Abstract
Enterocytozoon bieneusi is clinically the most significant of the microsporidia in humans, causing chronic diarrhea wasting and cholangitis in individuals with human immunodeficiency virus infection and AIDS. Little progress on this infection has been made because of the inability to propagate E. bieneusi in vitro and in vivo, which limits the source of parasite spores to the stools of infected human patients. Given the size and shape of the E. bieneusi spores (1.1 to 1.6 by 0.7 to 1.0 μm) and the lack of specific immune reagents, the identification and purification of large quantities of spores from feces are technically challenging. Consequently, diagnosis relies entirely on PCR, a labor-intensive approach that requires highly skilled personnel. We describe a method for the purification of E. bieneusi spores from human stools and the production of rabbit-specific antisera. Spores were purified by a combination of isopycnic Percoll gradient centrifugation and continuous sucrose gradient centrifugation. Specific polyclonal antibodies raised in mice and rabbits reacted by indirect immunofluorescence with E. bieneusi but not with Encephalitozoon spp., Candida albicans, Staphylococcus aureus, Escherichia coli, or other forms present in human stools.
Enterocytozoon bieneusi is a widespread enteric microsporidium which probably infects most mammals. The infection was first identified two decades ago in individuals with AIDS with severe symptoms of chronic diarrhea and wasting (10) and has remained a significant pathogen in this subpopulation ever since (5, 6, 18). E. bieneusi is recognized as the most common and clinically significant organism among the microsporidia which infect humans who have immunodeficiencies (18, 21) or who are receiving immunosuppressive therapy (13, 22). E. bieneusi is only rarely symptomatic in immunocompetent individuals, possibly contributing to traveler's diarrhea (11). It is an intracellular microorganism which appears to infect the epithelium of the upper small intestine and the hepatobiliary tract (26), causing chronic diarrhea and cholangitis. The number of E. bieneusi cases has diminished considerably in developed countries due to the use of antiretroviral therapy in people with human immunodeficiency virus infection and AIDS (12). The number of cases, however, in such individuals in developing countries, where antiretroviral therapy is either not available or not affordable, remains very high (4, 14, 23).
Scientific progress on E. bieneusi has been slow, largely because of a lack of in vitro and in vivo models for parasite propagation and laboratory investigations. While E. bieneusi did appear to infect cells in culture, the spore yields were low and continuous culture could not be maintained (25). Natural infections of immunologically competent and immunodeficient macaques have also been reported, and the distribution of infection and lesions are similar to those seen in infected humans (8, 9, 16, 17, 20). In macaques infected with simian immunodeficiency virus, lesions associated with E. bieneusi are localized in the cytoplasm of epithelial cells of the gallbladder, bile ducts, and small intestine, leading to proliferative cholecystitis, cholangiohepatitis, and enteropathy, which closely resemble the conditions seen in AIDS patients. We have successfully established E. bieneusi infections with spores of human origin in simian immunodeficiency virus-infected macaques (24) and immunosuppressed gnotobiotic piglets (15). In both models, however, the infection was asymptomatic and very mild, and the excretion of spores in the feces was sparse and intermittent. These models provided insufficient spores for laboratory investigations or for parasite purification and propagation in animals and in cell culture. The lack of sources of E. bieneusi spores has also limited the ability to generate immune diagnostic reagents. Consequently, at present diagnosis depends entirely on PCR, which is time-consuming and which requires sophisticated skills and equipment. Monoclonal antibodies (MAbs) specific for E. bieneusi have recently been described by investigators in Europe (2) but are not available to other investigators.
Here we describe a method for the concentration and purification of E. bieneusi spores from human stools and the production of highly efficient and specific polyclonal antibodies against E. bieneusi.
MATERIALS AND METHODS
Collection of stools with E. bieneusi.
Stools from individuals with chronic watery diarrhea were collected in disposable plastic containers at Mulago Hospital, Kampala, Uganda. The samples were screened for the presence of E. bieneusi spores by calcofluor white staining, and the results were confirmed by PCR. Positive watery stool samples were sieved to remove large particles, followed by centrifugation at 4,000 × g (Sorvall RC 3C Plus instrument) for 40 min in 50-ml centrifuge tubes. The pellets were resuspended in 10 ml of phosphate-buffered saline (PBS) and were stored at 4°C until further processing.
Calcofluor white staining.
