patients suspected of having OAT (Table 1). In addition, the mutation (in boldface) at codon 531 (TCG→TTG), which results in a high level of resistance to rifampin, was found in 3 of the 23 samples. On the other hand, 20 samples showed negative results. No amplicons were found by rpoB nested PCR, 16S rRNA gene PCR, or IS6110 PCR. These 20 DNA extracts were not observed to have an inhibitory effect on the PCR.
TABLE 1.
Rates of positivity by rpoB PCR-TA cloning and sequencing from clinical specimens
| Clinical material | No. of samples tested | No. (%) of samples M. tuberculosis positive | No. of mutated strains |
|---|---|---|---|
| Joint tissue | 31 | 18 (58.1) | 3a |
| Synovial fluid | 11 | 4 (36.4) | |
| Pus | 1 | 1 (100.0) | |
| Total | 43 | 23 (53.5) | 3 |
Mutation (in boldface) of codon 531 (TCG→TTG) was found.
DISCUSSION
The recent global resurgence of M. tuberculosis infection has been matched by a rapid increase in extrapulmonary TB, including joint and bone TB. Up to two-thirds of human immunodeficiency virus-infected patients have pulmonary and extrapulmonary disease or extrapulmonary disease only (17). Tubercle bacilli reach the joint space via the bloodstream and erode the joint space in, for example, the hip, knee, or ankle and cause OAT (24). Usually, a diagnosis of OAT depends on microbiological testing, such as smear or culture, and histological tissue examination. Several osteoarticular diseases, such as rheumatoid arthritis, should be differentiated from OAT. However, the differential diagnosis of M. tuberculosis arthritis and rheumatoid arthritis is problematic, especially in patients with joint biopsy samples that have nonspecific results by histological evaluation and that produce a negative culture result (24). Given this situation, a molecular biology-based method capable of detecting and identifying specific pathogens in tissues would be very useful, because not only could such a method sensitively detect and specifically identify the pathogens present, but it could also reduce the time required for testing compared the times required for microbiological identification procedures. In the present study, we applied an rpoB PCR-TA cloning-sequencing method to detect and identify tubercle bacilli. The usefulness of this method is supported by the inherent characteristics of the Mycobacterium-specific rpoB PCR, cloning, and the M. tuberculosis-specific rpoB sequence. Although samples were obtained from diseased tissues, many of them were negative by rpoB PCR. Because these negative samples could not be cultured, we confirmed the correctness of these negative results by using other molecular methods, such as rpoB nested PCR, 16S rRNA gene PCR, and IS6110 PCR.
However, 20 samples consistently produced negative results. Because the sample DNA extracts were not shown to have inhibitory effects on the PCR and other causes of the negative results were not identified by the clinical laboratory testing results, it was possible that the tubercle bacilli disappeared after they triggered an inflammatory change or that intact whole mycobacterial DNA did not exist locally in joint tissue. Thus, it might not be possible to detect M. tuberculosis by PCR in such samples, as was postulated previously (25, 27).
The unique HindII restriction site on the rpoB sequence of M. tuberculosis is useful for construct screening. Not knowing whether a given insert was M. tuberculosis DNA, we randomly selected transformed colonies for sequencing. If the detection of M. tuberculosis in a tissue sample is all that is required, double digestion of the construct is sufficient. However, the rpoB DNA insert must be sequenced to determine the presence of mutations related to rifampin resistance. Basically, any construct showing an uncleaved insert by double digestion should also be sequenced to rule out NTM. The HindII restriction site is not present in NTM rpoB DNA, and it has been reported that NTM are detectable in joint tissues (24). However, it is noteworthy that the undigested DNA inserts of our tested samples, which might be suspected of being NTM, were human DNAs or other unknown sequences by a sequence database search with the BLAST algorithm. These may be contaminants that have been introduced during PCR product purification.
IS6110 and 16S rRNA gene PCRs have been widely used to detect mycobacteria (2, 7). They have also been used to detect mycobacteria in joint biopsy samples (6, 26). The reported rates of positivity were 40 and 5.1% by IS6110 and 16S rRNA gene PCR, respectively (6, 26).
In our study, however, the rate of positivity was higher (53.5%). It is not clear whether this difference is due to the sensitivities of the methods used. The endemicity of M. tuberculosis infection in the area tested and the criteria used for patient selection may have affected the rate of positivity in important ways. In general, the high sensitivity of the IS6110 PCR due to the presence multiple copies of IS6110 in the M. tuberculosis complex (23) and the versatility of 16S rRNA gene PCR, which can detect many bacteria, including mycobacteria, by use of universal primers, might be considered useful. However, considering that the identification of pathogenic bacteria should be complemented by information regarding antimicrobial susceptibility, both PCR methods are inadequate, because they provide no information on susceptibility to antimicrobial agents. However, because rifampin resistance is related to rpoB mutations, rpoB PCR presents a useful method of determining rifampin resistance by analyzing amplicon sequences (9, 21). The most common mutations (65 to 85%) alter codon 526 or 531, which results in high levels of resistance to rifampin.
