Abstract
Infection with cytomegalovirus (CMV) remains a significant cause of morbidity and mortality among hematopoietic cell transplant (HCT) recipients. We describe two pediatric HCT recipients who developed persistent and severe drug-resistant CMV infections. CMV resistance to foscarnet and ganciclovir was detected after only 6 and 11 weeks of therapy, respectively. Viral pol mutations associated with drug resistance in these patients included T838A (a novel mutation) and D588N, which were shown by marker transfer to confer foscarnet and multidrug resistance, respectively. Each of these mutations significantly reduced in vitro replication of CMV, suggesting that they may decrease viral fitness. This finding was further supported by the disappearance of mutations upon withdrawal of antiviral pressure in one patient. Novel antivirals or combination therapy may be required for the treatment of drug-resistant CMV in HCT recipients and perhaps in other severely immunocompromised patients.
Immunocompromised hosts such as transplant recipients and AIDS patients are targets of cytomegalovirus (CMV) infection and its complications. There are only a few antivirals available to treat CMV disease: the commonly used nucleoside analog ganciclovir, the pyrophosphate analog foscarnet, and the nucleotide analog cidofovir. Valganciclovir, the prodrug of ganciclovir, is available in an oral formulation. Valacyclovir is effective when used for prophylaxis but not treatment (33). Resistance to antivirals may develop, complicating clinical management.
Most of the data on CMV resistance mutations and their clinical significance come from patients who are coinfected with CMV and human immunodeficiency virus (HIV) (15), but reports of CMV resistance in transplant recipients are increasing (12, 16, 19, 22, 36, 37). Hematopoietic cell transplant (HCT) recipients at risk for CMV infection (CMV-seropositive recipients and/or those with seropositive donors) are often exposed to long courses of prophylactic or preemptive antiviral therapy, which may lead to selection of resistance mutations. Additional risk factors for development of CMV drug resistance may include transplantation with T-cell-depleted grafts and graft-versus-host disease (11, 36).
Two CMV gene products are implicated in resistance to the available anti-CMV drugs: the CMV UL97 gene product, which is responsible for the first phosphorylation step of ganciclovir, a step necessary for its activity, and the UL54 (pol) gene product, the primary target of all available antivirals. Point mutations or deletions of portions of UL97 can lead to ganciclovir resistance. UL54 mutations, depending on their locations, can confer resistance to one or more antivirals (3, 7, 8).
Antiviral resistance can be determined by genotypic or phenotypic assays. Phenotypic assays measure the concentration of an antiviral agent necessary to inhibit viral replication. They require a viral isolate, thus prolonging the time necessary for completion of the test. Genotypic assays, which look for key resistance mutations in the UL97 and UL54 genes, can be performed in a timely fashion by using clinical specimens. Genotypic assays may be more sensitive than phenotypic assays (35) but may be limited by incomplete characterization of all the mutations that confer resistance.
In addition to the phenotypic characterization of all CMV mutations, determining how the mutations alter the fitness and ability of the virus to establish latency might help improve current therapeutic strategies. Certain resistance mutations in the HIV genome reduce fitness, attenuate pathogenicity, and potentiate the activity of other antivirals (9, 25, 27). It has been suggested that this may also be true for CMV (3, 14). In this report, we describe two HCT recipients who developed disease with drug-resistant CMV. The changing genotypic patterns of CMV in these hosts together with the in vitro growth characteristics conferred by the resistance mutations suggest decreased fitness of the resistant CMV.
(This work was presented in part at the 41st Annual Meeting of the Infectious Diseases Society of America, San Diego, Calif., 9 to 12 October 2003 [K. L. Springer et al., Abstr. 41st Annu. Meet. Infect. Dis. Soc. Am., abstr. 367, 2003]).
MATERIALS AND METHODS
Detection of CMV infection and disease.
CMV infection was defined by active viral replication at any site. Patients were monitored at least weekly after transplant by blood PCR, pp65 antigenemia, and culture. CMV disease was defined by the presence of consistent clinical symptoms and the finding of virus in the relevant organ or its secretions by histology or culture. CMV retinitis was diagnosed by ophthalmologic exam.
Phenotypic susceptibility.
Phenotypic susceptibility was determined by plaque reduction assay (PRA) performed as previously described (10), except that we used human embryonic lung fibroblast (HEL) monolayers and cell-associated virus. Control virus was stored in a glycerol-containing freezing medium, which insures consistent infectivity after thawing. CMV isolates were titered by inoculating 10-fold dilutions of the viral stocks into triplicate wells. After 6 to 10 days of incubation, when viral plaques were fully developed, plates were fixed with 10% formaldehyde solution and stained with 0.8% crystal violet, and plaques were counted by light microscopy. Thereafter, 100 PFU of each virus was inoculated into triplicate wells without antivirals and with serial dilutions of ganciclovir, foscarnet, and cidofovir. Plaques were counted as described above. The 50% inhibitory concentration (IC50) for each compound, defined as the antiviral concentration that reduced the number of plaques expected on the basis of untreated virus-inoculated wells by 50%, was determined by using a linear regression Excel program.