Calcofluor white (Remel) staining was performed according to the directions of the manufacturer. Five hundred microliters of calcofluor white reagent A (10% potassium hydroxide) was added to fecal smears, the contents were mixed gently, and then 500 μl of calcofluor white reagent B (0.1% calcofluor white) was added to each slide and the contents were mixed. The smears were stained for 1 min and rinsed with distilled water. The slides were dried, coverslips were mounted with aqueous mounting medium containing antifading compound [1,4-diazobicyclo (2,2,2)-octane (DABCO); Sigma, St. Louis, Mo.], and the slides were observed under a UV microscope at a wavelength of 395 to 415 nm. The spores appeared as bluish white or turquoise oval (or somewhat circular) halos.
PCR.
DNA was extracted from feces, and PCR was performed as described elsewhere (7). Briefly, 200 μl of feces was transferred to a 2-ml screw-cap conical tube containing 200 μl of glass beads (diameter, 0.5 mm; Biospec Products, Inc.), 400 μl of digestion buffer (100 mM NaCl, 25 mM EDTA, 10 mM Tris-HCl [pH 8.0], 1% sodium dodecyl sulfate), and 600 μl of Tris-buffered phenol-chloroform-isoamyl alcohol (pH 8.0; 25:24:1). The sample was then placed in a mini-bead beater (Biospec Products, Inc.) and homogenized at 5,000 rpm for 2 min. Samples were centrifuged in a microcentrifuge for 2 min at 16,000 × g (Biofuge instrument; Heraeus) and 300 μl of the supernatant was transferred to a new tube, in which it was adjusted to a concentration of 0.7 M with 5 M NaCl. Then, 40 μl of a mixture of 10% cetyltrimethylammonium bromide and 0.7 M NaCl was added. The sample was incubated for 10 min at 65°C. After incubation, the solution was extracted with an equal volume of chloroform and the DNA was recovered from the resulting supernatant by use of the Geneclean system (Bio 101) by the protocol of the manufacturer. One microliter of this preparation was used for PCR amplification by use of the rRNA gene sequence. The forward primer was EBITS3 (5′-GGTCATAGGGATGAAGAG), and the reverse primer was EBITS4 (5′-TTCGAGTTCTTTCGCGCTC). The cycling parameters were 35 cycles of 94°C for 30 s, 57°C for 30 s, and 72°C for 40. This reaction produced a fragment of 435 bp.
Purification of E. bieneusi spores with isopycnic Percoll and continuous sucrose gradients.
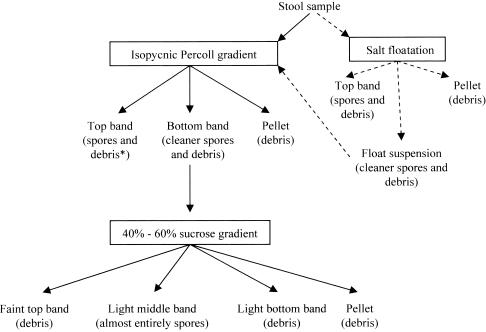
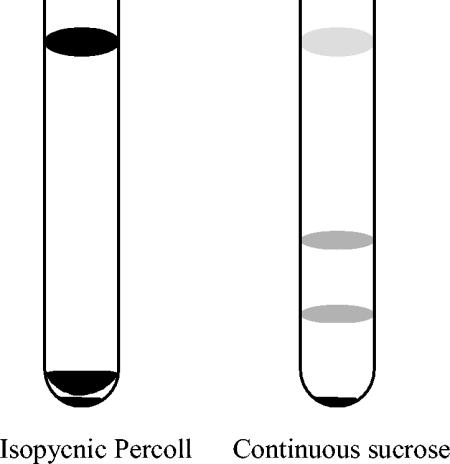
A combination of isopycnic Percoll gradient and continuous sucrose gradient (40 to 60% [wt/wt]) centrifugations was performed to purify the E. bieneusi spores from feces (Fig. 1). A stock of 100% isotonic Percoll was made by mixing 90 ml of Percoll (Sigma) with 10 ml of 10× PBS. Stock Percoll was sterilized by passage through a 0.22-μm-pore-size filter. One milliliter of fecal suspension containing E. bieneusi, as determined by PCR, was mixed with 9 ml of 80% Percoll (8 parts of 100% stock Percoll mixed with 2 parts of sterilized distilled water) and centrifuged in Beckman L-70 ultracentrifuge, which holds six tubes and which uses an SW 41 Ti rotor, at 11,000 rpm for 1 h at 10°C. The sample separated into two bands and a pellet (Fig. 2). Band 1 was at the top of the tube, and band 2 was just above the pellet at the bottom of the tube. Each band from all six tubes was pooled separately, and the pooled bands were mixed with 6 parts of PBS in 50-ml centrifuge tubes. Similarly, the pellets were also collected. The tubes were then centrifuged at 4,000 × g for 40 min. The supernatants were discarded, and the pellets were washed three times with PBS. The pellets were finally suspended in 1 ml of PBS. The bands and the pellet were analyzed for the presence of spores by calcofluor white staining. This completed one Percoll run. The bands but not the pellet contained spores. They were stored at 4°C. The two bands were further subjected to continuous sucrose gradient centrifugation. A total of nine Percoll runs were performed each time before continuous sucrose gradient centrifugation was performed. This provided 9 ml of material for each band.