Reports on multidrug-resistant M. tuberculosis infections are increasing (28), and most of the rifampin-resistant strains are also resistant to isoniazid. Therefore, rifampin resistance as a surrogate marker of multidrug resistance (20) supports the importance and usefulness of the rpoB PCR. However, compared to other routine PCR methods, the rpoB PCR-TA cloning method may be a laborious protocol, as it requires a sequencing unit and expert involvement in a clinical laboratory. Although it can be applied to sputa or other clinical samples, we suggest that the indications for the use of this method are limited to samples from patients with OAT, which are not common and which usually contain small amounts of bacilli.
In conclusion, although several difficulties are associated with the detection of M. tuberculosis in tissue specimens, rpoB PCR-TA cloning-sequencing offers a useful method for the detection and simultaneous identification of M. tuberculosis in specimens from patients with OAT.
Acknowledgments
This study was supported by a grant from the Korean Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea, and in part by the BK21 Project for Medicine, Dentistry, and Pharmacy.
REFERENCES
- 1.Aslanzadeh, J., M. de la Viuda, M. Fille, M. B. Smith, and H. Namdari. 1998. Comparison of culture and acid-fast bacilli stain to PCR for detection of Mycobacterium tuberculosis in clinical samples. Mol. Cell. Probes 12:207-211. [DOI] [PubMed] [Google Scholar]
- 2.Böddinghaus, B., T. Rogall, T. Flohr, H. Blöcker, and E. C. Böttger. 1990. Detection and identification of mycobacteria by amplification of rRNA. J. Clin. Microbiol. 28:1751-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boor, K. J., M. L. Duncan, and C. W. Price. 1995. Genetic and transcriptional organization of the region encoding the beta subunit of Bacillus subtillis RNA polymerase. J. Biol. Chem. 270:20329-20336. [DOI] [PubMed] [Google Scholar]
- 4.Cruciani, M., C. Scarparo, M. Malena, O. Bosco, G. Serpelloni, and C. Mengoli. 2004. Meta-analysis of BACTEC MGIT 960 and BACTEC 460 TB, with or without solid media, for detection of mycobacteria. J. Clin. Microbiol. 42:2321-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gingeras, T. R., G. Ghandour, E. Wang, A. Berno, P. M. Small, F. Drobniewski, D. Alland, E. Desmond, M. Holodniy, and J. Drenkow. 1998. Simultaneous genotyping and species identification using hybridization pattern recognition analysis of generic Mycobacterium DNA arrays. Genome Res. 8:435-448. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman, E. B., J. A. B. Campbell, and F. M. Leisegang. 2002. Tuberculosis of the knee. Clin. Orthop. 398:100-106. [DOI] [PubMed] [Google Scholar]
- 7.Hughes, M. S., R. A. Skuce, L. A. Beck, and S. D. Neill. 1993. Identification of mycobacteria from animals by restriction enzyme analysis and direct DNA cycle sequencing of polymerase chain reaction-amplified 16S rRNA gene sequences. J. Clin. Microbiol. 31:3216-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kempsell, K. E., C. J. Cox, A. A. McColm, J. A. Bagshaw, R. Reece, D. J. Veale, P. Emery, J. D. Isaacs, J. S. H. Gaston, and J. S. Crowe. 2001. Detection of Mycobacterium tuberculosis group organisms in human and mouse joint tissue by reverse transcriptase PCR: prevalence in diseased synovial tissue suggests lack of specific association with rheumatoid arthritis. Infect. Immun. 69:1821-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, B. J., K. H. Lee, B. N. Park, S. J. Kim, E. M. Park, Y. G. Park, G. H. Bai, S. J. Kim, and Y. H. Kook. 2001. Detection of rifampin-resistant Mycobacterium tuberculosis in sputa by nested PCR-linked single-strand conformation polymorphism and DNA sequencing. J. Clin. Microbiol. 39:2610-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, B. J., K. H. Lee, B. N. Park, S. J. Kim, G. H. Bai, S. J. Kim, and Y. H. Kook. 2001. Differentiation of mycobacterial species by PCR-restriction analysis of DNA (342 base pairs) of the RNA polymerase gene (rpoB). J. Clin. Microbiol. 39:2102-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, B. J., S. H. Lee, M. A. Lyu, S. J. Kim, G. H. Bai, G. T. Chae, E. C. Kim, C. Y. Cha, and Y. H. Kook. 1999. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB). J. Clin. Microbiol. 58:1714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, K. S., K. S. Ko, M. W. Chang, T. W. Hahn, S. K. Hong, and Y. H. Kook. 2003. Use of rpoB sequences for phylogenetic study of Mycoplasma species. FEMS Microbiol. Lett. 226:299-305. [DOI] [PubMed] [Google Scholar]