Genotypic testing.
DNA extracted from culture isolates or clinical specimens with a QIAamp DNA Mini kit (QIAGEN) was used for CMV UL97 and UL54 sequencing. The region encoding the C-terminal half of UL97, which contains the known drug resistance mutations, was amplified by using the primers 5′-CTGCTGCACAACGTCACGGTACATC-3′ (forward) and 5′-CTCCTCATCGTCGTCGTAGTCC-3′ (reverse), which produce a 1,038-bp product (24). The entire 3.7-kb CMV pol coding region was amplified by using flanking primers (forward, 5′-GTCAGCCTCTCACGGTCCGCTAT-3′; reverse, 5′-CTCAGTCTCAGCAGCATCATCAC-3′). PCR products generated with a GeneAMP XL PCR kit (Applied Biosystems) were sequenced without cloning, with primers internal to the amplified segment that produced overlapping coverage of the target sequences. Sequencing reactions were performed by dideoxy chain termination chemistry with an automated sequencer (ABI 377) and fluorescent dideoxy terminators (Applied Biosystems). Derived sequences of each isolate were aligned with the strain AD169 reference sequence with a sequence text editor. Amino acid differences were tabulated and compared with previously published pol resistance mutations (5, 17, 32).
Marker transfer.
The CMV pol gene mutations D588N and T838A which had not been previously fully characterized were individually transferred to a laboratory strain of CMV to determine the effect of these mutations on drug resistance. The chosen laboratory strain, T1472, was derived from Towne strain (ATCC VR977) and contains unique PmeI restriction sites within pol (3). A Bluescript-cloned DNA segment of Towne strain (nucleotides 74828 to 82550, based on the standard AD169 numbering scheme), modified with the same PmeI and other changes as in T1472, was further mutagenized by PCR to contain either mutation D588N or mutation T838A. Each plasmid containing D588N or T838A was linearized by restriction digestion (XbaI and EcoRI) and cotransfected into fibroblast cultures with PmeI-digested genomic T1472 DNA by the calcium phosphate coprecipitation method as previously described (6). Because of the targeted cleavage of the viral genomic DNA at the PmeI restriction sites, no drug selection was needed or used to recover infectious recombinant CMV containing the desired pol mutations from the transfected cultures. Extracellular virus from the transfected cultures was assessed by DNA sequencing to confirm the presence of the expected pol mutation and then plaque purified. The final viruses were resequenced in their entire pol coding sequences to exclude changes other than those intended and tested for drug susceptibility by PRA.
FFA.
The growth characteristics of the D588N and T838A mutants were compared with the parent T1472 strain by using the fluorescent focus assay (FFA) technique. Each viral strain was inoculated at a multiplicity of infection of 1 into duplicate HEL-containing tubes. Aliquots of the supernatants were inoculated daily into HEL-containing shell vials. Shell vials were centrifuged at 800 × g for 40 min and incubated at 37°C for 48 h. Coverslips were then fixed in cold acetone and stained with anti-pp72 mouse monoclonal antibody (Dupont) followed by an anti-mouse fluorescein isothiocyanate-conjugated antiserum (Bartels). The number of fluorescent foci (FF) was counted with a fluorescent microscope at ×40 magnification across ≥3 to 4 high-power fields (HPF) for each coverslip. The average number of FF per HPF was plotted for each time point to construct the viral growth curves. Due to limitations in counting accuracy, when the number of FF per HPF exceeded 100, an approximation was made.
RESULTS
Case histories.