FIG. 1.
Flowchart of the scheme used for purification of E. bieneusi spores from stools. Debris includes microorganisms other than E. bieneusi and food particles. Solid arrows, usual purification scheme; dashed arrows, spore enrichment step (salt floatation) used for those fecal samples that did not purify well when the samples were loaded on the Percoll gradient. Such fecal samples were first enriched by salt floatation, and then the float suspension part was loaded on the Percoll gradient for further purification.
FIG. 2.
Purification of E. bieneusi spores from human fecal samples with isopycnic Percoll and 40 to 60% (wt/wt) continuous sucrose gradients. The sample separated into two bands and a pellet on the isopycnic Percoll gradient. Band 1 was at the top of the tube, and band 2 was just above the pellet at the bottom of the tube. The bottom band of Percoll separated into three bands and a pellet on 40 to 60% (wt/wt) sucrose gradient. Band 1 (faint) was at the top of the tube, band 2 (light) was just below the middle of the tube, and band 3 (light) was near the bottom of the tube.
Nine milliliters of a continuous sucrose gradient (40 to 60% [wt/wt]) were prepared in the ultracentrifuge tubes with an SW41 Ti rotor by use of a Hoefer SG 30 gradient mixer (Amersham Biosciences) which was connected to a P-1 pump (Amersham Biosciences). The Hoefer SG 30 gradient mixer has two chambers. The chamber close to the outlet received 4.5 ml of 60% (wt/wt) sucrose, and the other chamber received the same volume of 40% (wt/wt) sucrose. Both chambers had magnetic bars that stirred the contents. While the contents were stirred, the connector channel between the chambers was opened and the gradient mixture was added to each ultracentrifuge tube by the P-1 pump at a speed setting of 40×. Each tube was overlaid with 1.5 ml of either the top band or the bottom band of the Percoll-purified material. The tubes were again centrifuged in a Beckman ultracentrifuge with an SW 41 Ti rotor at 25,000 rpm for 24 h at 10°C. The top band of the Percoll-purified material did not separate into clear bands, and material was generally suspended throughout the sucrose. However, the bottom band of the Percoll-purified material separated into three bands and a pellet (Fig. 2). The band at the top of the tube was faint and not easily visible. The middle and bottom bands were somewhat more visible. The three bands and the pellets from all six tubes were collected and separately mixed with 6 parts of PBS in 50-ml centrifuge tubes. The material was transferred to Beckman ultracentrifuge tubes and centrifuged in the Beckman ultracentrifuge with an SW 28 rotor at 25,000 rpm for 1 h at 10°C. The supernatant was discarded, and the pellets were washed three times with PBS in the microcentrifuge at 16,000 × g (Biofuge instrument; Heraeus) for 3 min. The pellets from all tubes were suspended in 1 ml of PBS. The bands and the pellet were analyzed for the presence of spores by calcofluor white staining and were stored at 4°C. The fraction containing the most spores was also analyzed by electron microscopy.
Enrichment of E. bieneusi spores by salt floatation.
Fecal samples that had more junk when they were separated on the Percoll gradient did not purify very well. E. bieneusi spores from such fecal samples were first enriched by salt floatation and were then loaded on the Percoll gradient. One volume of fecal suspension (5 ml) was mixed with 2 volumes of saturated sodium chloride solution (10 ml), and the mixture was centrifuged at 1,000 × g for 15 min at 10°C. The saturated salt solution was prepared by adding 195 g of NaCl to 500 ml of sterile water and then mixing the components by heating at 50°C on a stir plate. The saturated salt solution was brought to room temperature before use. After the solution was spun, a top band and a pellet were collected. The suspension between the pellet and the top band (float suspension) was also collected (Fig. 1). The suspension was pelleted and washed five times with distilled water and pelleted by centrifugation at 4,000 × g for 20 min at 10°C. After the final wash, each pellet was collected in 2 ml of PBS and analyzed for the presence of spores by calcofluor white staining. The float suspension contained the most spores. When the float suspension was subjected to isopycnic Percoll gradient centrifugation, it gave the same separation profile as described above for direct purification of the fecal sample on an isopycnic Percoll gradient.