- 13.Ko, K. S., S. K. Hong, K. H. Lee, H. K. Lee, M. Y. Park, H. Miyamoto, and Y. H. Kook. 2003. Detection and identification of Legionella pneumophila by PCR-restriction fragment length polymorphism analysis of the RNA polymerase gene (rpoB). J. Microbiol. Methods 54:325-337. [DOI] [PubMed] [Google Scholar]
- 14.Ko, K. S., H. K. Lee, M. Y. Park, K. H. Lee, Y. J. Yun, S. Y. Woo, H. Miyamoto, and Y. H. Kook. 2002. Application of RNA polymerase beta-subunit gene (rpoB) sequences for the molecular differentiation of Legionella species. J. Clin. Microbiol. 40:2653-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, S. H., B. J. Kim, J. H. Kim, K. H. Park, S. J. Kim, and Y. H. Kook. 2000. Differentiation of Borrelia burgdorferi sensu lato on the basis of RNA polymerase gene (rpoB) sequences. J. Clin. Microbiol. 38:2557-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim, C. Y., K. H. Lee, M. J. Cho, M. W. Chang, S. Y. Kim, N. H. Myong, W. G. Lee, K. H. Rhee, and Y. H. Kook. 2003. Detection of Helicobacter pylori in gastric mucosa of patients with gastroduodenal diseases by PCR-restriction analysis using the RNA polymerase gene (rpoB). J. Clin. Microbiol. 41:3387-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malavia, A. N., and P. P. Kotwal. 2003. Arthritis associated with tuberculosis. Best Pract. Res. Clin. Rheumatol. 17:319-343. [DOI] [PubMed] [Google Scholar]
- 18.Plikaytis, B. B., J. T. Crawford, C. L. Woodley, W. R. Butler, K. D. Eisenach, M. D. Cave, and T. M. Shinnick. 1993. Rapid, amplification-based fingerprinting of Mycobacterium tuberculosis. J. Gen. Microbiol. 139:1537-1542. [DOI] [PubMed] [Google Scholar]
- 19.Pras, E., H. R. Schumacher, D. L. Kastner, and R. L. Wilder. 1996. Lack of evidence of mycobacteria in synovial tissue from patient with rheumatoid arthritis. Arthritis Rheum. 39:2080-2081. [DOI] [PubMed] [Google Scholar]
- 20.Somoskovi, A., L. M. Parsons, and M. Salfinger. 2001. The molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosis. Respir. Res. 2:164-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Telenti, A., P. Imboden, F. Marchesi, D. Lowrie, S. Cole, M. J. Colston, L. Matter, K. Schopfer, and T. Bodmer. 1993. Detection of rifampin-resistance mutations in Mycobacterium tuberculosis. Lancet 341:647-650. [DOI] [PubMed] [Google Scholar]
- 22.Tenover, F. C., J. T. Crawford, R. E. Hjuebner, L. J. Geiter, C. R. Horsburgh, Jr., and R. C. Good. 1993. The resurgence of tuberculosis: is your laboratory ready. J. Clin. Microbiol. 31:767-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thierry, D., M. D. Cave, K. D. Eisenach, J. T. Crawford, J. H. Bates, B. Gicquel, and J. L. Guesdon. 1990. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 18:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuli, S. M. 2002. General principles of osteoarticular tuberculosis. Clin. Orthop. 398:11-19. [DOI] [PubMed] [Google Scholar]
- 25.Tunney, M. M., S. Patrick, M. D. Curran, G. Ramage, D. Hanna, J. R. Nixon, S. P. Gorman, R. I. Davis, and N. Anderson. 1999. Detection of prosthetic hip infection at restriction arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J. Clin. Microbiol. 37:3281-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Hijden, I. M., B. Wilbrink, L. M. Schouls, J. D. A. van Embden, F. C. Breedveld, and P. P. Tak. 1999. Detection of mycobacteria in joint samples from patients with arthritis using a genus-specific polymerase chain reaction and sequence analysis. Rheumatology 38:547-553. [DOI] [PubMed] [Google Scholar]
- 27.Wilbrink, B., I. M. van der Heijden, L. M. Schouls, J. D. A. van Embden, J. M. W. Hazes, F. C. Breedveld, and P. P. Tak. 1998. Detection of bacterial DNA in joint samples from patients with undifferentiated arthritis and reactive arthritis using polymerase chain reaction with universal 16S ribosomal RNA primer. Arthritis Rheum. 41:535-543. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. 2000. Tuberculosis. WHO fact sheet no. 104, revised August 2002. World Health Organization, Geneva, Switzerland. [Online.] http//www.who.int/int-fs/en/fact104.html. Accessed 11 November 2003.
- 29.Yamaoka, Y., H. M. Malaty, M. S. Osato, and D. Y. Graham. 2000. Conservation of Helicobacter pylori genotypes in different ethnic groups in Huston, Texas. J. Infect. Dis. 181:2083-2086. [DOI] [PubMed] [Google Scholar]