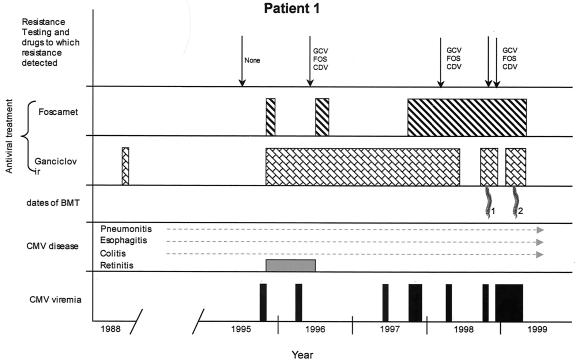
Patient 1 was diagnosed with severe congenital combined immunodeficiency-like syndrome at 8 months of age. Four months later, he received an HLA-matched allogeneic HCT from a sibling, which failed due to the lack of prior conditioning. He then developed severe disseminated CMV with pneumonitis, esophagitis, colitis, and possible cerebritis (Fig. 1). He received 3 mg of ganciclovir/kg intravenously (i.v.) three times daily (as per study protocol) but did not complete the treatment due to drug-associated neutropenia. He subsequently spent many years dealing with unrelenting CMV infection of the lungs and gastrointestinal tract, which resulted in chronic reactive airway disease and malabsorption. At 8 years of age, he developed CMV retinitis of both eyes. He was initially treated for 21 days with ganciclovir (6 mg/kg i.v twice daily) and foscarnet (90 mg/kg twice daily), followed by ganciclovir monotherapy (6 mg/kg i.v. daily). The foscarnet was continued for 6 weeks. CMV immunoglobulin (Ig) and i.v. gamma globulin were also given. Over the next few years, in spite of administration of ganciclovir suppressive therapy (5 mg/kg i.v. 3 to 5 times weekly), he was frequently admitted to hospital for flares of CMV disease (pneumonitis, colitis, and/or retinitis) for which he received higher doses of ganciclovir and/or foscarnet. Initial resistance testing in 1995 showed wild-type virus with no phenotypic resistance (Table 1). In 1996, a pol mutation (V812L) was detected in a blood isolate; although this mutation is known to confer low-level resistance to all three antivirals (8), the PRA did not detect reduced susceptibility. In early 1998, the UL97 mutation C592G was detected in a gastric isolate, and phenotypic testing of the same specimen showed partial or complete resistance to ganciclovir, foscarnet, and cidofovir. A new pol mutation, D588N, was detected after over a year of foscarnet therapy and years of ganciclovir therapy (November 1998). In November 1998, he underwent a nonmyeloablative HCT using his previous donor. CMV-activated CD4+ cells from the donor were also administered (30, 34), but attempts to grow donor CMV-specific CD8+ cells failed. The patient continued to have CMV DNA-emia and disease flares. Genotyping performed on a blood isolate approximately 2 months after transplantation revealed no change from the previous isolate. Due to lack of engraftment, he underwent a third HCT in March of 1999, preceded by a full myeloablative conditioning regimen. CMV-specific CD4+ T cells were again administered. At day 14 after this transplant, chimerism studies revealed only donor cells. The patient died on posttransplant day 23 of liver necrosis presumed due to Aspergillus fumigatus. Bronchoalveolar lavage cultures grew CMV and A. fumigatus, and CMV blood PCR remained positive at the time of death.
FIG. 1.
Clinical course for patient 1. Bars on the bottom row indicate positive blood tests for CMV viremia (culture, PCR, and/or antigenemia assay). Routine testing was not preformed prior to HCT 1. The patient had chronic progressive CMV disease of many organs throughout most of his life. For antiviral treatment, dosing is not displayed. GCV, ganciclovir; FOS, foscarnet; CDV, cidofovir.
TABLE 1.
CMV resistance studies of two hematopoietic cell transplant recipientsa
| Patient | Date (day after transplant)b | Source | UL97 resistance mutation(s) | UL54 resistance mutation(s) | UL54 polymorphisms | IC50 (μg/ml)
|
||
|---|---|---|---|---|---|---|---|---|
| GCV | FOS | CDV | ||||||
| 1 | 10/25/95 (before) | None | None | V526M, A647V, N685S, A688V, A885T, N898D, Y1047S | 2.8 | 115 | 1.3 | |
| 6/10/96 (before) | Blood | None | V812L | V526M, A647V, N685S, A688V, A885T, N898D | 4.0 | 100 | ND | |
| 3/27/98 (before) | Gastric biopsy | C592G | V812L | Same as 6/10/96 | 27.46 | 445 | 7.92 | |
| 11/6/98 (−4) | Blood | C592G | V812L, D588N | Same as 6/10/96 | 13.74 | 479 | 3.96 | |
| 12/28/98 (+48/−72) | Blood | C592G | V812L, D588N | Same as 6/10/96 | 27.5 | 577 | 7.92 | |
| 2 | 12/4/00 (+139) | Blood | M460V, C592G | None | S655L, N685S, A885T, N898D, T1121I, A1122T | ND | ND | ND |
| 9/17/01 (+426) | Respiratory | A594V | T838A | Same as 12/4/00 | ND | ND | ND | |
| 1/11/02 (+542) | Respiratory | A594V | T838A | Same as 12/4/00 | ND | ND | ND | |
| 5/12/02 (+664) | Respiratory | None | L802M | Same as 12/4/00 | 3.37 | 109.5 | 3.39 | |
GCV, ganciclovir; FOS, foscarnet; CDV, cidofovir; ND, not done.