Rabbit and mouse polyclonal antibodies against E. bieneusi.
An 8-week-old New Zealand White rabbit was primed by subcutaneous injection of 4 × 107 E. bieneusi spores emulsified in incomplete Freund's adjuvant (Difco Laboratories, Detroit, Mich.). The same dose emulsified in incomplete Freund's adjuvant (Difco Laboratories) was administered subcutaneously and intramuscularly 14 and 28 days later. Rabbit serum was collected 4 days after the third injection and was tested for the presence of antibodies against E. bieneusi by indirect immunofluorescence.
Four female BALB/c mice (age, 6 to 8 weeks) were primed by intraperitoneal injection of 2.5 × 107 E. bieneusi spores emulsified in complete Freund's adjuvant (Difco Laboratories). The same dose emulsified in incomplete Freund's adjuvant was administered intraperitoneally 14 and 28 days later. The mice were bled 4 days after the third injection to collect serum. The serum was tested for the presence of antibodies against E. bieneusi by immunofluorescence.
Immunofluorescence and confocal microscopy.
Fecal smears and smears of purified E. bieneusi spores were dried and heat fixed over a flame. The smears were blocked with 2% bovine serum albumin in PBS for 20 min at room temperature, washed with PBS, and then incubated with rabbit anti-E. bieneusi serum at various dilutions in PBS for 30 min at room temperature. Smears were washed and incubated with goat anti-rabbit immunoglobulin G (heavy and light chains) conjugated with Alexa 488 fluorescent dye (Molecular Probes, Eugene, Oreg.) at a dilution of 1/1,000 in PBS for 30 min at room temperature. The slides were washed and dried, and coverslips were mounted with aqueous mounting medium with antifading compound (DABCO; Sigma).
The slides were routinely examined with a regular fluorescent microscope (BX40; Olympus Optical Pvt. Ltd.). For capture of the final images, confocal microscopy with purified spores was performed with a TCS SP laser scanning microscope (Leica Microsystems, Exton, Pa.).
Both mouse and rabbit anti-E. bieneusi sera were analyzed for their cross-reactivities with cell culture-grown microsporidia of the genus Encephalitozoon (Encephalitozoon intestinalis, Encephalitozoon cuniculi, and Encephalitozoon hellem), Candida albicans, Staphylococcus aureus, and fecal Escherichia coli. The rabbit and mouse anti-E. bieneusi sera were also reacted with several human fecal smears known to be negative for E. bieneusi. Conversely, rabbit serum with antibodies raised against E. intestinalis was also reacted against E. bieneusi.
Electron microscopy.
E. bieneusi spores were fixed in 2.5% electron microscopy-grade glutaraldehyde (Polysciences) in 0.1 M sodium cacodylate buffer (pH 7.3) at room temperature for 1 h and stored in 0.1 M sodium cacodylate buffer until further processing. They were postfixed in 1% osmium tetroxide (Electron Microscopy Sciences) for 30 min in 0.1 M sodium cacodylate buffer containing 0.8% potassium ferricyanide (pH 7.3; Sigma) and washed in water five times for 10 min each time. This was followed by incubation with aqueous 1% uranyl acetate (Electron Microscopy Sciences) for 1 h. The samples were then dehydrated in a graded alcohol series and embedded in hard Spurr resin (Polysciences). Sections of 80 nm on Formvar-coated grids were stained with 6.25% uranyl acetate in 50% methanol for 5 min, followed by staining for 5 min in Sato lead stain. The grids were examined in a JEOL 1010 electron microscope.
RESULTS
Source of E. bieneusi.
Two stool samples containing large amounts of E. bieneusi spores were used and checked for the absence of other microsporidia by immunofluorescence with MAbs against Encephalitozoon.
Purification of spores.
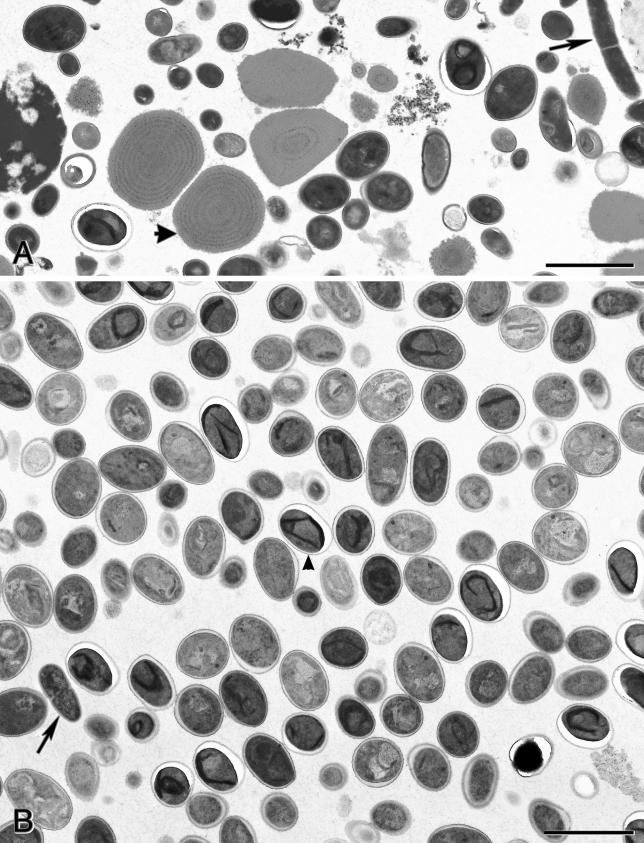
Two distinct populations of E. bieneusi spores were identified as two bands on isopycnic Percoll gradients. The top band contained about 60 to 80% of the total spores but was heavily contaminated with food particles and other microorganisms. It is possible that the top band contained empty spores. The bottom band, which contained the remaining 20 to 40% of the spores, was cleaner than the top band but was still significantly contaminated (Fig. 3). The pellet contained other microorganisms and debris.
FIG. 3.
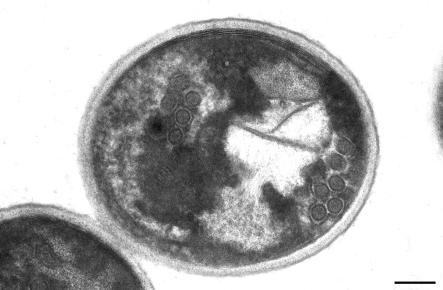
Transmission electron micrographs of material obtained after isopycnic Percoll and 40 to 60% (wt/wt) sucrose gradient centrifugations. (A) Material contained in band 2 (bottom band) of the isopycnic Percoll gradient centrifugation. Spores along with bacteria (arrow), possible food particles (arrowhead), and other contaminants are shown. (B) Material contained in the band just below the middle of the tube obtained following 40 to 60% (wt/wt) sucrose gradient centrifugation. Almost all microorganisms are spores (arrowhead), with some bacteria (arrow) and other contaminants (bottom right corner) detected. Bars, 2 μm.
When the bottom Percoll band was separated on 40 to 60% (wt/wt) sucrose, it resolved into three bands and a pellet. The top band was faint and mainly consisted of unidentified particles. The middle band contained more than 98% E. bieneusi spores (Fig. 3) and less than 2% other microorganisms and debris. Figure 4 shows the characteristic polar tube formation of the E. bieneusi spore in two rows with three coils in each row. The bottom band and pellet from the 40 to 60% (wt/wt) sucrose gradient contained few spores, together with other microorganisms and debris.
FIG. 4.
Transmission electron micrograph of a spore showing two rows of the polar tube with three coils in each row, a characteristic feature of the E. bieneusi spore. Bar, 200 nm.
Efficiency of purification.
The yield of purified spores after the use of a combination of Percoll and sucrose gradients was 9.1% of the starting material.
Immunofluorescence and confocal microscopy.
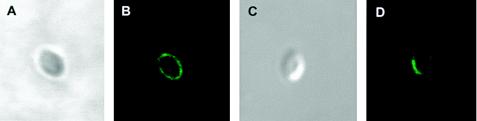
Both mouse and rabbit anti-E. bieneusi sera showed strong reactivities with the spore shell (Fig. 5). Mouse and rabbit sera had titers of antibodies against E. bieneusi of 1/64,000 and 1/8,000, respectively.
FIG. 5.
Confocal images of immunofluorescence staining of E. bieneusi spore wall with rabbit (B) and mouse (D) anti-E. bieneusi sera. (A and C) Differential interference contrast images of the images in panels B and D, respectively.
Cross-reactivity studies.
Neither mouse nor rabbit anti-E. bieneusi sera reacted with E. intestinalis, E. hellem, E. cuniculi, C. albicans, S. aureus, or E. coli by indirect immunofluorescence. The anti-E. bieneusi sera also did not react with anything in those human fecal smears that were negative for E. bieneusi and reacted with only E. bieneusi in those that were positive for E. bieneusi. Rabbit anti-E. intestinalis sera reacted with E. hellem and E. cuniculi but not with E. bieneusi (purified or in fecal smears).