Positive and negative (+ and −) numbers indicate days after the first transplant and days before the second transplant, respectively.
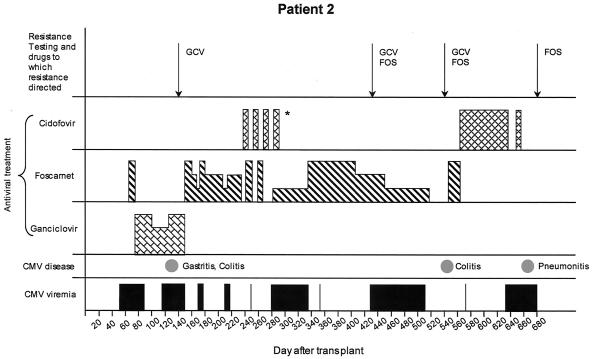
Patient 2 was an 18-year-old (year of birth, 1982) CMV-seropositive female with chronic myelogenous leukemia who underwent an unrelated HCT using a 5/6-HLA-class I-matched, HLA class II-identical cord blood unit. CMV saliva cultures from the donor baby were negative. She received 7 days of i.v. acyclovir (10 mg/kg i.v. three times daily) 6 weeks after transplantation for cutaneous herpes simplex virus infection. On day 56 after transplant (Fig. 2), she was admitted for CMV viral syndrome for which she received 4 days of foscarnet (90 mg/kg twice daily) treatment followed by ganciclovir (5 mg/kg twice daily) and CMV Ig treatment. The ganciclovir dose was reduced to once per day after 3 weeks as CMV PCR and antigenemia assays became negative. Viremia soon recurred with biopsy-proven CMV gastritis and colitis and failed to resolve after 4 weeks of full-dose ganciclovir and CMV Ig treatment. The first genotype (Table 1), performed on posttransplant day 139 on a blood sample, revealed UL97 mutations M460V and C592G, consistent with ganciclovir resistance (2, 32). Therapy was changed to foscarnet. Viremia recurred intermittently for several months, and her foscarnet dose was changed accordingly. She remained on immunosuppressants for chronic and acute graft-versus-host disease. Cidofovir was prescribed for gastrointestinal adenovirus infection, during which time foscarnet was administered on alternating weeks. She subsequently developed respiratory syncytial virus pneumonitis for which she received ribavirin (2 g inhaled three times daily for 7 days) and respiratory syncytial virus Ig (750 mg/kg i.v. daily for 2 days). Both infections resolved clinically and virologically.
FIG. 2.
Clinical course for patient 2. For CMV viremia, bars indicate positive blood tests (culture, PCR, and/or antigenemia assay). For CMV disease, spots indicate dates of diagnosis. For antiviral treatment, the height of the bar represents relative dosing (highest, induction; middle, maintenance; lowest, lower than maintenance). *Patient was treated with 5 mg of cidofovir every other week (for adenovirus infection), alternating with daily high-dose foscarnet during the other weeks. GCV, ganciclovir; FOS, foscarnet.
CMV recurred while on low-dose foscarnet (a dose lower than standard maintenance dosing), prompting genotyping of a respiratory isolate (day 426). Results revealed the A594V ganciclovir resistance mutation in UL97 and the novel T838A mutation in UL54. Cidofovir treatment was considered but was not restarted due to renal insufficiency. Viremia persisted, and she developed severe CMV colitis. Genotypic testing on day 542, again on a respiratory isolate, revealed no change from the previous isolate. On day 650 after transplant, she developed colonic pneumatosis and underwent ileostomy. She died 2 weeks later with respiratory failure and renal failure. Bronchoalveolar lavage specimen studies at the time of death were positive for both CMV and Pneumocystis carinii. CMV genotyping of this specimen revealed a new UL54 mutation, L802M, and disappearance of the previously detected resistance mutations. Phenotypic results are shown in Table 1.
Phenotypic characteristics of D588N- and T838A-containing viruses.
Among the foscarnet resistance mutations identified in these patients, V812L and L802M have been previously characterized (4, 8). T838A was identified for the first time in this study, and D588N was previously described but only partially characterized (28). Marker transfer experiments were done to create new recombinant viruses, T1911 and T1951, containing pol mutations D588N and T838A, respectively. PRA showed the following IC50 values (in μg/ml): for T1911 (D588N), 20.3 for ganciclovir, 461.8 for foscarnet, and 8.7 for cidofovir; for T1951 (T838A), 9.8 for ganciclovir, 347.9 for foscarnet, and 2.7 for cidofovir (Table 2). In the same runs, the T1472 wild-type CMV parent to the recombinant viruses showed IC50s of 5.4, 146.3, and 3.2 μg/ml, respectively. These data indicate that T838A confers resistance to foscarnet and that D588N confers resistance to ganciclovir, foscarnet, and cidofovir.