DISCUSSION
We describe here a method for purifying E. bieneusi spores from large volumes of watery diarrheic stools for the production of specific antibodies in mice and a rabbit. A method that uses a Percoll gradient step for purification of E. bieneusi spores from feces of individuals with AIDS has been described previously (2). The purified spores were, however, heavily contaminated with other microorganisms, which required treatment with a mixture of antibiotics (2). We found that incubation with antibiotics was insufficient to remove all other microbial contaminants, presumably due to the presence of antibiotic-resistant bacteria. A method that uses MAb-based immunoaffinity purification of E. bieneusi spores from human feces has also been described (1). The efficiency of spore recovery by that method was 1.02 to 1.71%. In contrast, the spore recovery efficiency of the purification procedures that we describe in this study is 9%.
The lack of specific antibodies against E. bieneusi makes the identification of this microsporidium in stools a difficult task, since the calcofluor white staining method is not specific for E. bieneusi. Calcofluor white labels chitin, which forms the endospore layer of the microsporidian spore shell of most species, and by calcofluor white staining the spores appear as bluish white or turquoise oval to almost circular halos. Chitin, however, is not limited to microsporidian spores and constitutes one of the three most abundant polysaccharides in nature, together with cellulose and starch. Chitin is also present in the cell wall of yeasts, which are often present in stools in abundance and which are easily confused with E. bieneusi spores. Therefore, we used PCR to confirm the presence of E. bieneusi. Since some members of the genus Encephalitozoon are also often present in the stools of individuals with AIDS, we used specific MAbs to exclude them from further processing.
We tried skipping the Percoll gradient step and purifying the salt floatation material directly on a continuous sucrose gradient. Although immunofluorescence of the band purified from the continuous sucrose gradient showed that the material was full of spores, electron microscopy revealed that material was contaminated with other microorganisms and that a significant percentage of the spores were empty.
Polyclonal antibodies against several species of microsporidia were shown to be cross-reactive by immunofluorescence (19). Recent studies have also shown that polyclonal antisera produced against E. cuniculi in rabbits reacted somewhat with E. bieneusi in deparaffinized tissue sections (27) and in stools by immunofluorescence (3, 28). However, in the present study, polyclonal antibodies raised against E. bieneusi in rabbit and mice reacted only with E. bieneusi by immunofluorescence. This included Encephalitozoon spp., yeasts, and all other gut microflora, suggesting the lack of antigenic cross-reaction between the spore wall antigens of E. bieneusi and Encephalitozoon spp. Conversely, rabbit polyclonal serum against E. intestinalis was cross-reactive with E. hellem and E. cuniculi but not with E. bieneusi. Two MAbs specific for E. bieneusi described recently (2) also failed to react with human intestinal parasites, yeasts, or bacteria (2, 4).
The purification procedure that we have described here and the availability of specific polyclonal antibodies will facilitate further laboratory and field investigations, including attempts to develop in vitro and in vivo models of E. bieneusi infection and identification of parasite surface protein. This study documents the first successful attempt to produce specific polyclonal rabbit and mouse anti-E. bieneusi sera. The polyclonal rabbit serum against E. bieneusi has proved extremely useful in studies being conducted by the Division of Infectious Diseases at the Tufts University School of Veterinary Medicine: (i) evaluation of the role of E. bieneusi in chronic diarrhea and wasting in children in Uganda with and without human immunodeficiency virus infection and AIDS and (ii) E. bieneusi infection and symptoms in relation to progressive immune dysfunction in AIDS in the macaque model.
Acknowledgments
The study was supported by grants R01AI43196, R21AI52792, and P01DK55510 from the National Institutes of Health.
We thank Najuka Florence and Evelyn and Joel Hanawalt for technical assistance. We also thank Karen Boisvert, New England Primate Research Center, for performing the confocal microscopy.