TABLE 2.
IC50 values for T1472 and mutation-containing derivativesa
| Strain | Mutation | IC50 (μg/ml) (SEM)
|
||
|---|---|---|---|---|
| Ganciclovir | Foscarnet | Cidofovir | ||
| T1472 | wt | 5.4 (1.1) | 146.3 (26.0) | 3.2 (0.7) |
| T1911 | D588N | 20.3 (1.8) | 461.8 (0.8) | 8.7 (1.3) |
| T1951 | T838A | 9.8 (NA) | 347.9 (NA) | 2.7 (NA) |
Data represent means (SEM). Values in boldface type represent ≥2-fold decreased sensitivity. Assays were repeated four times for T1472, two times for T1911, and one time for T1951. Triplicate wells were used for each antiviral dilution in all assays. SEM, standard error of the mean; wt, wild type; NA, not applicable.
Growth characteristics of D588N- and T838A-containing viruses.
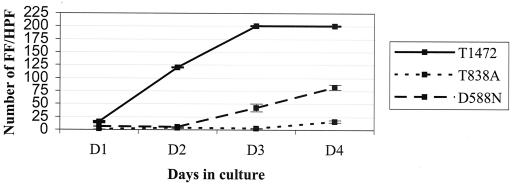
The effect of CMV pol mutations on viral fitness was assessed by comparing the growth characteristics of T1911 and T1951 mutants with the T1472 parent strain (Fig. 3). Growth curves showed that T838A decreased replication capacity by 1 order of magnitude compared to that of wild-type control virus. D588N also impaired growth, but to a lesser extent. These data demonstrate that viruses containing either the D588N or T838A mutation have reduced viral fitness in tissue culture.
FIG. 3.
Growth curves for the T1472 CMV strain and mutant viruses that use the T1472 backbone. Each virus was inoculated into duplicate tube tissue cultures at a multiplicity of infection of 1. Tissue culture supernatants were harvested at the indicated number of days of infection and inoculated into shell vials. FF were counted across four HPF. Means and standard errors of the means (SEM) of the number of FF/HPF are represented in the figure. This technique provides accurate counts up to 100 FF/HPF. Above this number, the number of FF/HPF was approximated to the nearest count, and SEM is not provided.
DISCUSSION
We describe two HCT recipients who died with persistent CMV infection complicated by resistance to antivirals. In our patients, resistance developed quickly (resistance was detected after only 6 weeks of foscarnet treatment in the first patient and after 11 weeks of ganciclovir treatment in the second). These findings are in accordance with those of other reports suggesting that resistance may develop more rapidly in severely immunocompromised children (37) than in HIV-infected patients, in whom resistance to ganciclovir was generally detected after ≥3 months of therapy (10, 20).
In this study, we characterized a new pol mutation, T838A, which is shown to confer resistance to foscarnet, by marker transfer. In addition, the D588N mutation, present in one of our patients, was also marker transferred and phenotypically characterized. In two independent experiments, D588N decreased ganciclovir susceptibility by 3.8-fold, foscarnet susceptibility by 3.2-fold, and cidofovir susceptibility by 2.7-fold. D588N was previously shown to confer foscarnet resistance only (28). Variations in the techniques and cell types used for marker transfer and viral phenotyping may account for the observed differences.
Our data contribute important information toward achieving full genotypic characterization of CMV antiviral resistance. Genotypic assays are advantageous in clinical practice because they provide rapid detection of resistance and can be more sensitive than phenotypic assays. As is illustrated here and in previous studies (35), PRA may fail to detect resistance associated with mutations conferring two- to fourfold decreased susceptibility and/or when the resistant strain virus represents less than 20% of the total viral population. In contrast, genotypic assays detect resistance mutations if mutations are present in ≥10% of the viral population. This underscores the importance of further improving genotypic resistance testing methods, particularly for transplant recipients—a group within which the incidence of CMV resistance has been increasing (19, 22, 23).
By marker transfer and FFA, we showed that viruses which contain either of the newly characterized D588N and T838A mutations exhibited reduced replication capacity. Previous studies have also suggested decreased fitness of CMV strains that contain drug-resistant mutations (1, 8, 13, 14, 18, 31). The lack of standardized growth phenotyping methodology precludes any direct comparison of the degree of growth attenuation of the mutants reported here with those previously reported. The extent to which mutations affect viral growth might be influenced by the location of the mutation and the experimental conditions. T838A and D588N, which are located in the highly conserved regions of the catalytic area of the CMV DNA polymerase, seemed to decrease replication capacity by 10- and 2-fold, respectively.