REFERENCES
- 1.Accoceberry, I., M. Thellier, A. Datry, I. Desportes-Livage, S. Biligui, M. Danis, and X. Santarelli. 2001. One-step purification of Enterocytozoon bieneusi spores from human stools by immunoaffinity expanded-bed adsorption. J. Clin. Microbiol. 39:1947-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accoceberry, I., M. Thellier, I. Desportes-Livage, A. Achbarou, S. Biligui, M. Danis, and A. Datry. 1999. Production of monoclonal antibodies directed against the microsporidium Enterocytozoon bieneusi. J. Clin. Microbiol. 37:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldras, A. M., J. M. Orenstein, D. P. Kotler, J. A. Shadduck, and E. S. Didier. 1994. Detection of microsporidia by indirect immunofluorescence antibody test using polyclonal and monoclonal antibodies. J. Clin. Microbiol. 32:608-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfa Cisse, O., A. Ouattara, M. Thellier, I. Accoceberry, S. Biligui, D. Minta, O. Doumbo, I. Desportes-Livage, M. A. Thera, M. Danis, and A. Datry. 2002. Evaluation of an immunofluorescent-antibody test using monoclonal antibodies directed against Enterocytozoon bieneusi and Encephalitozoon intestinalis for diagnosis of intestinal microsporidiosis in Bamako (Mali). J. Clin. Microbiol. 40:1715-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryan, R. T., A. Cali, R. L. Owen, and H. C. Spencer. 1991. Microsporidia: opportunistic pathogens in patients with AIDS. Prog. Clin. Parasitol. 2:1-26. [PubMed] [Google Scholar]
- 6.Bryan, R. T., and R. Weber. 1993. Microsporidia. Emerging pathogens in immunodeficient persons. Arch. Pathol. Lab. Med. 117:1243-1245. [PubMed] [Google Scholar]
- 7.Buckholt, M. A., J. H. Lee, and S. Tzipori. 2002. Prevalence of Enterocytozoon bieneusi in swine: an 18-month survey at a slaughterhouse in Massachusetts. Appl. Environ. Microbiol. 68:2595-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalifoux, L. V., A. Carville, D. Pauley, B. Thompson, A. A. Lackner, and K. G. Mansfield. 2000. Enterocytozoon bieneusi as a cause of proliferative serositis in simian immunodeficiency virus-infected immunodeficient macaques (Macaca mulatta). Arch. Pathol. Lab. Med. 124:1480-1484. [DOI] [PubMed] [Google Scholar]
- 9.Chalifoux, L. V., J. MacKey, A. Carville, D. Shvetz, K. C. Lin, A. Lackner, and K. G. Mansfield. 1998. Ultrastructural morphology of Enterocytozoon bieneusi in biliary epithelium of rhesus macaques (Macaca mulatta). Vet. Pathol. 35:292-296. [DOI] [PubMed] [Google Scholar]
- 10.Desportes, I., Y. Le Charpentier, A. Galian, F. Bernard, B. Cochand-Priollet, A. Lavergne, P. Ravisse, and R. Modigliani. 1985. Occurrence of a new microsporidan: Enterocytozoon bieneusi n. g., n. sp., in the enterocytes of a human patient with AIDS. J. Protozool. 32:250-254. [DOI] [PubMed] [Google Scholar]
- 11.Fournier, S., O. Liguory, V. Garrait, J. P. Gangneux, C. Sarfati, F. Derouin, and J. M. Molina. 1998. Microsporidiosis due to Enterocytozoon bieneusi infection as a possible cause of traveller's diarrhea. Eur. J. Clin. Microbiol. Infect. Dis. 17:743-744. [DOI] [PubMed] [Google Scholar]
- 12.Goguel, J., C. Katlama, C. Sarfati, C. Maslo, C. Leport, and J. M. Molina. 1997. Remission of AIDS-associated intestinal microsporidiosis with highly active antiretroviral therapy. AIDS 11:1658-1659. [PubMed] [Google Scholar]
- 13.Guerard, A., M. Rabodonirina, L. Cotte, O. Liguory, M. A. Piens, S. Daoud, S. Picot, and J. L. Touraine. 1999. Intestinal microsporidiosis occurring in two renal transplant recipients treated with mycophenolate mofetil. Transplantation 68:699-707. [DOI] [PubMed] [Google Scholar]
- 14.Gumbo, T., S. Sarbah, I. T. Gangaidzo, Y. Ortega, C. R. Sterling, A. Carville, S. Tzipori, and P. M. Wiest. 1999. Intestinal parasites in patients with diarrhea and human immunodeficiency virus infection in Zimbabwe. AIDS 13:819-821. [DOI] [PubMed] [Google Scholar]