The evolving drug resistance genotypes seen in case 2 reflect the interplay of changing antiviral therapy selecting for particular mutations and the biological fitness of the viruses containing those mutations (Table 1 and Fig. 2). As ganciclovir was discontinued after the day +139 specimen was collected, there was no longer selection pressure for UL97 mutations. After the day +542 specimen was collected, foscarnet was discontinued and replaced with cidofovir, thus decreasing the selection for the T838A pol mutation. Both the A594V and T838A mutations were later replaced with a presumably better-growing virus that was wild type in UL97 and contained a different pol mutation. Evolving CMV resistance genotypes have been reported previously (4), and the data reported here indicate that it may be a fairly common occurrence after changing CMV antiviral therapy, necessitating repeated testing to detect changes in viral genotypes and resistance profiles.
The clinical settings in which combination anti-CMV therapy should be used remain to be established. The observation of attenuated growth in viruses with resistant genotypes (such as the T838A and D588N pol mutations seen here) may help to explain the reported superior clinical efficacy of combination therapy compared with single-agent anti-CMV therapy (21), even when one of the agents appears to have lost its activity against CMV (29). Maintaining therapy with the drug to which resistance has developed could be beneficial if the drug selects for growth-impaired genotypes. Novel antivirals with different mechanisms of action, when they become available, may provide another option for the treatment of resistant CMV. Interestingly, combination therapy did not show advantages over single-agent therapy in preventing CMV disease (26). Future studies should consider not only the resistant genotypes being selected but also their potential effect on viral fitness. This may require the development of a more standardized method of comparing the growth effects of different CMV resistance mutations.
Acknowledgments
S.C. receives support from the National Institutes of Health (grant AI39938) and the Department of Veterans Affairs research funds.
We thank Julia Clark for technical assistance.
REFERENCES
- 1.Baldanti, F., M. R. Underwood, S. C. Stanat, K. K. Biron, S. Chou, A. Sarasini, E. Silini, and G. Gerna. 1996. Single amino acid changes in the DNA polymerase confer foscarnet resistance and slow-growth phenotype, while mutations in the UL97-encoded phosphotransferase confer ganciclovir resistance in three double-resistant human cytomegalovirus strains recovered from patients with AIDS. J. Virol. 70:1390-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou, S., S. Guentzel, K. R. Michels, R. C. Miner, and W. L. Drew. 1995. Frequency of UL97 phosphotransferase mutations related to ganciclovir resistance in clinical cytomegalovirus isolates. J. Infect. Dis. 172:239-242. [DOI] [PubMed] [Google Scholar]
- 3.Chou, S., N. S. Lurain, K. D. Thompson, R. C. Miner, and W. L. Drew. 2003. Viral DNA polymerase mutations associated with drug resistance in human cytomegalovirus. J. Infect. Dis. 188:32-39. [DOI] [PubMed] [Google Scholar]
- 4.Chou, S., G. Marousek, S. Guentzel, S. E. Follansbee, M. E. Poscher, J. P. Lalezari, R. C. Miner, and W. L. Drew. 1997. Evolution of mutations conferring multidrug resistance during prophylaxis and therapy for cytomegalovirus disease. J. Infect. Dis. 176:786-789. [DOI] [PubMed] [Google Scholar]
- 5.Chou, S., G. Marousek, D. M. Parenti, S. M. Gordon, A. G. LaVoy, J. G. Ross, R. C. Miner, and W. L. Drew. 1998. Mutation in region III of the DNA polymerase gene conferring foscarnet resistance in cytomegalovirus isolates from 3 subjects receiving prolonged antiviral therapy. J. Infect. Dis. 178:526-530. [DOI] [PubMed] [Google Scholar]
- 6.Chou, S., R. H. Waldemer, A. E. Senters, K. S. Michels, G. W. Kemble, R. C. Miner, and W. L. Drew. 2002. Cytomegalovirus UL97 phosphotransferase mutations that affect susceptibility to ganciclovir. J. Infect. Dis. 185:162-169. [DOI] [PubMed] [Google Scholar]