- 15.Kondova, I., K. Mansfield, M. A. Buckholt, B. Stein, G. Widmer, A. Carville, A. Lackner, and S. Tzipori. 1998. Transmission and serial propagation of Enterocytozoon bieneusi from humans and rhesus macaques in gnotobiotic piglets. Infect. Immun. 66:5515-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansfield, K. G., A. Carville, D. Hebert, L. Chalifoux, D. Shvetz, K. C. Lin, S. Tzipori, and A. A. Lackner. 1998. Localization of persistent Enterocytozoon bieneusi infection in normal rhesus macaques (Macaca mulatta) to the hepatobiliary tree. J. Clin. Microbiol. 36:2336-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansfield, K. G., A. Carville, D. Shvetz, J. MacKey, S. Tzipori, and A. A. Lackner. 1997. Identification of an Enterocytozoon bieneusi-like microsporidian parasite in simian-immunodeficiency-virus-inoculated macaques with hepatobiliary disease. Am. J. Pathol. 150:1395-1405. [PMC free article] [PubMed] [Google Scholar]
- 18.Molina, J. M., C. Sarfati, B. Beauvais, M. Lemann, A. Lesourd, F. Ferchal, I. Casin, P. Lagrange, R. Modigliani, F. Derouin, et al. 1993. Intestinal microsporidiosis in human immunodeficiency virus-infected patients with chronic unexplained diarrhea: prevalence and clinical and biologic features. J. Infect. Dis. 167:217-221. [DOI] [PubMed] [Google Scholar]
- 19.Niederkorn, J. Y., J. A. Shadduck, and E. Weidner. 1980. Antigenic cross-reactivity among different microsporidian spores as determined by immunofluorescence. J. Parasitol. 66:675-677. [PubMed] [Google Scholar]
- 20.Schwartz, D. A., D. C. Anderson, S. A. Klumpp, and H. M. McClure. 1998. Ultrastructure of atypical (teratoid) sporogonial stages of Enterocytozoon bieneusi (Microsporidia) in naturally infected rhesus monkeys (Macacca mulatta). Arch. Pathol. Lab. Med. 122:423-429. [PubMed] [Google Scholar]
- 21.Schwartz, D. A., I. Sobottka, G. J. Leitch, A. Cali, and G. S. Visvesvara. 1996. Pathology of microsporidiosis: emerging parasitic infections in patients with acquired immunodeficiency syndrome. Arch. Pathol. Lab. Med. 120:173-188. [PubMed] [Google Scholar]
- 22.Sing, A., K. Tybus, J. Heesemann, and A. Mathis. 2001. Molecular diagnosis of an Enterocytozoon bieneusi human genotype C infection in a moderately immunosuppressed human immunodeficiency virus-seronegative liver-transplant recipient with severe chronic diarrhea. J. Clin. Microbiol. 39:2371-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tumwine, J. K., A. Kekitiinwa, N. Nabukeera, D. E. Akiyoshi, M. A. Buckholt, and S. Tzipori. 2002. Enterocytozoon bieneusi among children with diarrhea attending Mulago Hospital in Uganda. Am. J. Trop. Med. Hyg. 67:299-303. [DOI] [PubMed] [Google Scholar]
- 24.Tzipori, S., A. Carville, G. Widmer, D. Kotler, K. Mansfield, and A. Lackner. 1997. Transmission and establishment of a persistent infection of Enterocytozoon bieneusi, derived from a human with AIDS, in simian immunodeficiency virus-infected rhesus monkeys. J. Infect. Dis. 175:1016-1020. [DOI] [PubMed] [Google Scholar]
- 25.Visvesvara, G., G. J. Leitch, N. J. Pieniazek, A. J. Da Silva, S. Wallace, S. B. Slemenda, R. Weber, D. A. Schwartz, L. Gorelkin, C. M. Wilcox, et al. 1995. Short-term in vitro culture and molecular analysis of the microsporidian, Enterocytozoon bieneusi. J. Eukaryot. Microbiol. 42:506-510. [DOI] [PubMed] [Google Scholar]
- 26.Weber, R., R. T. Bryan, D. A. Schwartz, and R. L. Owen. 1994. Human microsporidial infections. Clin. Microbiol. Rev. 7:426-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss, L. M., A. Cali, E. Levee, D. LaPlace, H. Tanowitz, D. Simon, and M. Wittner. 1992. Diagnosis of Encephalitozoon cuniculi infection by Western blot and the use of cross-reactive antigens for the possible detection of microsporidiosis in humans. Am. J. Trop. Med. Hyg. 47:456-462. [DOI] [PubMed] [Google Scholar]
- 28.Zierdt, C. H., V. J. Gill, and W. S. Zierdt. 1993. Detection of microsporidian spores in clinical samples by indirect fluorescent-antibody assay using whole-cell antisera to Encephalitozoon cuniculi and Encephalitozoon hellem. J. Clin. Microbiol. 31:3071-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]