- 7.Cihlar, T., M. D. Fuller, and J. M. Cherrington. 1998. Characterization of drug resistance-associated mutations in the human cytomegalovirus DNA polymerase gene by using recombinant mutant viruses generated from overlapping DNA fragments. J. Virol. 72:5927-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cihlar, T., M. D. Fuller, A. S. Mulato, and J. M. Cherrington. 1998. A point mutation in the human cytomegalovirus DNA polymerase gene selected in vitro by cidofovir confers a slow replication phenotype in cell culture. Virology 248:382-393. [DOI] [PubMed] [Google Scholar]
- 9.Deeks, S. G., T. Wrin, T. Liegler, R. Hoh, M. Hayden, J. D. Barbour, N. S. Hellmann, C. J. Petropoulos, J. M. McCune, M. K. Hellerstein, and R. M. Grant. 2001. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N. Engl. J. Med. 344:472-480. [DOI] [PubMed] [Google Scholar]
- 10.Drew, W. L., R. C. Miner, D. F. Busch, S. E. Follansbee, J. Gullett, S. G. Mehalko, S. M. Gordon, W. F. Owen, Jr., T. R. Matthews, W. C. Buhles, et al. 1991. Prevalence of resistance in patients receiving ganciclovir for serious cytomegalovirus infection. J Infect. Dis. 163:716-719. [DOI] [PubMed] [Google Scholar]
- 11.Eckle, T., P. Lang, L. Prix, G. Jahn, T. Klingebiel, R. Handgretinger, B. Selle, D. Niethammer, and K. Hamprecht. 2002. Rapid development of ganciclovir-resistant cytomegalovirus infection in children after allogeneic stem cell transplantation in the early phase of immune cell recovery. Bone Marrow Transplant. 30:433-439. [DOI] [PubMed] [Google Scholar]
- 12.Eckle, T., L. Prix, G. Jahn, T. Klingebiel, R. Handgretinger, B. Selle, and K. Hamprecht. 2000. Drug-resistant human cytomegalovirus infection in children after allogeneic stem cell transplantation may have different clinical outcomes. Blood 96:3286-3289. [PubMed] [Google Scholar]
- 13.Emery, V. C., A. V. Cope, E. F. Bowen, D. Gor, and P. D. Griffiths. 1999. The dynamics of human cytomegalovirus replication in vivo. J. Exp. Med. 190:177-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emery, V. C., and P. D. Griffiths. 2000. Prediction of cytomegalovirus load and resistance patterns after antiviral chemotherapy. Proc. Natl. Acad. Sci. USA 97:8039-8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erice, A. 1999. Resistance of human cytomegalovirus to antiviral drugs. Clin. Microbiol. Rev. 12:286-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erice, A., N. Borrell, W. Li, W. J. Miller, and H. H. Balfour, Jr. 1998. Ganciclovir susceptibilities and analysis of UL97 region in cytomegalovirus (CMV) isolates from bone marrow recipients with CMV disease after antiviral prophylaxis. J. Infect. Dis. 178:531-534. [DOI] [PubMed] [Google Scholar]
- 17.Erice, A., C. Gil-Roda, J. L. Perez, H. H. Balfour, Jr., K. J. Sannerud, M. N. Hanson, G. Boivin, and S. Chou. 1997. Antiviral susceptibilities and analysis of UL97 and DNA polymerase sequences of clinical cytomegalovirus isolates from immunocompromised patients. J. Infect. Dis. 175:1087-1092. [DOI] [PubMed] [Google Scholar]
- 18.Gerna, G., A. Sarasini, E. Percivalle, M. Zavattoni, F. Baldanti, and M. G. Revello. 1995. Rapid screening for resistance to ganciclovir and foscarnet of primary isolates of human cytomegalovirus from culture-positive blood samples. J. Clin. Microbiol. 33:738-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamprecht, K., T. Eckle, L. Prix, C. Faul, H. Einsele, and G. Jahn. 2003. Ganciclovir-resistant cytomegalovirus disease after allogeneic stem cell transplantation: pitfalls of phenotypic diagnosis by in vitro selection of an UL97 mutant strain. J. Infect. Dis. 187:139-143. [DOI] [PubMed] [Google Scholar]
- 20.Jabs, D. A., C. Enger, J. P. Dunn, M. Forman, et al. 1998. Cytomegalovirus retinitis and viral resistance: ganciclovir resistance. J. Infect. Dis. 177:770-773. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson, M. A. 1997. Treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 337:105-114. [DOI] [PubMed] [Google Scholar]
- 22.Julin, J. E., J. H. van Burik, W. Krivit, C. Webb, C. J. Holman, H. B. Clark, and H. H. Balfour, Jr. 2002. Ganciclovir-resistant cytomegalovirus encephalitis in a bone marrow transplant recipient. Transpl. Infect. Dis. 4:201-206. [DOI] [PubMed] [Google Scholar]
- 23.Limaye, A. P., L. Corey, D. M. Koelle, C. L. Davis, and M. Boeckh. 2000. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet 356:645-649. [DOI] [PubMed] [Google Scholar]
- 24.Lurain, N. S., A. Weinberg, C. S. Crumpacker, and S. Chou. 2001. Sequencing of cytomegalovirus UL97 gene for genotypic antiviral resistance testing. Antimicrob. Agents Chemother. 45:2775-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Picado, J., A. V. Savara, L. Sutton, and R. T. D'Aquila. 1999. Replicative fitness of protease inhibitor-resistant mutants of human immunodeficiency virus type 1. J. Virol. 73:3744-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattes, F. M., E. G. Hainsworth, A. M. Geretti, G. Nebbia, G. Prentice, M. Potter, A. K. Burroughs, P. Sweny, A. F. Hassan-Walker, S. Okwuadi, C. Sabin, G. Amooty, V. S. Brown, S. C. Grace, V. C. Emery, and P. D. Griffiths. 2004. A randomized, controlled trial comparing ganciclovir to ganciclovir plus foscarnet (each at half dose) for preemptive therapy of cytomegalovirus infection in transplant recipients. J. Infect. Dis. 189:1355-1361. [DOI] [PubMed] [Google Scholar]
- 27.Miller, V., T. Stark, A. E. Loeliger, and J. M. Lange. 2002. The impact of the M184V substitution in HIV-1 reverse transcriptase on treatment response. HIV Med. 3:135-145. [DOI] [PubMed] [Google Scholar]
- 28.Mousavi-Jazi, M., L. Schloss, W. L. Drew, A. Linde, R. C. Miner, J. Harmenberg, B. Wahren, and M. Brytting. 2001. Variations in the cytomegalovirus DNA polymerase and phosphotransferase genes in relation to foscarnet and ganciclovir sensitivity. J. Clin. Virol. 23:1-15. [DOI] [PubMed] [Google Scholar]
- 29.Mylonakis, E., W. M. Kallas, and J. A. Fishman. 2002. Combination antiviral therapy for ganciclovir-resistant cytomegalovirus infection in solid-organ transplant recipients. Clin. Infect. Dis. 34:1337-1341. [DOI] [PubMed] [Google Scholar]
- 30.Riddell, S. R., P. Reusser, and P. D. Greenberg. 1991. Cytotoxic T cells specific for cytomegalovirus: a potential therapy for immunocompromised patients. Rev. Infect. Dis. 13(Suppl. 11):S966-S973. [DOI] [PubMed] [Google Scholar]
- 31.Sarasini, A., F. Baldanti, M. Furione, E. Percivalle, R. Brerra, M. Barbi, and G. Gerna. 1995. Double resistance to ganciclovir and foscarnet of four human cytomegalovirus strains recovered from AIDS patients. J. Med. Virol. 47:237-244. [DOI] [PubMed] [Google Scholar]
- 32.Smith, I. L., J. M. Cherrington, R. E. Jiles, M. D. Fuller, W. R. Freeman, and S. A. Spector. 1997. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J. Infect. Dis. 176:69-77. [DOI] [PubMed] [Google Scholar]
- 33.Squifflet, J. P., and C. Legendre. 2002. The economic value of valacyclovir prophylaxis in transplantation. J. Infect. Dis. 186(Suppl. 1):S116-S122. [DOI] [PubMed] [Google Scholar]
- 34.Walter, E. A., P. D. Greenberg, M. J. Gilbert, R. J. Finch, K. S. Watanabe, E. D. Thomas, and S. R. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038-1044. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg, A., D. A. Jabs, S. Chou, B. K. Martin, N. S. Lurain, M. S. Forman, and C. Crumpacker. 2003. Mutations conferring foscarnet resistance in a cohort of patients with acquired immunodeficiency syndrome and cytomegalovirus retinitis. J. Infect. Dis. 187:777-784. [DOI] [PubMed] [Google Scholar]
- 36.Wolf, D. G., N. S. Lurain, T. Zuckerman, R. Hoffman, J. Satinger, A. Honigman, N. Saleh, E. S. Robert, J. M. Rowe, and Z. Kra-Oz. 2003. Emergence of late cytomegalovirus central nervous system disease in hematopoietic stem cell transplant recipients. Blood 101:463-465. [DOI] [PubMed] [Google Scholar]
- 37.Wolf, D. G., I. Yaniv, A. Honigman, I. Kassis, T. Schonfeld, and S. Ashkenazi. 1998. Early emergence of ganciclovir-resistant human cytomegalovirus strains in children with primary combined immunodeficiency. J. Infect. Dis. 178:535-538. [DOI] [PubMed] [Google Scholar]